Abstract
Animal models have proved valuable to investigate the pathogenesis of dry eye disease, identify therapeutic targets and the efficacy of candidate therapeutics for dry eye. Pharmacological inhibition of the lacrimal functional unit and exposure of the mouse eye to desiccating stress was found to activate innate immune pathways, promote dendritic cell maturation and initiate an adaptive T cell response to ocular surface antigens. Disease relevant mediators and pathways have been identified through use of genetically altered mice, specific inhibitors and adoptive transfer of desiccating stress primed CD4+ T cells to naïve recipients. Findings from mouse models have elucidated the mechanism of action of cyclosporine A and the rationale for developing lifitegrast, the two currently approved therapeutics in the US.
Introduction
The development of reliable animal models which are relevant to human disease provides a valuable research tool. A key use of these models is to identify “attackable” targets and develop candidate therapeutics based on this knowledge.
Findings in dry eye dogs that the ocular surface (conjunctiva) and lacrimal glands are replete with infiltrating lymphocytes was a key discovery that inflammation causes dry eye disease.1 This phenomenon is also seen in salivary and lacrimal gland biopsies from patients with Sjögren’s syndrome, who experience signature symptoms of dry eye and dry mouth due to mucosal dryness. This gave the first indication that dry eye is an immune based inflammatory disease of the Lacrimal Functional Unit (LFU).
Lacrimal Functional Unit
The LFU, a concept we put forth for the first time in 1998 consists anatomically of the ocular surface (conjunctival and corneal epithelia), the lacrimal glands, meibomian glands and the interconnecting innervatjon.2 Also included in this unit are the hormonally responsive and resident immune cells in these tissues. The LFU is essential in maintaining a normal homeostatic environment on the ocular surface which includes maintaining a smooth optically clear cornea and secretion of a tear film of normal composition to protect the ocular surface from damaging inflammation and infection and to promote rapid healing after trauma.3,4
Stimulation of the highly innervated ocular surface results in afferent nerve traffic through the ophthalmic branch of the trigeminal nerve (V) into the central nervous system where the signal is integrated by both autonomic and cortical input. This then engenders efferent nerve traffic through the Facial Nerve (VII) which innervates the main and accessory lacrimal glands (Wolfring and Krauss), the mucin secreting conjunctival goblet cells, as well as the lipid secreting meibomian glands.
Desiccating Stress Model of Dry Eye
While canine dry eye is an important clinical entity, these dogs are difficult to acquire and expensive to maintain. Additionally, when studying immune based inflammatory diseases, mice are the animals of choice due to the availability of genetically altered strains and specific reagents. We therefore developed a model in which the LFU is inhibited with systemic anticholinergic agent in mice that are exposed to a dry, drafty environment.5 This is the first model which demonstrated the initiation of chronic autoimmunity from an environmental insult.6 Mice are given three daily subcutaneous injections of scopolamine, a short acting anti-cholinergic in order inhibit tear secretion by the lacrimal glands and conjunctival goblet cells. They are then maintained in a low humidity environment (20%) and exposed to an air movement for 5–10 days.5
The immunological premise of the model
Exposure of susceptible ocular surface tissues to a desiccating stress results initially in an innate immune response. This stress activates NFK-β and MAPK innate signaling pathways in immune cells which reside on within the ocular surface tissues, as well as surface epithelial cells causing them to secrete copious amounts of “defensive” cytokines, chemokines and matrix metalloproteinases (MMPs) in response to a perceived threat.7–13 This response is rapid but non-specific.
The Afferent Arm of the Adaptive Immune Response
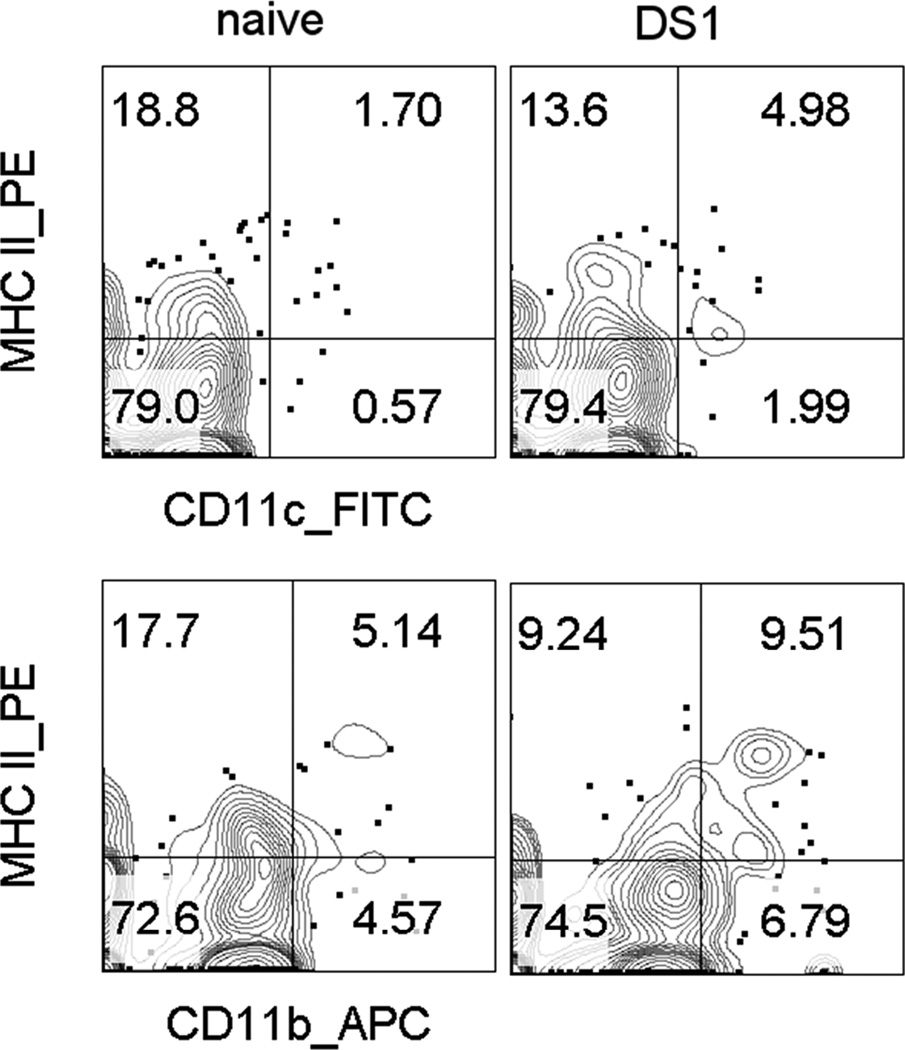
This acute innate phase is followed by the more specific adaptive immune response. Antigens exposed on the ocular surface are taken up and processed by resident dendritic cells and macrophages (Antigen Presenting Cells, APC’s). These cells internalize the antigens, process them and then express them on their surface using MHCII. Exposure to innate cytokines stimulate expression of dendritic cell maturation markers that initiate their migration from the ocular surface to specific draining lymph nodes.14 We have observed doubling of the number if CD11b+ and CD11c+ dendritic cells in the draining cervical lymph nodes 1 day after exposure to desiccating stress. (Figure 4) These APC’s encounter a population of naïve T-cells (Th0) in the lymph nodes. Through the local environment and the presence of a specific antigen, these naïve T cells are primed and targeted. This generates a response which is more specific to the perceived threat.
Figure 4.
Flow cytometry scatter plots of two dendritic cell (DC) populations (A. CD11c+ and B. CD11b+) in the cervical lymph nodes that drain the eye in unstressed naïve mice (left) and after 1 day of desiccating ocular surface stress (right). The percentage of these dendritic cells with higher levels of major histocompatibility complex II (MHCII), a DC maturation marker increases approximately 2 fold in both populations, indicating that exposure to dry eye causes changes acute changes in dendritic cell status in the draining nodes.
The Efferent Arm of the Adaptive Immune Response
The primed and targeted T cells exit the lymph nodes and travel via the circulation to the site of the antigen (inflammation). There, they exit the blood vessel and enter the tissue within the LFU, become fully activated after encountering antigen loaded DCs and secrete cytokines (IL-17, IFN-γ) to initiate and maintain an immune based inflammation. This process is referred to as “homing” and is not yet totally understood. IL-17 stimulates production of MMPs that disrupt cornea barrier function, while IFN-γ causes goblet cell secretory dysfunction and cornea and conjunctival epithelial apoptosis.15–17
Specific Experiments and Relevant Learnings
How can we be sure that the infiltrating T cells are specific to the disease and not just some sort of inflammatory epiphenomenon?
The experiment conducted to answer this question is based on the immunological tenet that if cells are responsible for a disease, the disease can be transferred to a naïve animal solely by transferring that population of cells. This is referred to as Adoptive Transfer. We confirmed the pathogenicity of desiccating stressed primed CD4+ T cells by inducing dry eye in mice and then isolating all of the CD4+ T helper cells (pro-inflammatory cells) from the draining lymph nodes and injecting them intra-peritoneally into a naïve “nude” recipient mouse (a mouse that lacks its own T cells).
Within 48 hours, all of the transferred cells appeared in tissues of the LFU (conjunctiva, cornea and lacrimal glands) of the recipient mouse and the mouse manifested signs of dry eye, including conjunctival goblet cell loss and corneal barrier disruption.18
People are exposed to adverse environments regularly, why don’t they get dry eye?
In contrast, if we performed an adoptive transfer into a normal immunologically intact wild-type mouse (ie: not a nude mouse), the recipient did not get dry eye and showed no signs of inflammation. We attribute this to presence of T regulatory cells which are responsible for suppressing the transferred pathogenic T cells. This is one mechanism we use to eliminate an inappropriate T cell response and prevent development of autoimmunity. To demonstrate the suppressive effect of endogenous T regulatory cells, we performed another experiment and treated the normal recipients with an anti-CD25 antibody which eliminates T regulatory cells – the mice did come down with dry eye.
How can you be sure that this is an antigen driven disease? Could the antigen be a target for a therapeutic?
-
In order to answer these questions we performed two experiments. They both involved the use of the compound clodronate which eliminates antigen presenting cells (APCs) on the ocular surface. This was formulated in liposomes in the laboratory of Dr. Jerry Niederkorn. We injected them subconjunctivally into mice at three time points both before and after the initiation of desiccating stress.
By eliminating the ability of these mice to process antigen, we hypothesized that the mice would not develop an adaptive immunological inflammation. In fact, that is what we found. No evidence of ocular inflammation was found in these mice even though they had been exposed to the adverse environment.14
-
In the second experiment we performed the adoptive transfer of CD4+ T cells from the clodronate treated mice. The CD4+ T-cells we transferred were not able to induce any inflammation in the recipient which indicated that they were not given any processed antigen which would promote activation, cytokine production and elicit inflammation in the recipient.14
The results of the previous two sets of experiments have taught us that dry eye is a T cell based inflammatory disease, that these T cells are primed and targeted by ocular surface antigens and produce cytokines such as IL-17 and IFN-γ.
Correlation of Mouse Model with Human Dry Eye Disease
The findings from mouse model experiments and their relevance to pathogenesis if human dry eye disease has been confirmed in studies performed in human patient with tear dysfunction. Major similarities in pathogenesis and therapeutic response between mouse models and human patients with dry eye disease are presented in the Table.
Table.
Comparisons between mouse model and human dry eye disease
| Disease marker/parameter Therapeutic response | Mouse Model | Human Dry Eye Disease |
|---|---|---|
| Increased innate inflammatory mediators (cytokines, chemokines, MMPs) |
10–12 | 19–21 |
| Increased ICAM1 expression in lacrimal gland and conjunctiva |
22,23 | 24 |
| Increased epithelial apoptosis |
1,25 | 26 |
| Conjunctival goblet cell loss | 15,27 | 28,29 |
| Dendritic cell maturation | 14 | 30,31 |
| CD4 T cell infiltration | 15,32 | 33 |
| Increased Th cytokines | 15,16 | 29 |
| Improved corneal barrier with corticosteroids |
34 | 35–37 |
| Improved conjunctival goblet cells with cyclosporine |
38 | 39,40 |
The numbers in the table refer to relevant references.
Figure 1.

This is a depiction of the Lacrimal Functional Unit (LFU). This is the mechanism by which a normal tear film environment is maintained on the ocular surface. 1 — (yellow) Stimulation of the ocular surface epithelium and nerve endings results in afferent nerve traffic through the ophthalmic branch of the Trigeminal Nerve (V1). 2 — Integration of this signal within the central nervous system (both autonomic and cortical). 3— (red- sympathetic, yellow-parasympathetic) Efferent nerve traffic through the Facial Nerve (VII) to innervate the lacrimal glands (main and accessory), meibomian glands and conjunctival goblet cells.
Figure 2.
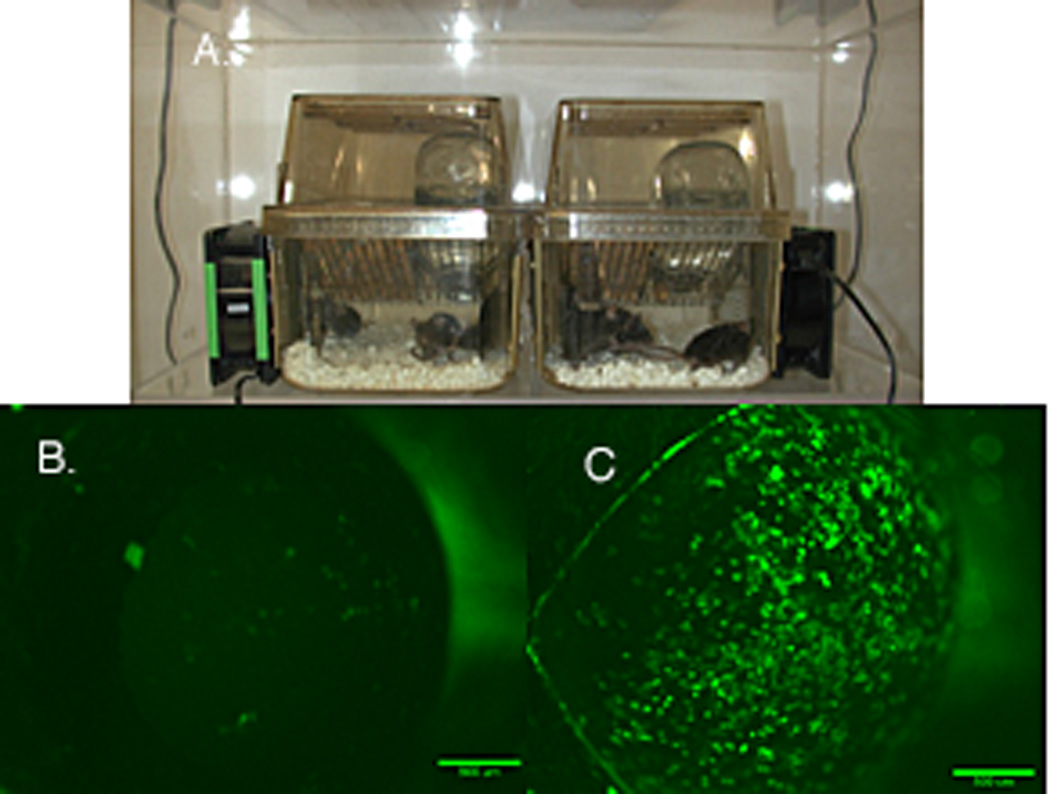
Mouse desiccating stress model. A. Mice are maintained in an environment of 20–25% humidity. Air movement is induced using computer fans. Mice are given subcutaneous injections of scopolamine (anti-cholinergic) to decrease secretions. B. Fluorescein staining of the normal mice cornea. C Fluorescein staining of the cornea of a mouse after 5 days of this protocol.
Figure 3. of Ocular Surface Immune Response.
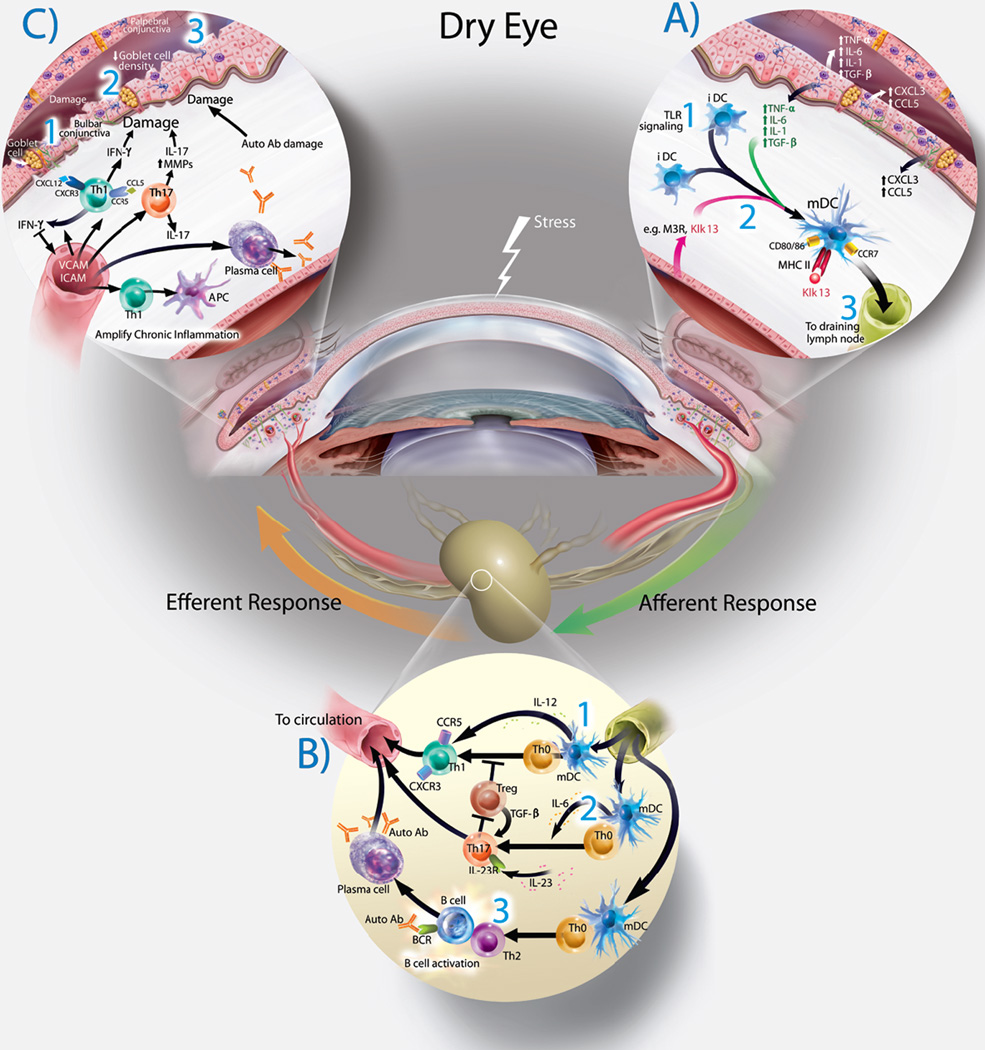
A— Exposure of the ocular surface to desiccating stress or pathogen associated molecular products (PAMPS) initiates an initial non-specific innate immune response with secretion of multiple cytokines and chemokines. Initiation of Adaptive Immune Response— The Afferent Arm of the Immune Response (right side): 1— Initial Toll-Like Receptor (TLR) signaling and antigen exposure on the ocular surface results in antigen uptake and processing by immature dendritic cells (iDS’s). 2— As the dendritic cells mature (mDC’s), antigens are processed and presented to the cell surface. These cell utilize the chemokine receptor CCR7 to leave the ocular surface and travel to the regional draining lymph nodes via ocular surface lymphatics.
B— Lymph Node (Bottom). The dendritic cells arrive in the lymph node and encounters a population of naive T-cells (Th0). The fate of these naive T cells is determined by the antigens, local environment and the specific cytokines secreted. 1— Th-1 cells are generated by DC secretion of IL-12. 2— Th-17 cells are generated by DC secretion of IL-6 and IL-23. 3— Th2 cells are generated by secretion of TSLP. Th-2 cells interact and activate B-cells which can mature (exposure to auto-antibodies) which mature the B-cells into antibody secreting Plasma Cells. All of these primed and targeted T cells will “home” via the systemic circulation to the LFU as part of the Efferent Arm of the Immune Response (left side).
C— Once these cells arrive at the ocular surface, they encounter adhesion molecules, leave the vessels and are activated by the constant presence of specific antigen. These cells then secrete a multitude of cytokines (IFN-γ, IL-17, etc. and MMPs). This creates the chronic immune response found in dry eye.
Acknowledgments
This study was supported by: NIH Grant EY11915, NIH Core Grants EY002520 & EY020799, an unrestricted grant from Research to Prevent Blindness, New York, NY
Footnotes
Conflicts of Interest: none
References
- 1.Gao J, Schwalb TA, Addeo JV, Ghosn CR, Stern ME. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: the effect of topical Cyclosporin A therapy. Cornea. 1998;17(6):654–663. doi: 10.1097/00003226-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Experimental eye research. 2004;78(3):409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. American journal of ophthalmology. 2011;152(6):900 e901–909.e901. doi: 10.1016/j.ajo.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Investigative ophthalmology & visual science. 2002;43(3):632–638. [PubMed] [Google Scholar]
- 6.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. International reviews of immunology. 2013;32(1):19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Paiva CS, Pangelinan SB, Chang E, et al. Essential role for c-Jun N-terminal kinase 2 in corneal epithelial response to desiccating stress. Arch Ophthalmol. 2009;127(12):1625–1631. doi: 10.1001/archophthalmol.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye & contact lens. 2005;31(5):186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 9.Guzman M, Keitelman I, Sabbione F, Trevani AS, Giordano MN, Galletti JG. Desiccating-stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clinical and experimental immunology. 2015 doi: 10.1111/cei.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Investigative ophthalmology & visual science. 2007;48(6):2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 11.Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26(5):579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 12.Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Investigative ophthalmology & visual science. 2006;47(8):3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 13.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. The American journal of pathology. 2005;166(1):61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaumburg CS, Siemasko KF, De Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. Journal of immunology (Baltimore, Md : 1950) 2011;187(7):3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 15.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Investigative ophthalmology & visual science. 2007;48(6):2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 16.De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal immunology. 2009;2(3):243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coursey TG, Tukler Henriksson J, Barbosa FL, de Paiva CS, Pflugfelder SC. Interferon-gamma-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjogren Syndrome. The American journal of pathology. 2016 doi: 10.1016/j.ajpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren's Syndrome-like lacrimal keratoconjunctivitis. Journal of immunology (Baltimore, Md : 1950) 2006;176(7):3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 19.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. American journal of ophthalmology. 2009;147(2):198–205. e191. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Investigative ophthalmology & visual science. 2010;51(2):643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Investigative ophthalmology & visual science. 2009;50(7):3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Morgan G, Tieu DD, Schwalb TA, Ngo M, Stern ME. ICAM-1: its role in the pathophysiology of immune activation in the MRL/LPR mouse. Advances in experimental medicine and biology. 2002;506(Pt B):777–781. doi: 10.1007/978-1-4615-0717-8_109. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjogrens syndrome-like MRL/lpr mice. Experimental eye research. 2004;78(4):823–835. doi: 10.1016/j.exer.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjogren's syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Investigative ophthalmology & visual science. 1994;35(9):3493–3504. [PubMed] [Google Scholar]
- 25.Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Investigative ophthalmology & visual science. 2003;44(1):124–129. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- 26.Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Investigative ophthalmology & visual science. 2001;42(1):90–95. [PubMed] [Google Scholar]
- 27.Corrales RM, de Paiva CS, Li DQ, et al. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Investigative ophthalmology & visual science. 2011;52(6):3492–3499. doi: 10.1167/iovs.10-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104(2):223–235. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 29.Pflugfelder SC, De Paiva CS, Moore QL, et al. Aqueous Tear Deficiency Increases Conjunctival Interferon-gamma (IFN-gamma) Expression and Goblet Cell Loss. Investigative ophthalmology & visual science. 2015;56(12):7545–7550. doi: 10.1167/iovs.15-17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baudouin C, Brignole F, Becquet F, Pisella PJ, Goguel A. Flow cytometry in impression cytology specimens. A new method for evaluation of conjunctival inflammation. Investigative ophthalmology & visual science. 1997;38(7):1458–1464. [PubMed] [Google Scholar]
- 31.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea. 2013 doi: 10.1097/ICO.0b013e318299549c. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, De Paiva CS, Su Z, Volpe EA, Li DQ, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Experimental eye research. 2014;118:117–124. doi: 10.1016/j.exer.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118(11):1489–1496. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 34.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Experimental eye research. 2006;83(3):526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. American journal of ophthalmology. 2004;138(3):444–457. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05% Eye & contact lens. 2014;40(5):289–296. doi: 10.1097/ICL.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 37.Moore QL, De Paiva CS, Pflugfelder SC. Effects of dry eye therapies on environmentally induced ocular surface disease. American journal of ophthalmology. 2015 doi: 10.1016/j.ajo.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005;24(1):80–85. doi: 10.1097/01.ico.0000133994.22392.47. [DOI] [PubMed] [Google Scholar]
- 39.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 40.Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27(1):64–69. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]