Abstract
The formation of the mandibular condylar cartilage (MCC) and its subchondral bone is an important but understudied topic in dental research. The current concept regarding endochondral bone formation postulates that most hypertrophic chondrocytes undergo programmed cell death prior to bone formation. Under this paradigm, the MCC and its underlying bone are thought to result from 2 closely linked but separate processes: chondrogenesis and osteogenesis. However, recent investigations using cell lineage tracing techniques have demonstrated that many, perhaps the majority, of bone cells are derived via direct transformation from chondrocytes. In this review, the authors will briefly discuss the history of this idea and describe recent studies that clearly demonstrate that the direct transformation of chondrocytes into bone cells is common in both long bone and mandibular condyle development and during bone fracture repair. The authors will also provide new evidence of a distinct difference in ossification orientation in the condylar ramus (1 ossification center) versus long bone ossification formation (2 ossification centers). Based on our recent findings and those of other laboratories, we propose a new model that contrasts the mode of bone formation in much of the mandibular ramus (chondrocyte-derived) with intramembranous bone formation of the mandibular body (non-chondrocyte-derived).
Keywords: cartilage, osteocyte, osteoblast, temporomandibular joint, cell transdifferentiation, osteogenesis
Endochondral Bone Formation: The Current Concept
It Has Been Widely Accepted that Endochondral Bone Formation Requires the Cell Death of Hypertrophic Chondrocytes Prior To the Invasion of Bone Marrow-Derived Mesenchymal Cells
Endochondral ossification, in which a cartilage matrix is replaced by bone, is a fundamental process of hard tissue biology. It is the process by which many bones are formed, as well as the primary way that long bones increase in length. The cartilage and the underlying bone are thought to be linked through the deepest layers of the hypertrophic chondrocytes, which are surrounded by a mineralized matrix. Based on decades of histomorphological and immunohistochemical observations, the process of endochondral ossification is generally accepted to be initiated through the programmed cell death (apoptosis) of hypertrophic chondrocytes. This is followed by the resorption of the transverse mineralized matrix by MMP-13 and MMP-9, and then by the invasion of angiogenic and osteoblastic cells from the underlying bone marrow (Kronenberg 2003; Mackie et al. 2011). In this paradigm, chondrogenesis serves chiefly as a means for the production of hypertrophic chondrocytes, which, in turn, initiate subchondral bone formation (carried out by other cells); this ultimately renders chondrogenesis and osteogenesis as largely separate processes. In fact, it has been suggested that chondrocyte apoptosis actively triggers or catalyzes the cellular events that result in bone formation (Gibson 1998). However, although apoptosis is repeatedly demonstrated in the deepest hypertrophic chondrocyte layer (Aizawa et al. 1997; Farnum and Wilsman 1987), the extent of this phenomenon has been a matter of debate.
Endochondral Bone Formation: An Alternative Paradigm
The Possibility that Chondrocytes Can Transform Directly into Osteoblasts Has Been Suggested by Morphological and Immunohistochemical Studies
For decades, there have been reports contending that at least some hypertrophic chondrocytes can transform directly into bone cells, rather than undergoing programmed cell death, as assumed in the conventional paradigm. Although the concept that chondrocytes can be transformed into osteoblasts in organ culture dates back at least to the 1930s (references in Kahn and Simmons 1977), a number of morphological studies in the late 1980s and 1990s only hinted at the possibility of this transformation. Using electron microscopic images of the rat mandibular condylar cartilage (MCC), Yoshioka and Yagi (1988) demonstrated that some of the hypertrophic chondrocytes in the deepest tissue layer appeared to have been released from their lacunae into the primary spongiosa. Moreover, a number of other studies showed that hypertrophic chondrocytes secreted substances such as alkaline phosphatase, osteocalcin, osteopontin, and bone sialoprotein, which were all thought to be restricted to osteoblasts (Cancedda et al. 1992; Gerstenfeld and Shapiro 1996; Roach 1992; Roach et al. 1995). More recently, inhibiting vascular invasion through the placement of a membrane filter in a rabbit growth plate will induce the proliferation in hypertrophic chondrocytes alongside the expression of osteocalcin, osteonectin, and collagen 1, resulting in a tissue that resembles chondroid bone (Enishi et al. 2014). However, the foregoing studies were all morphological in scope, and potentially only a “snapshot” of a single moment in tissue development, with there being no possibility of following individual cells over time. In an effort to circumvent these limitations and confirm cell lineage, Kahn and Simmons (Kahn and Simmons 1977) performed interspecies grafting of quail bone and limb rudiments onto chick chorioallantoic membrane (CAM), and found that the bone formed was clearly derived from the quail graft, providing evidence for the transformation of chondrocytes into bone-secreting cells, in some instances, while still in their original lacunae. While intriguing, these findings are not regarded as sufficiently rigorous or conclusive to challenge the current concepts surrounding the process of endochondral ossification.
Cell Lineage Studies Demonstrate Conclusively that Chondrocytes Transform Directly into Bone Matrix-Secreting Cells in the Long Bone Growth Plate
The Cre-ERt2 loxP system is an extremely powerful genetic cell lineage tracing technique used to study the fate of chondrocytes, and has been used by several laboratories to confirm the cellular transformation of chondrocytes into bone cells during long bone development and growth (Ono et al. 2014; Yang et al. 2014a, b; Zhou et al. 2014). This system requires 2 transgenic lines: the Cre and Rosa26-fluorescent protein lines. In the Cre-ERt2 line, the fusion of the recombinase (Cre) and the tamoxifen-responsive estrogen receptor (ERt2) is driven by a tissue-specific promoter and expressed in the cytoplasm of cells. Injections with tamoxifen (which bind to the Cre-ERt2) will translocate the Cre recombinase to the nucleus to initiate recombination. In the Rosa26 line, this ubiquitous promoter drives a super-stop sequence—flanked by loxP sites—that lies upstream of a fluorescent protein sequence, such as tomato. Crossing these lines along with tamoxifen induction will permanently and fluorescently label the cells in which the Cre was initially active, as well as in their daughter cells.
Most of the relevant studies have previously generated mice expressing Cre-ERt2 controlled by the endogenous Col10a1 gene, thereby targeting hypertrophic chondrocytes. In each case, the investigators found that hypertrophic chondrocytes linked to a fluorescent reporter came to resemble Col10a1-expressing osteoblasts and were visible on bony trabeculae, in the endosteum, and eventually in the primary spongiosa expressing. This transformation was seen in the developing long bones, as well as in the postnatal growth plates in adult mice, suggesting that the direct transformation of hypertrophic chondrocytes to bone cells is a relatively widespread phenomenon. Importantly, it was found (Jing et al. 2015) that, although some hypertrophic chondrocytes undergo apoptosis, they collectively expressed high levels of the anti-apoptotic protein, BCL2. In addition, alkaline phosphatase immunoreactivity was strong in hypertrophic chondrocytes, and the BrdU data showed that some hypertrophic chondrocytes undergo cell division. Thus, hypertrophic chondrocytes resemble metabolically active cells that secrete a marker for bone cells, rather than simply being inert, metabolically inactive cells waiting to undergo apoptosis. Interestingly, Zhou et al. (2014) remarked that chondrocyte-derived bone cells were never observed in intramembranous bone, where bone cells differentiate directly from mesenchymal precursors with no cartilage intermediate.
Hypertrophic Chondrocytes Also Transform into Bone Cells in Fracture Callus and Cartilage Grafts
If this transformation is indeed a universal property of hypertrophic chondrocytes, it would be expected to occur in other cartilages, such as in those that form secondarily or by chance. One example of this type of cartilage is that which forms after a bone fracture. Since bone formation in the fracture callus takes place in a fashion similar to endochondral bone formation, some osteoblasts appearing during fracture healing should derive from hypertrophic chondrocytes. Indeed Zhou et al. (2014) found that many of the cells in the ossifying fracture callus, which were not contiguous with the growth plate chondrocytes, showed the fluorescent marker, indicating their derivation from Col10a1-secreting hypertrophic chondrocytes. Similar transformational events have been observed when using hypertrophic chondrocyte grafts to heal bone defects, which typically have problems with producing well-integrated, vascularized bone. Cell lineage techniques have shown that cells derived from hypertrophic chondrocyte grafts placed within bone injury sites in the tibia expressed Col1a1 and Osx, indicating their transformation to osteogenic cells (Yang et al. 2014b). Similar experiments employing cartilage grafts to heal tibial defects (Bahney et al. 2014) or mandibular fractures (Wong et al. 2016) produced well-vascularized and integrated bone tissue, confirmed by lineage tracing to be graft-derived. The results from both of these bone defect models and the fracture repair model affirm that the transformation of hypertrophic chondrocytes directly into bone cells occurs in contexts other than normal growth and development.
As in Long Bones, Hypertrophic Chondrocytes in the Mandibular Condyle Undergo Direct Transformation into Bone Cells
The MCC is a site of growth and articulation, combining the growth capability of the limb growth plate with the articular function of articular cartilage. However, the MCC has a very different developmental origin from that of limb cartilage and, accordingly, a different structure and growth pattern (Petrovic et al. 1975; Silbermann et al. 1987). The MCC is considered a “secondary” cartilage, because it develops in proximity to the intramembranous bone of the mandibular ramus much later than “primary” cartilages of the limbs and cranial base. This developmental difference is reflected in its superficial layer, which comprises a perichondrium with undifferentiated (prechondroblastic) cells that secrete a type I collagen-rich matrix rather than type II collagen matrix, typical of chondrocytes (Mizoguchi et al. 1990; Silbermann et al. 1987). Under normal functional conditions, it is these undifferentiated cells—rather than the chondrocytes in the deeper layers—that proliferate and mature to effect growth at the MCC (Luder et al. 1988; Petrovic et al. 1975). However, immobilization of the mandible or removal of the MCC from its normal functional environment produces a relatively rapid conversion of these prechondroblastic cells to an osteoblastic phenotype (Duterloo and Wolters 1971; Petrovic et al. 1975). Therefore, we wondered if and to what extent hypertrophic chondrocyte transformation would occur in this developmentally distinct and phenotypically malleable cartilage.
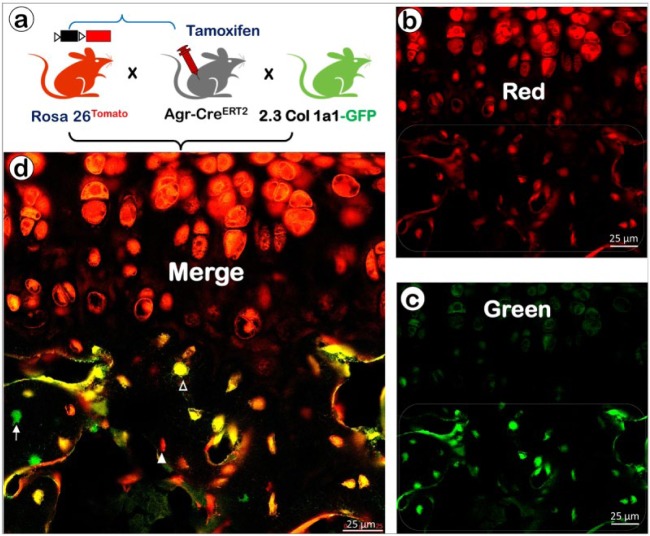
To study the fate of the chondrocytes in the postnatal MCC, we generated triple mice by crossing mice expressing aggrecan-CreERT2 (Agr-CreERT2; Akiyama et al. 2005; Henry et al. 2009) with mice expressing 2.3Col1a1-GFP (as a marker of osteoblasts) and mice expressing Rosa26tdTomato reporter, followed by a one-time tamoxifen induction at day 14. These crossings activated the red tomato reporter in all cells that expressed aggrecan (nearly all of them were chondrocytes) and all cells derived from them, although some perichondrial and periosteal cells also underwent recombination. The 2.3Col1a1-GFP line gave rise to a green-fluorescing color, and these cells were Col1a1-expressing bone cells. When all of the mouse lines were bred together, the red and green colors became superimposed, leading to yellow fluorescence (combination of red and green). This result was indicative of the transformation of aggrecan-expressing cells into bone cells expressing Col1a1.
We found that the trabecular bone just above the MCC contained a mixture of aggrecan-expressing cells (red), Col1a1-expressing cells (green) and cells expressing both aggrecan and Col1a1 (yellow), with red predominating; this mixture persisted deeper into the ramus, with the predominant color gradually changing to green (Jing et al. 2015). The large number of aggrecan-expressing cells in the subchondral bone was unexpected, representing cells that began as chondrocytes and then subsequently transformed into bone cells. We interpreted the persistence of red in these cells (rather than yellow) as being indicative of transformed cells that had not yet begun to secrete enough Col1a1 (green) to appear yellow, as in the superimposed image (Fig. 1). It was interesting that there were so few green-colored marrow-derived bone cells in the bone immediately adjacent to the cartilage, with their numbers increasing only in the ramus inferior to the condylar neck.
Figure 1.
Cellular co-localization of the chondrocyte-derived tomato marker and a 2.3 Col1a1-GFP osteoblast-specific marker in the growth plate and underlying trabecular bone. (a) Cartoon illustrating the cross that generates triple mice containing Agr-CreERT2, Rosa26tdTomato, and 2.3Col1a1-GFP, with tamoxifen induction at postnatal week 2 and at week 6 (harvest) for confocal imaging. Confocal images from red channel (b), green channel (c), and merged channels (d), in which the green (arrow) indicates a non-chondrocyte-derived bone cell, red (solid arrowhead) reflects a chondrocyte-derived cell that produces little collagen with no GFP activation, and yellow indicates a chondrocyte-derived bone cell that produces tomato (reflecting aggrecan activity) and type I collagen with GFP activation (open arrowhead; Agr-CreERT2; 2.3Col1a1-GFP).
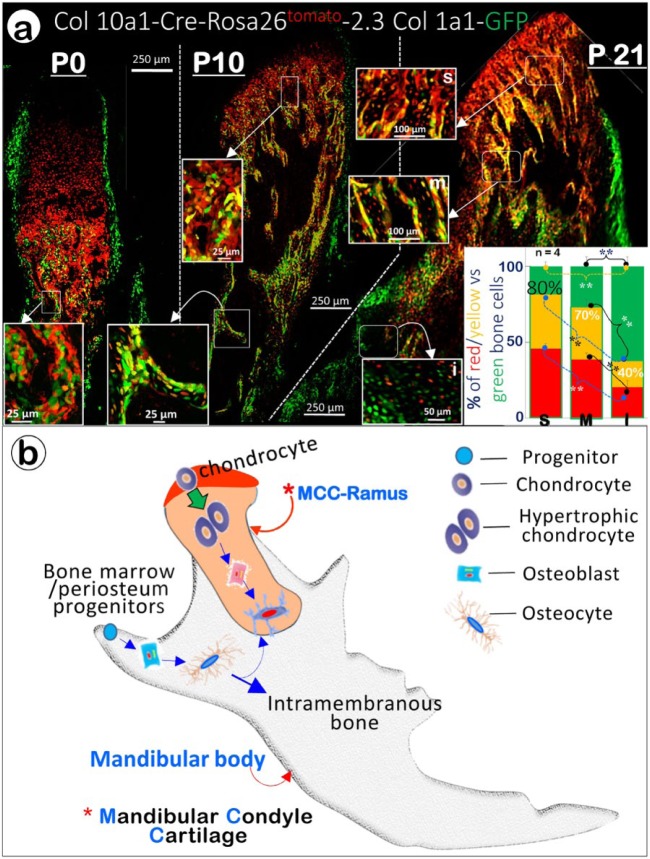
Chondrocyte-Derived Bone Cells Are Responsible for Forming the Bone of the Condylar Neck and Upper Ramus of the Mandible
Because our previous results reflected the transformation of all cells expressing aggrecan, they necessarily included some cells (e.g., in the perichondrium) that were not yet chondrocytes. Accordingly, we used the BAC Col10a1-Cre transgenic line, in which the Cre cDNA was inserted into the second exon of Col10a1 in the bacterial artificial chromosome (BAC RP23-192A7) containing the entire murine Col10a1 gene, together with over 200-kb of flanking sequences (Gebhard et al. 2008). We then repeated the triple crossings (Col10a1-Cre, Rosa26tdTomato and 2.3Col1a1-GFP) to trace the hypertrophic chondrocyte-derived bone cells. To appreciate the extent to which the chondrocyte-derived bone cells accounted for the bone of the ramus, we examined confocal images of the mice at birth (P0), at 10 d old (P10), and at 21 d old (P21). In the newborns, the subchondral bone was populated mostly by a mass of Col10a1-expressing cells (chondrocyte-derived bone cells in red color), with scattered Col1a1-positive cells (osteoblasts in green color), and essentially no cells that expressed both Col10a1 and Col1a1 (chondrocyte-derived bone cells in yellow color). By P10, recognizable trabecular bone was apparent, with Col10a1-expressing cells once again predominating as deep as the cartilage, giving way to a mixture of mostly Col10a1-expressing cells and those carrying both Col10a1 and Col1a1 in the somewhat more inferior trabeculae. The cells expressing both Col10a1 and Col1a1 (yellow) were more numerous than pure Col10a1-expressing cells (red) in the deeper trabeculae as well as the cortical bone of the condylar neck. The Col1a1-positive cells were sparse near the cartilage, but increased in number in the most inferior part of the condylar neck/ramus intersection (Fig. 2a). This trend continued in the P21 mice, with more trabeculae apparent. Because prechondroblastic cells also secrete aggrecan (Fukada et al. 1999), the pool of cells giving rise to bone cells was not purely comprised of chondrocytes. However, these images suggest that chondrocyte-derived bone cells are the primary agents of bone formation not only in the immediate subchondral bone but also in the entire condylar neck and upper ramus (Fig. 2b).
Figure 2.
Chondrocyte-derived bone cells contribute to condylar neck and mandibular ramus bone formation. (a) Qualitative and quantitative demonstration of the direct transformation of condylar chondrocytes into bone in Col10a1-Cre (activated in hypertrophic chondrocytes at ~E15.5), 2.3Col1a1-GFP (activated in bone cells), and Rosa26tdTomato triple transgenic mice. The merged P01 (postnatal day 1, left panel) confocal image reveals Col1a1-positive cells (green) in perichondrium and periosteum on the periphery, Col10a1-expressing cells (red) in the top center, and bone cells derived from cartilage or bone marrow (red/yellow; green) in the lower center. The P10 confocal image in the center panel shows a mix of different-colored bone cells in the subchondral bone (white arrows; top center and the enlarged inset on top right), trabecular bone (enlarged insert on left), and periosteum. In the right panel (P21), the low-magnification confocal image illustrates the 3 areas used to quantify bone cell origin: yellow/red marks cells derived from chondrocytes and green marks those originating from non-chondrocyte progenitor cells. Enlarged representative confocal images were used for quantitation in each area (superior, s; mid, m; inferior, i); and the results are presented in the bar graphs in the lower right panel. The figure is modified from Jing et al. 2015. (b) We propose that the mandibular bone is formed by 2 types of bone cells: the chondrocyte-derived bone cell for condylar neck and the upper ramus (endochondral bone origin) and the bone marrow- and periosteum-derived progenitor cell for the surface of the ramus and body of the mandible (intramembranous bone origin).
The Majority of Subchondral Bone in the Mandibular Condyle is Formed by Direct Transformation of Chondrocytes into Bone Cells
To quantify this distribution of Col 10a1-expressing cells (red), Col1a1-expressing cells (green), and chondrocyte-derived bone cells (yellow) expressing both Col10a1 and Col1a1, we stratified the bone inferior to the MCC into 3 regions of increasing distance from the MCC. This procedure revealed a clear gradient in the transformed cell distribution: around 80% of the bone cells in the area immediately adjacent to the MCC were either red or yellow, with this number dropping slightly to 70% in the intermediate region, and 40% in the lowest region of the ramus (Fig. 2a, lower right). Thus, the Col1a1-positive bone cells predominated only in the most inferior portion of the ramus. A greater fraction of the total chondrocyte-derived bone cells expressing both Col10a1 and Col1a1 were found in the middle zone than in the superior zone, but a significant number of Col10a1-expressing cells that had not yet started to express appreciable amounts of Col1a1 were evident in the middle and inferior zones. Overall, these quantitative data provide definitive evidence that the subchondral bone and the bone of the condylar neck are largely formed by chondrocyte-derived bone cells.
In Both the Long Bones and Mandibular Condyle, Chondrocytes Can Transform into Osteoblasts or Osteocytes
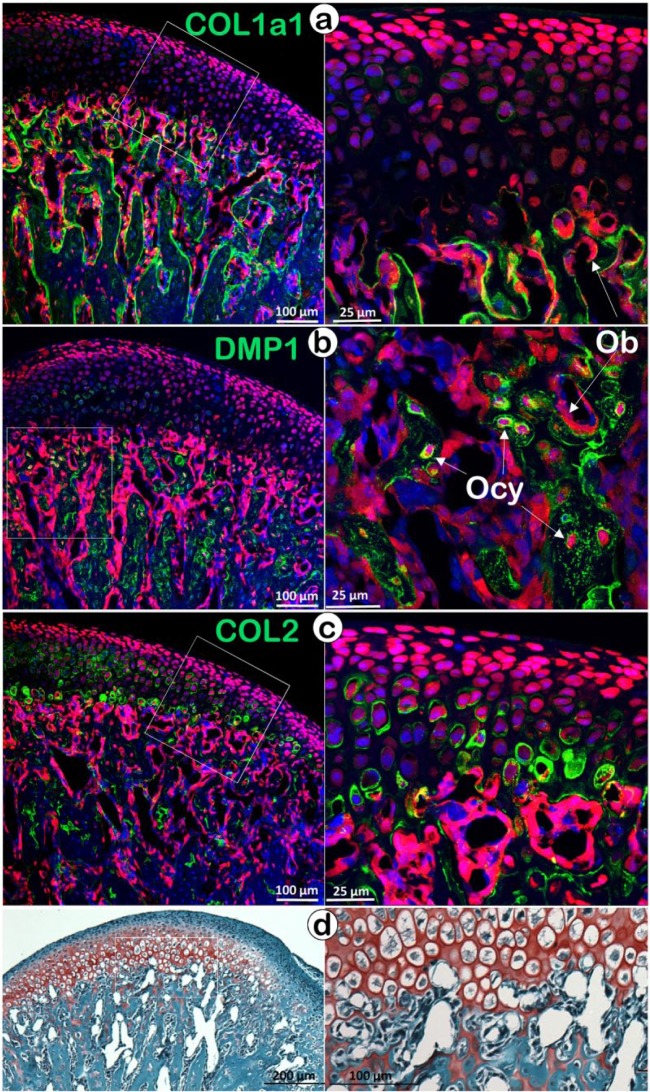
Confocal imaging that combines the tomato reporter (red color) and an immunostained matrix protein (green color, co-localized) is a powerful way to trace aggrecan-secreting chondrocytes and their daughter cells (or transformed cells, red color). For example, in the COL1a1-immunostained mandibular condyle at a time point 2 wk after tamoxifen injection, the perichondrium cells appeared green, and this is in agreement with light microscopic immunohistochemical studies (Mizoguchi et al. 1990; Silbermann et al. 1987). However, the subchondral bone cells were red (chondrocyte-derived) and produced a pericellular matrix of type I collagen (green color; Fig. 3a). Note that some aggrecan-expressing (red) cells could be seen in the perichondrium and periosteum. In the subchondral bone, these red cells clearly lined the surface of bony trabeculae and stained for COL1a1 but not for DMP1, a marker for osteocytes (Fig. 3b), suggesting that these cells are osteoblasts. On the other hand, most osteocytes in the bone matrix showed immunoreactivity for DMP1, indicating their chondrocytic origin (Fig. 3b). Similarly, another report studying cell lineages in long bones (Yang et al. 2014b) has identified chondrocyte-derived, osteocyte-like cells using immunostaining for sclerostin (SOST). In contrast, the COL2-immunostained condyle demonstrates the expected immunostaining in the cartilage matrix but not the upper perichondrium or the periosteum at the lower left (Fig. 3c). Importantly, there are numerous patches of COL2 immunoreactivity in the subchondral bone (the cartilage “remnants” demonstrated by Safranin O staining). For many years, the presence of cartilage remnants in subchondral bone or even in cortical bone has been puzzling. The cell lineage tracing data seem to support the notion that the replacement of cartilage matrices by bone matrices takes place more slowly than the more rapid cell transformation.
Figure 3.
Co-localization of the tomato reporter (red) with different immunostained markers (green color) in mice containing Rosa26tdTomato–Aggrecan CreERT2. The reporter was activated at P14 (postnatal day 14) by tamoxifen. Mice were harvested at P28, and viewed with immunostaining for COL1a1 (early bone marker, a), or DMP1 (late bone marker, b), or COL2 (chondrocyte marker, c); (d) Safranin O-stained section showing the cartilage remnants in the subchondral bone area to trace the fate of chondrocyte-derived bone cells. Ob, osteoblast; Ocy, osteocyte.
The cellular pathways taken by transforming chondrocytes have not been well delineated. As noted by Zhou et al. (2014), it is unclear whether hypertrophic chondrocytes might first dedifferentiate prior to transforming into osteoblasts or, if once transformed, a subset of osteoblasts go on to become osteocytes. However, we have observed chondrocyte-derived bone cells that exhibit immunostaining for DMP1 at very short intervals following tamoxifen administration, suggesting that a direct pathway from hypertrophic chondrocytes to osteocytes may be possible for some cells (a type of “fast track” to accelerate bone cell transformation).
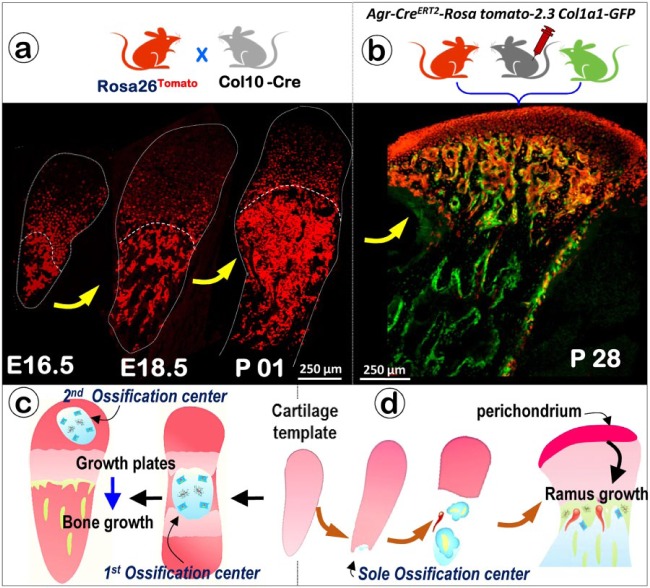
Cell Lineage Tracing Demonstrates 1 Ossification Center in the MCC-Ramus Complex versus 2 Ossification Centers in the Limbs
It is well documented that there are 2 ossification centers in limb development: the primary center appears in the embryonic stage, and the secondary ossification occurs after birth. In contrast, it has not been explicitly shown when and how ossification occurs in the condyle and upper mandibular ramus. Taking advantage of the cell lineage tracing technique, we generated 2 compound mice: Rosa26tdTomato and Col10a1-Cre (activated from E14.5 throughout the adult stage); Rosa26tdTomato, Agr-CreERT2 and 2.3 Col1a1-GFP. We first showed that the initially chondrocyte-transformed bone cells at the inferior portion of the MCC spread in one direction to fill the entire upper ramus with the exception of the surface bone (Fig. 4a, b; Jing et al. 2015). This unique ossification process contrasts with the bi-directional long bone ossification process. It also demonstrates that, while the mandibular body is formed intramembranously from marrow- and periosteum-derived precursors adjacent to Meckel’s cartilage (Oka et al. 2007; Ramaesh and Bard 2003), most of the ramus bone can be traced to chondrocyte-derived bone cells (Figs. 2, 4d). This suggests a new paradigm: the mandible is formed as a mosaic of bone cells derived from at least 2 sources.
Figure 4.
The condyle ossifies at a single ossification center versus 2 ossification centers in the limb. (a) Cartoon illustrating the cross that generates the compound mouse containing Col10a1-Cre and Rosa26tdTomato. The confocal images show a gradually expanding ossification area at stages E16.5, E18.5 and P01. (b) Cartoon illustrating the cross that generates the compound mouse containing Agr-CreERT2, Rosa26tdTomato, and 2.3Col1a1-GFP, with tamoxifen induction at postnatal day 14 (P14) and harvested at P28 for confocal imaging. (c) Cartoon illustrating the ossification process in long bone, in which the first ossification center is formed during the embryonic stage and the second ossification center appears after birth, with the growth plate vital for bone growth. (d) Cartoon illustrating the ossification process in the condyle, in which the single ossification center is initiated below and continues to expand during growth. For condylar development, the perichondrium plays a role similar to that of the growth plate.
Implications for Research and Clinical Practice
The accepted concept asserts that endochondral bone formation is preceded by widespread apoptosis of hypertrophic chondrocytes, followed by bone formation by cells derived almost exclusively from marrow and periosteum. While apoptosis of hypertrophic chondrocytes surely takes place, we have shown that apoptotic chondrocytes in the deepest layers of the MCC are relatively few (Jing et al. 2015), casting doubt on the extent of this phenomenon. Meanwhile, estimates of the percent of hypertrophic chondrocyte-derived cells that become osteoblasts or osteocytes in limb cartilage range anywhere from 20% to 30% (Yang et al. 2014a), 60% to 70% (Zhou et al. 2014), or 80% (Yang et al. 2014b), depending on the study. Our quantitative data fit within the high end of this spectrum, suggesting that around 80% of bone cells within the bone near the cartilage-bone interface are cartilage-derived. However, our approach to quantifying successively deeper regions of the condylar neck allowed us to characterize a gradient of bone cell derivation in which 80% of bone cells are cartilage-derived in the immediately subchondral (superior) zone, 70% in the next, deeper (middle) zone, and only 40% in the deepest (inferior) zone. While comparisons of percentages of chondrocyte-derived bone cells among different tissues should be quantified using standard methodology, the published percentages are often half or greater, suggesting that the transformation of chondrocytes accounts for a significant portion of bone cells. Although it is clear that some bone is formed by bone cells derived from the marrow and periosteum, the results of our work in condyle and the work of others in the long bone and fracture callus suggest that bone formed by cells transformed from chondrocytes is far from anomalous, and may often be the norm. Moreover, a recent study (Park et al. 2015) has used lineage tracing techniques to demonstrate that hypertrophic chondrocytes in the lowest zone of limb cartilages give rise to small cells that, when isolated by FACS, express osteogenic genes such as Col1a1, osteocalcin, and Runx2. As the authors note, these cells may correspond to the “dark” chondrocytes observed in ultrastructural studies, which differ from classic apoptotic chondrocytes. At the very least, their results and the number of cell lineage studies demonstrating the direct transformation of hypertrophic chondrocytes to bone cells suggest that there may be multiple pathways instrumental in endochondral bone formation.
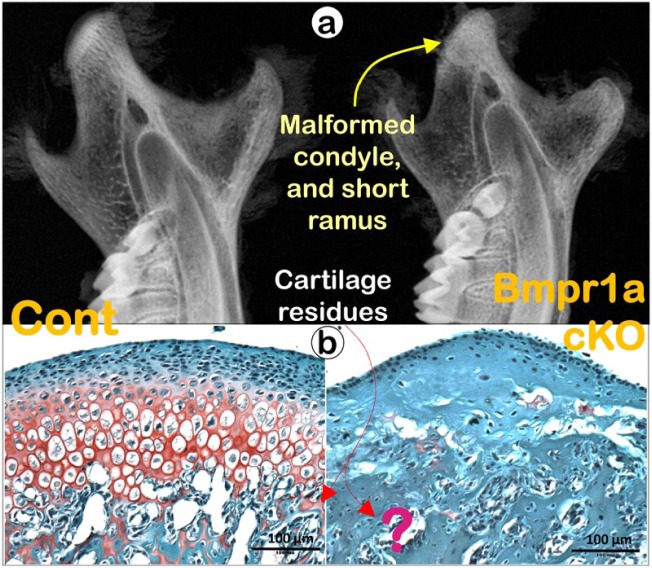
These studies open a new window into how endochondral bone formation is regulated, since they strongly suggest that chondrogenesis and osteogenesis, far from being separate processes, are, in reality, sequential phases of the same process linked by chondrocytes transforming into bone cells. Based on the cell transformation data from our laboratory and those of others, it is reasonable to postulate that virtually any gene that exerts a regulatory effect on cartilage may also affect bone formation. For example, the targeted deletion of Bone Matrix Protein Receptor 1a (Bmpr1a) in chondrocytes by Aggrecan-CreERT2 leads to not only severe defects in chondrogenesis but also complete cessation of endochondral bone formation (Fig. 5), with the absence of any cartilage ‘remnants’ in the subchondral bone (Jing et al. 2014). Thus, the cell lineage technique can be used to compare cell transformation in mice with conditional knockout (a loss of function study) or constitutive activation (a gain of function study) of a gene to reveal the phenotypic change in both cell types. Perhaps bone phenotypes should now be scrutinized for mutations that primarily affect cartilage. Furthermore, studying cellular transformation will pave the way for exciting new approaches to the stimulation of new bone formation for tissue regeneration (Bahney et al. 2014).
Figure 5.
Deletion of Bmpr1a (the key receptor for BMP2 and BMP4) in chondrocytes leads to a lack of condylar cartilage and a short ramus. (a) Representative radiographs from the Bmpr1a cKO mice (a crossing of Aggrecan-CreERT2 and Bmpr1a loxP with tamoxifen induction at postnatal day 3 and the animal harvested at day 21) reveal not only a major defect in the condylar cartilage, but a short ramus as well (right panel); and (b) Safranin O-stained images demonstrate a lack of active chondrogenesis, and poorly formed subchondral bone with no cartilage residues in the Bmpr1a cKO mice. These data support a strong dependence on subchondral bone formation for healthy chondrogenesis, in which BMP signaling plays a key role.
Author Contributions
R.J. Hinton, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; Y. Jing, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J. Jing, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; J.Q. Feng, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was partially supported from NIH grants DE025014 and DE025659 to JQF.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aizawa T, Kokubun S, Tanaka Y. 1997. Apoptosis and proliferation of growth plate chondrocytes in rabbits. J Bone Joint Surg Br. 79(3):483–486. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, et al. 2005. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 102(41):14665–14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahney CS, Hu DP, Taylor AJ, Ferro F, Britz HM, Hallgrimsson B, Johnstone B, Miclau T, Marcucio RS. 2014. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res. 29(5):1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda F, Gentili C, Manduca P, Cancedda R. 1992. Hypertrophic chondrocytes undergo further differentiation in culture. J Cell Biol. 117(2):427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duterloo HS, Wolters JM. 1971. Experiments on the significance of articular function as a stimulating chondrogenic factor for the growth of secondary cartilages of the rat mandible. Trans Eur Orthod Soc. 1971:103–115. [PubMed] [Google Scholar]
- Enishi T, Yukata K, Takahashi M, Sato R, Sairyo K, Yasui N. 2014. Hypertrophic chondrocytes in the rabbit growth plate can proliferate and differentiate into osteogenic cells when capillary invasion is interposed by a membrane filter. PLoS One. 9(8):e104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum CE, Wilsman NJ. 1987. Morphologic stages of the terminal hypertrophic chondrocyte of growth plate cartilage. Anat Rec. 219(3):221–232. [DOI] [PubMed] [Google Scholar]
- Fukada K, Shibata S, Suzuki S, Ohya K, Kuroda T. 1999. In situ hybridisation study of type I, II, X collagens and aggrecan mRNas in the developing condylar cartilage of fetal mouse mandible. J Anat. 195(Pt 3):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard S, Hattori T, Bauer E, Schlund B, Bosl MR, de Crombrugghe B, von der Mark K. 2008. Specific expression of Cre recombinase in hypertrophic cartilage under the control of a BAC-Col10a1 promoter. Matrix Biol. 27(8):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld LC, Shapiro FD. 1996. Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem. 62(1):1–9. [DOI] [PubMed] [Google Scholar]
- Gibson G. 1998. Active role of chondrocyte apoptosis in endochondral ossification. Microsc Res Tech. 43(2):191–204. [DOI] [PubMed] [Google Scholar]
- Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. 2009. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis. 47(12):805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Hinton RJ, Mishina Y, Liu Y, Zhou X, Feng JQ. 2014. Critical role of Bmpr1a in mandibular condyle growth. Connect Tissue Res. 55(Suppl 1):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, de Crombrugghe B, Hinton RJ, Feng JQ. 2015. Chondrocytes directly transform into bone cells in mandibular condyle growth. J Dent Res. 94(12):1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn AJ, Simmons DJ. 1977. Chondrocyte-to-osteocyte transformation in grafts of perichondrium-free epiphyseal cartilage. Clin Orthop Relat Res. 129:299–304. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature. 423(6937):332–336. [DOI] [PubMed] [Google Scholar]
- Luder HU, Leblond CP, von der Mark K. 1988. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 182(3):197–214. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Tatarczuch L, Mirams M. 2011. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 211(2):109–121. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I, Nakamura M, Takahashi I, Kagayama M, Mitani H. 1990. An immunohistochemical study of localization of type I and type II collagens in mandibular condylar cartilage compared with tibial growth plate. Histochemistry. 93(6):593–599. [DOI] [PubMed] [Google Scholar]
- Oka K, Oka S, Sasaki T, Ito Y, Bringas P, Jr, Nonaka K, Chai Y. 2007. The role of TGF-beta signaling in regulating chondrogenesis and osteogenesis during mandibular development. Dev Biol. 303(1):391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono N, Ono W, Nagasawa T, Kronenberg HM. 2014. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 16(12):1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Gebhardt M, Golovchenko S, Perez-Branguli F, Hattori T, Hartmann C, Zhou X, deCrombrugghe B, Stock M, Schneider H, et al. 2015. Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biol Open. 4(5):608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic A, Stutzmann J, Oudet C, McNamara JJ. 1975. Control processes in the postnatal growth of the condylar cartilage of the mandible. In: McNamara JA. editor. Determinants of mandibular form and growth. Ann Arbor (MI): Center for Human Growth and Development, University of Michigan, p. 101–154. [Google Scholar]
- Ramaesh T, Bard JB. 2003. The growth and morphogenesis of the early mouse mandible: a quantitative analysis. J Anat. 203(2):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach HI. 1992. Trans-differentiation of hypertrophic chondrocytes into cells capable of producing a mineralized bone matrix. Bone Miner. 19(1):1–20. [DOI] [PubMed] [Google Scholar]
- Roach HI, Erenpreisa J, Aigner T. 1995. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 131(2):483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbermann M, Reddi AH, Hand AR, Leapman RD, Von der Mark K, Franzen A. 1987. Further characterisation of the extracellular matrix in the mandibular condyle in neonatal mice. J Anat. 151:169–188. [PMC free article] [PubMed] [Google Scholar]
- Wong S, Hu D, Miclau T, Bahney C, Marcucio R. 2016. Trans differentiation of chondrocytes to osteoblasts during endochondral ossification in the healing mandible. FASEB J. 30(Suppl 1):1039.11(Abstract). [Google Scholar]
- Yang G, Zhu L, Hou N, Lan Y, Wu XM, Zhou B, Teng Y, Yang X. 2014a. Osteogenic fate of hypertrophic chondrocytes. Cell Res. 24(10):1266–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. 2014b. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA. 111(33):12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka C, Yagi T. 1988. Electron microscopic observations on the fate of hypertrophic chondrocytes in condylar cartilage of rat mandible. J Craniofac Genet Dev Biol. 8(3):253–264. [PubMed] [Google Scholar]
- Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. 2014. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10(12):e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]