Abstract
In vitro maturation (IVM) is emerging as a popular technology at the forefront of fertility treatment and preservation. However, standard in vitro culture (IVC) conditions usually increase reactive oxygen species (ROS), which have been implicated as one of the major causes for reduced embryonic development. It is well-known that higher than physiological levels of ROS trigger granulosa cell apoptosis and thereby reduce the transfer of nutrients and survival factors to oocytes, which leads to apoptosis. ROS are neutralized by an elaborate defense system that consists of enzymatic and non-enzymatic antioxidants. The balance between ROS levels and antioxidants within IVM media are important for maintenance of oocytes that develop to the blastocyst stage. The effects of antioxidant supplementation of IVM media have been studied in various mammalian species. Therefore, this article reviews and summarizes the effects of ROS on oocyte quality and the use of antioxidant supplementations for IVM, in addition to its effects on maturation rates and further embryo development.
Keywords: Oxidative Stress, Reactive Oxygen Species, Antioxidant, In Vitro Maturation
Introduction
In vitro embryo production (IVEP) allows the production of a high and inexpensive number of embryos to conduct basic research and apply emerging biotechnologies such as cloning and transgenesis. IVEP is a three-step methodology that comprises the following procedures: i. In vitro maturation (IVM) of oocytes recovered directly from follicles, ii. In vitro fertilization (IVF) or co-incubation of capacitated spermatozoa with in vitro matured oocytes, and iii. In vitro culture (IVC) of zygotes up to the blastocyst stage. According to reports, IVM is the key factor that determines the proportion of oocytes which develop to the blastocyst stage.
IVM of oocytes is a complex process influenced by the interplay of regulatory factors that include gonadotrophins and a growing list of secreted molecules, the biochemical state of the oocyte, and interactions between the oocyte and cumulus cells (1- 5). Therefore, the in vitro advancement of an oocyte from the diplotene stage of prophase I [germinal vesicle (GV)] to metaphase II (MII), along with cytoplasmic maturation that encloses a broad set of still ill-defined cellular events are essential for fertilization and early development of the embryo (6-8).
Although substantial progress has been made to improve the efficiency of an IVM protocol, however, there is a lack of consistency in the success rate of conventional in vitro matured oocytes compared to in vivo matured oocytes. Multiple factors likely contribute to the overall poor quality of in vitro matured oocytes. One of the important factors may be oxidative stress (OS). The generation of pro-oxidants such as reactive oxygen species (ROS) is an invariable phenomenon in the culture condition. It is possible that OS also influences oocyte development in vitro. On the other hand, ROS are considered signal molecules in oocyte physiology and their impact on maturation promoting factor (MPF) destabilization has recently been reported (9-11).
Oocyte protection against ROS may play important roles in pre-implantation embryonic development. On the other hand, antioxidants are ROS scavengers, thereby helping to maintain the oocyte’s oxidant/antioxidant balance. The effects of antioxidant supplementation to IVM media have been studied in various mammalian species (12-14). Our purpose was to incorporate the role of ROS in oocyte physiology, impact of OS in downfall of oocyte quality (15, 16), and the role of enzymatic as well as non-enzymatic antioxidants in reducing ROS levels and deterioration of oocyte quality under IVC conditions. This review article summarized the effects of ROS, the use of antioxidant supplementations for IVM, and its effects on maturation rates. In this systematic review, we used IVM, OS, ROS, and antioxidant as keywords from scientific databases between 1990 and 2016. After a review of all abstracts, we included strong, reliable research in this report.
Production of reactive oxygen species and generation of oxidative stress
OS is caused by an imbalance between pro-oxidants and antioxidants (17). This ratio could change with increased levels of pro-oxidants, such as ROS, or a decrease in antioxidant defense mechanisms (18-20). ROS represents a wide class of molecules that indicate the collection of free radicals (hydroxyl ion, superoxide, etc.), non-radicals (ozone, single oxygen, lipid peroxides, hydrogen peroxide) and oxygen derivatives (21). They are highly reactive and unstable. Hence, ROS can react with nucleic acids, lipids, proteins, and carbohydrates to acquire an electron and become stable. These reactions induce a cascade of subsequent chain reactions that eventually result in cell damage (22-24). ROS can diffuse and pass through cell membranes and alter most types of cellular molecules (nucleic acids, proteins, and lipids), leading to mitochondrial alterations (25), meiotic arrest in the oocytes (26), embryonic block, and cell death (27). On the other hand, OS occurs when increased ROS levels which disrupt cellular redox circuits, result in disturbances of redox-regulated cellular processes and/or oxidatively damage cellular macromolecules (28).
Oxidative stress and in vitro maturation
Under physiological conditions, the oocytes are major sources of ROS because they use oxygen to produce energy through mitochondrial oxidative phosphorylation. Their ROS production is increased during IVM when compared to in vitro maturation (13, 29). Increased levels of ROS beyond the physiological range that may lead to OS can result in deterioration in oocyte quality and thereby affect reproductive outcomes (30). A better understanding of the OS status and its regulation during IVM is needed. However, one must also consider whether and how OS may influence the process of IVM. This section focuses on reports that refer to mechanistic roles for OS in oocyte maturation, especially with respect to key features of nuclear and cytoplasmic events within the oocyte.
Reactive oxygen species and nuclear and cytoplasmic maturation
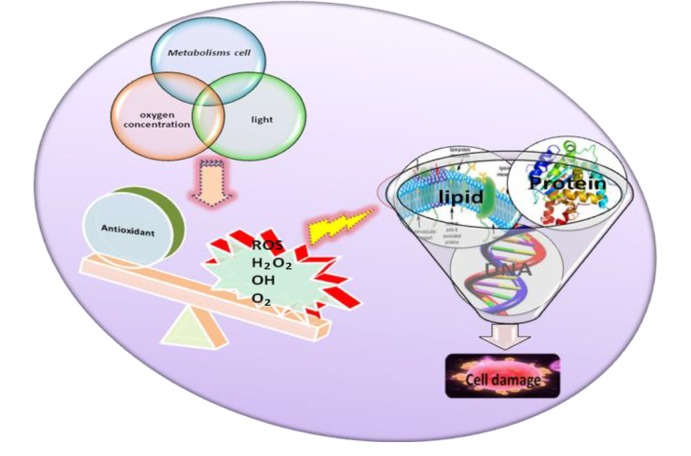
Increased levels of ROS associated with induce cell cycle arrest in human oocytes as well as in mouse embryos (31). A multitude of key factors regulate the generation of ROS in the media and include various cellular metabolic reactions, oxygen concentration, light, oocyte handling, and general physicochemical parameters that may have a negative impact on oocyte physiology by inducing apoptosis (Fig .1). One of the major constituent that may alter developmental responses in the oocyte is relevant to OS since light is known to result in an imbalance of pro- and antioxidants in somatic cells and embryos. Similarly, a relationship has been shown in a mouse model between a type of light commonly used in the laboratory with increased ROS concentrations and compromised embryonic and fetal development (32). Oxygen tension is another important difference between the in vivo and in vitro environments for the oocyte culture. Toxic effects of atmospheric oxygen concentration under standard culture conditions and the beneficial effects of lower O2 concentrations (5-7%) on developmental competence of oocytes in vitro have been reported in mice (33, 34), hamsters (35, 36), rats (37), sheep and cattle (38-40), and humans (41-43).
Fig.1.
The possible factors that induce generation of reactive oxygen species (ROS) in the oocyte. The imbalance between ROS and antioxidants, the impact of high levels of ROS, and the resulting oxidative stress (OS) on meiotic arrest and apoptosis in oocytes.
The conditions of an IVC generate ROS, which could exert some beneficial effects if the ROS levels remain under physiological levels (44). The tonic generation of ROS triggers meiotic resumption from diplotene as well as the MII arrest stage in several mammalian species (44, 45). It has been reported that levels of ROS beyond the physiological range could induce destabilization of maturation MPF, reduce survival factors, and trigger mitochondria-mediated apoptosis of oocytes (15, 46). The biphasic role of ROS must be sufficiently discussed in order to update OS and its impact on oocyte quality (15). The beneficial role of ROS comes from the observations that non-enzymatic antioxidants, such as ascorbic acid and 3-tert-butyl-4-hydroxyanisole (BHA), inhibit spontaneous meiotic resumption from diplotene arrest (47). These results suggest a beneficial threshold level for ROS.
Antioxidants
Antioxidants scavenge ROS, which helps maintain the cell oxidant/antioxidant balance. On the other hand, antioxidants are the compounds which either suppress the formation of ROS or oppose their actions. There are two types of antioxidants: enzymatic and non-enzymatic (Table 1).
Table 1.
List of studies that show the effects of antioxidant supplements that improve in vitro maturation
| Antioxidant | Experimental model |
|---|---|
| Enzymatic antioxidants | |
| Superoxide dismutase (SOD) | Mouse |
| Thioredoxin | Porcine |
| Catalase (CAT) | Bovine |
| Sericin | Bovine |
| Non-enzymatic antioxidants | |
| Glutathione (GSH) | Hamster, pig, ovine, |
| Bovine and equine | |
| Cysteamine | Canine, mice, goats, porcine |
| Vitamin C (Ascorbic acid) | Mouse |
| Vitamin E and trolox | - |
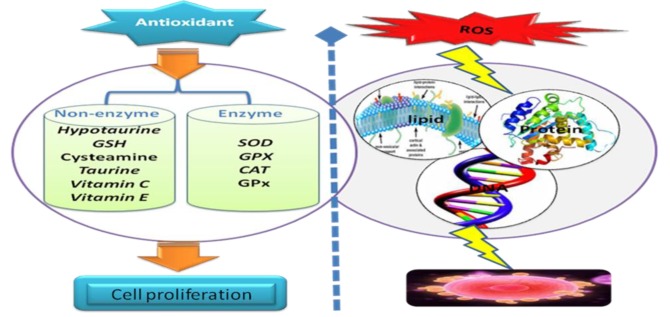
Enzymatic antioxidants neutralize excess ROS and prevent it from damaging the cellular structure. Enzymatic antioxidants are composed of superoxide dismutase (SOD), catalase (CAT), various peroxidases and peroxiredoxins (PRDXs), including glutathione peroxidases (GPXs), which can convert peroxides to water and alcohol (48). SOD enzymes catalyze the dismutation of superoxide anion (O2-) into O2 and HO2O2 while CAT converts HO2O2 to O2 and H2O. The enzyme SOD exists as three isoenzymes (49): SOD1, SOD2, and SOD3. SOD1 contains Cu and zinc (Zn) as metal co-factors in the cytosol. SOD2 is a mitochondrial isoform that contains manganese (Mn), whereas SOD3 encodes the extracellular form. Nutrients such as Se, Cu, and Zn are required for the activities of some antioxidant enzymes, although they have no antioxidant actions. Non-enzymatic antioxidants are composed of glutathione (GSH), vitamin C, taurine, hypotaurine, vitamin E, Zn, selenium (Se), beta carotene, and carotene (47). GSH is a tripeptide thiol compound with many important functions in intracellular physiology and metabolism. One of the most important roles of GSH is to maintain the redox state in cells which protects them against harmful effects effects caused by oxidative injuries. The protective action of GSH against ROS is facilitated by the interactions with its associated enzymes, such as GPx and GSH reductase (Fig .2).
Fig.2.
The presence of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidases (GPx), and catalase (CAT) as well as non-enzymatic antioxidants, such as vitamin E and C (ascorbic acid), glutathione (GSH), uric acid, and albumin in the oocytes. Excess amounts of reactive oxygen species (ROS) may be involved in oxidative stress (OS) of oocytes and granulosa cells.
Vitamin C (ascorbic acid) is a known redox catalyst that can reduce and neutralize ROS (50). Based on its chemical structure, ascorbic acid is an electron donor and therefore a reducing agent. It has two different biochemical roles-antioxidant and enzymatic cofactor. Ascorbic acid is maintained through reactions with GSH and can be catalyzed by protein disulfide isomerase and glutaredoxins. Cysteamine is a low-molecular weight amino acid that contains thiol (51). The addition of cysteamine not only enhances the GSH content in MII oocytes but also protects the membrane lipids and proteins due to indirect radical scavenging properties (52). The concentrations of many amino acids, including taurine and hypotaurine are non-enzymatic antioxidants that help maintain the redox status in oocytes (53).
Vitamin E (α-tocopherol) is a lipid soluble vitamin with antioxidant activity. It consists of eight tocopherols and tocotrienols. Vitamin E may directly destroy free radicals such as peroxyl and alkoxyl (ROO•) generated during ferrous ascorbate-induced lipid peroxidation (LPO), thus it is suggested as a major chain breaking antioxidant (54). Hyaluronan, melatonin, tea and sericin are known to act as indispensable antioxidants in IVEP. They can block the release of pro-oxidant factors released as a result of OS (12, 55, 56).
Hyaluronan, an essential component of the extracellular matrix and non-sulfated glycosaminoglycan may play an important role in meiotic resumption of oocytes (57). The hormone melatonin (N-acetyl-5-metoxy tryptamine) is an antioxidant that, unlike GSH and vitamins C and E, is produced by mammals. In contrast to other antioxidants, however, melatonin cannot undergo redox cycling. Once oxidized, it is unable to return to its reduced state because of the formation of stable end-products after the reaction (14). As an antioxidant, green tea has been shown to improve IVM and embryo development of sheep COCs to the blastocyst stage in IVM medium (58). Sericin a water-soluble globular protein (protein hydrolysate) is derived from the silkworm Bombyx mori. This protein represents a family of proteins whose molecular mass ranges from 10 to 310 kDa (59). Dash et al. (60) have reported that sericin might provide a protective effect on fibroblasts by promoting endogenous antioxidant enzymes in vitro.
Antioxidant supplements and improving in vitro maturation
The addition of enzymatic antioxidants such as SOD, CAT, and thioredoxin are effective for pre-embryo development as scavengers of ROS and serving embryos a low OS condition in mice (61, 62), porcine (63), and bovines (64). Sericin, an antioxidant protein, improves embryo development (60, 65) and is a critical supplement for oocyte maturation (12, 56).
A series of non-enzymatic antioxidants protect oocytes against ROS damage during oocyte maturation. GSH is one of the naturally synthesized antioxidants that protect cells from ROS toxicity and regulate the intracellular redox balance (66). The intracellular level of GSH increases during oocyte maturation in hamsters (67), pigs (68), ovine (69), bovines (70), and equines (71). Recent reports have shown that addition of low molecular weight thiol compounds, such as cysteamine and b-mercaptoethanol to IVM media improved the cytoplasmic maturation of oocytes and embryo development by increasing GSH synthesis (66, 72, 73).
Cysteamine supplementation during IVM reportedly improved nuclear maturation rates in canines (74), mice (75), goats (76), and porcine (77). Although, other studies in goats (78), pigs (79), horses (13), buffalos (80), and cattle (81) did not show any increase in nuclear maturation rates. Addition of cysteamine to the IVM medium improved embryo development to the blastocyst stage in mammalian oocytes (82).
Ascorbate is concentrated in granulosa cells, theca cells, luteal cells, and oocytes (28). Choi et al. (83) reported a beneficial role for vitamin C in protecting spindle structures of MII mouse oocytes and chromosomal alignment against an oxidant (hydrogen peroxide)-induced damage. It is suggested that the effect of vitamin C is associated mainly with its capability to promote ooplasmic maturation during IVM. The beneficial role of ROS comes from the observations that non-enzymatic antioxidants, such as ascorbic acid, inhibit spontaneous meiotic resumption from diplotene arrest. We have presented a number of these observations. Tatemoto et al. (84), Kere et al. (85), and Córdova el al. (86) found that the addition of vitamin C to the oocyte maturation medium exerted no effect on the maturation rates of oocytes. Similarly, antioxidants such as vitamin E and trolox had no effect on oocyte maturation, but other antioxidants such as propyl gallate and 2,4,5-trihydroxybutrophenone inhibited the spontaneous resumption of meiosis (87). Together, these studies emphasized the beneficial roles of ROS during IVM at certain concentrations (low level).
Conclusion
It is well-known that high levels of ROS beyond the physiological range could induce MPF destabilization, reduce survival factors, and trigger apoptosis in oocytes of several mammalian species. Antioxidants are the main defense factors against OS induced by ROS. Many reports suggest that antioxidant supplementation of IVM media improves cytoplasmic maturation by alleviating OS during oocyte maturation via increasing GSH storage, and contributes to further protect the embryo against oxidative aggressions during its early developmental stages. On the other hand, supplementation by antioxidants during IVC improves oocyte quality by reducing ROS levels and apoptotic factors. However, some of the non-antioxidants such as ascorbic acid and 2, 4, 5-trihydroxybutrophenone do not improve oocyte maturation; rather, they inhibit spontaneous resumption of meiosis. Improvements to culture conditions are complex challenges that depend not only on the choice of an antioxidant but also on its concentration, the medium and its components, the species, and the dynamic changes of the specific requirements of the oocyte according to its developmental stage. Future efforts should be placed on understanding the involvement of ROS in oocyte apoptosis and for guiding antioxidant-based strategies to selectively control ROS-induced damage without compromising the physiological functions of these species.
Acknowledgments
This study was supported financially by Kermanshah University of Medical Sciences. There is no conflict of interest in this study.
References
- 1.Canipari R. Oocyte-granulosa cell interactions. Hum Reprod Update. 2000;6(3):279–289. doi: 10.1093/humupd/6.3.279. [DOI] [PubMed] [Google Scholar]
- 2.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122(6):829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 3.Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev. 2002;61(3):414–424. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- 4.Combelles CM, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod. 2005;20(5):1349–1358. doi: 10.1093/humrep/deh750. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67(1):6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Smitz J, Nogueira D, Albano C, Cortvrindt R, Devroey P. Improving in vitro maturation of oocytes in the human taking lessons from experiences in animal species. Reprod Domest Anim. 2001;36(1):11–17. doi: 10.1046/j.1439-0531.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 7.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121(1):51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 8.Ali A, Benkhalifa M, Miron P. In-vitro maturation of oocytes: biological aspects. Reprod Biomed Online. 2006;13(3):437–446. doi: 10.1016/s1472-6483(10)61450-2. [DOI] [PubMed] [Google Scholar]
- 9.Premkumar KV, Chaube SK. Increased level of reactive oxygen species persuades postovulatory aging-mediated spontaneous egg activation in rat eggs cultured in vitro. In Vitro Cell Dev Biol Anim. 2016;52(5):576–588. doi: 10.1007/s11626-016-0007-3. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari M, Prasad S, Tripathi A, Pandey AN, Singh AK, Shrivastav TG, et al. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. Reactive Oxygen Species. 2016;1(2):110–116. [Google Scholar]
- 11.Chaube SK, Shrivastav TG, Prasad S, Tiwari M, Tripathi A, Pandey AN, et al. Clomiphene citrate induces ROSmediated apoptosis in mammalian oocytes. Open J Apoptosis. 2014;3(3):52–58. [Google Scholar]
- 12.Aghaz F, Hajarian H, Shabankareh HK, Abdolmohammadi A. Effect of sericin supplementation in maturation medium on cumulus cell expansion, oocyte nuclear maturation, and subsequent embryo development in Sanjabi ewes during the breeding season. Theriogenology. 2015;84(9):1631–1635. doi: 10.1016/j.theriogenology.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Deleuze S, Goudet G. Cysteamine supplementation of in vitro maturation media: a review. Reprod Domest Anim. 2010;45(6):e476–482. doi: 10.1111/j.1439-0531.2010.01587.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues-Cunha MC, Mesquita LG, Bressan F, Collado MD, Balieiro JC, Schwarz KR, et al. Effects of melatonin during IVM in defined medium on oocyte meiosis, oxidative stress, and subsequentembryo development. Theriogenology. 2016;86(7):1685–1694. doi: 10.1016/j.theriogenology.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:26–26. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari M, Prasad S, Tripathi A, Pandey AN, Ali I, Singh AK, et al. Apoptosis in mammalian oocytes: a review. Apoptosis. 2015;20(8):1019–1025. doi: 10.1007/s10495-015-1136-y. [DOI] [PubMed] [Google Scholar]
- 17.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, et al. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol. 2007;171(4):11681179–11681179. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21(3):219–222. doi: 10.1097/gco.0b013e32832924ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A, Prabakaran SA. Mechanism, measurement and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43(11):963–974. [PubMed] [Google Scholar]
- 22.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45(5):314–320. [PubMed] [Google Scholar]
- 23.Szczepańska M, Koźlik J, Skrzypczak J, Mikołajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79(6):1288–1293. doi: 10.1016/s0015-0282(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 24.Pierce JD, Cackler AB, Arnett MG. Why should you care about free radicals? RN. 2004;67(1):38–42. [PubMed] [Google Scholar]
- 25.Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26(3-4):463–471. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Yamagata Y, Sugino N, Takayama H, Kato H. Nitric oxide inhibits oocyte meiotic maturation. Biol Reprod. 2002;67(5):1588–1592. doi: 10.1095/biolreprod.102.005264. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto S, Minami N, Yamada M, Imai H. Excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev. 2000;56(4):520–526. doi: 10.1002/1098-2795(200008)56:4<520::AID-MRD10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27–27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha-Frigoni NA, Leão BC, Dall'Acqua PC, Mingoti GZ. Improving the cytoplasmic maturation of bovine oocytes matured in vitro with intracellular and/or extracellular antioxidants is not associated with increased rates of embryo development. Theriogenology. 2016;86(8):18971905–18971905. doi: 10.1016/j.theriogenology.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Chaube SK, Shrivastav TG, Tiwari M, Prasad S, Tripathi A, Pandey AK. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. Springerplus. 2014;3:464–464. doi: 10.1186/2193-1801-3-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, et al. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res. 2009;43(3):287–294. doi: 10.1080/10715760802695985. [DOI] [PubMed] [Google Scholar]
- 32.Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci USA. 2007;104(36):14289–14293. doi: 10.1073/pnas.0706687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auerbach S, Brinster RL. Effect of concentration on the development of two-cell mouse embryos. Nature. 1968;217:465–466. doi: 10.1038/217465a0. [DOI] [PubMed] [Google Scholar]
- 34.Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86(4 Suppl):1252-1265, e1-36. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Mckiernan SH, Bavister BD. Environmental variables influencing in vitro development of hamster 2-cell embryos to the blastocyst stage. Biol Reprod. 1990;43(3):404–413. doi: 10.1095/biolreprod43.3.404. [DOI] [PubMed] [Google Scholar]
- 36.Bavister BD. Role of oviductal secretions in embryonic growth in vivo and in vitro. Theriogenology. 1988;29(1):143–154. [Google Scholar]
- 37.Kishi J, Noda Y, Narimoto K, Umaoka Y, Mori T. Block to development in cultured rat 1-cell embryos is overcome using medium HECM-1. Hum Reprod. 1991;6(10):14451448–14451448. doi: 10.1093/oxfordjournals.humrep.a137286. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JG, Simpson AC, Pugh PA, Donnelly PE, Tervit HR. Effect of oxygen concentration on in-vitro development of preimplantation sheep and cattle embryos. J Reprod Fertil. 1990;89(2):573–578. doi: 10.1530/jrf.0.0890573. [DOI] [PubMed] [Google Scholar]
- 39.Harlow GM, Quinn P. Foetal and placental growth in the mouse after pre-implantation development in vitro under oxygen concentrations' of 5 and 20% Aust J Biol Sci. 1979;32(3):363–369. doi: 10.1071/bi9790363. [DOI] [PubMed] [Google Scholar]
- 40.Wolfenson D, Roth Z, Meidan R. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim Reprod Sci. 2000;60-61:535–547. doi: 10.1016/s0378-4320(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 41.Ciray HN, Aksoy T, Yaramanci K, Karayaka I, Bahceci M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertil Steril. 2009;91(4):1459–1461. doi: 10.1016/j.fertnstert.2008.07.1707. [DOI] [PubMed] [Google Scholar]
- 42.Dumoulin JC, Meijers CJ, Bras M, Coonen E, Geraedts JP, Evers JL. Effect of oxygen concentration on human invitro fertilization and embryo culture. Hum Reprod. 1999;14(2):465–469. doi: 10.1093/humrep/14.2.465. [DOI] [PubMed] [Google Scholar]
- 43.Kovačič B, Vlaisavljević V. Influence of atmospheric versus reduced oxygen concentration on development of human blastocysts in vitro: a prospective study on sibling oocytes. Reprod Biomed Online. 2008;17(2):229–236. doi: 10.1016/s1472-6483(10)60199-x. [DOI] [PubMed] [Google Scholar]
- 44.Tiwari M, Chaube SK. Moderate increase of reactive oxygen species triggers meiotic resumption in rat follicular oocytes. J Obstet Gynaecol Res. 2016;42(5):536–546. doi: 10.1111/jog.12938. [DOI] [PubMed] [Google Scholar]
- 45.Pandey AN, Tripathi A, PremKumar KV, Shrivastav TG, Chaube SK. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem. 2010;111(3):521–528. doi: 10.1002/jcb.22736. [DOI] [PubMed] [Google Scholar]
- 46.Chaube S, Khatun S, Misra S, Shrivastav T. Calcium ionophore-induced egg activation and apoptosis are associated with the generation of intracellular hydrogen peroxide. Free Radic Res. 2008;42(3):212–220. doi: 10.1080/10715760701868352. [DOI] [PubMed] [Google Scholar]
- 47.Pandey AN, Chaube SK. A moderate increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. Biores Open Access. 2014;3(4):183–191. doi: 10.1089/biores.2014.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28–28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tripathi A, PremKumar KV, Pandey AN, Khatun S, Mishra SK, Shrivastav TG, et al. Melatonin protects against clomiphene citrate-induced generation of hydrogen peroxide and morphological apoptotic changes in rat eggs. Eur J Pharmacol. 2011;667(1-3):419–424. doi: 10.1016/j.ejphar.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Balboula AZ, Yamanaka K, Sakatani M, Kawahara M, Hegab AO, Zaabel SM, et al. Cathepsin B activity has a crucial role in the developmental competence of bovine cumulus-oocyte complexes exposed to heat shock during in vitro maturation. Reproduction. 2013;146(4):407–417. doi: 10.1530/REP-13-0179. [DOI] [PubMed] [Google Scholar]
- 51.Uysal O, Bucak MN. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen. Acta Vet Brno. 2007;76(3):383–390. [Google Scholar]
- 52.Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Agarwal A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999;161(6):1831–1834. [PubMed] [Google Scholar]
- 53.Premkumar KV, Chaube SK. RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. In Vitro Cell Dev Biol Anim. 2014;50(7):640–647. doi: 10.1007/s11626-014-9749-y. [DOI] [PubMed] [Google Scholar]
- 54.Bansal AK, Bilaspuri GS. Antioxidant effect of vitamin E on motility, viability and lipid peroxidation of cattle spermatozoa under oxidative stress. Anim Sci Pap Rep. 2009;27(1):5–14. [Google Scholar]
- 55.Aghaz F, Hajarian H, KaramiShabankareh H. In vitro culture medium (IVC) supplementation with sericin improves developmental competence of ovine zygotes. Reprod Biol. 2016;16(1):87–90. doi: 10.1016/j.repbio.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Aghaz F, Hajarian H, Shabankareh HK. Enhanced in vitro developmental competence of sheep embryos following sericin supplementation of the in vitro maturation and in vitro culture media. Small Rumin Res. 2016;136(6):257260–257260. [Google Scholar]
- 57.Prasad S, Tiwari M, Tripathi A, Pandey AN, Chaube SK. Changes in signal molecules and maturation promoting factor levels associate with spontaneous resumption of meiosis in rat oocytes. Cell Biol Int. 2015;39(6):759–769. doi: 10.1002/cbin.10444. [DOI] [PubMed] [Google Scholar]
- 58.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea-a review. J Am Coll Nutr. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 59.Tao W, Li M, Xie R. Preparation and structure of porous silk sericin materials. Macromol Mater Eng. 2005;290(3):188–194. [Google Scholar]
- 60.Dash R, Acharya C, Bindu P, Kundu SC. Antioxidant potential of silk protein sericin against hydrogen peroxideinduced oxidative stress in skin fibroblasts. BMB Rep. 2008;41(3):236–421. doi: 10.5483/bmbrep.2008.41.3.236. [DOI] [PubMed] [Google Scholar]
- 61.Legge M, Sellens MH. Free radical scavengers ameliorate the 2-cell block in mouse embryo culture. Hum Reprod. 1991;6(6):867–871. doi: 10.1093/oxfordjournals.humrep.a137442. [DOI] [PubMed] [Google Scholar]
- 62.Natsuyama S, Noda Y, Yamashita M, Nagahama Y, Mori T. Superoxide dismutase and thioredoxin restore defective p34cdc2 kinase activation in mouse two-cell block. Biochim Biophys Acta. 1993;1176(1-2):90–94. doi: 10.1016/0167-4889(93)90182-o. [DOI] [PubMed] [Google Scholar]
- 63.Ozawa M, Nagai T, Fahrudin M, Karja NWK, Kaneko H, Noguchi J, et al. Addition of glutathione or thioredoxin to culture medium reduces intracellular redox status of porcine IVM/IVF embryos, resulting in improved development to the blastocyst stage. Mol Reprod Dev. 2006;73(8):998–1007. doi: 10.1002/mrd.20533. [DOI] [PubMed] [Google Scholar]
- 64.Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;59(3):939–949. doi: 10.1016/s0093-691x(02)01125-1. [DOI] [PubMed] [Google Scholar]
- 65.Isobe T, Ikebata Y, Onitsuka T, Wittayarat M, Sato Y, Taniguchi M, et al. Effect of sericin on preimplantation development of bovine embryos cultured individually. Theriogenology. 2012;78(4):747–752. doi: 10.1016/j.theriogenology.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 66.You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology. 2010;74(5):777–785. doi: 10.1016/j.theriogenology.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol. 1988;125(1):181–186. doi: 10.1016/0012-1606(88)90070-x. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod. 1993;49(1):89–94. doi: 10.1095/biolreprod49.1.89. [DOI] [PubMed] [Google Scholar]
- 69.Matos DG, Gasparrini B, Pasqualini SR, Thompson JG. Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology. 2002;57(5):1443–1451. doi: 10.1016/s0093-691x(02)00643-x. [DOI] [PubMed] [Google Scholar]
- 70.de Matos DG, Furnus CC, Moses DF, Martinez AG, Matkovic M. Stimulation of glutathione synthesis of in vitro matured bovine oocytes and its effect on embryo development and freezability. Mol Reprod Dev. 1996;45(4):451–457. doi: 10.1002/(SICI)1098-2795(199612)45:4<451::AID-MRD7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 71.Luciano AM, Goudet G, Perazzoli F, Lahuec C, Gérard N. Glutathione content and glutathione peroxidase expression in in vivo and in vitro matured equine oocytes. Mol Reprod Dev. 2006;73(5):658–666. doi: 10.1002/mrd.20469. [DOI] [PubMed] [Google Scholar]
- 72.Choe C, Shin YW, Kim EJ, Cho SR, Kim HJ, Choi SH, et al. Synergistic effects of glutathione and.β.-mercaptoethanol treatment during in vitro maturation of porcine oocytes on early embryonic development in a culture system supplemented with L-cysteine. J Reprod Dev. 2010;56(6):575–582. doi: 10.1262/jrd.09-214h. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura BN, Fielder TJ, Hoang YD, Lim J, McConnachie LA, Kavanagh TJ, et al. Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology. 2011;152(7):28062815–28062815. doi: 10.1210/en.2011-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hossein MS, Kim MK, Jang G, Oh HJ, Koo O, Kim JJ, et al. Effects of thiol compounds on in vitro maturation of canine oocytes collected from different reproductive stages. Mol Reprod Dev. 2007;74(9):1213–1220. doi: 10.1002/mrd.20674. [DOI] [PubMed] [Google Scholar]
- 75.Chen N, Liow SL, Yip WY, Tan LG, Ng SC. Influence of cysteamine supplementation and culture in portable dry-incubator on the in vitro maturation, fertilization and subsequent development of mouse oocytes. Theriogenology. 2005;63(8):2300–2310. doi: 10.1016/j.theriogenology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Urdaneta A, Jiménez-Macedo AR, Izquierdo D, Paramio MT. Supplementation with cysteamine during maturation and embryo culture on embryo development of prepubertal goat oocytes selected by the brilliant cresyl blue test. Zygote. 2003;11(04):347–354. doi: 10.1017/s0967199403002405. [DOI] [PubMed] [Google Scholar]
- 77.Bing Y, Nagai T, Rodriguez-Martinez H. Effects of cysteamine, Fsh and estradiol-17 beta on in vitro maturation of porcine oocytes. Theriogenology. 2001;55(4):867–876. doi: 10.1016/s0093-691x(01)00449-6. [DOI] [PubMed] [Google Scholar]
- 78.Zhou P, Wu YG, Li Q, Lan GC, Wang G, Gao D, et al. The interactions between cysteamine, cystine and cumulus cells increase the intracellular glutathione level and developmental capacity of goat cumulus-denuded oocytes. Reproduction. 2008;135(5):605–611. doi: 10.1530/REP-08-0003. [DOI] [PubMed] [Google Scholar]
- 79.Song K, Lee E. Modification of maturation condition improves oocyte maturation and in vitro development of somatic cell nuclear transfer pig embryos. J Vet Sci. 2007;8(1):81–87. doi: 10.4142/jvs.2007.8.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singhal S, Prasad S, Singh B, Prasad JK, Gupta HP. Effect of including growth factors and antioxidants in maturation medium used for in vitro culture of buffalo oocytes recovered in vivo. Anim Reprod Sci. 2009;113(1-4):44–50. doi: 10.1016/j.anireprosci.2008.05.078. [DOI] [PubMed] [Google Scholar]
- 81.Balasubramanian S, Rho GJ. Effect of cysteamine supplementation of in vitro matured bovine oocytes on chilling sensitivity and development of embryos. Anim Reprod Sci. 2007;98(3):282–292. doi: 10.1016/j.anireprosci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Wani A, Khan M, Sofi K, Lone F, Malik A, Bhat F. Effect of cysteamine and epidermal growth factor (EGF) supplementation in maturation medium on in vitro maturation, fertilization and culturing of embryos in sheep. Small Rumin Res. 2012;106:160–164. [Google Scholar]
- 83.Choi WJ, Banerjee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factoralpha-induced alterations in metaphase II mouse oocyte spindle structure. Fertil Steril. 2007;88(4):1220–1231. doi: 10.1016/j.fertnstert.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 84.Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alphaglucoside during in vitro maturation. Biol Reprod. 2001;65(6):1800–1806. doi: 10.1095/biolreprod65.6.1800. [DOI] [PubMed] [Google Scholar]
- 85.Kere M, Siriboon C, Lo NW, Nguyen NT, Ju JC. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J Reprod Dev. 2013;59(1):78–84. doi: 10.1262/jrd.2012-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Córdova B, Morató R, Izquierdo D, Paramio T, Mogas T. Effect of the addition of insulin-transferrin-selenium and/ or L-ascorbic acid to the in vitro maturation of prepubertal bovine oocytes on cytoplasmic maturation and embryo development. Theriogenology. 2010;74(8):1341–1348. doi: 10.1016/j.theriogenology.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Takami M, Preston SL, Toyloy VA, Behrman HR. Antioxidants reversibly inhibit the spontaneous resumption of meiosis. Am J Physiol. 1999;276(4 Pt 1):684–688. doi: 10.1152/ajpendo.1999.276.4.E684. [DOI] [PubMed] [Google Scholar]