Abstract
Mammalian breast adipose tissue is replaced by a milk-secreting gland during pregnancy; the reverse process takes place upon interruption of lactation. Morphological and bromodeoxyuridine studies provide indirect evidence that mouse mammary adipocytes transform into secretory epithelial cells during pregnancy and revert to adipocytes after lactation. By using the Cre-loxP recombination system we show that the mammary gland of whey acidic protein (WAP)-Cre/R26R mice, in which secretory epithelial cells express the lacZ gene during pregnancy, contains labeled adipocytes during involution. Conversely, adipocyte P2-Cre/R26R mice, in which adipocytes are labeled before pregnancy, contain labeled secretory epithelial cells during pregnancy. We conclude that reversible adipocyte-to-epithelium and epithelium-to-adipocyte transdifferentiation occurs in the mammary gland of adult mice during pregnancy and lactation.
Keywords: Cre/LoxP recombination system, X-Gal, morphology
The evolution of the mammary gland through pregnancy, lactation, and weaning is one of the most interesting instances of tissue plasticity in adult mammals. In these physiological conditions, the gland undergoes dramatic, hormonally regulated events that cause massive changes in its two main components: glandular epithelium and adipose tissue. In virgin females, most of the resting gland consists of adipocytes, but during pregnancy and lactation ≈90% of the organ is occupied by secretory epithelial cells. After lactation, rapid and massive adipogenesis restores the prevalence of adipose tissue (1, 2).
When we examined postlactation adipogenesis with an electron microscope, we obtained images suggesting epithelium-to-adipocyte transdifferentiation in the involuting mammary gland and adipocyte-to-epithelium transdifferentiation during pregnancy. Furthermore, the electron microscope ruled out the presence of dormant adipocytes in the gland during lactation. BrdUrd injections demonstrated the absence of adipocyte proliferation during postlactation adipogenesis and the low rate of epithelial proliferation during pregnancy. Finally, by using the Cre-loxP system to drive the expression of β-gal in secretory epithelium and adipocytes under control of cell type-specific promoters (3, 4), we confirmed that alveolar epithelial cells can transdifferentiate into white adipocytes during mammary gland involution and that white adipocytes can transdifferentiate into alveolar epithelial cells during pregnancy.
Materials and Methods
Animals. ROSA26 and R26R mice were purchased from and genotyped as described by The Jackson Laboratory. Whey acidic protein (WAP)-Cre (5) and adipocyte-binding protein 2 (aP2)-Cre (6) mice were kindly provided by C. Dickson (London Research Institute, London) and B.B.K., respectively. ROSA26 mice, in which expression of β-gal seems to be ubiquitous (7), were used as positive controls. Double transgenic mice were derived from crosses between R26R hemizygous mice and either WAP-Cre or aP2-Cre hemizygous mice. Mice were genotyped for the presence of Cre by PCR analysis using the primers Cre1 (5′-ATGTCCAATTTACTGACC-3′) and Cre2 (5′-CGCCGCATAACCAGTGAAAC-3′), which yielded a 356-bp product.
WAP-Cre/R26R mice were killed at the following points: early (11 days) and late (18 days) pregnancy, lactation (10 days), early involution (18 h), and late involution (10 days and 5 months). aP2-Cre/R26R mice were studied in virgin and late-pregnancy (18 days) conditions. All experiments were performed in compliance with Italian institutional guidelines.
Cre-Mediated Recombination Analysis. For molecular biology, specimens were carefully dissected, rapidly frozen in liquid nitrogen, and stored at –80°C. Cre-mediated recombination was detected by PCR using genomic DNA isolated from a variety of tissues. To recognize the Cre-recombined allele, we used two primer sets, F2 (5′-GGTTGAGGACAAACTCTTCGC-3′) and R2 (5′-GTCGTTTTACAACGTCGTGACT-3′) to detect the newly formed gene and F1 (5′-TCTTTTTGTCAAGACCGACCTGT-3′) and R1 (5′-GTCCAGATCATCCTGATCGACA-3′) to amplify the neo gene.
RT-PCR. Total RNA was extracted from a variety of tissues by using TRIzol (GIBCO/BRL). Samples were treated with DNA-free (Ambion, Huntingdon, U.K.) to remove possible contaminating DNA; 1.5 μg of RNA was reverse-transcribed by using AMV reverse transcriptase (Takara Bio Europe, Gennevilliers, France) and then subjected to PCR analysis with the following primers: Cre1 (5′-ATGTCCAATTTACTGACC-3′) and Cre2 (5′-CGCCGCATAACCAGTGAAAC-3′); lacZ1 (5′-GTCGTTTTACAACGTCGTGAC-3′) and lacZ2 (5′-GTCGTTTTACAACGTCGTGACT-3′); and actin1 (5′-GTGGGCCGCTCTAGGCACCAA-3′) and actin2 (5′-CTCTTTGATGTCACGCACGATTTC-3′). RT-PCR products were electrophoresed on 2% agarose gel and analyzed by Southern blotting.
β-Gal Histochemistry. Animals were perfused intracardially with 2% formaldehyde/0.25% glutaraldehyde in PBS, pH 7.3. Inguinal mammary glands (fourth and fifth) and other tissues were dissected out and fixed in the same fixative (for up to 90 min). After an overnight wash in PBS, tissues were preincubated in 2 mM MgCl2/0.01% sodium deoxycholate/0.02% Nonidet P-40 in PBS, pH 8.5, for 2 h at room temperature, then incubated in X-Gal solution: 5 mM potassium ferrocyanide containing 1 mg/ml X-Gal (Sigma; as 40 mg/ml solution in dimethylformamide), at 30°C for 24 h (WAP-Cre/R26R) and 5 h (aP2-Cre/R26R). PBS (pH 8.5) was used to avoid detection of endogenous β-galactoside, active at pH values of <5. After X-Gal incubation, tissues were postfixed in 4% formaldehyde in 0.1 M phosphate buffer, dehydrated, and embedded in paraffin. Some small tissue fragments were resin-embedded and processed for electron microscopy. For morphometric analysis, representative sections of the fourth mammary gland underwent X-Gal staining in standardized conditions. For each condition, three animals were studied. In each section, 1,000 epithelial and 1,000 adipose cells were randomly selected, and cells containing X-Gal crystals were counted at high magnification. Results are given as mean ± SEM.
Immunohistochemistry. Immunohistochemistry was carried out on paraffin-embedded tissues with the avidin–biotin–peroxidase (ABC) method by using the following primary antibodies: polyclonal rabbit anti S-100, 1:1,000 dilution (DAKO); polyclonal rabbit anti-perilipin, 1:300 dilution [kindly provided by A. Greenberg (Tufts University, Boston)]; polyclonal rabbit anti-aP2, 1:400 dilution [kindly provided by D. Bernlohr (University of Minnesota, Minneapolis) and K. Kristiansen (University of Southern Denmark, Odense)]; polyclonal rabbit anti-β-casein, 1:500 dilution [kindly provided by I. Barash (Volcani Center, Bet-Dagan, Israel)]; and polyclonal rabbit anti-cytokeratin, wide spectrum screening, 1:300 dilution (DAKO).
Transmission Electron Microscopy. Specimens were fixed in 2% glutaraldehyde/2% formaldehyde in 0.1 M phosphate buffer, postfixed in osmium tetroxide, dehydrated in acetone, and embedded in epoxy resin. Thin sections were obtained with an MTX ultramicrotome (RMC, Tucson, AZ) and examined with a transmission electron microscope (CM10, Philips, Eindhoven, The Netherlands).
BrdUrd Experiments. Five-month-old mice forced-weaned at 10 days of lactation were injected i.p. with 50 μg of BrdUrd (Sigma) per g of body weight and killed after 13 h. Pregnant mice were BrdUrd-injected at days 12 and 15 and killed at day 18 of pregnancy. They were anesthetized and perfused intracardially with 4% formaldehyde in 0.1 M phosphate buffer. The inguinal glands and some fragments of the small intestine were dissected, and incorporated BrdUrd was detected by immunohistochemistry (see above) with a mouse monoclonal anti-BrdUrd antibody, 1:100 dilution (Sigma).
Results
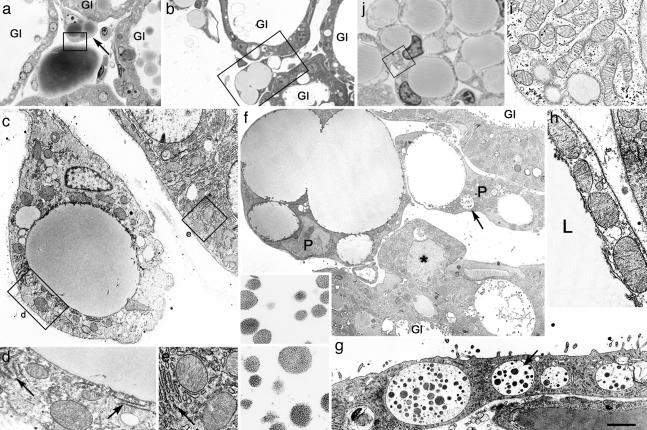
Adipocyte Precursors During Postlactation Involution Exhibit Epithelial Features. Light microscopic examination of the mammary gland on day 1 of postlactation involution (6–18 h after litter removal) showed that the connective tissue septa intervening between the secretory alveoli contained numerous, multilocular adipocyte precursors, characterized by multiple lipid droplets (Fig. 1 a and b) and positive for S-100, a marker of preadipocytes (data not shown) (8, 9). Under electron microscope examination, however, the complement of cytoplasmic organelles of these cells differed from that typical of white adipocyte precursors (10, 11): it was reminiscent of that contained in the adjacent alveolar epithelium. Stacks of profiles of the rough endoplasmic reticulum were a distinctive feature, as well as large, cristae-rich mitochondria with dense matrix (Fig. 1 c–e), quite dissimilar from the elongate mitochondria of developing adipocytes (Fig. 1 i and j). Especially striking was the fact that 15 ± 5% (mean ± SEM, n = 3) of the adipocyte precursors exhibited secretory vacuoles containing granules of milk protein, identical to those found in lactating alveolar epithelial cells (Fig. 1 f and g). These multilocular adipocyte precursors appeared healthy, with none of the nuclear or cytoplasmic changes typical of apoptosis. Furthermore, they were negative for uncoupling protein 1 (data not shown), thus ruling out the possibility that they represented brown adipocytes.
Fig. 1.
During postlactation involution, adipocyte precursors among mammary gland alveoli show epithelial features and differ from typical white adipocyte precursors. (a–h) Day 1 of postlactation involution. (a and b) Light microscopy of unilocular and multilocular adipocyte precursors among mammary gland alveoli (Gl). (c) Electron microscopy of the adipocyte precursor indicated by arrow in a; part of the alveolar epithelium is also visible. (d and e) Enlargements of the areas boxed in c, showing similarities of mitochondria and stacked rough endoplasmic reticulum (RER) (arrows) in the two cell types. (f) Electron microscopy of the area boxed in b: adipocyte precursors (P), one of which contains vacuoles (arrow) filled with milk protein secretory granules (enlarged in Inset) identical to those found in alveolar epithelium (compare with g). An alveolar epithelial cell protruding toward the interstitium (asterisk) is also visible. Gl, glands. (g) Electron microscopy of the alveolar epithelium with milk protein secretory granules (indicated by arrow and enlarged in Inset). (h) Electron microscopy of the area boxed in a: mitochondria of the mature unilocular adipocyte (at left) are large and similar to those of the adipocyte precursor (at right) and of mammary gland alveolar epithelium (compare with e). L, lipid droplet. (i and j) Developing mammary gland of a 12-h-old mouse. (i) The area boxed in j. Under the electron microscope, typical adipocyte precursors show the classic elongated mitochondria, which are distinctly different from those observed in mammary gland adipocyte precursors during postlactation adipogenesis (compare with d). [Scale bar: 10 (a), 12.5 (b), 1.2 (c), 0.8 (d, e, and i), 2.4 (f), 0.3 (Inset in f and g), 2 (g), 1 (h), and 7 (j) μm.]
These findings, together with the absence of ultrastructural features suggesting adipocytic differentiation in endothelial cells, fibroblasts, or pericytes, seemed to indicate that alveolar epithelial cells were transdifferentiating into white adipocytes, contributing to the rapid fat tissue restoration that takes place during weaning. Consistent with this hypothesis was the observation that organelles normally found in secretory epithelial cells were also present in more differentiated, nearly unilocular adipocytes (Fig. 1h). Finally, we noted epithelial cells apparently isolated in the interstitium or protruding into the interstitial space from the periphery of mammary alveoli (Fig. 1f and data not shown). Notably, none of these cells showed ultrastructural signs of apoptosis.
Postlactation Adipogenesis Is Not Caused by Refilling of Slimmed Adipocytes and Is Independent of Cell Proliferation. To test the widely held hypothesis that completely delipidized (slimmed) adipocytes lie among epithelial structures during lactation and become again filled with lipids during epithelial involution (12, 13), we compared the structure of the mammary gland of lactating mice with that of fasted (for 48 h), nonpregnant animals. Slimmed adipocytes retained positivity for perilipin, a marker of differentiated adipocytes (ref. 14 and data not shown). They packed the connective tissue spaces among the glandular alveoli in the fasted animals (see Fig. 5 b and c, which is published as supporting information on the PNAS web site), but neither slimmed nor any other type of dormant adipocytes were observed in the lactating mice (Fig. 5a). Furthermore, in mice fed after fasting, white adipocytes exhibited the distinctive morphology of the early stages of lipid refilling (Fig. 5 d and e), with peripheral cytoplasmic digitations, an appearance notably different from that of adipocyte precursors during postlactation adipogenesis (compare with Fig. 1c).
To clarify whether the massive increase in the number of mammary gland white adipocytes during postlactation involution was the result of a process of cell proliferation, BrdUrd was administered to lactating mice at the time of litter removal, and the animals were killed 12 h later. Neither alveolar epithelial cells nor white adipocytes were labeled (Fig. 5f), suggesting that postlactation adipogenesis is not the result of cell proliferation.
Postlactation Adipocytes and Adipocyte Precursors Express the Reporter Gene in WAP-Cre/R26R Double Transgenic Mice. To support the idea that the white adipocytes of the involuting mammary gland derive from epithelial alveolar cells, we resorted to the Cre-loxP DNA recombination system (3, 4). Mice carrying the WAP-Cre transgene, in which expression of the gene for Cre recombinase is controlled by the WAP promoter, were crossed with mice carrying the ROSA26 reporter (R26R) transgene (4), in which ubiquitous expression of the reporter gene lacZ is blocked by a loxP-flanked stop sequence. WAP is a milk protein specifically produced by mammary alveolar epithelial cells during pregnancy (15). DNA recombination analysis confirmed that Cre-mediated recombination took place in the mammary gland of double transgenic pregnant WAP-Cre/R26R mice, but not in lung, intestine, liver, and heart and skeletal muscle, nor in the mammary gland of virgin mice (see Fig. 6, which is published as supporting information on the PNAS web site). Accordingly, RT-PCR analysis showed that Cre and lacZ gene expression was restricted to the mammary gland of pregnant WAP-Cre/R26R mice (see Fig. 7, which is published as supporting information on the PNAS web site).
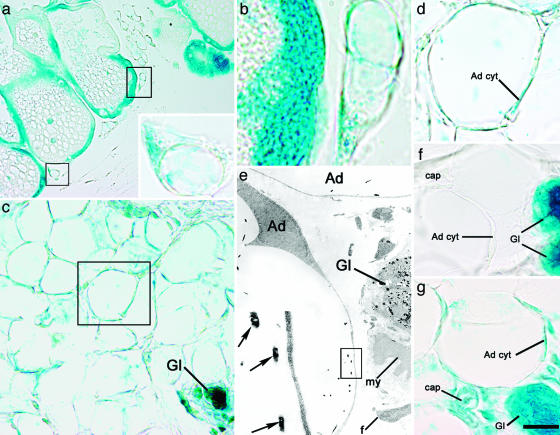
Cre-mediated excision is cell-heritable, and labeled cells and their progeny should continue to express the reporter gene at later stages even if Cre is no longer expressed. As a consequence, by visualizing the activity of β-gal, mammary epithelial cells and any other cell type eventually deriving from them should be labeled. By using X-Gal as a substrate, β-gal-positive cells were detected in glandular epithelial cells at all stages examined, early and late pregnancy, lactation, and postlactation (Fig. 2a), in agreement with previous reports (5, 15). The percentage of β-gal-positive epithelial cells was 47 ± 21% (mean ± SEM, n = 3) in early pregnancy and rose to 98 ± 0.7% (n = 3) during lactation and postlactation, thus suggesting that recombination took place in almost all epithelial cells during these stages of mammary development (5). All of the other cell types in the gland (myoepithelial cells, fibroblasts, vascular and blood cells, lymphocytes, and macrophages scattered in the interstitium) were negative, as were all tissues from control organs [lung, skeletal muscle, heart, and liver (data not shown)]. In keeping with the finding that white adipocytes never activate the WAP promoter (5), visceral white adipocytes and those observed in the mammary gland before and during pregnancy (Fig. 2f) were always β-gal-negative. In contrast, the adipocyte precursors detected among the alveoli during postlactation adipogenesis were positive (Fig. 2 a Inset and b), as were most of the adipocytes of fully involuted postlactation glands studied 10 days (Fig. 2 c and d) and 5 months (data not shown) after litter removal. Under the electron microscope, dense precipitates with the typical morphology of X-Gal crystals were present in alveolar milk-secreting epithelial cells during pregnancy, lactation, and postlactation. Crystals were also found in developing and fully differentiated adipocytes, although exclusively after lactation (Fig. 2e). Of note, postlactation β-gal-positive adipocytes were also positive for aP2 and perilipin (see Fig. 8, which is published as supporting information on the PNAS web site), which confirmed that these cells were indeed fully differentiated fat cells.
Fig. 2.
Postlactation adipocytes express the gene reporter driven by the alveolar epithelial cell-specific WAP promoter. (a–f) Mammary gland of WAP-Cre/R26R mouse, β-gal histochemistry. (a and b) On day 1 of postlactation involution, epithelial alveolar cells of the gland as well as adipocyte precursors (boxed) stain for X-Gal. (a Inset) Enlargement of the lower of the two boxed areas in a. (b) Enlargement of the upper of the two boxed areas in a. (c–e) On day 10 of postlactation involution, atrophic glands and most adipocytes are positive for X-Gal. (c) Gl, atrophic glands. (d) Enlargement of the area boxed in c. The cytoplasm of the adipocyte (Ad cyt) is X-Gal-positive. (e) By electron microscopy, typical X-Gal crystals are present only in adipocytes (Ad) and the epithelial component of the atrophic gland (Gl). Note the absence of crystals in other cell types. my, Myoepithelial cell; f, fibroblast. (e Inset) Enlargement of boxed area in e showing the typical crystals (arrows). (f) On day 9 of pregnancy, only the epithelial component of the mammary gland (Gl) is positive, whereas adipocytes (Ad cyt) and the other cell types [such as capillary (cap)] do not stain for X-Gal (negative control). (g) In a ROSA26 mouse (positive control) on day 1 of postlactation involution, all of the cell types found in the mammary gland were positive. Ad cyt, adipocyte cytoplasm; cap, capillary; Gl, gland. [Scale bar: 17 (a), 4 (a Inset), 2 (b), 9 (c), 4 (d, f, and g), 2 (e), and 0.4 (e Inset) μm.]
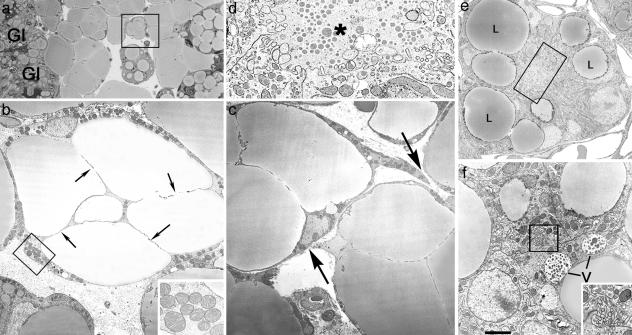
Mammary Gland Adipocytes Acquire Epithelial Features During Pregnancy. In mid-to-late pregnancy, when most of the lobuloalveolar portion of the gland develops, the white adipocytes located near developing alveoli exhibited unusual features. “Compartmentalization” of the cytoplasm, suggesting partition of the large, single-lipid droplet into smaller droplets, was frequently observed (Fig. 3a). At this stage, these multilocular adipocytes showed a thicker cytoplasm containing a richer complement of organelles, including larger mitochondria than neighboring unilocular adipocytes (Fig. 3b). In addition, these multilocular adipocytes had irregular outlines with slender peripheral projections, as though they were in the process of joining with one another (Fig. 3c). More importantly, clusters of cells were found containing large lipid droplets and occasional vacuoles that surrounded a small lumen crowded with microvilli (Fig. 3 d and e). In their cytoplasm, large lipid droplets coexisted with vacuoles containing structures resembling milk protein granules (Fig. 3f). Thus, the electron microscopic profile was consistent with the possibility that, during pregnancy, fully differentiated white adipocytes were transdifferentiating into secretory cells and coalesced into glandular alveoli. Furthermore, when mice were twice injected with BrdUrd during the phase of alveolar development (days 9 and 14 of pregnancy), very few (8 ± 3%; mean ± SEM, n = 3) alveolar epithelial cells were labeled, suggesting a limited role of cell proliferation in gland development, as previously noted by others (16, 17).
Fig. 3.
During pregnancy, adipocytes of the mammary gland exhibit epithelial features. Mouse mammary gland on day 18 of pregnancy. (a) Light microscopy. (b–f) Electron microscopy. (a) Adipocytes near the newly formed alveoli (Gl) show lipid droplet compartmentalization. (b) Electron microscopy of the area squared in a. Adipocytes show a thickened peripheral cytoplasm containing unusually large mitochondria (enlarged in Inset) and thin cytoplasmic processes (arrows) that seem to subdivide the lipid droplet. (c) Transdifferentiating adipocytes also exhibit cytoplasmic projections (arrows), suggesting a tendency to become joined and to give rise to early alveolar structures (see d–f). (d) Enlargement of the boxed area in e, showing a small lumen (asterisk) with microvilli. (e) Early alveolar structure containing large lipid droplets (L). (f) Vacuoles (V) containing milk protein secretory granules are present in some early alveolar structures (see the lumen crowded with microvilli in the boxed area, which is enlarged in Inset). [Scale bar: 15 (a), 2.5 (b), 0.5 (b Inset), 2.5 (c), 0.5 (d), 2 (e), 1.2 (f), and 0.4 (f Inset) μm.]
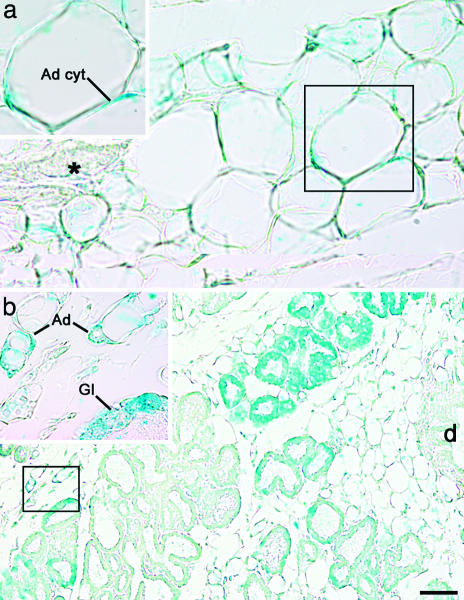
During Pregnancy, Mammary Alveolar Epithelial Cells Express the Reporter Gene in aP2-Cre/R26R Double Transgenic Mice. To provide further evidence that white adipocytes transdifferentiate into mammary epithelial cells during pregnancy, we again resorted to the Cre-loxP DNA recombination system. After confirming the observation by Specht et al. (18) that aP2 protein (19) is never expressed in mammary epithelial cells (data not shown), we crossed R26R mice with aP2-Cre mice, which selectively express Cre recombinase in the adipose tissue (7). In aP2-Cre/R26R virgin mice, DNA recombination was detected in the mammary gland, largely consisting of adipose tissue, and in white and brown fat depots, but not in liver or lung (Fig. 6 and data not shown). Accordingly, RT-PCR showed that Cre and lacZ genes were expressed in mammary fat (Fig. 7). Finally, after X-Gal histochemistry, both light and electron microscopy demonstrated that in the mammary gland of virgin mice only adipocytes stained for β-gal (Fig. 4a). Elsewhere in the body, specific staining was present in white and brown adipocytes and absent in other tissues [liver, lung, skeletal muscle, and heart (data not shown)]. In contrast, during late pregnancy (day 18), mammary epithelial alveolar cells also expressed β-gal (Fig. 4b). Morphometric analysis showed that 67 ± 4% (mean ± SEM, n = 3) of epithelial cells were positive, suggesting that most of them derive from adipocytes. Of note, β-gal-positive epithelial cells were also positive for cytokeratins and β-casein (see Fig. 9, which is published as supporting information on the PNAS web site), two markers of fully differentiated alveolar epithelial cells.
Fig. 4.
During pregnancy, mammary alveolar epithelial cells express the gene reporter driven by the adipocyte-specific aP2 promoter. Mammary gland of aP2-Cre/R26R mouse, β-gal histochemistry. (a) In virgin mice only adipocytes stain for X-Gal. Note the absence of staining in the epithelial portion of the gland (asterisk). Ad cyt, adipocyte cytoplasm. (b) On day 18 of pregnancy, adipocytes (Ad) as well as many alveolar epithelial cells (Gl) are positive for X-Gal. d, Main duct. (Insets) Enlargements of the corresponding boxed areas. [Scale bar: 10 (a), 8 (Insets), and 30 (b) μm.]
Discussion
Transdifferentiation describes the transformation of a fully differentiated cell type into another, directly or with intervening cell division, and therefore differs from development or differentiation of stem or progenitor cells (3, 20–22). Most of the examples of transdifferentiation reported in the literature, however, were observed in experimental and/or pathological conditions and predate the recent discovery of stem cell precursors in adult tissues and organs (23). Thus, toxic or pathological stimuli and/or the presence of stem cells may explain the appearance of hepatocytes in the rodent pancreas in response to a copper-deficient diet (24, 25) or insular overexpression of keratinocyte growth factor (26). Similar mechanisms may be responsible for transdifferentiation of hepatic to exocrine pancreatic cells after treatment with polychlorinated biphenils (27) and transplantation of pancreatic epithelial cells (28), as well as for the conversion of kidney tubular epithelial cells into fibroblasts during experimental fibrosis (29) and of Bowman's capsule epithelial cells into myofibroblasts during experimental glomerulonephritis (30). On the other hand, the term “transdifferentiation” can hardly be applied to the differentiation of embryonic endothelial cells into cardiomyocytes in various experimental conditions (31) or to the switch from muscular to adipose phenotype in skeletal muscle under disruption of Wnt signaling (32). In contrast with the examples quoted above, we show that transdifferentiation may take place in normal, adult animals as part of a physiological process, and that it may be reversible.
It is classically described (33) and has recently been confirmed (34) that during pregnancy mammary gland secretory alveoli develop from stem cells of the duct system. Nevertheless, our data suggest a substantial role for adipocyte transdifferentiation. Clearly, both processes take place, their relative contributions to mammary gland development during pregnancy and lactation probably varying in different species and in different physiological or pathological conditions. A-ZIP/F-1 transgenic mice lacking differentiated white fat (35) were reported to show an apparently normal development of the mammary gland during pregnancy (36); however, a role for their poorly differentiated s.c. adipocytes in the adipocyte-to-epithelial transdifferentiation process remains to be investigated.
In conclusion, our results indicate that direct and reversible transdifferentiation is a physiological process that takes place in the adult, normal animal. Unraveling the molecular events responsible for this unusual cell behavior may shed light on new mechanisms that control gene expression in differentiated cells. The present data also foreshadow the possibility that adipose–glandular transdifferentiation occurs in the human mammary gland.
Supplementary Material
Acknowledgments
We thank E. Raviola for stimulating criticism during the experimental part of the work and for helpful comments on the manuscript; and D. Accili and M. O. Carruba for critically reading the manuscript. This work was supported by grants from Italian Ministry of the University (Cofinanziamento Bando 2001, to S.C. and E.N., and FIRB 2001, to S.C.), Italian Ministry of Health (Ricerca Finalizzata Bando 2002, to E.N.), Fondazione Cassa di Risparmio di VR, VC, BL, e AN (Verona, Vicenza, Belluno, and Ancona, respectively; Bando 2003 to S.C.), and European Union DLARFID Project Contract QLK1 2001-00183 (to S.C.).
Author contributions: M. Morroni, A.G., M.C.Z., R.B., R.D.M., C.T., C.P., M.M.L., and M. Marelli performed research; M. Morroni, A.G., M.C.Z., B.B.K., E.N., and S.C. analyzed data; A.G. and S.C. wrote the paper; B.B.K. contributed new reagents/analytic tools; and S.C. designed research.
Abbreviations: WAP, whey acidic protein; aP2, adipocyte-binding protein 2.
References
- 1.Hennighausen, L. & Robinson, G. W. (2001) Dev. Cell 1, 467–475. [DOI] [PubMed] [Google Scholar]
- 2.Silberstein, G. B. (2001) Microsc. Res. Tech. 52, 155–162. [DOI] [PubMed] [Google Scholar]
- 3.Tosh, D. & Slack, J. M. W. (2002) Nat. Rev. Mol. Cell Biol. 3, 187–194. [DOI] [PubMed] [Google Scholar]
- 4.Soriano, P. (1999) Nat. Genet. 21, 70–71. [DOI] [PubMed] [Google Scholar]
- 5.Wagner, K.-U., Wall, R. J., St-Onge, L., Gruss, P., Wynshaw-Boris, A., Garret, L., Li, M., Furth, P. A. & Hennighausen, L. (1997) Nucleic Acids Res. 25, 4323–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abel, E. D., Peroni, O., Kim, J. K., Kim, Y.-B., Boss, O., Hadro, E., Minnemann, T., Shulman, G. I. & Kahn, B. B. (2001) Nature 409, 729–733. [DOI] [PubMed] [Google Scholar]
- 7.Zambrowicz, B. P., Immoto, A., Fiering, S., Herzenberg, L. A., Kerr, W. G. & Soriano, P. (1997) Proc. Natl. Acad. Sci. USA 94, 3789–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cinti, S., Cigolini, M., Morroni, M. & Zingaretti, M. C. Z. (1989) Anat. Rec. 224, 466–472. [DOI] [PubMed] [Google Scholar]
- 9.Anbazhagan, R. & Gusterson, B. A. (1995) Anat. Rec. 241, 129–135. [DOI] [PubMed] [Google Scholar]
- 10.Napolitano, L. (1963) J. Cell Biol. 18, 663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cinti, S. (1999) The Adipose Organ (Editrice Kurtis, Milan).
- 12.Blanchette-Mackie, E. J., Dwyer, N. K., Barber, T., Coxey, R. A., Takeda, T., Rondinone, C. M., Theodorakis, J. L., Greenberg, A. S. & Londos, C. (1995) J. Lipid Res. 36, 1211–1226. [PubMed] [Google Scholar]
- 13.Elias, J. J., Pitelka, D. R. & Armstrong, R. C. (1973) Anat. Rec. 177, 533–548. [DOI] [PubMed] [Google Scholar]
- 14.Neville, M. C., Medina, D., Monks, J. & Hovey, R. C. (1998) J. Mammary Gland Biol. Neoplasia 3, 109–116. [DOI] [PubMed] [Google Scholar]
- 15.Robinson, G. W., McKnight, R. A., Smith, G. H. & Hennighausen, L. (1995) Development (Cambridge, U.K.) 121, 2079–2090. [DOI] [PubMed] [Google Scholar]
- 16.Traurig, H. H. (1967) Anat. Rec. 159, 239–246. [DOI] [PubMed] [Google Scholar]
- 17.Joshi, K., Ellis, J. T. B., Hughes, C. M., Monaghan, P. & Neville, A. M. (1986) Lab. Invest. 54, 52–61. [PubMed] [Google Scholar]
- 18.Specht, B., Bartetzko, N., Hohoff, C., Kuhl, H., Franke, R., Borchers, T. & Spener, F. (1996) J. Biol. Chem. 271, 19943–19949. [DOI] [PubMed] [Google Scholar]
- 19.Graves, R. A., Tontonoz, P., Platt, K. A., Ross, S. R. & Spiegelman, B. M. (1992) J. Cell. Biochem. 49, 219–224. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi, G. & Kodama, R. (1993) Curr. Opin. Cell Biol. 5, 1023–1028. [DOI] [PubMed] [Google Scholar]
- 21.Beresford, W. A. (1990) Cell Differ. Dev. 29, 81–93. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y. & Rao, M. S. (2003) J. Cell. Biochem. 88, 29–40. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs, E. & Segre, J. A. (2000) Cell 100, 143–155. [DOI] [PubMed] [Google Scholar]
- 24.Rao, M. S., Dwivedi, R. S., Subbarao, V., Usman, M. I., Scarpelli, D. G., Nemali, M. R., Yeldandi, A., Thangada, S., Kumar, S. & Reddy, J. K. (1988) Biochem. Biophys. Res. Commun. 156, 131–136. [DOI] [PubMed] [Google Scholar]
- 25.Rao, M. S., Dwivedi, R. S., Yeldandi, A., Subbarao, V., Tan, X. D., Usman, M. I., Thangada, S., Nemali, M. R., Kumar, S. & Scarpelli, D. G. (1989) Am. J. Pathol. 134, 1069–1086. [PMC free article] [PubMed] [Google Scholar]
- 26.Krakowski, M. L., Kritzik, M. R., Jones, E. M., Krahl, T., Lee, J., Arnush, M., Gu, D. & Sarvetnick, N. (1999) Am. J. Pathol. 154, 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao, M. S., Bendayan, M., Kimbrough, R. D. & Reddy, J. K. (1986) J. Histochem. Cytochem. 34, 197–201. [DOI] [PubMed] [Google Scholar]
- 28.Dabeva, M. D., Hwang, S. G., Vasa, S. R., Hurston, E., Novikoff, P. M., Hixson, D. C., Gupta, S. & Shafritz, D. A. (1997) Proc. Natl. Acad. Sci. USA 94, 7356–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwano, M., Plieth, D., Danoff, T. M., Xue, C., Okada, H. & Neilson, E. G. (2002) J. Clin. Invest. 110, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujigaki, Y., Sun, D. F., Fujimoto, T., Suzuki, T., Goto, T., Yonemura, K., Morioka, T., Yaoita, E. & Hishida, A. (2002) Nephron 92, 203–212. [DOI] [PubMed] [Google Scholar]
- 31.Condorelli, G., Borello, U., De Angelis, L., Latronico, M., Strabella, D., Coletta, M., Galli, R., Balconi, G., Follenzi, A., Frati, G., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10733–10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross, S. E., Hemati, N., Longo, K. A., Bennet, C. N., Lucas, P. C., Erickson, R. L. & MacDougald, O. A. (2000) Science 289, 950–953. [DOI] [PubMed] [Google Scholar]
- 33.Smith, G. H. & Medina, D. A. (1988) J. Cell Sci. 89, 173–183. [DOI] [PubMed] [Google Scholar]
- 34.Rijnkels, M. & Rosen, J. M. (2001) J. Cell Sci. 114, 3147–3153. [DOI] [PubMed] [Google Scholar]
- 35.Moitra, J., Mason, M. M., Olive, M., Krylov, D., Gavrilova, O., Marcus-Samuels, B., Feigenbaum, L., Lee, E., Aoyama, T., Eckhaus, M., et al. (1998) Genes Dev. 12, 3168–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couldrey, C., Moitra, J., Vinson, C., Anver, M., Nagashima, K. & Green, J. (2002) Dev. Dyn. 223, 459–468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.