Abstract
Objectives
We sought to investigate whether and what carotid plaque characteristics predict systemic cardiovascular outcomes in patients with clinically established atherosclerotic disease.
Background
Advancements in atherosclerosis imaging have allowed assessment of various plaque characteristics, some of which are more directly linked to the pathogenesis of acute cardiovascular events compared to plaque burden.
Methods
As part of an event-driven clinical trial (AIM-HIGH), subjects with clinically established atherosclerotic disease underwent multicontrast carotid MRI to detect plaque tissue composition and high-risk features. Prospective associations between MRI measurements and the AIM-HIGH primary endpoint (fatal and non-fatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, and symptom-driven revascularization) were analyzed using Cox proportional hazard models.
Results
Of 232 subjects recruited, 214 (92.2%) with diagnostic image quality constituted the study population (mean age: 61±9 years; males: 82%; statin use: 94%). During a median follow-up of 35.1 months, 18 (8.4%) subjects reached the AIM-HIGH endpoint. High lipid content (hazard ratio per one-standard-deviation increase in % lipid core volume: 1.57, p=0.002) and thin/ruptured fibrous cap (hazard ratio: 4.31, p=0.003) in carotid plaques were strongly associated with the AIM-HIGH endpoint. Intraplaque hemorrhage had a low prevalence (8%) and was marginally associated with the AIM-HIGH endpoint (hazard ratio: 3.00, p=0.053). High calcification content (hazard ratio per one-standard-deviation increase in % calcification volume: 0.66, p=0.20), plaque burden metrics, and clinical risk factors were not significantly associated with the AIM-HIGH endpoint. The associations between carotid plaque characteristics and the AIM-HIGH endpoint changed little after adjusting for clinical risk factors, plaque burden, or AIM-HIGH randomized treatment assignment.
Conclusions
Among patients with clinically established atherosclerotic disease, carotid plaque lipid content and fibrous cap status were strongly associated with systemic cardiovascular outcomes. Markers of carotid plaque vulnerability may serve as novel surrogate markers for systemic atherothrombotic risk.
Keywords: magnetic resonance imaging, carotid artery, atherosclerosis, cardiovascular events, vulnerable plaque, surrogate marker
Introduction
Imaging markers of atherosclerosis, which are traditionally focused on measuring plaque burden, have proven clinical utility in refining cardiovascular risk assessment for individual patients (1,2). Nonetheless, improved understanding of the pathophysiology of myocardial infarction and ischemic stroke directs our attention to high-risk plaques featuring typically thin fibrous cap and large underlying lipid cores, which are considered the true pathological substrate for acute cardiovascular events (3). As such, imaging measurements on high-risk plaque characteristics, compared to those on plaque burden, may potentially serve as more effective biomarkers for atherothrombotic risk in research and clinical practice. Advancements in cardiovascular imaging have enabled identification of high-risk plaque characteristics in the carotid artery. Echolucency of carotid plaques on B-mode ultrasound indicates high lipid content and is used as a feature of high-risk lesions (4). Multidetector computed tomography angiography has good performance in detecting vascular calcification but may also identify large lipid cores as low attenuation regions (5,6). Magnetic resonance imaging (MRI) offers high soft tissue contrast and is increasingly used to evaluate plaque tissue composition and high-risk features (7-12). Although some carotid plaque characteristics have been shown to pose increased risk for ipsilateral stroke (13-15) and are being used in evaluating drug efficacy and defining plaque progression (16-19), data that directly address the association between high-risk carotid plaque characteristics and systemic cardiovascular outcomes remain scarce.
In a prospective cohort study incorporated within the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial (20,21), we investigated whether and what carotid plaque characteristics predict systemic cardiovascular outcomes. The use of multicontrast MRI enabled us to examine a wide spectrum of carotid plaque characteristics. Cardiovascular outcomes as determined in an event-driven clinical trial provided a unique opportunity to test novel imaging markers against rigorously adjudicated clinical events, among subjects undergoing contemporary intensive medical therapy.
Methods
Study Population
The AIM-HIGH Study is a multicenter event-driven clinical trial that assessed the effect of extended-release niacin in patients with atherosclerotic cardiovascular disease and dyslipidemia (20,21). Participants were males and females, aged 45 or older, who had: 1) established vascular disease, defined as documented coronary artery, cerebrovascular, or peripheral artery disease; and 2) well-controlled low-density lipoprotein (LDL) cholesterol but low high-density lipoprotein (HDL) cholesterol (20). An MRI ancillary study (clinicaltrials.gov: NCT00880178) was integrated into the main study, which recruited AIM-HIGH subjects who had no contraindications for MRI or administration of gadolinium contrast (e.g. metal implants, claustrophobia, glomerular infiltration rate < 60 ml/min/1.73 m2) and were willing to participate. Of 447 available AIM-HIGH subjects at 21 clinical sites, 232 met inclusion criteria of the MRI sub-study and underwent MRI at 10 imaging sites (22). Institutional review board approval was obtained at all participating sites. Enrolled subjects provided written informed consent.
Carotid MRI
Carotid MRI was performed at 3T using commercially available carotid phased-array surface coils (GE: 6-channel, Neocoil LLC, Pewaukee, Wisconsin; Philips: 8-channel, Shanghai Chenguang Medical Technologies, Shanghai, China). To comprehensively characterize carotid plaques, a multicontrast protocol was standardized and implemented on both GE and Philips platforms, including 3D time-of-flight, T1, T2, proton-density, and magnetization-prepared rapid acquisition gradient-echo (MPRAGE). Detailed parameters and reproducibility of the MRI protocol have been previously reported (22,23). Each sequence acquired a contiguous stack of cross-sectional images centered on the common carotid bifurcation (acquired in-plane resolution: 0.625 × 0.625 mm2; slice thickness: 2 mm). Post-contrast T1-weighted images were acquired at about 5 minutes after contrast injection (Magnevist, Bayer Healthcare). Total acquisition time was approximately 45 minutes.
Blinded image review was performed in a core lab using a custom-designed image analysis software package (CASCADE, University of Washington, Seattle, Washington) (24). Image analysis was limited to the index side, defined as the carotid artery with patent lumen, or if both were patent, the one with the larger (based on maximum thickness), de novo plaque. The various contrast-weightings were aligned using the common carotid bifurcation as a fiducial marker. Lumen, outer wall, and plaque components were outlined using a semi-automated contouring tool in CASCADE based on multicontrast review criteria (Figure 1) that have been validated previously (7-9). Calcification was classified as areas that were hypointense on all contrast weightings. For juxtaluminal calcifications, accurate detection requires matching and comparing bright-blood (time-of-flight) and black-blood images (Figure 1). Lipid-rich necrotic core (LRNC) was then classified as non-calcified areas that had no- or little-enhancement on post-contrast T1-weighted images using the pre-contrast images as reference. Presence of IPH was recorded if hyperintense signals were noted on MPRAGE images (10). Presence of thin/ruptured fibrous cap was recorded if the integrity of fibrous cap was compromised (thin cap: partially invisible with smooth surface; ruptured cap: partially invisible with surface irregularity or juxtaluminal hyperintensity on time-of-flight images) on time-of-flight, post-contrast T1-weighted, or T2-weighted images (11,12). Presence of ulceration was recorded if there was distinct depression below luminal surface that shows similar signals to flowing blood. Presence of calcified nodule, as described by Virmani et al (3), was recorded if a piece of calcification protrudes into the lumen.
Figure 1. Identification of carotid plaque characteristics on multi-contrast MRI.
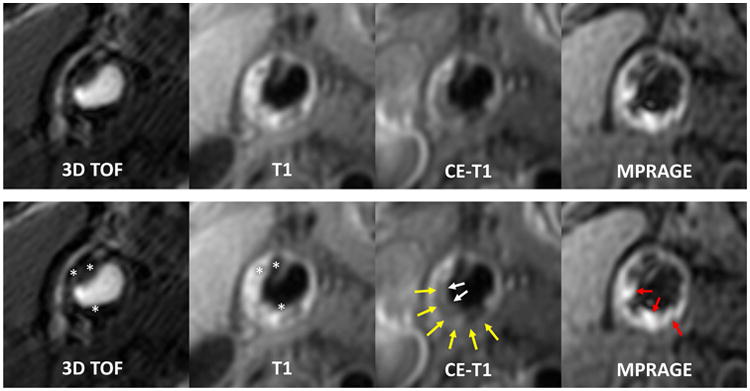
Upper panel: multicontrast images of a representative carotid plaque with all the common plaque characteristics. Lower panel: markers for specific plaque characteristics are added. Areas that are hypointense on all contrast weightings indicate calcification (asterisks); non-calcified areas that have no- or little-enhancement on post-contrast images indicate LRNC (yellow arrows); hyperintense signals on MPRAGE indicate IPH (red arrows). The absence of fibrous cap signals (LRNC is immediately adjacent to lumen) on TOF and CE-T1W indicates the presence of thin/ruptured fibrous cap (white arrows). CE-T1W = contrast enhanced T1-weighted; LRNC = lipid-rich necrotic core; T1W = T1-weighted; T2W = T2-weighted; TOF = time-of-flight.
Continuous measurements were calculated based on plaque segmentation, which are thought to be pathophysiologically relevant to cardiovascular outcomes according to experience from previous studies (16,17): 1) plaque burden, including maximum wall thickness, maximum % wall area (wall area / total vessel area), and % wall volume (wall volume / total vessel volume); 2) plaque composition, including absolute and % volume (component volume / wall volume) of calcification and LRNC, using component-containing slices only and assigned zero if not detected (16,17). Inter-scan reproducibility of plaque measurements derived from multicontrast MRI review using CASCADE has been previously reported, with intraclass correlation coefficient ranging from 0.87 to 0.99 for all morphological and compositional measurements (23,25). Furthermore, the previously proposed definitions of high-risk plaques that include heterogeneous plaque types were also studied (7,22,26): the American Heart Association (AHA) Type VI lesions include plaques with surface disruption (i.e. cap rupture, ulceration, and calcified nodule), IPH, or mural thrombus; the Carotid Atherosclerosis Score Type 4 lesions (CAS-4) include American Heart Association Type VI lesions as well as those with maximum % LRNC area (LRNC area / wall area) >40%. Individual plaque types included in Type VI lesions were not examined alone because of low prevalence (<10%) except IPH, which was shown previously to predict systemic cardiovascular outcome (27,28).
Clinical risk factors and follow-up of cardiovascular events
In the AIM-HIGH study, all subjects were taking simvastatin with or without ezetimibe to achieve the LDL cholesterol target of 40-80 mg/dl (1.0-2.1 mmol/L). Regular clinical follow-up visits were arranged to monitor safety and adjust dosage as needed, which was previously reported in detail (20). Depending on study assignment, subjects were on extended-release niacin 1500-2000 mg/d or its active placebo. Clinical management beyond lipid-modifying treatment was consistent with current standard-of-care and at the discretion of local physicians. Demographics and information on traditional risk factors were collected at study entry and updated during follow-up visits. A clinical risk score was calculated based on the previously published Framingham risk model for predicting risk of recurrent events in patients with history of coronary heart disease or stroke (29).
In order to leverage the high-quality outcome data available from the main study, the MRI sub-study was designed a priori to use the AIM-HIGH primary endpoint, which was the composite of the first occurrence of fatal or non-fatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, symptom-driven coronary or cerebrovascular revascularization. All recorded events were adjudicated by the AIM-HIGH Clinical Events Committee. After the databases were locked, individual participant data on clinical profile, carotid plaque measurements, and incident events were linked. Time-to-event (or end of follow-up) was modified to start from MRI scan instead of study enrollment as in the main study.
Statistical Analysis
Patient characteristics are presented as mean±standard deviation (SD) for continuous variables, and count (percentage) for categorical variables. Associations between MRI characteristics and incident clinical events were quantified using hazard ratios (HRs) by Cox proportional hazard models. Kaplan-Meier curves of event-free survival grouped by individual carotid features were used to visualize associations and estimate absolute event-free survival rates at 3 years. As a sensitivity analysis, leave-one-out regression diagnostics (dfbetas) were performed to detect influential observations that changed the regression coefficients by more than 0.5 standard errors. Models without influential observations were run to test the robustness of statistically significant findings. For carotid plaque characteristics, exploratory multivariate analysis was performed to evaluate their associations with incident clinical events after adjusting separately for the Framingham risk score, maximum wall thickness, or AIM-HIGH randomized treatment assignment. Data analyses were performed using R 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as p<0.05 and all tests were two-tailed.
Results
Demographics, risk profile, and clinical outcomes
Of the 232 subjects recruited, 214 (92.2%) with acceptable diagnostic image quality constituted the study cohort (Table 1). Mean age was 61.2±8.6 years and 82% were males. Detailed clinical characteristics of the MRI cohort and their association with carotid plaque characteristics have been reported in a previous publication (22).
Table 1. Patient characteristics and associations with systemic cardiovascular outcomes.
| All subjects | AIM-HIGH primary endpoint* | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| (N=214) | Yes (N=18) | No (N=196) | HR† | (95% CI) | p-value | |
| Clinical characteristics | ||||||
| Age, years | 61.2±8.6 | 63.5±9.8 | 61.0±8.5 | 1.26 | (0.79-2.00) | 0.3 |
| Male sex | 82% | 94% | 81% | 3.98 | (0.53-29.89) | 0.2 |
| Caucasian | 88% | 100% | 87% | NA | NA | NA |
| Body mass index, kg/m2 | 30.1±4.5 | 29.0±6.0 | 30.2±4.4 | 0.75 | (0.45-1.24) | 0.3 |
| Smoker | ||||||
| Never | 29% | 33% | 29% | 1.00 (ref.) | ||
| Former | 53% | 56% | 53% | 0.92 | (0.33-2.52) | 0.9 |
| Current | 17% | 11% | 18% | 0.57 | (0.12-2.85) | 0.5 |
| Hypertension | 83% | 89% | 83% | 1.65 | (0.38-7.19) | 0.5 |
| Diabetes mellitus | 25% | 17% | 26% | 0.59 | (0.17-2.04) | 0.4 |
| Coronary artery disease | 95% | 94% | 95% | 1.00 | (0.13-7.52) | 1.0 |
| Cerebrovascular disease | 16% | 17% | 16% | 0.99 | (0.29-3.42) | 1.0 |
| Total cholesterol, mg/dl | 146±31 | 144±21 | 146±32 | 0.94 | (0.58-1.52) | 0.8 |
| LDL cholesterol, mg/dl | 77±27 | 79±16 | 76±28 | 1.08 | (0.70-1.68) | 0.7 |
| HDL cholesterol, mg/dl | 35±6 | 34±5 | 35±6 | 0.90 | (0.57-1.42) | 0.6 |
| Triglycerides, mg/dl | 174±66 | 158±52 | 176±67 | 0.70 | (0.39-1.27) | 0.2 |
| Systolic blood pressure, mmHg | 128±17 | 132±16 | 127±17 | 1.27 | (0.82-1.99) | 0.3 |
| Diastolic blood pressure, mmHg | 75±10 | 76±10 | 75±10 | 1.05 | (0.67-1.66) | 0.8 |
| Framingham risk score, % | 8.3±2.7 | 8.8±2.0 | 8.2±2.7 | 1.19 | (0.76-1.86) | 0.4 |
| On extended-release niacin | 44% | 33% | 45% | 0.65 | (0.24-1.73) | 0.4 |
| Carotid plaque burden | ||||||
| Maximum wall thickness, mm | 2.7±1.4 | 3.3±2.1 | 2.7±1.3 | 1.43 | (0.96-2.11) | 0.08 |
| Maximum % wall area, % | 48±15 | 54±27 | 48±14 | 1.34 | (0.92-1.94) | 0.12 |
| Percent wall volume, % | 41.8±7.1 | 42.5±7.5 | 41.7±7.1 | 1.12 | (0.72-1.74) | 0.60 |
| Carotid plaque characteristics | ||||||
| LRNC volume, mm3 | 40±99 | 130±206 | 31±78 | 1.43 | (1.16-1.75) | <0.001 |
| % LRNC volume, % | 6.6±9.6 | 14±15 | 5.9±8.7 | 1.57 | (1.22-2.01) | 0.002 |
| Calcification volume, mm3 | 12±24 | 6±11 | 13±25 | 0.60 | (0.26-1.41) | 0.2 |
| % calcification volume, % | 2.8±4.2 | 1.7±2.4 | 2.9±4.3 | 0.66 | (0.35-1.27) | 0.2 |
| Intraplaque hemorrhage | 8% | 22% | 7% | 3.00 | (0.99-9.13) | 0.053 |
| Thin/ruptured fibrous cap | 14% | 39% | 11% | 4.31 | (1.67-11.1) | 0.003 |
| High-risk plaque classifications | ||||||
| AHA Type-VI‡ | 11% | 22% | 10% | 2.36 | (0.77-7.17) | 0.13 |
| CAS-4§ | 12% | 28% | 11% | 2.79 | (0.99-7.85) | 0.051 |
The AIM-HIGH primary endpoint was the composite of the first occurrence of fatal or non-fatal myocardial infarction or ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven coronary or cerebrovascular revascularization.
For continuous variables, hazard ratios are presented per one-standard-deviation increase.
AHA Type VI lesions include plaques with surface disruption (i.e. cap rupture, ulceration, and calcified nodule), IPH, or mural thrombus.
CAS-4 lesions include AHA Type VI lesions as well as those with maximum % LRNC area (LRNC area / wall area) >40%.
Descriptive statistics are presented as mean±standard deviation or percentage. AHA = American Heart Association; CAS = Carotid Atherosclerosis Score; CI = confidence interval; HDL = high-density lipoprotein; HR = hazard ratio; IPH = intraplaque hemorrhage; LDL = low-density lipoprotein; LRNC = lipid-rich necrotic core.
During a median follow-up of 35.1 months (range: 1.2 to 57.7), 18 (8.4%) subjects reached the AIM-HIGH primary endpoint, including myocardial infarction (n=6), ischemic stroke (n=2), hospitalization for acute coronary syndrome (n=2), symptom-driven coronary revascularization (n=7), and symptom-driven cerebrovascular revascularization (n=1). None of the traditional risk factors or AIM-HIGH randomized treatment assignment (i.e. on extended-release niacin) were significantly associated with the AIM-HIGH primary endpoint (Table 1), nor was the Framingham risk score, which aggregates multiple risk factors including age, sex, smoking, blood pressures, lipid levels, and diabetes status.
Carotid plaque characteristics and systemic cardiovascular outcomes
Despite a high rate of long-term statin use (<1y: 22%, 1-5y: 36%, >5y: 36%), calcification, LRNC, IPH, and thin/ruptured fibrous cap were detected by carotid MRI in 48%, 52%, 8% and 14% of the subjects, respectively. Twenty-three (11%) subjects had AHA Type VI lesions, the majority of which were because of IPH alone (n=6), cap rupture alone (n=2), or both (n=12). The remaining three were because of presence of ulceration in the absence of remnant LRNC (ulceration overlying a remnant LRNC was considered as cap rupture) or calcified nodule. CAS-4 lesions had a slightly higher prevalence of 12%, with the addition of three cases due to maximum % LRNC area >40%.
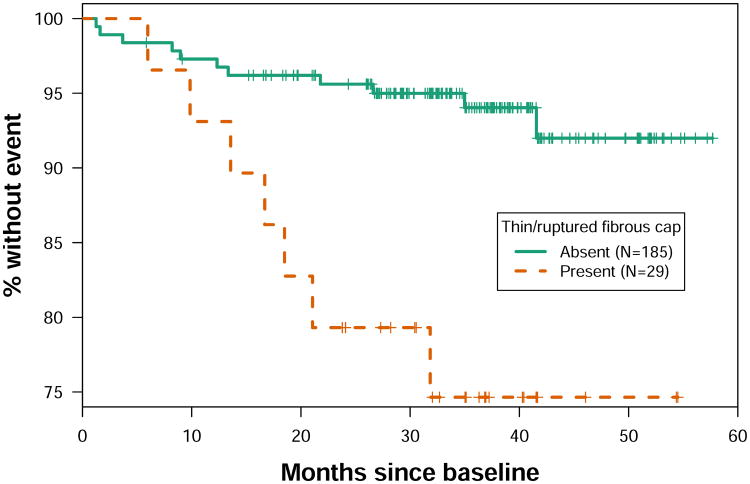
Notably, subjects with thin/ruptured fibrous cap had 4.31-fold (95% CI: 1.67 to 11.12, p=0.003) increased risk for the AIM-HIGH primary endpoint (Table 1). The estimated 3-year primary event-free survival rate was 74.6% for subjects with thin/ruptured fibrous cap versus 94.0% for those without (Figure 2). Plaque lipid content was also significantly associated with the AIM-HIGH primary endpoint (HR per 1-SD increase in % LRNC volume: 1.57, 95% CI: 1.22 to 2.01, p=0.002) whereas the association of plaque calcification content was not statistically significant and in the other direction (HR per 1-SD increase in % calcification volume: 0.66, 95% CI: 0.35 to 1.27, p=0.2) (Table 1). The association of IPH with the AIM-HIGH primary endpoint was positive but did not reach statistical significance (HR: 3.00, 95% CI: 0.99 to 9.13, p=0.053). There was no significant association between the AIM-HIGH primary endpoint and AHA Type VI (HR: 2.36, 95% CI: 0.77 to 7.17, p=0.13) or CAS-4 (HR: 2.79, 95% CI: 0.99 to 7.85, p=0.051) lesions that include heterogeneous plaque types (Table 1). The three subjects that were included in the category of Type VI lesions because of complications other than IPH or cap rupture remained event-free during follow-up. None of the plaque burden measurements were significantly associated with the primary endpoint, though there was a trend for maximum wall thickness (HR per 1-SD increase: 1.43, 95% CI: 0.96 to 2.11, p=0.08).
Figure 2. Kaplan-Meier estimates of event-free survival.
Kaplan-Meier curves show event-free survival in the group with (red line) and without (green line) thin/ruptured fibrous cap. The corresponding hazard ratio is 4.31 (95% CI: 1.67 to 11.12, p=0.003) for the AIM-HIGH primary endpoint (fatal or non-fatal myocardial infarction or ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven coronary or cerebrovascular revascularization). The estimated 3-year primary event-free survival rate was 74.6% for subjects with thin/ruptured fibrous cap versus 94.0% for those without.
Exploratory multivariate analysis
The associations between carotid plaque characteristics and the AIM-HIGH primary endpoint changed little in multivariate analyses adjusting separately for the Framingham risk score, maximum wall thickness, or AIM-HIGH randomized treatment assignment (Table 2). Thin/ruptured fibrous cap remained significantly associated with the AIM-HIGH primary endpoint after each adjustment with HRs ranging from 4.04 to 4.61 (p<0.02 for each). Similarly, % LRNC volume also remained significantly associated with the primary endpoint after each adjustment with HRs ranging from 1.56 to 1.99 per 1-SD increase (p<0.03 for each).
Table 2. Exploratory multivariate analysis of associations between carotid plaque characteristics and AIM-HIGH primary endpoint.
| Adjusted for Framingham risk score* | Adjusted for maximum wall thickness* | Adjusted for AIM-HIGH treatment arm* | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| HR† | (95% CI) | p-value | HR† | (95% CI) | p-value | HR† | (95% CI) | p-value | |
| LRNC volume, mm3 | 1.42 | (1.15-1.74) | 0.001 | 1.58 | (1.06-2.35) | 0.02 | 1.44 | (1.17-1.77) | <0.001 |
| % LRNC volume, % | 1.56 | (1.14-2.13) | 0.005 | 1.99 | (1.06-3.73) | 0.03 | 1.62 | (1.21-2.16) | 0.001 |
| Calcification volume, mm3 | 0.58 | (0.24-1.40) | 0.2 | 0.45 | (0.18-1.15) | 0.10 | 0.60 | (0.26-1.39) | 0.2 |
| % calcification volume, % | 0.65 | (0.33-1.26) | 0.2 | 0.53 | (0.27-1.06) | 0.07 | 0.67 | (0.35-1.27) | 0.2 |
| Intraplaque hemorrhage | 2.80 | (0.89-8.82) | 0.08 | 2.07 | (0.50-8.60) | 0.3 | 3.53 | (1.13-11.05) | 0.03 |
| Thin/ruptured fibrous cap | 4.18 | (1.57-11.14) | 0.004 | 4.04 | (1.23-13.22) | 0.02 | 4.61 | (1.77-11.98) | 0.002 |
| AHA Type-VI‡ | 2.33 | (0.74-7.31) | 0.15 | 1.47 | (0.35-6.12) | 0.6 | 2.51 | (0.82-7.68) | 0.11 |
| CAS-4§ | 2.79 | (0.97-8.06) | 0.06 | 2.01 | (0.52-7.73) | 0.3 | 3.04 | (1.07-8.63) | 0.04 |
All models are bivariate, with one plaque characteristic variable and a single adjustment variable. Adjustment was made individually for the Framingham risk score for recurrent events in patients with coronary heart disease or stroke (29), maximum wall thickness, and treatment assignment in AIM-HIGH.
For continuous variables, hazard ratios are presented per one-standard-deviation increase.
AHA Type VI lesions include plaques with surface disruption (i.e. cap rupture, ulceration, and calcified nodule), IPH, or mural thrombus.
CAS-4 lesions include AHA Type VI lesions as well as those with maximum % LRNC area (LRNC area / wall area) >40%.
AHA = American Heart Association; CAS = Carotid Atherosclerosis Score; CI = confidence interval; HR = hazard ratio; IPH = intraplaque hemorrhage; LRNC = lipid-rich necrotic core.
Discussion
By leveraging the rigorously adjudicated outcome data of a contemporary clinical trial, we evaluated MRI measurements of carotid plaque characteristics as surrogate markers for systemic cardiovascular outcomes. Among subjects with clinically established atherosclerotic disease receiving intensive medical therapy, high plaque lipid content and thin/ruptured fibrous cap were shown to identify those with substantially worse short- to intermediate-term prognosis. IPH had a low prevalence and was marginally associated with poor prognosis. In contrast, although calcium deposition is traditionally pursued as a marker for atherosclerosis, high plaque calcification content was not associated with poor prognosis. The observed associations between carotid plaque characteristics and systemic cardiovascular outcomes were not driven by traditional risk factors, plaque burden, or AIM-HIGH treatment assignment, which did not significantly predict the primary endpoint by themselves and had little influence on the associations of plaque characteristics with outcomes in multivariate analyses.
The field of cardiovascular medicine has seen a longstanding quest to use imaging measures of carotid atherosclerosis as surrogate markers in clinical management of atherosclerotic disease. However, whether the emerging imaging measures of carotid plaque characteristics, some of which were shown to predict ipsilateral cerebrovascular events and thought to reflect plaque vulnerability (13-15), can be used as novel surrogate markers remain elusive due to the limited data that are currently available. The concept is supported in a histopathological study, in which Hellings et al (27) reported that carotid plaque features including IPH and neovasculature identified in endarterectomy specimens were associated with future cardiovascular events in 818 patients who underwent carotid endarterectomy. Compared to the previous observation in highly selected patients undergoing carotid surgery, our findings provide prospective evidence in a broader group of patients with clinically established atherosclerotic disease that carotid plaque characteristics were strongly associated with systemic cardiovascular outcomes whereas traditional risk factors and plaque burden were not. Further evidence can be seen from two focused studies which respectively reported that echolucent carotid plaques on ultrasound (indicating high lipid content) and hyper-intense signals on MRI (indicating IPH) predicted coronary events in patients with existing coronary artery disease (28,30). Therefore, it has become increasing clear that imaging measurements of carotid plaque characteristics can serve as novel surrogate markers and provide useful information for understanding systemic atherothrombotic risk in individual patients, particularly in patients with established atherosclerotic cardiovascular disease where the predictive value of traditional risk factors and carotid plaque burden could be more limited compared to low-risk populations.
As compared to previous studies, the use of a standardized multicontrast MRI protocol allowed us to examine a spectrum of carotid plaque characteristics in the same cohort and perform quantitative assessment on plaque tissue composition. Notably, this is one of the first studies highlighting the prognostic value of thin-cap fibroatheroma in terms of systemic cardiovascular outcomes. Approximately 25% of those with thin/ruptured fibrous cap on carotid MRI experienced the AIM-HIGH primary endpoint in 3 years by Kaplan-Meier analysis whereas the event-free survival at 3 years was 94% among those without. This finding supports the pathophysiological concept that high-risk individuals harbor multiple vulnerable plaques and that intensive medical treatment should play a primary role in clinical management. Quantification of plaque lipid content provided another promising imaging marker that informs about systemic atherothrombotic risk. Recently, there have been studies using imaging measures of plaque lipid content for testing drug efficacy in clinical trials in order to get an early signal of drug efficacy or reveal the underlying mechanisms of clinical treatment (16,17,31). These efforts are supported by findings from the present study. On the other hand, plaque calcification content in extracranial carotid artery was not associated with future risk for systemic cardiovascular events in this cohort, questioning the value of surrogate artery calcification in identifying high-risk patients. Unlike lipid content, it is possible that calcification is more reflective of plaque burden but not plaque vulnerability. Prior investigations have stressed the importance of distinguishing the patterns of plaque calcification. Macrocalcification has been hypothesized as a feature of stable plaques whereas microcalcification has been associated with plaque inflammation (32). Alternatively, increasing evidence suggests that statins promote plaque calcification while reducing the risk for clinical events (33). The predictive value of calcification, measured as total volume, could be limited in intensively treated patients. Despite the unique strength of merging imaging data on novel plaque measurements and clinical outcome data in the setting of a contemporary clinical trial, there are several notable limitations of the present study. First, because of the small number of clinical events, our findings should be considered hypothesis-generating. It primarily tested the hypothesis that imaging measures of carotid plaque vulnerability have potential for understanding systemic atherothrombotic risk in individuals. C-statistic and net reclassification index were not performed so that we could not quantify the incremental predictive value of carotid plaque characteristics beyond clinical risk models and established imaging markers (e.g. carotid intima-media thickness and coronary artery calcium score). Accordingly, the implications are mainly pathophysiological and do not immediately translate into changes of clinical practice. Some of the clinical events were revascularizations rather than hard events. However, the inclusion of symptom-driven revascularization was made a priori to be consistent with the parent AIM-HIGH study. These revascularizations were performed following new-onset symptoms in an effort to prevent more severe clinical complications. Including these events would be consistent with the hypothesis that carotid plaque vulnerability indicates increased systemic atherothrombotic risk. Another limitation was that all patients in this study were already considered candidates for intensive medical therapy prior to imaging. Even though a subgroup with very poor prognosis was identified, it is currently unclear how we can utilize this information to improve their clinical outcomes. Nonetheless, in light of the high prevalence of vulnerable plaque characteristics despite contemporary intensive medical therapy, the development of novel agents that are able to stabilize plaques are needed to reduce the residual risk.
Conclusions
By leveraging the rigorously adjudicated outcome data of the AIM-HIGH trial, we found that among patients with clinically established atherosclerotic disease, high-risk carotid plaque features despite intensive medical therapy, including thin/ruptured fibrous cap, high lipid content, and marginally IPH, were associated with systemic cardiovascular outcomes independent of traditional risk factors and plaque burden. In contrast, high calcification content did not indicate poor prognosis. Markers of carotid plaque vulnerability may serve as novel surrogate markers for systemic atherothrombotic risk.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge
Among patients with clinically established atherosclerotic disease, high lipid content and thin/ruptured fibrous cap in carotid plaques identifies those with substantially worse short- to intermediate-term prognosis whereas plaque calcification content, plaque burden, and traditional risk factors did not.
Competency in Patient Care and Procedural Skills
Patients with high-risk carotid plaques represent a unique population which could benefit from more intensive lifestyle intervention and novel medical therapy.
Acknowledgments
We appreciate support from the AIM-HIGH Study, Principal Investigators: B. Gregory Brown, MD, PhD (retired); Jeffrey L. Probstfield, MD; William E. Boden, MD.
We would like to thank the investigators who were instrumental in recruiting subjects and obtaining the MR images. Participating institutions, investigators and coordinators are listed in Appendix.
Financial support: This study was supported by NIH R01 HL088214, R01 HL089504, and R01 HL103609. Carotid coils were provided by GE Healthcare and Philips Healthcare.
Disclosures: Mr. Hippe received research grants from Philips Healthcare and GE Healthcare. Dr. Balu received research grant from Philips Healthcare. Dr. Yuan received research grants from Philips Healthcare and is a member of Radiology Medical Advisory Network of Philips Healthcare. Dr. Hatsukami received research grant from Philips Healthcare. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations
- AHA
American Heart Association
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes Trial
- CAS
Carotid Atherosclerosis Score
- CI
confidence interval
- HDL
high-density lipoprotein
- HR
hazard ratio
- IPH
intraplaque hemorrhage
- LDL
low-density lipoprotein
- LRNC
lipid-rich necrotic core
- MPRAGE
magnetization-prepared rapid acquisition gradient-echo
- MRI
magnetic resonance imaging
- SD
standard deviation
Appendix. Site location, PI and coordinator information
21 AIM-HIGH Sites (by site #)
9 - Duke University - John Guyton, Shubi Khan; 10 -University of Calgary - Todd Anderson, Bev Madden; 14/30 - University of Pennsylvania / Philadelphia VA - Richard Dunbar, Dalia Roberts, Monica Williams; 15 - University of Southern California - Colletti, Andrea Contreras; 21 - University of Western Ontario -William Kostuk, Cathy Bone; 43 - University of Maryland - Michael Miller, Abby Roberts; 47 - University of Washington Cardiology - Xue-Qiao Zhao, Kevin O'Brien, Suzanne Williams; 48 - University of Washington NW Lipid Research Center - Robert Knopp, Pathmaja Paramsothy, Alice Dowdy, Barbara Twaddell; 49 - Long Beach VA - Moti Kahyap, Olaf Fallye, Sunil Kakadia; 51 - Puget Sound VA - Kenneth Lehmann, Julie LaGuire; 53 -Vancouver General Hospital - Anthony Fung, Rebecca Fox, Linda Axen; 55 - Wake Forest University Endocrinology - Robin Crouse, Donna Davis; 56 - Wake Forest University Geriatrics - Jamehl Demons, Tricia Wittmer; 58 - Wake Forest University Cardiology - David Herrington, Vickie Wayne, Lynda Doomy; 62 - Baylor College of Medicine - Peter Jones, Terry Techmanski, Diane Tanksley; 71 - Cardiovascular Consultants - Chris Geohas, Rose Prasad, Annie Laborin; 74 - Mayo Clinic - Stephen Kopecky, Cindy Woltman, Dawn Shelstad; 77 - Johns Hopkins University - Peter Kwiterovich, Kathleen Byrne; 78 - Heart Health Institute - Patrick Ma, Maureen McRae, Donna Louch; 79 - Methodist Hospital - Alan Hoffman, Mary Rangel; 86 - Kelsey Research Foundation - Haroon-Ur Harry Rashid, Stacy Meadows.
10 MRI Centers (alphabetical)
BAR - Barrows Neurological Institute - Jim Pipe, Sharmeen Joomun; BAY - Baylor School of Medicine - Joel Morrisett, Karima Ghazzaly; CAL -University of Calgary - Richard Frayne, Brian O'Brien, Frances Raymond; JHU - Johns Hopkins University - Bruce Wasserman, Rena Geckle; MAY - Mayo Clinic - John Huston, Mandie Maroney-Smith; ROB - Robarts Research Institute - Brian Rutt, Cyndi Harper Little; UBC - University of British Columbia - Alex MacKay, Linda Chandler; UOW - University of Washington - Chun Yuan, Baocheng Chu, Niranjan Balu; USC - University of Southern California - Patrick Colletti, Samuel Valencerina; WFU - Wake Forest University - J. Robin Crouse, J. Greg Terry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SJ. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 2.Baber U, Mehran R, Sartori S, et al. Prevalence, Impact, and Predictive Value of Detecting Subclinical Coronary and Carotid Atherosclerosis in Asymptomatic Adults: The BioImage Study. J Am Coll Cardiol. 2015;65:1065–74. doi: 10.1016/j.jacc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death - A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 4.Gronholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001;104:68–73. doi: 10.1161/hc2601.091704. [DOI] [PubMed] [Google Scholar]
- 5.de Weert TT, Ouhlous M, Meijering E, et al. In Vivo Characterization and Quantification of Atherosclerotic Carotid Plaque Components With Multidetector Computed Tomography and Histopathological Correlation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:2366–72. doi: 10.1161/01.ATV.0000240518.90124.57. [DOI] [PubMed] [Google Scholar]
- 6.Wintermark M, Jawadi SS, Rapp JH, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol. 2008;29:875–82. doi: 10.3174/ajnr.A0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–73. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi RA, U-King-Im JM, Graves MJ, et al. MRI-derived measurements of fibrous-cap and lipid-core thickness: the potential for identifying vulnerable carotid plaques in vivo. Neuroradiology. 2004;46:738–43. doi: 10.1007/s00234-004-1247-6. [DOI] [PubMed] [Google Scholar]
- 9.Wasserman BA, Sharrett AR, Lai S, et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA) Stroke. 2008;39:329–35. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 10.Ota H, Yarnykh VL, Ferguson MS, et al. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology. 2010;254:551–63. doi: 10.1148/radiol.09090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ota H, Yu W, Underhill HR, et al. Hemorrhage and Large Lipid-Rich Necrotic Cores Are Independently Associated With Thin or Ruptured Fibrous Caps An In vivo 3T MRI Study. Arterioscler Thromb Vasc Biol. 2009;29:1696–701. doi: 10.1161/ATVBAHA.109.192179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underhill HR, Yuan C, Yarnykh VL, et al. Predictors of surface disruption with MR imaging in asymptomatic carotid artery stenosis. AJNR Am J Neuroradiol. 2010;31:487–93. doi: 10.3174/ajnr.A1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takaya N, Yuan C, Chu BC, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI - initial results. Stroke. 2006;37:818–23. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Baradaran H, Schweitzer AD, et al. Carotid Plaque MRI and Stroke Risk: A Systematic Review and Meta-analysis. Stroke. 2013;44:3071–7. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 15.Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–91. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Zhao XQ, Dong L, Hatsukami T, et al. MR imaging of carotid plaque composition during lipid-lowering therapy: a prospective assessment of effect and time course. J Am Coll Cardiol Img. 2011;4:977–86. doi: 10.1016/j.jcmg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Balu N, Hippe DS, et al. Subclinical carotid atherosclerosis: short-term natural history of lipid-rich necrotic core--a multicenter study with MR imaging. Radiology. 2013;268:61–8. doi: 10.1148/radiol.13121702. [DOI] [PubMed] [Google Scholar]
- 18.van Gils MJ, Vukadinovic D, van Dijk AC, Dippel DW, Niessen WJ, van der Lugt A. Carotid atherosclerotic plaque progression and change in plaque composition over time: a 5-year follow-up study using serial CT angiography. Am J Neuroradiol. 2012;33:1267–73. doi: 10.3174/ajnr.A2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneyama T, Sun J, Hippe DS, et al. In vivo semi-automatic segmentation of multicontrast cardiovascular magnetic resonance for prospective cohort studies on plaque tissue composition: initial experience. Int J Cardiovasc Imaging. 2016;32:73–81. doi: 10.1007/s10554-015-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: Impact on Global Health outcomes (AIM-HIGH) Am Heart J. 2011;161:471–7. doi: 10.1016/j.ahj.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 22.Zhao XQ, Hatsukami TS, Hippe DS, et al. Clinical Factors Associated With High-Risk Carotid Plaque Features as Assessed by Magnetic Resonance Imaging in Patients With Established Vascular Disease (from the AIM-HIGH Study) Am J Cardiol. 2014;114:1412–9. doi: 10.1016/j.amjcard.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Zhao XQ, Balu N, et al. Carotid magnetic resonance imaging for monitoring atherosclerotic plaque progression: a multicenter reproducibility study. Int J Cardiovasc Imaging. 2015;31:95–103. doi: 10.1007/s10554-014-0532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerwin WS, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18:371–8. doi: 10.1097/rmr.0b013e3181598d9d. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Yarnykh VL, Hatsukami TS, et al. Scan-rescan reproducibility of carotid atherosclerotic plaque morphology and tissue composition measurements using multicontrast MRI at 3T. J Magn Reson Imaging. 2010;31:168–76. doi: 10.1002/jmri.22014. [DOI] [PubMed] [Google Scholar]
- 26.Xu D, Hippe DS, Underhill HR, et al. Prediction of high-risk plaque development and plaque progression with the carotid atherosclerosis score. JACC Cardiovasc Imaging. 2014;7:366–73. doi: 10.1016/j.jcmg.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellings WE, Peeters W, Moll FL, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121:1941–50. doi: 10.1161/CIRCULATIONAHA.109.887497. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi T, Yamada N, Higashi M, Goto Y, Naito H. High-Intensity Signals in Carotid Plaques on T1-Weighted Magnetic Resonance Imaging Predict Coronary Events in Patients With Coronary Artery Disease. J Am Coll Cardiol. 2011;58:416–22. doi: 10.1016/j.jacc.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 29.D'Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000;139:272–81. doi: 10.1067/mhj.2000.96469. [DOI] [PubMed] [Google Scholar]
- 30.Honda O, Sugiyama S, Kugiyama K, et al. Echolucent carotid plaques predict future coronary events in patients with coronary artery disease. J Am Coll Cardiol. 2004;43:1177–84. doi: 10.1016/j.jacc.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 31.Kini AS, Baber U, Kovacic JC, et al. Changes in Plaque Lipid Content After Short-Term Intensive Versus Standard Statin TherapyThe YELLOW Trial (Reduction in Yellow Plaque by Aggressive Lipid-Lowering Therapy) J Am Coll Cardiol. 2013;62:21–9. doi: 10.1016/j.jacc.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 32.Pugliese G, Iacobini C, Blasetti FC, Menini S. The dark and bright side of atherosclerotic calcification. Atherosclerosis. 2015;238:220–30. doi: 10.1016/j.atherosclerosis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Puri R, Nicholls SJ, Shao M, et al. Impact of Statins on Serial Coronary Calcification During Atheroma Progression and Regression. J Am Coll Cardiol. 2015;65:1273–82. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.