Abstract
Objectives:
Diabetes management imposes considerable demands on patients. Treatment method used has an impact on treatment satisfaction. We aim to examine the relationship between treatment satisfaction and health perception with the method used for treatment of type 1 diabetes mellitus in children and adolescents.
Subjects and method:
We have interviewed patients with type 1 diabetes mellitus using questionnaires to assess treatment satisfaction and health perception. Patients were divided into three groups based on treatment used: multiple daily injection, insulin pump and sensor-augmented pump therapy. Comparison of scores was done between the groups.
Results:
A total of 72 patients were enrolled (36 males). Mean age (standard deviation) was 11.4 (4.4) years and duration of diabetes of 4.9 (3.5) years. Mean (standard deviation) HbA1c was 8.1 (1.2). Median (range) duration of sensor use was 17.7 (3–30) days/month. Mean scale for treatment satisfaction and health perception questions was 25.3, 29.7 and 31.7 and 60, 79.7 and 81 for the multiple daily injection, pump and sensor-augmented pump, respectively (p = 0.00). Significant difference was seen between the multiple daily injection and both other groups. Sensor-augmented pump group scored higher than the pump group. However, the difference was not statistically significant. Duration of sensor use showed no correlation with treatment satisfaction.
Conclusion:
The method used for diabetes treatment has an impact on patients’ satisfaction and health perception in children and adolescents with type 1 diabetes mellitus. Insulin pump users have a higher treatment satisfaction and better health perception than those on multiple daily injection. Augmenting pump therapy with sensor use adds value to treatment satisfaction without correlation with the duration of the sensors use.
Keywords: Satisfaction, health, type 1 diabetes, children, insulin, pump
Introduction
Diabetes management imposes considerable demands on patients including dietary control, adherence to insulin regime and close glucose monitoring. These daily tasks are difficult to comply with particularly in children and adolescents who are going through major physical, cognitive and psychological changes and have a risk-taking behavior.1 There is considerable evidence that children and adolescents with type 1 diabetes mellitus (T1DM) experience poorer health-related quality of life than their healthy peers.2 It is shown that increased flexibility in daily life is one of the most important benefits for improving quality of life for children with T1DM and their parents.3 Quality of life for young people with T1DM centers on two major domains: treatment satisfaction and health perception. These two domains are directly related to various issues in diabetes management including lifestyle flexibility, anxiety over hypoglycemia and hyperglycemia, feasibility of correcting abnormal glucose readings and covering extra meals and snacks with insulin.4 The observation of improved treatment satisfaction can be seen with or without improved quality of life and the overall treatment satisfaction in diabetes management appears to be related to the method used for treatment.5,6
In the recent decades, success of particular diabetes treatment method has been linked not only to diabetes metabolic outcome but also to its impact on patient’s satisfaction and quality of life. Various instruments have been used to measure treatment satisfaction. Quality of life is often measured by Bradley Diabetes Treatment Satisfaction Questionnaire (BDTSQ).7 Other customized questionnaires have also been used.
Health perception is defined as perception of physical, mental and social well-being. It can be assessed by rating of personal health status. Good health perception is shown to be strongly related to better metabolic control in children and adolescents with T1DM.8
Diabetes management requires a great patient engagement and participation. Accordingly, more comprehensive understanding and appreciation of children’s and parents’ treatment satisfaction are required. Better understanding of patients’ needs and attitude are critical for both the medical and psychological care of T1DM. We aim to examine the relationship between treatment satisfaction and health perception with the method used for treatment of T1DM in children and adolescents.
Patients and methods
A questionnaire was designed to assess treatment satisfaction and health perception with three different methods of insulin treatment: multiple daily injection (MDI), insulin pump therapy and sensor-augmented pump therapy. The questionnaire consisted of two parts. The first part contained eight items related to treatment satisfaction over various aspects of diabetes impact and management and the second part showing a scale for assessing health perception.
The eight items assessing treatment satisfaction analyzed four subscales: perceived general management (Q1–3), feeling toward hypo and hyperglycemia (Q4–5), perceived frequency of use of the treatment method to correct high blood glucose or give extra insulin for snacks (Q6–7) and perceived compatibility of the treatment method with the lifestyle related to dietary habits (Q8). The response to each question was assessed by a scale ranging from 1 to 5. The higher the score, the higher the satisfaction. Each scale is indicated in three forms. First is a drawn symbol explained by text (two thumbs up for strongly agree, one thumb up for agree, a balance for neutral, one thumb down for disagree and two thumbs down for strongly disagree). The scale is also indicated arithmetically by numbers from 1 to 5 with 1 is the lowest and 5 the highest (Table 1).
Table 1.
Questionnaire used to assess treatment satisfaction.
| Statement |
Strongly agree |
Agree |
Neutral |
Disagree |
Strongly disagree |
|
|---|---|---|---|---|---|---|
| Symbols Numerical scale |
 5 5 |
 4 4 |
 3 3 |
 2 2 |
 1 1 |
|
| 1 | I feel generally good with my diabetes treatment | |||||
| 2 | My treatment modality is easy and convenient | |||||
| 3 | I feel confident with dealing with my diabetes | |||||
| 4 | I am not worried about hypoglycemia | |||||
| 5 | I am not worried about hyperglycemia | |||||
| 6 | I use correction doses when my blood sugar is high | |||||
| 7 | I inject/bolus with snacks | |||||
| 8 | My treatment does not restrict my dietary habits |
Each symbol corresponds to the arithmetic scale indicated with the number underneath.
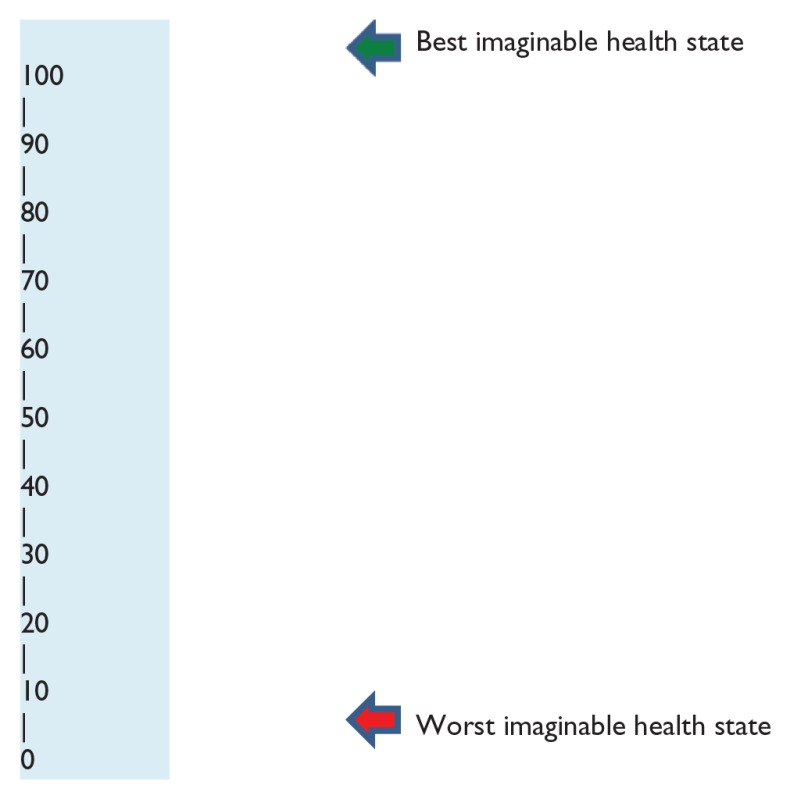
Health perception was assessed by a scale of 0–100 with 0 indicating worst imaginable health and 100 as an indicator of best health possible. The higher the scale chosen, the higher the subjective health perception (Table 2).
Table 2.
Subjective Health Perception Scale.
| Analog scale | |
|---|---|
| |
Data were collected using the customized questionnaire during the patients’ follow-up visit at the outpatient diabetes clinic in the endocrine department. Approval from the Ethics and Research Committee at Mafraq hospital was obtained to run the study.
Patients were approached by the study team and are given information about the study. Those who consented were enrolled in the study.
The questionnaire format and symbol meanings were explained to the patients by the study coordinator (T.H.). Answers to the questionnaire were obtained through interviews, which were conducted in a private room at the department with each lasting about 30–40 min. Data about age and duration of diabetes were obtained. Duration of sensor use in the sensor-augmented pump group in days per month was noted from the latest insulin pump download. Capillary HbA1c was done on the day of the visit if there was no record of HbA1c within the previous 4 weeks.
Statistical methods
Statistical data analysis was performed using SPSS, version 22. One-way analysis of variance (ANOVA) with post hoc Bonferroni-corrected pairwise comparison tests was used to compare means between groups. Non-parametric Spearman’s correlation coefficients were used to examine the associations between selected variables. A two-tailed statistical significance level of 0.05 was chosen for all analyses.
Power calculation
Sample size is calculated based on the requirement of detecting a difference between any two groups of one standard deviation (SD) with a power of 80% and a significance level of 5%. After Bonferroni correction, this implies a significance level of 1.67% for comparison of any two groups. Using the program openepi.com, a sample size of at least 42 for comparison of any two groups is required provided that the proportions are approximately 50:50 of the total sample size.
Results
In all, 72 patients were enrolled in the study. Male-to-female ratio was 1:1. Mean age (SD) for all participants was 11.4 (4.4) years with a mean (SD) duration of diabetes of 4.9 (3.5) years. Mean (SD) HbA1c was 8.1 (1.2). Breakdown of age, gender, duration of diabetes and HbA1c for each study group is illustrated in Table 3. Median (range) duration of sensor use in the sensor-augmented therapy group was 17.7 (3–30) days/month. Assessment of treatment satisfaction based on analysis of the questionnaire questions is detailed below and summarized in Table 4.
Table 3.
Demographic description for the study group.
| Total (72) | MDI (23) | Pump (30) | Pump/sensor (19) | p Value |
|---|---|---|---|---|
| Gender (36 M) | 12 (M) | 13 (M) | 11 (M) | 0.59 |
| Age (years) | 10.5 (4.6) | 12.3 (4) | 11.2 (4.8) | 0.34 |
| Duration | 3.73 (3) | 4.8 (2.8) | 6.5 (4.7) | 0.043 |
| HbA1c | 8.5 (1.3) | 8.0 (1.2) | 7.9 (1.2) | 0.007 |
MDI: multiple daily injection; M: male; SD: standard deviation.
Data are expressed as mean and SD.
Table 4.
Treatment satisfaction and health perception analysis: overall mean and mean scale by individual study question for the three study groups.
| MDI (no. 23) | Pump (no. 30) | Pump/sensor (no. 19) | p Value | |
|---|---|---|---|---|
| Treatment satisfaction | ||||
| Total mean | 25.3 | 29.7 | 31.7 | 0.00 |
| Mean scale/question | ||||
| Q1 | 3.56 | 4.26 | 4.4 | 0.003 |
| Q2 | 3.91 | 4.68 | 4.76 | .000 |
| Q3 | 2.63 | 2.69 | 3.36 | 0.49 |
| Q4 | 2.52 | 2.57 | 3.20 | 0.097 |
| Q5 | 3.95 | 4.31 | 4.56 | 0.07 |
| Q6 | 2.5 | 4.1 | 4.15 | 0.00 |
| Q7 | 2.87 | 3.00 | 3.10 | 0.82 |
| Q8 | 3.39 | 4.15 | 4.16 | 0.012 |
| Mean Health Perception Scale | ||||
| MDI | Pump | Pump/sensor | p Value | |
| 60 | 79.7 | 81 | 0.00 | |
MDI: multiple daily injection.
Overall mean of scale for treatment satisfaction questions was 25.3, 29.7 and 31.7 for the MDI, pump and sensor-augmented pump, respectively. The difference was statistically significant with a p value of 0.00. On post hoc Bonferroni analysis, the difference shown was statistically significant between the MDI and both the pump and the pump and sensor (p = 0.004 and 0.00, respectively). However, the difference was not significant between the pump and the sensor-augmented pump groups (p = 0.36).
Q1. I feel generally good with my diabetes treatment
Mean scale for this question was 3.56, 4.26 and 4.4 for the MDI, pump and sensor-augmented pump groups, respectively. ANOVA testing showed a statistically significant difference in the answers between the study groups (p = 0.003).
Q2. My treatment modality is easy and convenient
There was a statistically significant difference in the mean scale answer for this question in the three study groups with the MDI group showing the lowest mean scale p = 0.000.
Q3. I feel confident with dealing with my diabetes
The overall feeling on the confidence of diabetes management was assessed in this question, which showed a higher confidence in the pump and the sensor-augmented pump groups compared to the MDI. The difference was statistically significant with a p value of 0.012.
Q4. I am not worried about hypoglycemia
In responding to the question related to concerns about hypoglycemia, the sensor-augmented pump group scored the highest with a statistically significant difference (p = 0.049).
Q5. I am not worried about hyperglycemia
Similar to Q3, the sensor-augmented pump therapy group scored higher in response to this question. However, there was no statistical significance in the difference between groups.
Q6. I use correction doses when my blood sugar is high
The sensor-augmented group used more correction boluses for high glucose reflected by the higher mean scale for this question. The difference between the three groups was not statistically significant.
Q7. I inject/bolus with snacks
The MDI group scored markedly lower in answering this question. Both the pump and the sensor-augmented pump had a higher mean scale with a p value of 0.000.
Q8. My treatment does not restrict my dietary habits
A lower mean scale was obtained from the MDI group in answering this question. The difference in mean scale was not statistically significant in the three groups.
Health perception
Overall mean of scale for health scale was 60, 79.7 and 81 for the MDI, pump and sensor-augmented pump, respectively. The difference was statistically significant with a p value of 0.00. On post hoc Bonferroni analysis, the difference shown was statistically significant between the MDI and both the pump and the pump and sensor (p = 0.00). However, the difference was not significant between the pump and the sensor-augmented pump groups (p = 1.0).
Treatment satisfaction and use of continuous glucose monitoring system
A total of 19 patients used continuous glucose monitoring system (CGMS). This group scored highest among the three studied groups in majority of the treatment satisfaction questions as well as the health perception. In this group, the duration of use of sensors ranged between 3 and 10 days per month with a median of 17.7 days. There was no correlation between the treatment satisfaction score and the health perception with the duration of CGMS use. p value was 0.25 and 0.48 for both variables.
Discussion
Treatment satisfaction plays an important role in the success of treatment used. In a randomized crossover open trial in children with T1DM, patients expressed a higher treatment satisfaction from pump therapy than MDI, although there was no difference in quality of life between the two different methods of treatment.5 Several other studies comparing pump therapy and MDI have demonstrated increased quality of life after the transition to pump therapy.6 Furthermore, Barnard et al.9 showed that switching from MDI or older version of pump therapy to more advanced later generation form of pumps is associated with increased treatment satisfaction. Cherubini et al.10 suggested that pump therapy might be useful for patients perceiving a poor health-related quality of considering the favorable impact reported by pump users.
Data from studies of children with recent diagnosis of diabetes showed that they are more satisfied with pump treatment than with MDI.5 We found a statistically significant difference in the overall mean score for treatment satisfaction in our three study groups. Mean score increased from 25.3 in the MDI group to 29.7 and 31.7 in the pump and the sensor-augmented pump groups (p = 0.00).
Self-efficacy and confidence in ability to manage diabetes is an important factor for treatment satisfaction. It is shown that patients on insulin pumps have higher diabetes self-efficacy compared to those on MDI treatment.11 Insulin pump therapy improves diabetes self-efficacy and engagement in children, thereby improving diabetes self-management.3 In our cohort, we saw a higher mean score in pump and sensor-augmented pump users in areas related to the general feelings about method of treatment, ease of use of treatment method and confidence in dealing with diabetes (Questions 1, 2 and 3, Table 4).
The added flexibility in pump function is proven to be an important benefit of insulin pump therapy.12 With the pump, patients have the benefit of flexibility to make various adjustments to suit exercise, various types and timings of food, variable dose and different basal rates and patterns required.4 Insulin pumps offer greater ease with meal coverage and hyperglycemia correction. This is particularly the case outside home (personal observation). We found that more patients on pumps are satisfied with their treatment in this regard, as they have higher mean score for satisfaction related to feasibility of use of correction boluses or bolusing for snacks (Questions 6 and 7 in Table 4).
Other possible reasons for superiority of treatment satisfaction of pump therapy over MDI are decreased sense of physical and dietary restrictions.4 We found a statistically significant difference between the study groups in relation to the impact of method of treatment used on diet restriction with those on pump scoring higher in Question 8 (Table 4).
Garmo et al.13 showed significant improvement in treatment satisfaction and perceived frequency of symptomatic hypoglycemia and hyperglycemic events after changing from MDI to Continous Subcutaneous Insulin Infusion (CSII). In a group of 47 young people with T1DM, treatment satisfaction has increased when switching to insulin pumps from MDI. This was clearly associated with reduction in the rate of hypoglycemia and hyperglycemia.14 Our pump users showed significant satisfaction difference with their treatment method compared to MDI users in relation to hypoglycemia and hyperglycemia (Questions 4 and 5, Table 4).
Improved treatment satisfaction with pump therapy might be due to improved metabolic control on this treatment modality. Several meta-analyses suggest that insulin pump therapy and Continuous Glucmose Monitoring (CGM) improve glycemic control.15 Furthermore, it is shown that health-related quality of life and health perception are higher among patients with better glycemic control.16 However, in a randomized controlled trial in newly diagnosed children with T1DM, health satisfaction is shown to be higher in the pump group compared to the MDI group despite that there was no difference in metabolic control.17 Our study group on pump treatment has a lower HbA1c compared to the MDI group and their higher treatment satisfaction might be, at least in part, attributed to the better metabolic control.
Integrating diabetes technology, such as insulin pump therapy and CGM, into a child’s treatment plan may help children improve their treatment satisfaction and glycemic control while preventing severe hypoglycemia and diabetic ketoacidosis (DKA).3 Children and parents find that CGMS helps improving treatment satisfaction and enhance ease of diabetes care.18 In addition, children and parents favor CGMS mainly for hypoglycemia prevention and improvement of diabetes control.18 A large multicentric randomized controlled study by the Juvenile Diabetes Research Foundation (JDRF) which compared real-time CGM versus conventional treatment group showed high treatment satisfaction in users.19 In our cohort, users of sensor-augmented pump therapy scored highest in expressing ease of use of the treatment and reassurance related to hypoglycemia prevention (Questions 2 and 4, Table 4).
The DirecNet study found that higher self-management scores are associated with more frequent CGM use.20 In the JDRF study, CGM satisfaction was higher in patients who used CGM 6 or more days per week compared to those who wore it less.19 Our group had 19 patients on sensor-augmented therapy. Their duration of using the sensor ranged from 3 to 30 days per month. We did not find correlation between the duration of CGMS use and treatment satisfaction. Many patients and parents acknowledge the benefit of CGMS; however, disadvantages of the CGMS use are also highlighted by patients with cost and lack of insurance cover being major issues.21 In our center, false alarms and skin reaction are the major disadvantages in the CGMS use (unpublished data). Accordingly, use of CGMS might be improving diabetes care and prevention of acute complication but might not necessarily improve the treatment satisfaction.
Limitations of our study include the relatively low number of patients particularly in the sensor-augmented pump patients. In addition, it would be useful to compare the health perception scores in a group of healthy controls.
Conclusion
We conclude that the method used for diabetes treatment has an impact on patients’ satisfaction and health perception in children and adolescents with T1DM. Insulin pump users have a higher treatment satisfaction and better health perception than those on MDI. Significant difference was seen particularly toward diabetes management ability, ease of use of treatment method, ease of hyperglycemia correction and diet flexibility between treatment groups. Augmenting pump therapy with CGM adds value to treatment satisfaction in the group studied without correlation with the duration of the sensors use.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Mafraq Hospital Research & Ethics Committee (MAF-REC-08/2014_03).
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from parents of all subjects in addition to subjects’ assents before the study.
References
- 1. Scaramuzza A, De Palma A, Mameli C, et al. Adolescents with type 1 diabetes and risky behaviour. Acta Paediatr 2010; 99: 1237–1241. [DOI] [PubMed] [Google Scholar]
- 2. Grootenhuis MA, Koopman HM, Verrips EGH, et al. Health-related quality of life problems of children aged 8–11 years with a chronic disease. Dev Neurorehabil 2007; 10: 27–33. [DOI] [PubMed] [Google Scholar]
- 3. Hirose M, Beverly EA, Weinger K. Quality of life and technology: impact on children and families with diabetes. Curr Diab Rep 2012; 12(6): 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med 2012; 366: 1616–1624. [DOI] [PubMed] [Google Scholar]
- 5. Weintrob N, Benz Aquen H, Galatzer A, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics 2003; 112: 559–564. [DOI] [PubMed] [Google Scholar]
- 6. Maiorino MI, Bellastella G, Petrizzo M, et al. Treatment satisfaction and glycemic control in young type 1 diabetic patients in transition from pediatric health care: CSII versus MDI. Endocrine 2014; 46(2): 256–262. [DOI] [PubMed] [Google Scholar]
- 7. Bradley C, Lewis KS, Knight G. A measure of treatment satisfaction designed specially for people with insulin-dependent diabetes. Diabetic Med 1988; 5(3): 235–242. [DOI] [PubMed] [Google Scholar]
- 8. Keller M, Attia R, Beltrand J, et al. Insulin regimens, diabetes knowledge, quality of life, and HbA1c in children and adolescents with type 1 diabetes. Pediatr Diabetes. Epub ahead of print 10 May 2016. DOI: 10.1111/pedi.12397. [DOI] [PubMed] [Google Scholar]
- 9. Barnard KD, Bromba M, de Lange M, et al. High reported treatment satisfaction in people with type 1 diabetes switching to latest generation insulin pump regardless of previous therapy. J Diabetes Sci Technol 2015; 9(2): 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cherubini V, Gesuita R, Bonfanti R, et al. Health-related quality of life and treatment preferences in adolescents with type 1 diabetes. The VIPKIDS study. Acta Diabetol 2014; 51(1): 43–51. [DOI] [PubMed] [Google Scholar]
- 11. Battaglia MR, Alemzadeh R, Katte H, et al. Brief report: disordered eating and psychosocial factors in adolescent females with type 1 diabetes mellitus. J Pediatr Psychol 2006; 31: 552–556. [DOI] [PubMed] [Google Scholar]
- 12. Low KG, Massa L, Lehman D, et al. Insulin pump use in young adolescents with type 1 diabetes: a descriptive study. Pediatr Diabetes 2005; 6: 22–31. [DOI] [PubMed] [Google Scholar]
- 13. Garmo A, Pettersson-Frank B, Ehrenberg A. Treatment effects and satisfaction in diabetic patients changing from multiple daily insulin injections to CSII. Practical Diabetes Int 2004; 21(1): 7–12. [Google Scholar]
- 14. Al Hayek AA, Asirvatham AR, Al Darwish M. Efficacy of insulin pump therapy on diabetes treatment satisfaction and glycemic control among patients with type 1 diabetes mellitus in Saudi Arabia: a prospective study. Diabetes Ther 2015; 6: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monami M, Lamanna C, Marchionni N, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol 2010; 47: 77–81. [DOI] [PubMed] [Google Scholar]
- 16. Huang G, Palta M, Allen C, et al. Self-rated health among young people with type 1 diabetes in relation to risk factors in a longitudinal study. Am J Epidemiol 2004; 159: 364–372. [DOI] [PubMed] [Google Scholar]
- 17. Skogsberg L, Fors H, Hanas R, et al. Improved treatment satisfaction but no difference in metabolic control when using continuous subcutaneous insulin infusion vs. multiple daily injections in children at onset of type 1 diabetes mellitus. Pediatr Diabetes 2008; 9: 472–479. [DOI] [PubMed] [Google Scholar]
- 18. Cemeroglu AP, Stone R, Kleis L, et al. Use of a real-time continuous glucose monitoring system in children and young adults on insulin pump therapy: patients’ and caregivers’ perception of benefit. Pediatr Diabetes 2010; 11: 182–187. [DOI] [PubMed] [Google Scholar]
- 19. Beck RW, Lawrence JM, Laffel L, et al. Quality-of-life measures in children and adults with type 1 diabetes: Juvenile Diabetes Research Foundation Continuous Glucose Monitoring randomized trial. Diabetes Care 2010; 33: 2175–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diabetes Research in Children Network (DirecNet) Study Group. Psychological aspects of continuous glucose monitoring in pediatric type 1 diabetes. Pediatr Diabetes 2006; 7: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patton SR, Clements MA. Continuous glucose monitoring versus self-monitoring of blood glucose in children with type 1 diabetes—are there pros and cons for both? Endocrinol 2012; 8(1): 27–29. [PMC free article] [PubMed] [Google Scholar]


