Many lizards frequently eat fruits and flowers, but few are strictly herbivorous (1, 2). For >30 years, biologists have perpetuated the notion that herbivory in lizards required large body size, based largely on a set of physiological arguments centered on thermal requirements for digestion of plants and the observation that the few studied herbivorous lizards were relatively large in body size (3). From the outset, the argument was fundamentally flawed, because most known large-bodied herbivorous lizards are members of a strictly herbivorous clade, the Iguanidae. Consequently, a single origin of herbivory from a large-bodied ancestor accounts for much of the association between herbivory and large size in lizards. Within other lizard clades, herbivorous species are not among the largest (e.g., Teiidae, Cnemidophorus murinus, Cnemidophorus arubensis, and Dicrodon guttulatum; and Varanidae, Varanus olivaceus). Even when the few noniguanian origins of herbivory are added, the number of origins pales in comparison with those identified in this issue of PNAS by Espinoza et al. (4) in a single iguanian clade, the Liolaemidae. More importantly, the multiple origins identified by Espinoza et al. derive from small-bodied ancestors in relatively cool environments, running counter to the notion that herbivory in ectotherms requires large body size and warm environments. Why is this such an important finding, and how does it change the way we think about the evolution of feeding and diets in lizards?
Squamate Feeding Strategies
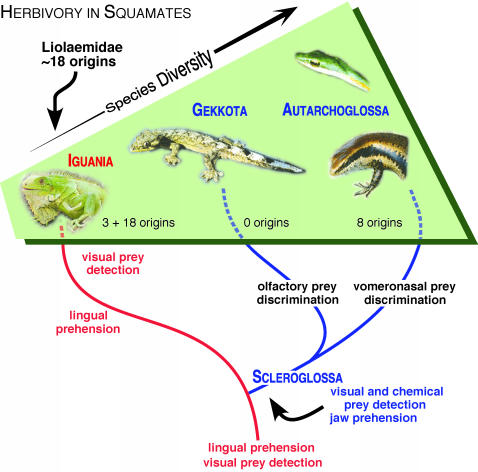
To fully appreciate the significance of this finding, it is necessary to recount the evolution of feeding strategies in squamate reptiles (Fig. 1). Squamate ancestors used visual cues to detect and discriminate prey, the tongue to capture and manipulate prey (lingual prehension), and an ambush foraging mode (2, 5–7). During late Triassic or early Jurassic, the most important divergence in the history of squamates produced one clade, Iguania, which retained ancestral traits, and another, Scleroglossa, which, rather than using the tongue for prey capture, used the jaws (jaw prehension). The scleroglossan skull was much more kinetic, facilitating prey capture, and the tongue was freed from involvement in prey prehension (5). Scleroglossa soon diverged, with one clade, Gekkota, using the tongue to clean its facial scales and, in some cases, the spectacle over the eye. These lizards developed an olfactory chemical discrimination system (8–10). Members of the other clade, Autarchoglossa, used the tongue to carry chemical cues from the environment to their highly developed vomeronasal organs (5, 8). For ancestral iguanians, prey that moved were the primary targets. For ancestral gekkotans and autarchoglossans, moving prey were detected visually, but nonmoving and hidden prey were detected chemically. Moreover, chemosensory systems allowed members of these clades to discriminate prey based on quality and production of defensive chemicals (6). Comparisons between these clades clearly reveal a major dietary shift deep in squamate history. Although most carnivorous and omnivorous lizards eat a diversity of prey (1), iguanians (excluding herbivores) primarily eat ants, other hymenopterans, and beetles, whereas scleroglossans eat more orthopterans, spiders, and insect larvae (6). Increases in activity levels and, in some autarcholglossans, activity at elevated body temperatures were associated with changes in foraging and possibly the kinds of prey eaten (11). Several autarchoglossan clades specialized on vertebrates, termites, snails, and other prey not frequently eaten by iguanians. New opportunities opened up, and a remarkable diversification followed. The evolution of several limbless lizard clades, including one frequently referred to as “snakes” (12), is but one of many examples. Success of this diversification becomes obvious when comparing numbers of species in each major clade. Iguania contains ≈1,230, Gekkota contains ≈975, and Autarchoglossa contains ≈5,000 species. Snakes comprise ≈2,900 of autarchoglossans, none of which is herbivorous.
Fig. 1.
Evolution of prey detection, prey prehension, and herbivory in squamate reptiles. Numbers of origins for herbivory are taken from Espinoza et al. [ref. 4; at least two more are known in Autarchoglossa; one in the family Teiidae (Dicrodon) and at least one in Varanidae (V. olivaceus)]. Nevertheless, the number of origins (at least 18) in the Liolaemidae (a subclade of the Iguania) far exceeds those in all other lizards combined. The traditional phylogeny is shown (2, 5, 6).
Traits related to feeding in iguanian ancestors (visual prey detection, lingual prehension, and ambush foraging) render it even more remarkable that herbivory evolved repeatedly in the Liolaemidae. Most prior known origins of herbivory occurred in the Autarchoglossa, in which typical behavior includes moving about in search of prey, discriminating prey chemically, and grasping prey with the jaws. The shift from digesting arthropods, vertebrates, and other animals to digesting plants is not a small one but should have been more easily attained in lizard clades living primarily in tropical and desert habitats, where thermal conditions facilitating gut fermentation abound. Autarchoglossan diversity is greatest in such regions (2, 6). Nevertheless, it now appears that origins of herbivory are much more frequent in an iguanian clade than among autarchoglossans.
Within Iguania, two subclades diversified more than all others: Polychrotidae (mostly the genus Anolis) and Liolaemidae. Anolis have proven to be excellent models for evolutionary studies, partially because ecomorphs have evolved independently on different Caribbean islands. Studies of these lizards have revealed that morphology responds to changes in microhabitat use (13, 14). Liolaemid lizards have been long overlooked as models for ecological and evolutionary studies, even though they diversified at high elevations in southern South America, producing species that occupy nearly every imaginable terrestrial microhabitat (15–18). The high diversity of liolaemids in southern South America (168 species, with 157 in the genus Liolaemus) suggests an evolutionary history quite different from that of other iguanians, and herein may lie a hypothesis explaining the high diversity and frequent evolution of herbivory.
Many Paths to Herbivory
A recent study posits that diversification and the ability of autarchoglossans to harvest prey unavailable to iguanians provided them with a competitive advantage and enabled them to dominate terrestrial environments throughout the world (6). Iguanians tend to use elevated perches, and many species are arboreal in environments containing autarchoglossans. Gekkotans, for the most part, are nocturnal, thus avoiding interactions with autarchoglossans as well as iguanians. Autarchoglossans reach their greatest diversity in warm environments (tropical rainforests, tropical savannas, deserts), likely because they can attain body temperatures necessary to support their relatively high activity levels. Their diversity drops off rapidly with increased elevation and latitude. Although portions of the history of iguanians and autarchoglossans may have been independent, much of it was not (2, 6). However, liolaemids diversified at high elevations at high latitudes, largely in the absence of autarchoglossans, possibly releasing them from competition and predation by autarchoglossans. Even though herbivorous liolaemids may have slightly higher body temperatures than their carnivorous or omnivorous relatives (4), high body temperature alone may not be sufficient for digestion of plant materials. Increased activity periods may be necessary as well. The herbivorous teiid lizard C. murinus (an autarchoglossan) maintains body temperatures similar to other teiid lizards but remains active much longer to facilitate digestion of plants (19). Risk associated with extended activity is reduced because the island on which it lives (Bonaire) contains few predators. The high elevation and high latitude habitat of most liolaemids may have provided an environment with few competitors and predators (in particular, a lack of autarchoglossans) throughout much of their evolutionary history; thus, diversification into terrestrial microhabitats and extended activity times with reduced risk may have set the stage for repeated evolution of herbivory. The ability of small liolaemid lizards to heat rapidly if combined with extended activity may be sufficient to select for herbivory, particularly in environments in which arthropod food resources are limited. Some iguanians developed the ability to discriminate plant chemicals by using vomerofaction, even though insectivorous iguanians have not (20). High frequency of evolutionary events leading to herbivory, coupled with a background of ecological diversity among liolaemid lizards, makes them ideal models for evolutionary, ecological, physiological, and behavioral studies aimed at understanding adaptive radiation. Thus, in a more general context, the discovery of multiple origins of herbivory in liolaemids is a starting point for what promises to be exciting new directions in lizard ecology.
High body temperature may not be sufficient for digestion of plants; increased activity periods may be necessary as well.
See companion article on page 16819.
References
- 1.Cooper, W. E., Jr., & Vitt, L. J. (2002) J. Zool. 257, 487–517. [Google Scholar]
- 2.Pianka, E. R. & Vitt, L. J. (2003) Lizards: Windows to the Evolution of Diversity (Univ. of California Press, Berkeley).
- 3.Pough, F. H. (1973) Ecology 54, 837–844. [Google Scholar]
- 4.Espinoza, R. E., Wiens, J. J. & Tracy, C. R. (2004) Proc. Natl. Acad. Sci. USA 101, 16819–16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwenk, K. (2000) in Feeding, ed. Schwenk, K. (Academic, San Diego), pp. 175–291.
- 6.Vitt, L. J., Pianka, E. R., Cooper, W. E., Jr., & Schwenk, K. (2003) Am. Nat. 162, 44–60. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, W. E., Jr. (1997) Behav. Ecol. Sociobiol. 41, 257–265. [Google Scholar]
- 8.Cooper, W. E., Jr. (1995) Anim. Behav. 50, 973–985. [Google Scholar]
- 9.Schwenk, K. (1993) J. Zool. 229, 289–302. [Google Scholar]
- 10.Schwenk, K. (1993) Brain Behav. Evol. 41, 124–137. [DOI] [PubMed] [Google Scholar]
- 11.Huey, R. B. & Pianka, E. R. (1981) Ecology 62, 991–999. [Google Scholar]
- 12.Greene, H. W. (1997) Snakes: The Evolution of Mystery in Nature (Univ. of California Press, Berkeley).
- 13.Losos, J. B. (1992) Syst. Biol. 41, 403–420. [Google Scholar]
- 14.Losos, J. B., Warheit, K. I. & Schoener, T. W. (1997) Nature 387, 70–73. [Google Scholar]
- 15.Cei, J. M., ed. (1986) Mus. Reg. Sci. Nat. Torino Monogr. 4.
- 16.Cei, J. M., ed. (1993) Mus. Reg. Sci. Nat. Torino Monogr. 14.
- 17.Fuentes, E. R. (1976) Ecology 57, 3–17. [Google Scholar]
- 18.Fuentes, E. R. & Jaksíc, J. M. (1980) Oecologia 46, 45–48. [DOI] [PubMed] [Google Scholar]
- 19.Vitt, L. J., Caldwell, J. P., Sartorius, S. S., Cooper, W. E., Jr., Baird, T. A., Baird, T. D. & Peréz-Mellado, V. (2004) Funct. Ecol., in press.
- 20.Cooper, W. E., Jr., & Alberts, A. C. (1991) J. Chem. Ecol. 17, 135–146. [DOI] [PubMed] [Google Scholar]