Abstract
Dry eye affects millions of people worldwide and causes eye well recognized risk factors for dry eye. Anatomical and inflammation-induced age-related changes affect all components of the lacrimal gland functional unit, inclusive of lacrimal gland, conjunctiva, meibomian gland and compromise ocular surface health. There is increased evidence that inflammation plays a role in dry eye. This review will summarize the current knowledge about aging and dry eye, inclusive of lessons learned from animal models and promising therapies.
Introduction
Tear dysfunction is one of the most common problems encountered by eye doctors. Tear dysfunction has a reported prevalence ranging from 2 to 14.2%.1–3 It has been found to increase with age from the 4th to 8th decade of life and is more prevalent in women than men throughout this period.4, 5 Tear dysfunction results from disease of one or more components of the lacrimal functional unit that consists of the tear producing glands and their neural connections. Many patients over the age of 40 with tear dysfunction have evidence of meibomian gland disease (MGD) as an underlying cause, particularly in patients with an unstable tear film, but normal tear production and tear volume. This review will focus on epidemiological aspects of aging, possible mechanisms for age-related dry eye and discuss some potential therapies along with lessons learned from animal models of aging.
1. Aging as a risk factor for Dry Eye
Aging is a significant risk factor for dry eye
Large epidemiological studies from the Women’s Health Study and Physician’s Health noted that dry eye prevalence increases in women and men every five years after the age of 50, with greater prevalence in women compared to men.1, 3–8 Age and female sex have been found to be the greatest risk factors for dry eye. This is supported by the clinical findings of decreased tear production in women through the 6th decade of life.9, 10 The corneal surface irregularity in dry eye degrades visual function by decreasing contrast sensitivity and functional visual acuity.11, 12 The presence of dry eye was found to significantly impact the ability to perform daily activities such as reading, using a computer and driving.13 Using time trade-off techniques, Schifman and others calculated that severe dry eye had the same impact on quality-of-life as moderate to severe angina.14, 15 With an increasing aged population and with the increased life expectancy, it is expected that dry eye will continue to be one of the main reasons for ophthalmologic visits.16 Therefore, better understanding specifically of age-related dry eye and therapies tailored to this specific population are much needed.
The lacrimal gland (LG) suffers significantly with aging. Various histopathologic changes were observed in the human main lacrimal gland such as acinar atrophy; periacinar fibrosis; periductal fibrosis; interlobular ductal dilatation; interlobular ductal proliferation; lymphocytic infiltration; and fatty infiltration.17–20 Several histopathologic differences exist between the palpebral and orbital lobes. Diffuse fibrosis and diffuse atrophy in orbital lobes were more frequently observed in women than in men.20, 21 Rodents are frequently used in aging research as their LG suffer aging-related changes similar to humans (see review by Rocha and colleagues).22–25
Age-related eyelid alterations include lid laxity, meibomian gland atrophy and orifice metaplasia, decrease in tear volume with increased tear break-up time and dry eye.26–31 In the young healthy eyelid, the meibomian gland orifices are located anterior to the mucocutaneous junction that can be identified as Marx’s line, a physiological line of staining with diagnostic dyes, such as fluorescein, lissamine green, and rose bengal. It has been reported that Marx’s line migrate anteriorly with aging.32 MG have androgen receptors and respond to androgen stimulation31, 33–37 and MGD is more frequent in men than women.28, 29, 38, 39 MGD may occur by itself or in association with aqueous-tear deficiency (ATD). The inflammatory cytokine milieu both in tears and the conjunctiva cells collected by impression cytology are different than ATD.40–44 ATD patients have significant goblet cell loss, increased expression of IFN-γ and IL-17A compared to normal controls, but none of the aforementioned changes are present in MGD patients.44
Another frequent age-related disorder is conjunctivochalasis, a condition where the conjunctiva folds and becomes redundant and may obstruct the puncta and cause ocular discomfort. It is often associated with age45–48 and it is discussed in more details in the companion article by Gumus and colleagues.
There is still controversy if aging leads to dry-eye or if dry eye is an age-related disease that has a totally different mechanism than aging per se.49 For example, age as a risk factor for goblet cell loss remains controversial.23, 50–54 It has becoming more apparent that similar to autoimmune dry eye, age-related dry eye has significant inflammation and a complex immune response that overtime leads to profound LG and ocular surface alterations (Figure 1).22–25, 28, 51, 55–58
Figure 1.
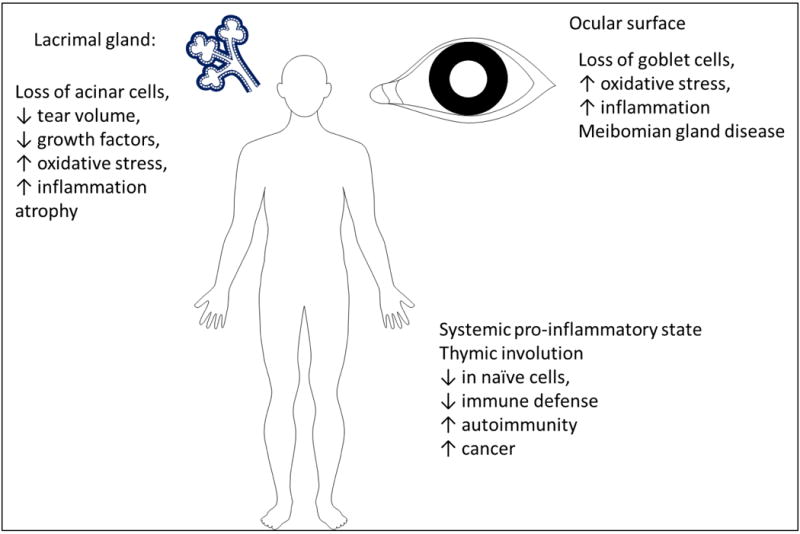
Aging induces profound systemic changes but also changes the lacrimal gland functional unit.
2-Age-related co-morbidities and iatrogenic dry eye
Aging is often accompanied by co-morbidities such as cardiovascular diseases, type 2 diabetes, depression, glaucoma and other ocular diseases. Some of these co-morbidities themselves or the medications that are used to manage them, may have a deleterious secondary effect on the ocular surface. An example is diabetes mellitus that affects retina, LG and corneal nerves and it is frequently associated with dry eye.59–61 Some of the age-related co-morbidities alters ocular surface homeostasis secondary to the systemic or ocular therapies that are required to control the original morbidity and some of these medications may have secondary ocular drying effects. For example, anti-hypertensive, anti-histaminics and antidepressants are some of well-recognized classes of drugs that have a drying effect on the ocular surface.3, 5–8, 56, 62 For a complete list of medications that have been noted to have a drying effect or to aggravate dry eye, please refer to review by Fraunfelder and colleagues62, 63 and visit the National Eye Drug registry (http://www.eyedrugregistry.com/about.html).
Patients with glaucoma, a disease with greater incidence in elderly patients, often complain about ocular irritation.64, 65 Although a direct effect of the compounds used to treat glaucoma cannot be excluded, benzalkonium chloride, a widely used eyedrop preservative due to its potent antibacterial action, has been recognized as toxic to the ocular surface. Benzalkonium chloride enhances drug penetration, partially due to secondary epithelial barrier breakage and toxicity to corneal epithelium.65–68 Recent studies have shown that switching to a preservative –free solution improved ocular surface related-symptoms with no loss efficacy of anti-glaucoma drugs, promising relief to a great number of patients with secondary dry eye due to glaucoma eyedrops.69–71
3-Possible mechanisms leading to age-related dry eye
All organisms start to age as soon as they are born. While some age-related changes are celebrated as milestones, (i.e. first baby steps and words, first definitive tooth, first mustache shaving in boys; first child, etc), the decline in health status and vitality that often accompanies aging is a nuisance for many. There is also an economical burden to the society and caregivers as life expectancy increases as well as susceptibility to a great number of diseases, such as cancer, cardiovascular diseases, autoimmunity and frailty.
The immune system also undergoes aging, named immunosenescence, a decline in the immune function characterized by a decrease in naïve T cells, chronic inflammation, hyperimmunoglobulinemia, autoimmunity, poor response to vaccines, and increased susceptibility to infections.72–75 A typical feature of aging is a chronic, low-grade inflammatory status, named inflammaging76, characterized by a general increase in the production of proinflammatory cytokines. Increased serum inflammatory mediators have been found associated with Alzheimer’s disease, dementia, Parkinson’s disease and type 2 diabetes.76–78 The changes in the immune system have profound consequences for survival and are reliable predictors of morbidity and mortality in the elderly.75
Other age-related cellular/metabolic theories regarding aging include increased oxidative stress, increased DNA damage, alteration of DNA repair, increased protein glycation, telomere shortening and decreased proteasome function to name a few.79, 80 Although an extensive body of evidence has accumulated in these areas, the precise mechanism(s) that control aging are still poorly understood. Every cell ages, but not at the same rate; age-related changes may or may not accompany pathological aging and every person is unique and so its own aging. The same is true for aging-related dry eye.
There is increasing evidence that dry eye is accompanied by inflammation. In the last 20 years there was a shift on dry eye paradigm, from merely a disease of decreased tears to a disease where inflammation and autoimmunity play an important role. Increased levels of inflammatory and T cell related mediators such as IL-1β, IL-6, TNF-α, IL-17, IFN-γ have been noted in conjunctiva and tear fluid of dry eye patients compared to control subjects.12, 40, 44, 81–90 The activation of the local immune environment is another universal feature of dry eye, as increased expression of HLA-DR+ cells are a frequent finding, which has been used as an indicator of therapeutic response in clinical trials.81, 91–100 Other features of dry eye include increased production of matrix metalloproteinases (MMPs), increased levels of chemokines and proteins involved in oxidative stress, increased squamous metaplasia of the ocular surface epithelium, loss of goblet cells and increased endoplasmic reticulum stress.12, 81, 83, 85, 96, 97, 101–117
Dry eye is a chronic disease but acute exacerbations are frequent when performing activities such as driving, viewing a video display for long periods of time and shopping in stores with high flow AC. Dry eye patients live and work in controlled drafty and/or low humidity (desiccating stress) environmental conditions, and the irritation and ocular surface disease118–122 they experience from this environment may decrease their productivity and quality of life.123, 124 Exposure to desiccating environmental conditions has been shown to activate stress-sensing cells/pathways on the ocular surface, to stimulate ocular surface epithelial cells to synthesize and secrete of pro-inflammatory cytokines and MMPs that can be detected in the epithelial cells themselves.42, 85 This acute stress related inflammation can sensitize corneal nociceptors and cause surface epithelial changes, such as breakdown of epithelial tight junctions resulting in corneal barrier dysfunction, clinically observed as increased uptake of fluorescein. The environmental changes related to dry eye are discussed in more details in the companion article by Calonge and colleagues.
4-Animal models of age-related dry eye
A variety of animal models have been described that have been used to study pathogenesis of dry eye. The desiccating stress dry eye model that uses a combination of low humidity, environmental stress, and pharmacological cholinergic blockade has become a standard model in the research field. It has been used in more than 65 publications since its first description.125 This model recapitulates several features of human dry eye, inclusive of corneal barrier dysfunction, goblet cell loss and production of cytokines by the ocular surface epithelium.126–144 The desiccating stress mostly uses the inbred C57BL/6 mouse strain, while Balb/c mice have also been used with slightly different disease kinetics.142 Recent studies have shown that aged C57BL/6 mice are a valuable tool to study age-related dry eye, as these mice develop age-related MGD, corneal staining, goblet cell loss and conjunctival lymphocytic infiltration and dacryoadenitis.23, 145, 146 Similarly to desiccated mice and human Sjögren Syndrome (SS) patients, there is an increase production of MMP-9 and T-cell related cytokines such as IL-17, IFN-γ, on the aged ocular surface and an influx of CD4+ and CD8+ T cells into the aged LG23, suggesting that multiple inciting mechanism may converge to produce clinically significant dry eye independent of the initiating factor. Inflammation may be a common denominator in these situations. Lessons learned from animal models of dry eye are discussed in more details in the companion article by Stern and Pflugfelder. Pathogenic studies that utilized aged mice are summarized and briefly discussed in the next paragraphs.
Female sex is a risk factor for dry eye. Similar to the human studies4, 147, female C57BL/6 mice develop greater corneal barrier disruption than age-matched male mice, although other features of dry eye such as low goblet cell density and LG infiltration were similar in both sexes.23 This was accompanied by greater levels of IL-17A and IFN-γ compared to young mice. IL-17 stimulates MMPs- 3 and -9, which have been shown to break tight junction proteins and disrupt corneal barrier function.85, 148–151 Dry eye affects quality of life7, 14 and quality of vision, and decreases contrast sensitivity.12, 13 We can speculate that greater corneal disease in female patients may bring more women to the eye clinic than men, although other factors such as sex hormones and their influences on the corneal disease cannot be excluded.
Aged CD4+ T cells are spontaneously autoreactive. A common feature observed in SS subjects, SS animal models and desiccating stressed mice is increased infiltration of CD4+ T cells in conjunctiva and LG biopsies.152–157 This has also been observed in aged C57BL/6 mice.23 Furthermore, adoptive transfer of aged CD4+ T cells induced greater lymphocytic infiltration of the conjunctiva and LG and greater goblet cell loss than adoptive transfer of young donors, demonstrating that aged CD4+T cells participate in age-related dry eye.23 These studies indicate that aging promotes generation of autoreactive T cells; however, the precise mechanism of generation of these autoreactive T cells remains unknown as wells as the antigen. It is possible that a combination of factors such as aging of the immune system, lack of peripheral tolerance or accumulated exposure of an antigen over a life’s period maybe at play. It is possible that all three could be acting synergistically.
Interferon-γ participates in age-related goblet cell loss. The conjunctiva is one of the goblet-cell richest tissues in all body, just trailing the intestinal tract. Goblet cells are very sensitive to the ocular environment and besides producing mucins, have been implicated in immunomodulation on the ocular surface.158–161 The Th-2 signature cytokine IL-13 has an important role in goblet cell survival and homeostasis.162, 163 IFN-γ, on the other hand, has been shown to negatively impact conjunctival goblet cells.112, 130, 157, 164 This is true for both DS-induced or aged-induced upregulation of IFN-γ, as IFN-γKO mice are partially resistant to aged-related goblet cell loss.23, 57, 157
Aged regulatory T cells are dysfunctional. Using a variation of the nonobese diabetic mouse that does not develop diabetes, Coursey and colleagues demonstrated that dacryoadenitis in aged NOD.B10.H2b mice is accompanied by a significant increase in frequency of regulatory T cells (Tregs) in the LG, despite increased LG pathology compared to young mice of the same strain.25 This is in agreement with reports about biopsies of salivary glands from SS patients where increased frequency of Foxp3+ cells correlated with more severe disease.165, 166 Despite being dysfunctional and unable to suppress T effector cells in vitro, these aged murine Tregs do not loose Foxp3+ expression, but acquire the ability to produce IFN-γ and IL-17. Furthermore, adoptive transfer of aged T regs or aged T effectors resulted in similar higher inflammatory lacrimal keratoconjunctivitis scores compared to young Tregs and T effectors, suggesting loss of in vivo suppression ability and acquisition of an effector phenotype.25
Oxidative stress in aged LG impairs tear secretion. Reactive oxygen species generated from normal metabolism must be counteracted by anti-oxidant defenses to prevent damage to lipids, DNA and mitochondria. Mice that have increased mitochondrial oxidative stress or decrease in anti-oxidative pathways with aging have altered LG homeostasis, leading to decreased LG tear volume and increased corneal staining.167–170 In support of the animal studies, decreased expression of anti-oxidant enzymes and increased expression of oxidative stress markers have been noted in conjunctiva of SS patients.108–111 Elevated tear concentration of hexanoyl-lysine, a lipid oxidative stress marker, correlated significantly with staining scores and inflammatory cell density in confocal microscopy study evaluating SS and controls.108 Although it is unclear if oxidative stress occurs as a cause or consequence of dry eye, Deng and colleagues recently showed that hyperosmolarity can increase oxidative stress in cultured epithelial cells.171
Aged meibomian glands become atrophic. Histologic analysis of aged human eyelids have shown many morphological alterations including cystic dilatation of acini and/or ducts, atrophy of acini, thickening of acini basement membrane, granulation tissue, and lipogranulomatous inflammation.26 Aged C57BL/6 mice also develop age-related MGD, characterized by a dropout in acini volume, and increased expression of cytokines, and increased meibocyte differentiation.145, 146, 172, 173 It is postulated that a decrease in acini volume maybe secondary to loss of stem cells within the meibomian gland and not necessary to duct keratinization, as no increase in Keratin 10 nor SPRR1-a was noted in aged C57BL/6 MG.145, 173 In elegant studies using immunofluorescent computed tomography and 3D reconstruction, no duct keratinization was observed in aged meibomian glands from C57BL/6 mice, further corroborating their findings.145
5. Treatment of age-related dry eye
There are only two FDA-approved drugs to treat dry eye. Cyclosporine, a T cell modulator, has been extensively used for more than a decade to treat all forms of dry eye.92, 99, 106, 117, 174–177 Lifitegrast, a novel inhibitor of lymphocyte function-associated antigen-1 (LFA-1) which is involved in migration of T cells from the vessels, has just received approval to treat signs and symptoms of dry eye in July 2016. Results from the Phase 2 and 3 clinical trials showed promising results.178–180 For reviews about current dry eye therapies please see the published reviews.181–183 Other new therapies for dry eye are reviewed in the companion article by Gumus and Pflugfelder.
A non-pharmacological approach to improve dry eye is the use of supplements. Polyunsaturated fatty acids such as omega-6 (linolenic acid) and omega-3 (α-linolenic acid) have gained popularity as oral supplements in dry eye disease as they decrease inflammatory mediators and decrease activation of dendritic cells.95, 184–188
A more holistic approach to aging-dry eye has been proposed and it involves the use of calorie restriction (CR) and supplementation with vitamins and anti-oxidants.189 Calorie restriction (CR) without malnutrition has emerged in the 21st century as a potential strategy to prevent age-related diseases. Although first published in 1935 by McCay and Colleagues,190 CR has gained interest after studies showing prolonged life span, decreased cardiovascular diseases and incidence of tumors in rodents.191–194 These results were duplicated in non-human primates,195, 196 generating great interest of the scientific community in this aging intervention. A massive body of evidence has been accumulated since then, demonstrating that CR induces profound metabolic, hormonal and molecular changes. For a detailed review about CR inclusive of NIH-sponsored CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) clinical trial in humans, please see the review by Most and colleagues.197 In ophthalmology, CR has been shown to substantially decrease infiltration of submandibular glands in aged autoimmune mice prone mice.198 This was accompanied by a significant decrease in inflammatory mediators such as TNF-α and IL-6 while an increase in TGF-β1 was noted.198 In aged rats, CR for 6 months significantly improved tear secretion, LG acinar morphology and decreased expression of lipid oxidation markers 8-hydroxy-2 deoxyguanosine (8-OHdG) and 4-hydroxynonenal (HNE) that relates to oxidative stress.199
Conclusions
Despite intensive research, the true nature of aging remains elusive. Not all organisms age well or will age at the same rate. However, aging involves complex biochemical, molecular, immune mechanisms that may be interacting. Inflammation on the ocular surface participates in age-related dry eye. Understanding the mechanisms of aging will enable early interventions and prevent end-stage organ atrophy and ocular surface disease.
Acknowledgments
Supported by Research to Prevent Blindness, The Oshman Foundation, William Stamps Farish Fund and the Hamill Foundation.
References
- 1.Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 2.Schein OD, Tiesch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997;104:1395–1401. doi: 10.1016/s0161-6420(97)30125-0. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 5.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127:763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schein OD, Hochberg MC, Munoz B, et al. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999;159:1359–1363. doi: 10.1001/archinte.159.12.1359. [DOI] [PubMed] [Google Scholar]
- 7.Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2003;31:229–232. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 9.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 10.Lamberts DW, Foster CS, Perry HD. Schirmer test after topical anesthesis and the tear menius height in normal eyes. ArchOphthalmol. 1979;97:1082–1085. doi: 10.1001/archopht.1979.01020010536004. [DOI] [PubMed] [Google Scholar]
- 11.Maeda N, Sato S, Watanabe H, et al. Prediction of letter contrast sensitivity using videokeratographic indices. AmJOphthalmol. 2000;129:759–763. doi: 10.1016/s0002-9394(00)00380-9. [DOI] [PubMed] [Google Scholar]
- 12.Chotikavanich S, de Paiva CS, D-Q L, et al. Production and Activity of Matrix Metalloproteinase-9 on the Ocular Surface Increase in Dysfunctional Tear Syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. AmJOphthalmol. 2007;143:409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. OculSurf. 2006;4:155–161. doi: 10.1016/s1542-0124(12)70043-5. [DOI] [PubMed] [Google Scholar]
- 15.Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419. doi: 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 16.Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. AmJManagCare. 2008;14:S102–S106. [PubMed] [Google Scholar]
- 17.Bukhari AA, Basheer NA, Joharjy HI. Age, gender, and interracial variability of normal lacrimal gland volume using MRI. Ophthal Plast Reconstr Surg. 2014;30:388–391. doi: 10.1097/IOP.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 18.El-Fadaly AB, El-Shaarawy EA, Rizk AA, Nasralla MM, Shuaib DM. Age-related alterations in the lacrimal gland of adult albino rat: a light and electron microscopic study. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2014;196:336–351. doi: 10.1016/j.aanat.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Nasu M, Matsubara O, Yamamoto H. Post-mortem prevalence of lymphocytic infiltration of the lacrymal gland: a comparative study in autoimmune and non-autoimmune diseases. J Pathol. 1984;143:11–15. doi: 10.1002/path.1711430104. [DOI] [PubMed] [Google Scholar]
- 20.Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging Ophthalmology. 1995;102:678–686. doi: 10.1016/s0161-6420(95)30971-2. [DOI] [PubMed] [Google Scholar]
- 21.Obata H. Anatomy and histopathology of the human lacrimal gland. Cornea. 2006;25:S82–S89. doi: 10.1097/01.ico.0000247220.18295.d3. [DOI] [PubMed] [Google Scholar]
- 22.Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008;6:162–174. doi: 10.1016/s1542-0124(12)70177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClellan AJ, Volpe EA, Zhang X, et al. Ocular Surface Disease and Dacryoadenitis in Aging C57BL/6 Mice. Am J Pathol. 2014;184:631–643. doi: 10.1016/j.ajpath.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rios JD, Horikawa Y, Chen LL, et al. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. ExpEye Res. 2005;80:477–491. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coursey TG, Bian F, Zaheer M, Pflugfelder SC, Volpe EA, de Paiva CS. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21:S70–74. doi: 10.1097/01.ico.0000263122.45898.09. [DOI] [PubMed] [Google Scholar]
- 27.Chhadva P, McClellan AL, Alabiad CR, Feuer WJ, Batawi H, Galor A. Impact of Eyelid Laxity on Symptoms and Signs of Dry Eye Disease. Cornea. 2016;35:531–535. doi: 10.1097/ICO.0000000000000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alghamdi YA, Mercado C, McClellan AL, Batawi H, Karp CL, Galor A. Epidemiology of Meibomian Gland Dysfunction in an Elderly Population. Cornea. 2016;35:731–735. doi: 10.1097/ICO.0000000000000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25:651–655. doi: 10.1097/01.ico.0000227889.11500.6f. [DOI] [PubMed] [Google Scholar]
- 30.Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–342. doi: 10.1097/00003226-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan DA, Sullivan BD, Evans JE, et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi M, Kutsuna M, Uno T, Zheng X, Kodama T, Ohashi Y. Marx line: fluorescein staining line on the inner lid as indicator of meibomian gland function. Am J Ophthalmol. 2006;141:669–675. doi: 10.1016/j.ajo.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan DA, Rocha EM, Ullman MD, et al. Androgen regulation of the meibomian gland. Adv Exp Med Biol. 1998;438:327–331. doi: 10.1007/978-1-4615-5359-5_46. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan DA, Sullivan BD, Ullman MD, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41:3732–3742. [PubMed] [Google Scholar]
- 35.Sullivan DA, Wickham LA, Rocha EM, et al. Androgens and dry eye in Sjogren’s syndrome. Ann N Y Acad Sci. 1999;876:312–324. doi: 10.1111/j.1749-6632.1999.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 36.Toda I, Wickham LA, Rocha EM, da Silveira LA, Sullivan DA. Gender- and androgen-related impact on the expression of proto-oncogenes and apoptotic factors in lacrimal and salivary glands of mouse models of Sjogren’s syndrome. Adv Exp Med Biol. 1998;438:447–452. doi: 10.1007/978-1-4615-5359-5_62. [DOI] [PubMed] [Google Scholar]
- 37.Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand. 2000;78:146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- 38.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Azcarate PM, Venincasa VD, Feuer W, Stanczyk F, Schally AV, Galor A. Androgen deficiency and dry eye syndrome in the aging male. Invest Ophthalmol Vis Sci. 2014;55:5046–5053. doi: 10.1167/iovs.14-14689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. MolVis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Garcia MJ, Gonzalez-Saiz A, de la Fuente B, et al. Exposure to a controlled adverse environment impairs the ocular surface of subjects with minimally symptomatic dry eye. Invest OphthalmolVisSci. 2007;48:4026–4032. doi: 10.1167/iovs.06-0817. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Miguel A, Teson M, Martin-Montanez V, et al. Dry eye exacerbation in patients exposed to desiccating stress under controlled environmental conditions. AmJOphthalmol. 2014;157:788–798. doi: 10.1016/j.ajo.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Reinoso R, Calonge M, Castellanos E, et al. Differential cell proliferation, apoptosis, and immune response in healthy and evaporative-type dry eye conjunctival epithelia. Invest Ophthalmol Vis Sci. 2011;52:4819–4828. doi: 10.1167/iovs.10-6073. [DOI] [PubMed] [Google Scholar]
- 44.Pflugfelder SC, De Paiva CS, Moore QL, et al. Aqueous Tear Deficiency Increases Conjunctival Interferon-gamma (IFN-gamma) Expression and Goblet Cell Loss. Invest Ophthalmol Vis Sci. 2015;56:7545–7550. doi: 10.1167/iovs.15-17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998;43:225–232. doi: 10.1016/s0039-6257(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 46.Chhadva P, Alexander A, McClellan AL, McManus KT, Seiden B, Galor A. The impact of conjunctivochalasis on dry eye symptoms and signs. Invest Ophthalmol Vis Sci. 2015;56:2867–2871. doi: 10.1167/iovs.14-16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gumus K, Pflugfelder SC. Increasing prevalence and severity of conjunctivochalasis with aging detected by anterior segment optical coherence tomography. Am J Ophthalmol. 2013;155:238–242. doi: 10.1016/j.ajo.2012.07.014. e232. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y, Sheha H, Tseng SC. Conjunctivochalasis interferes with tear flow from fornix to tear meniscus. Ophthalmology. 2013;120:1681–1687. doi: 10.1016/j.ophtha.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Stern ME. Dry eye–is this a disease or a natural consequence of aging? Archivos de la Sociedad Espanola de Oftalmologia. 2005;80:125–131. [PubMed] [Google Scholar]
- 50.Paschides CA, Petroutsos G, Psilas K. Correlation of conjunctival impression cytology results with lacrimal function and age. Acta Ophthalmol (Copenh) 1991;69:422–425. doi: 10.1111/j.1755-3768.1991.tb02016.x. [DOI] [PubMed] [Google Scholar]
- 51.Huang AJ, Tseng SC, Kenyon KR. Morphogenesis of rat conjunctival goblet cells. Invest OphthalmolVisSci. 1988;29:969–975. [PubMed] [Google Scholar]
- 52.Zhu W, Hong J, Zheng T, Le Q, Xu J, Sun X. Age-related changes of human conjunctiva on in vivo confocal microscopy. Br J Ophthalmol. 2010;94:1448–1453. doi: 10.1136/bjo.2008.155820. [DOI] [PubMed] [Google Scholar]
- 53.Vujkovic V, Mikac G, Kozomara R. Distribution and density of conjunctival goblet cells. Med Pregl. 2002;55:195–200. doi: 10.2298/mpns0206195v. [DOI] [PubMed] [Google Scholar]
- 54.Wei A, Hong J, Sun X, Xu J. Evaluation of age-related changes in human palpebral conjunctiva and meibomian glands by in vivo confocal microscopy. Cornea. 2011;30:1007–1012. doi: 10.1097/ICO.0b013e31820ca468. [DOI] [PubMed] [Google Scholar]
- 55.Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152:377–384. doi: 10.1016/j.ajo.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galor A, Zheng DD, Arheart KL, et al. Dry eye medication use and expenditures: data from the medical expenditure panel survey 2001 to 2006. Cornea. 2012;31:1403–1407. doi: 10.1097/ICO.0b013e31823cc0b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volpe EA, Henriksson JT, Wang C, et al. Interferon-gamma deficiency protects against aging-related goblet cell loss. Oncotarget. 2016 doi: 10.18632/oncotarget.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You IC, Bian F, Volpe EA, de Paiva CS, Pflugfelder SC. Age-related conjunctival disease in the C57BL/6.NOD-Aec1Aec2 Mouse Model of Sjogren Syndrome develops independent of lacrimal dysfunction. Invest Ophthalmol Vis Sci. 2015 Mar 10; doi: 10.1167/iovs.14-15668. 2015. pii: IOVS-14-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Achtsidis V, Eleftheriadou I, Kozanidou E, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care. 2014;37:e210–211. doi: 10.2337/dc14-0860. [DOI] [PubMed] [Google Scholar]
- 60.Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8:10. doi: 10.1186/1471-2415-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aljarousha M, Badarudin NE, Che Azemin MZ. Comparison of Dry Eye Parameters between Diabetics and Non-Diabetics in District of Kuantan, Pahang. Malays J Med Sci. 2016;23:72–77. [PMC free article] [PubMed] [Google Scholar]
- 62.Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. Journal of ophthalmology. 2012;2012:285851. doi: 10.1155/2012/285851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fraunfelder FT, Meyer SM. Corneal complications of ocular medications. Cornea. 1986;5:55–59. [Google Scholar]
- 64.Baudouin C, Renard JP, Nordmann JP, et al. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. 2012;0 doi: 10.5301/ejo.5000181. [DOI] [PubMed] [Google Scholar]
- 65.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 66.De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40:619–630. [PubMed] [Google Scholar]
- 67.Pauly A, Meloni M, Brignole-Baudouin F, Warnet JM, Baudouin C. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: early detection of toxic damage. Invest Ophthalmol Vis Sci. 2009;50:1644–1652. doi: 10.1167/iovs.08-2992. [DOI] [PubMed] [Google Scholar]
- 68.Uematsu M, Kumagami T, Kusano M, et al. Acute corneal epithelial change after instillation of benzalkonium chloride evaluated using a newly developed in vivo corneal transepithelial electric resistance measurement method. Ophthalmic Res. 2007;39:308–314. doi: 10.1159/000109986. [DOI] [PubMed] [Google Scholar]
- 69.Uusitalo H, Chen E, Pfeiffer N, et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 2010;88:329–336. doi: 10.1111/j.1755-3768.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 70.Aihara M, Otani S, Kozaki J, et al. Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J Glaucoma. 2012;21:60–64. doi: 10.1097/IJG.0b013e3181fc8129. [DOI] [PubMed] [Google Scholar]
- 71.Walimbe T, Chelerkar V, Bhagat P, Joshi A, Raut A. Effect of benzalkonium chloride-free latanoprost ophthalmic solution on ocular surface in patients with glaucoma. Clin Ophthalmol. 2016;10:821–827. doi: 10.2147/OPTH.S102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. AutoimmunRev. 2004;3:401–406. doi: 10.1016/j.autrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 73.Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging cell. 2004;3:151–160. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- 74.Canaday DH, Parker KE, Aung H, Chen HE, Nunez-Medina D, Burant CJ. Age-dependent changes in the expression of regulatory cell surface ligands in activated human T-cells. BMC Immunol. 2013;14:45. doi: 10.1186/1471-2172-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Capri M, Monti D, Salvioli S, et al. Complexity of anti-immunosenescence strategies in humans. ArtifOrgans. 2006;30:730–742. doi: 10.1111/j.1525-1594.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 76.Franceschi C, BonaFe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. AnnNYAcadSci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 77.Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. MechAgeing Dev. 2000;121:37–46. doi: 10.1016/s0047-6374(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 78.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. CurrOpinHematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Cohen AA. Complex systems dynamics in aging: new evidence, continuing questions. Biogerontology. 2016;17:205–220. doi: 10.1007/s10522-015-9584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sergiev PV, Dontsova OA, Berezkin GV. Theories of aging: an ever-evolving field. Acta naturae. 2015;7:9–18. [PMC free article] [PubMed] [Google Scholar]
- 81.Pisella PJ, Brignole F, Debbasch C, et al. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. 2000;107:1841–1849. doi: 10.1016/s0161-6420(00)00347-x. [DOI] [PubMed] [Google Scholar]
- 82.Jones DT, Monroy D, Ji Z, Pflugfelder SC. Alterations of ocular surface gene expression in Sjogren’s syndrome. Adv Exp Med Biol. 1998;438:533–536. doi: 10.1007/978-1-4615-5359-5_75. [DOI] [PubMed] [Google Scholar]
- 83.Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjogren’s syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest OphthalmolVisSci. 1994;35:3493–3504. [PubMed] [Google Scholar]
- 84.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 85.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 87.Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26:431–437. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 88.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 89.Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. MolVis. 2012;18:797–802. [PMC free article] [PubMed] [Google Scholar]
- 90.Lam H, Blieden L, de Paiva CS, Farley WJ, Stern ME, Pflugfelder SC. Tear Cytokine Profiles in Dysfunctional Tear Syndrome. Am J Ophthalmol. 2009;147:198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Epstein SP, Gadaria-Rathod N, Wei Y, Maguire MG, Asbell PA. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp Eye Res. 2013;111:95–104. doi: 10.1016/j.exer.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baudouin C, Brignole F, Pisella PJ, De Jean MS, Goguel A. Flow cytometric analysis of the inflammatory marker HLA DR in dry eye syndrome: results from 12 months of randomized treatment with topical cyclosporin A. AdvExpMedBiol. 2002;506:761–769. doi: 10.1007/978-1-4615-0717-8_107. [DOI] [PubMed] [Google Scholar]
- 93.Brignole F, Pisella PJ, De Saint JM, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest OphthalmolVisSci. 2001;42:90–95. [PubMed] [Google Scholar]
- 94.Brignole F, Pisella PJ, Goldschild M, De Saint JM, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest OphthalmolVisSci. 2000;41:1356–1363. [PubMed] [Google Scholar]
- 95.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea. 2013 doi: 10.1097/ICO.0b013e318299549c. [DOI] [PubMed] [Google Scholar]
- 96.Tsubota K, Fukagawa K, Fujihara T, et al. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest OphthalmolVisSci. 1999;40:28–34. [PubMed] [Google Scholar]
- 97.Tsubota K, Fujihara T, Saito K, Takeuchi T. Conjunctival epithelium expression of HLA-DR in dry eye patients. Ophthalmologica. 1999;213:16–19. doi: 10.1159/000027387. [DOI] [PubMed] [Google Scholar]
- 98.Rolando M, Barabino S, Mingari C, Moretti S, Giuffrida S, Calabria G. Distribution of Conjunctival HLA-DR Expression and the Pathogenesis of Damage in Early Dry Eyes. Cornea. 2005;24:951–954. doi: 10.1097/01.ico.0000157421.93522.00. [DOI] [PubMed] [Google Scholar]
- 99.Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. ArchOphthalmol. 2000;118:1489–1496. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 100.Baudouin C, Liang H, Bremond-Gignac D, et al. CCR 4 and CCR 5 expression in conjunctival specimens as differential markers of T(H)1/T(H)2 in ocular surface disorders. JAllergy ClinImmunol. 2005;116:614–619. doi: 10.1016/j.jaci.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 101.Li S, Nikulina K, DeVoss J, et al. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008;49:34–41. doi: 10.1167/iovs.07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawasaki S, Kawamoto S, Yokoi N, et al. Up-regulated gene expression in the conjunctival epithelium of patients with Sjogren’s syndrome. Exp Eye Res. 2003;77:17–26. doi: 10.1016/s0014-4835(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 103.Pflugfelder SC, de Paiva CS, Moore QL, et al. Aqueous Tear Deficiency Increases Conjunctival Interferon-gamma (IFN-g) Expression and Goblet Cell Loss. Invest Ophthalmol Vis Sci. 2015;56(12):7545–50. doi: 10.1167/iovs.15-17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest OphthalmolVisSci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uchino Y, Mauris J, Woodward AM, et al. Alteration of galectin-3 in tears of patients with dry eye disease. Am J Ophthalmol. 2015;159:1027–1035.e1023. doi: 10.1016/j.ajo.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gurdal C, Genc I, Sarac O, Gonul I, Takmaz T, Can I. Topical cyclosporine in thyroid orbitopathy-related dry eye: clinical findings, conjunctival epithelial apoptosis, and MMP-9 expression. Curr Eye Res. 2010;35:771–777. doi: 10.3109/02713683.2010.490320. [DOI] [PubMed] [Google Scholar]
- 107.Aragona P, Aguennouz MH, Rania L, et al. Matrix Metalloproteinase 9 and Transglutaminase 2 Expression at the Ocular Surface in Patients with Different Forms of Dry Eye Disease. Ophthalmology. 2015;122:62–71. doi: 10.1016/j.ophtha.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 108.Wakamatsu TH, Dogru M, Matsumoto Y, et al. Evaluation of lipid oxidative stress status in Sjogren syndrome patients. Invest Ophthalmol Vis Sci. 2013;54:201–210. doi: 10.1167/iovs.12-10325. [DOI] [PubMed] [Google Scholar]
- 109.Choi W, Lian C, Ying L, et al. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr Eye Res. 2016;41:1143–1149. doi: 10.3109/02713683.2015.1098707. [DOI] [PubMed] [Google Scholar]
- 110.Cejkova J, Ardan T, Jirsova K, et al. The role of conjunctival epithelial cell xanthine oxidoreductase/xanthine oxidase in oxidative reactions on the ocular surface of dry eye patients with Sjogren’s syndrome. Histol Histopathol. 2007;22:997–1003. doi: 10.14670/HH-22.997. [DOI] [PubMed] [Google Scholar]
- 111.Cejkova J, Ardan T, Simonova Z, et al. Decreased expression of antioxidant enzymes in the conjunctival epithelium of dry eye (Sjogren’s syndrome) and its possible contribution to the development of ocular surface oxidative injuries. Histol Histopathol. 2008;23:1477–1483. doi: 10.14670/HH-23.1477. [DOI] [PubMed] [Google Scholar]
- 112.Coursey TG, Henriksson JT, Barbosa FL, de Paiva CS, Pflugfelder SC. Interferon-gamma-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjogren Syndrome. Am J Pathol. 2016:S0002–9440. 30005–30000. doi: 10.1016/j.ajpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lemp MA, Dohlman CH, Kuwabara T, Holly FJ, Carroll JM. Dry eye secondary mucus deficiency. TransAmOphthalmolSoc. 1971;75:1223–1227. [PubMed] [Google Scholar]
- 114.Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14:299–302. [PubMed] [Google Scholar]
- 115.Dohlman CH, Friend J, Kalevar V, Yagoda D, Balazs E. The glycoprotein (mucus) content of tears from normals and dry eye patients. ExpEye Res. 1976;22:359–365. doi: 10.1016/0014-4835(76)90228-1. [DOI] [PubMed] [Google Scholar]
- 116.Terry MA. Dry eye in the elderly. Drugs Aging. 2001;18:101–107. doi: 10.2165/00002512-200118020-00003. [DOI] [PubMed] [Google Scholar]
- 117.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. ArchOphthalmol. 2002;120:330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 118.Lu P, Chen X, Liu X, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008;27:545–551. doi: 10.1097/ICO.0b013e318165b1b7. [DOI] [PubMed] [Google Scholar]
- 119.Guo B, Lu P, Chen X, Zhang W, Chen R. Prevalence of dry eye disease in Mongolians at high altitude in China: the Henan eye study. Ophthalmic Epidemiol. 2010;17:234–241. doi: 10.3109/09286586.2010.498659. [DOI] [PubMed] [Google Scholar]
- 120.Gupta N, Prasad I, Himashree G, D’Souza P. Prevalence of dry eye at high altitude: a case controlled comparative study. High AltMedBiol. 2008;9:327–334. doi: 10.1089/ham.2007.1055. [DOI] [PubMed] [Google Scholar]
- 121.Madden LC, Tomlinson A, Simmons PA. Effect of humidity variations in a controlled environment chamber on tear evaporation after dry eye therapy. Eye Contact Lens. 2013;39:169–174. doi: 10.1097/ICL.0b013e318283dfc6. [DOI] [PubMed] [Google Scholar]
- 122.Rios JL, Boechat JL, Gioda A, dos Santos CY, de Aquino Neto FR, Lapa e Silva JR. Symptoms prevalence among office workers of a sealed versus a non-sealed building: associations to indoor air quality. EnvironInt. 2009;35:1136–1141. doi: 10.1016/j.envint.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 123.Su SB, Wang BJ, Tai C, Chang HF, Guo HR. Higher prevalence of dry symptoms in skin, eyes, nose and throat among workers in clean rooms with moderate humidity. JOccupHealth. 2009;51:364–369. doi: 10.1539/joh.q8002. [DOI] [PubMed] [Google Scholar]
- 124.Yamada M, Mizuno Y, Shigeyasu C. Impact of dry eye on work productivity. ClinicoeconOutcomesRes. 2012;4:307–312. doi: 10.2147/CEOR.S36352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest OphthalmolVisSci. 2002;43:632–638. [PubMed] [Google Scholar]
- 126.de Paiva CS, Jones DB, Stern ME, et al. Altered Mucosal Microbiome Diversity and Disease Severity in Sjogren Syndrome. Sci Rep. 2016;6:23561. doi: 10.1038/srep23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simmons KT, Xiao Y, Pflugfelder SC, de Paiva CS. Inflammatory Response to Lipopolysaccharide on the Ocular Surface in a Murine Dry Eye Model. Invest Ophthalmol Vis Sci. 2016;57:2443–2451. doi: 10.1167/iovs.15-18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.You IC, Coursey TG, Bian F, Barbosa FL, de Paiva CS, Pflugfelder SC. Macrophage Phenotype in the Ocular Surface of Experimental Murine Dry Eye Disease. Arch Immunol Ther Exp (Warsz) 2015 doi: 10.1007/s00005-015-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krauss AH, Corrales RM, Pelegrino FS, Tukler-Henriksson J, Pflugfelder SC, de Paiva CS. Improvement of Outcome Measures of Dry Eye by a Novel Integrin Antagonist in the Murine Desiccating Stress Model. Invest Ophthalmol Vis Sci. 2015;56:5888–5895. doi: 10.1167/iovs.15-17249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X, de Paiva CS, Su Z, Volpe EA, Li DQ, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014;118:117–124. doi: 10.1016/j.exer.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coursey TG, Bohat R, Barbosa FL, Pflugfelder SC, de Paiva CS. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J Immunol. 2014;193:5264–5272. doi: 10.4049/jimmunol.1400016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gandhi NB, Su Z, Zhang X, et al. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. J Leukoc Biol. 2013 doi: 10.1189/jlb.1012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoSOne. 2013;8:e78508. doi: 10.1371/journal.pone.0078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang X, Volpe EA, Gandhi NB, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoSOne. 2012;7:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stern ME, Schaumburg CS, Siemasko KF, et al. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest OphthalmolVisSci. 2012;53:2062–2075. doi: 10.1167/iovs.11-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schaumburg CS, Siemasko KF, de Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 137.Corrales RM, de Paiva CS, Li DQ, et al. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest Ophthalmol Vis Sci. 2011;52:3492–3499. doi: 10.1167/iovs.10-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beardsley RM, de Paiva CS, Power DF, Pflugfelder SC. Desiccating stress decreases apical corneal epithelial cell size–modulation by the metalloproteinase inhibitor doxycycline. Cornea. 2008;27:935–940. doi: 10.1097/ICO.0b013e3181757997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoon KC, de Paiva CS, Qi H, et al. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. JAutoimmun. 2008;30:212–221. doi: 10.1016/j.jaut.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoon KC, de Paiva CS, Qi H, et al. Expression of th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest OphthalmolVisSci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 141.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating Stress Induces T Cell-Mediated Sjogren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 142.Corrales RM, Stern ME, de Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 143.de Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 144.de Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. ExpEye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 145.Parfitt GJ, Xie Y, Geyfman M, Brown DJ, Jester JV. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD) Aging (Albany NY) 2013;5:825–834. doi: 10.18632/aging.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nien CJ, Paugh JR, Massei S, Wahlert AJ, Kao WW, Jester JV. Age-related changes in the meibomian gland. Exp Eye Res. 2009;89:1021–1027. doi: 10.1016/j.exer.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Uchino M, Dogru M, Uchino Y, et al. Japan Ministry of Health study on prevalence of dry eye disease among Japanese high school students. Am J Ophthalmol. 2008;146:925–929.e922. doi: 10.1016/j.ajo.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 148.Cortez DM, Feldman MD, Mummidi S, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38. Am J Physiol Heart Circ Physiol. 2007;293:H3356–H3365. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 149.Sylvester J, Liacini A, Li WQ, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chondrocytes. Cell Signal. 2004;16:469–476. doi: 10.1016/j.cellsig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 150.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol VisSci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 151.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Paiva CS, Hwang CS, Pitcher JD, III, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. 2010;49:246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rahimy E, Pitcher JD, III, Pangelinan SB, et al. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. 2010;177:744–753. doi: 10.2353/ajpath.2010.091116. Epub 2010 Jun 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cha S, Brayer J, Gao J, et al. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60:552–565. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 155.Cha S, Nagashima H, Brown VB, Peck AB, Humphreys-Beher MG. Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjogren’s syndrome) on a healthy murine background. Arthritis Rheum. 2002;46:1390–1398. doi: 10.1002/art.10258. [DOI] [PubMed] [Google Scholar]
- 156.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 157.de Paiva CS, Villarreal AL, Corrales RM, et al. Dry Eye-Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-{gamma} Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 158.Xiao Y, Coursey TG, Li DQ, de Paiva CS, Tukler Henriksson J, Pflugfelder SC. Conjunctival Goblet Cells modulate dendritic cell maturation and retinoic acid producing capacity. Invest Ophthalmol Vis Sci. 2016;57 [Google Scholar]
- 159.Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, Dartt DA, Masli S. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediators Inflamm. 2013;2013:636812. doi: 10.1155/2013/636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garcia-Posadas L, Hodges RR, Li D, et al. Interaction of IFN-gamma with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2016;9:206–217. doi: 10.1038/mi.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015;10:e0120284. doi: 10.1371/journal.pone.0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tukler Henriksson J, Coursey TG, Corry DB, De Paiva CS, Pflugfelder SC. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cultured Conjunctival Goblet Cells. Invest Ophthalmol Vis Sci. 2015;56:4186–4197. doi: 10.1167/iovs.14-15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de Paiva CS, Raince JK, McClellan AJ, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4:397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang X, Chen W, de Paiva CS, et al. Desiccating Stress Induces CD4(+) T-Cell-Mediated Sjogren’s Syndrome-Like Corneal Epithelial Apoptosis via Activation of the Extrinsic Apoptotic Pathway by Interferon-gamma. Am J Pathol. 2011;179:1807–1814. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sarigul M, Yazisiz V, Bassorgun CI, et al. The numbers of Foxp3 + Treg cells are positively correlated with higher grade of infiltration at the salivary glands in primary Sjogren’s syndrome. Lupus. 2010;19:138–145. doi: 10.1177/0961203309348234. [DOI] [PubMed] [Google Scholar]
- 166.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos NM, Moutsopoulos HM. Foxp3+ T-regulatory cells in Sjogren’s syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. AmJPathol. 2008;173:1389–1396. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kojima T, Dogru M, Ibrahim OM, et al. Effects of Oxidative Stress on the Conjunctiva in Cu, Zn-Superoxide Dismutase-1 (Sod1)-Knockout Mice. Invest Ophthalmol Vis Sci. 2015;56:8382–8391. doi: 10.1167/iovs.15-18295. [DOI] [PubMed] [Google Scholar]
- 168.Ibrahim OM, Dogru M, Matsumoto Y, et al. Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PLoS One. 2014;9:e99328. doi: 10.1371/journal.pone.0099328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kojima T, Wakamatsu TH, Dogru M, et al. Age-related dysfunction of the lacrimal gland and oxidative stress: evidence from the Cu,Zn-superoxide dismutase-1 (Sod1) knockout mice. Am J Pathol. 2012;180:1879–1896. doi: 10.1016/j.ajpath.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 170.Uchino Y, Kawakita T, Miyazawa M, et al. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS One. 2012;7:e45805. doi: 10.1371/journal.pone.0045805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Deng R, Hua X, Li J, et al. Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS One. 2015;10:e0126561. doi: 10.1371/journal.pone.0126561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jester BE, Nien CJ, Winkler M, Brown DJ, Jester JV. Volumetric reconstruction of the mouse meibomian gland using high-resolution nonlinear optical imaging. Anatomical record (Hoboken, NJ: 2007) 2011;294:185–192. doi: 10.1002/ar.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Parfitt GJ, Brown DJ, Jester JV. Transcriptome analysis of aging mouse meibomian glands. Mol Vis. 2016;22:518–527. [PMC free article] [PubMed] [Google Scholar]
- 174.Gunduz K, Ozdemir O. Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjogren’s syndrome. Acta Ophthalmol(Copenh) 1994;72:438–442. doi: 10.1111/j.1755-3768.1994.tb02792.x. [DOI] [PubMed] [Google Scholar]
- 175.Kiang E, Tesavibul N, Yee R, Kellaway J, Przepiorka D. The use of topical cyclosporin A in ocular graft-versus-host-disease. Bone Marrow Transplant. 1998;22:147–151. doi: 10.1038/sj.bmt.1701304. [DOI] [PubMed] [Google Scholar]
- 176.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 177.Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group Ophthalmology. 2000;107:967–974. doi: 10.1016/s0161-6420(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 178.Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a Novel Integrin Antagonist for Treatment of Dry Eye Disease. The ocular surface. 2016;14:207–215. doi: 10.1016/j.jtos.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 179.Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol. 2016;10:1083–1094. doi: 10.2147/OPTH.S110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121:475–483. doi: 10.1016/j.ophtha.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 181.Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopinski P. Immunomodulation on the ocular surface: a review. Central-European journal of immunology. 2016;41:195–208. doi: 10.5114/ceji.2016.60995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wan KH, Chen LJ, Young AL. Efficacy and Safety of Topical 0.05% Cyclosporine Eye Drops in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-analysis. The ocular surface. 2015;13:213–225. doi: 10.1016/j.jtos.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 183.Marshall LL, Roach JM. Treatment of Dry Eye Disease. Consult Pharm. 2016;31:96–106. doi: 10.4140/TCP.n.2016.96. [DOI] [PubMed] [Google Scholar]
- 184.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. AmJClinNutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 185.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. NEnglJMed. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 186.Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis) TransAm OphthalmolSoc. 2008;106:336–356. [PMC free article] [PubMed] [Google Scholar]
- 187.Barabino S, Rolando M, Camicione P, et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22:97–101. doi: 10.1097/00003226-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 188.Mostofsky E, Wilker EH, Schwartz J, et al. Short-term changes in ambient temperature and risk of ischemic stroke. CerebrovascDisExtra. 2014;4:9–18. doi: 10.1159/000357352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsubota K, Kawashima M, Inaba T, et al. The antiaging approach for the treatment of dry eye. Cornea. 2012;31(Suppl 1):S3–S8. doi: 10.1097/ICO.0b013e31826a05a8. [DOI] [PubMed] [Google Scholar]
- 190.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. J Nutrition. 193510:63. [PubMed] [Google Scholar]
- 191.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fontana L, Villareal DT, Weiss EP, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial AmJPhysiol EndocrinolMetab. 2007;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 193.Riesen WH, Herbst EJ, et al. The effect of restricted caloric intake on the longevity of rats. Am J Physiol. 1947;148:614–617. doi: 10.1152/ajplegacy.1947.148.3.614. [DOI] [PubMed] [Google Scholar]
- 194.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 195.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature communications. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing research reviews. 2016 doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chandrasekar B, McGuff HS, Aufdermorte TB, Troyer DA, Talal N, Fernandes G. Effects of calorie restriction on transforming growth factor beta 1 and proinflammatory cytokines in murine Sjogren’s syndrome. Clin Immunol Immunopathol. 1995;76:291–296. doi: 10.1006/clin.1995.1128. [DOI] [PubMed] [Google Scholar]
- 199.Kawashima M, Kawakita T, Okada N, et al. Calorie restriction: A new therapeutic intervention for age-related dry eye disease in rats. Biochem Biophys Res Commun. 2010;397:724–728. doi: 10.1016/j.bbrc.2010.06.018. [DOI] [PubMed] [Google Scholar]


