Abstract
Sarcolipin (SLN) and phospholamban (PLN) are effective inhibitors of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). These homologous proteins differ at their N and C termini: the C-terminal Met-Leu-Leu in PLN is replaced by Arg-Ser-Tyr-Gln-Tyr in SLN. The role of the C-terminal sequence of SLN tagged N-terminally with the FLAG epitope (NF-SLN) in endoplasmic reticulum (ER) retention was investigated by transfecting human embryonic kidney-293 cells with cDNAs encoding NF-SLN or a series of NF-SLN mutants in which C-terminal amino acids were deleted progressively. Immunofluorescence and immunoblotting of transfected cells by using anti-FLAG antibodies indicated that NF-SLN and PLN tagged at its N terminus with the FLAG epitope, even when overexpressed, were restricted to the ER. However, C-terminal truncation deletions of SLN, which lacked RSYQY, were not localized to ER and did not inhibit Ca2+-dependent Ca2+ uptake by SERCA. The shortest deletion constructs, NF-SLN 1-22 and NF-SLN 1-23, did not express stable protein products. However, all NF-SLN cDNA constructs, including NF-SLN 1-22 and NF-SLN 1-23, were expressed stably and localized to the ER when they were coexpressed with SERCA2a. These results show that NF-SLN subcellular distribution depends on SERCA coexpression and on its luminal, C-terminal RSYQY sequence. By using immunoprecipitation and MS, glucose-regulated protein 78/BiP and glucose-regulated protein 94 were identified as proteins that interact with NF-SLN through the RSYQY sequence. Thus, in the absence of SERCA, retention of NF-SLN in the ER is mediated through its association with other components through the C-terminal RSYQY sequence.
Sarco(endo)plasmic reticulum Ca2+-ATPases (SERCAs) are 110-kDa integral membrane proteins that transport Ca2+ ions actively from the cytosol to the lumen of the sarco(endo)plasmic reticulum. In cardiac muscle, SERCA2a can associate with a 52-aa transmembrane protein, phospholamban (PLN). The PLN–SERCA2a complex has a lower apparent affinity for Ca2+, but the inhibited complex is disrupted by phosphorylation of PLN or elevation of cytosolic Ca2+, thereby reversing SERCA2a inhibition (1). Sarcolipin (SLN) is a 31-aa protein (2, 3) that copurifies with SERCA1a in fast-twitch skeletal muscle (4). Like PLN, SLN is an effective inhibitor of SERCA molecules (5–7). The two proteins share significant sequence identity and gene structure and are clearly homologous members of a gene family (3, 5).
When both SLN and PLN are coexpressed with SERCA1a or SERCA2a, superinhibition results: the apparent Ca2+ affinity is reduced by nearly 1 pCa unit, in contrast to reductions in the order of 0.17–0.35 pCa units for SLN or PLN alone (5, 6, 8). Biochemical analyses has shown that PLN and SLN form a very stable PLN–SLN binary complex when expressed at equal levels (6, 7). This complex can interact with SERCA molecules and the additional binding sites supplied by the binary complex make the inhibited ternary complex more stable than either PLN–SERCA or SLN–SERCA binary complexes (7, 9).
Molecular models show that either PLN or SLN can fit into a groove formed by M2, M4, M6, and M9 in SERCA, whereas the SLN–PLN binary complex fits more snugly into the same groove, accounting for the greater stability of the ternary complex (7). In the SLN-containing complexes, the aromatic residues Tyr-29 and Tyr-31 are predicted to interact with other aromatic residues in the luminal loop connecting M1 and M2 of SERCA, leaving hydrophilic amino acids Arg-27, Ser-28, and Gln-30 exposed to the luminal space. Consistent with this model, the SLN double-point mutation, Y29E/Y31E, loses its ability to inhibit SERCA (3).
Endoplasmic reticulum (ER) proteins are concentrated and retained in the ER by two major pathways: exclusion of ER proteins from entering newly forming transport vesicles; and retrieval of proteins that escape from the ER (10, 11). The well characterized KDEL motif in the C-terminal region of soluble ER proteins, together with the KDEL receptor protein, Erd2p, mediate the retrieval of ER proteins from the Golgi apparatus (12). For integral ER membrane proteins, the dilysine motif KKxx or xKxK, located near the C terminus, interacts with coat protein I, whereas the diarginine motif xRRx, located near the N terminus, is involved in ER retention (10–14).
Because the RSYQY sequence may play a role in retaining SLN in the ER/SR membrane, we postulated that mutations in the C-terminal domain would affect the sorting or retention of mutant SLN proteins. Alanine scanning mutagenesis and truncation scanning at the C terminus of SLN tagged N-terminally with a FLAG epitope (NF-SLN) was carried out and the subcellular location of the mutants was determined after they were expressed in human embryonic kidney (HEK)-293 cells, alone or in the presence of SERCA2a. Their ability to inhibit SERCA activity was also investigated. Amino acids Trp-22 to Tyr-31 were found to play a critical role in the stability of NF-SLN and its retention in the ER When NF-SLN was expressed alone in HEK-293 cells, the chaperone proteins, glucose-regulated protein (GRP)78/BiP and GRP94, were found to bind to the C-terminal RSYQY sequence in NF-SLN. However, coexpression of SERCA2a with the truncated forms of NF-SLN rescued ER retention.
Materials and Methods
Materials and Cell Culture. FLAG antibody (M2) was obtained from Sigma and GRP78/BiP and GRP94 antibodies were purchased from Affinity BioReagents (Golden, CO). Monoclonal antibody IID8 against SERCA2 was a gift from K. Campbell (University of Iowa, Iowa City) and polyclonal antibody against GRP75 was a gift from R. Wadhwa and S. Kaul (National Institute of Advanced Industrial Science and Technology, Tokyo). cDNA encoding NF-SLN, a protein with a FLAG epitope (MDYKDDDDK) fused to the N terminus of rabbit SLN, was inserted into the pMT2 vector (5). NF-SLN and wild-type SLN have been shown to be equally inhibitory to SERCA activity (5). As a control in some experiments, the FLAG epitope was also fused to the N terminus of full-length PLN (5). Alanine scanning mutagenesis and truncation scanning at the C terminus of NF-SLN (Fig. 1), the culture of HEK-293 cells, transfection with cDNAs, microsome isolation, and Ca2+ transport assays have been described in earlier publications (5, 8, 15). Immunoprecipitations (IPs) were performed as described (5, 8, 15) (Fig. 1).
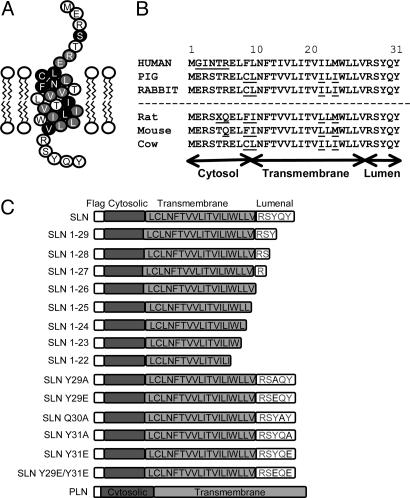
Fig. 1.
Sequence comparison of SLN from different species and schematic diagram of NF-SLN and NF-PLN expression constructs. (A) Model of rabbit SLN amino acid sequence. Note the presence of a short cytosolic domain, transmembrane sequence, and a C-terminal/luminal sequence. Black circles represent amino acids identical to PLN and gray circles represent amino acids conserved between PLN and SLN. (B) SLN amino acid sequences for human, pig, and rabbit. Sequences for rat, mouse, and cow are deduced from National Center for Biotechnology Information cDNA sequences. Note the strong conservation of the C-terminal sequence from Trp-23 to Tyr-31. (C) SLN and PLN expression constructs were fused N-terminally with a FLAG epitope. NF-SLN point- and truncation-deletion mutants were generated subsequently by site-directed mutagenesis.
Immunofluorescence. Plasmid DNA was transfected as described (16). Fixed cells were incubated with either the anti-FLAG antibody (final concentration of 2 μg/ml) alone, or together with the IID8 antibody against SERCA2 (2 μg/ml final), overnight at 4°C. Appropriate FITC- and tetramethylrhodamine B isothiocyanate-conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA).
Images at each wavelength (488 and 568 nm) were collected separately by using a Leica TCS SP laser scanning confocal system (Leica, Richmond Hill, ON, Canada). For quantification, a minimum of 200 cells in 10 random fields of view from at least four independent experiments were examined under the ×40 microscope objective and classified qualitatively according to their immunofluorescent staining patterns as either normal ER immunostaining or abnormal/no ER immunostaining. Normal was characterized by strong ER localization, as seen in cells transfected with NF-SLN and PLN tagged N-terminally with a FLAG epitope (NF-PLN). These cells showed well defined perinuclear staining and an equally well defined cytosolic meshwork with no plasma membrane staining. Abnormal or no ER staining was characterized by additional immunofluorescence at the plasma membrane, by increased diffuse staining in the cytosol, or by lack of a clear perinuclear pattern, or all three.
RNA Extraction and RT-PCR. Total RNA was extracted from cells by using TRIpure reagent (Roche Molecular Biochemicals, Indianapolis). Total RNA (100 ng) was used for RT-PCR as described (17–19). NF-SLN cDNAs of 81 bp were amplified specifically by using a primer which amplified the last 15 nt of FLAG and the first 15 nt of rabbit SLN (5′-GACGACGATGACAAGATGGAGCGATCCACC-3′) and a primer with reverse complementarity to SLN amino acids 17–22 (5′-AATAAGGGATCACTGTAATAAGGAC-3′) (3).
Cardiac Lysates and Multidimensional Protein Identification Technology Analysis of IP Complexes. Mouse ventricles were isolated, washed in PBS, then homogenized for 30 sec in 250 mM sucrose, 50 mM Tris·HCl (pH 7.4)/1 mM PMSF/20 μg/ml aprotinin by using a Polytron homogenizer set at maximum speed. Lysates were isolated after 15 min of centrifugation at 14,000 × g, and the supernatant was collected and used for IPs. Samples (250 μl) from wild-type and NF-SLN-transgenic mice were subjected to IP by using 10 μg of M2 antibody conjugated to 50 μl of protein G-Sepharose, and incubated overnight with 500 μl of Seize-X Protein G IP buffer (Pierce), 7.5 μl of Nonidet P-40, and 7.5 μl of activated sodium orthovanadate. Samples were thoroughly washed with IP buffer to reduce background and eluted with 190 μl of Seize-X elution buffer (Pierce). One hundred fifty micrograms of protein were analyzed by tandem MS essentially as described (20, 21).
Statistics. Data are presented as mean ± SE. In all cases, results shown are from a minimum of three separate experiments. Comparisons were by Student's t test as appropriate. P values of <0.05 were considered significant.
Results
Previously, we have shown that NF-SLN and NF-PLN inhibit Ca2+ uptake by SERCA, but the NF-SLN Y29E/Y31E mutant does not (5). Transfection of cDNA constructs encoding NF-SLN and NF-PLN into HEK-293 cells revealed that NF-SLN and NF-PLN were largely confined to the ER, showing clear perinuclear localization, together with a highly organized web-like distribution throughout the cell (ref. 5 and Fig. 6, which is published as supporting information on the PNAS web site). However, the NF-SLN Y29E/Y31E mutant showed very little perinuclear staining, very diffuse cytosolic staining, and pronounced plasma membrane staining. Immunoblot analyses of whole-cell lysates and subcellular fractions confirmed the expression of NF-PLN, NF-SLN, and NF-SLN Y29E/Y31E proteins, and little difference was observed in the expression of NF-SLN and NF-SLN Y29E/Y31E. Immunoblot analyses of whole-cell extracts of 5-day-differentiated cultures of the mouse skeletal muscle cell line C2C12 transfected with NF-PLN, NF-SLN, or NF-SLN Y29E/Y31E constructs showed that expression was comparable with that observed in HEK-293 cells (results not shown).
Because equivalent amounts of plasmid DNAs were transfected into HEK-293 cells and transfection efficiency did not vary significantly among experiments, the number of fluorescent cells on the coverslip was used as a quantitative measure of the stable expression of NF-SLN variant proteins in the cell (Table 1). We counted 1,254 ± 151 and 1,031 ± 61 fluorescent cells in NF-PLN- and NF-SLN-transfected cultures, respectively. Under identical conditions, we detected only 37 ± 12 and 28 ± 7 fluorescent cells for NF-SLN 1-22- and NF-SLN 1-23-transfected cultures, respectively (P < 0.05). Although the stable expression level for NF-SLN 1-24 was closer to control levels at 772 ± 57 fluorescent cells, the difference was statistically significant (P < 0.05). The numbers of cells expressing a fluorescent product for all other NF-SLN point mutants and longer truncation mutants fell within a range between 873 ± 62 and 1,025 ± 85 cells per coverslip (P > 0.05).
Table 1. Effects of NF-SLN wild-type and mutant constructs on cellular localization and Ca2+ uptake.
| cDNA construct | KCa, pCa units | ΔKCa, pCa units | Fluorescent cells per coverslip | Flag localization in ER, percent total | Colocalization between SERCA and FLAG, percent of total cells | Fluorescent cells per coverslip when cotransfected with SERCA |
|---|---|---|---|---|---|---|
| Serca2a alone | 6.39 ± 0.04 | — | — | — | — | — |
| NFSLN | 5.94 ± 0.07* | -0.45 | 1,031 ± 61 | 85.5 ± 5.4 | 92.4 ± 2.8 | 916 ± 30 |
| NFSLN 1-22 | 6.39 ± 0.06** | -0.00 | 37 ± 12** | ND** | 89.3 ± 3.6 | 892 ± 46 |
| NFSLN 1-23 | 6.36 ± 0.04** | -0.03 | 28 ± 7** | ND** | 88.4 ± 3.8 | 886 ± 17 |
| NFSLN 1-24 | 6.27 ± 0.02*,** | -0.12 | 772 ± 57** | 40.0 ± 8.0** | 92.9 ± 1.6 | 889 ± 49 |
| NFSLN 1-25 | 6.17 ± 0.07*,** | -0.22 | 797 ± 65 | 24.1 ± 4.5** | 92.9 ± 2.2 | 836 ± 22 |
| NFSLN 1-26 | 6.17 ± 0.04*,** | -0.22 | 900 ± 76 | 24.1 ± 12.1** | 89.4 ± 2.9 | 892 ± 39 |
| NFSLN 1-27 | 5.95 ± 0.04* | -0.44 | 925 ± 59 | 42.9 ± 5.6** | 91.7 ± 4.0 | 936 ± 18 |
| NFSLN 1-28 | 5.98 ± 0.05* | -0.41 | 873 ± 62 | 91 ± 9.8 | 92.4 ± 1.0 | 948 ± 40 |
| NFSLN 1-29 | 6.03 ± 0.05* | -0.36 | 920 ± 35 | 86 ± 4.3 | 92.9 ± 0.9 | 978 ± 43 |
| NFSLN Y29E/Y31E | 6.26 ± 0.03*,** | -0.13 | 984 ± 18 | 27.9 ± 8.9** | 88.5 ± 3.5 | 914 ± 16 |
Microsomes were isolated from transfected cells and were analyzed for calcium transport over Ca2+ concentrations from pCa 5 to 8 to obtain KCa. KCa (pCa) is the negative logarithm of the Ca2+ concentration required to attain the half-maximal Ca2+ uptake rate. Transfected cells were also processed for immunofluorescence, and the number of cells per coverslip was determined and expressed in relation to normal ER localization or colocalization with SERCA. Data are mean ± SEM. *, P < 0.05 versus SERCA2a; **, P < 0.05 versus NFSLN. ND, not determined.
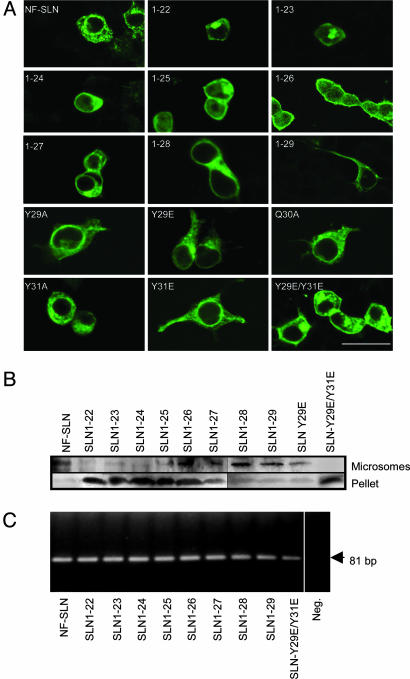
Confocal microscopy showed NF-SLN to be located in the ER with perinuclear and defined cellular staining patterns (Fig. 2) that were typical of normal ER staining patterns (5). In cultures transfected with SLN 1-22 and 1-23, the staining pattern was not typical of ER localization, but was punctate, with aggregates distributed in a region immediately adjacent to the nucleus (Fig. 2). In cells transfected with SLN 1-24, 1-25, or 1-26, there was very little ER immunostaining, but immunofluorescence was diffuse in the cytosol and enriched at the plasma membrane (Fig. 2). Within the series of NF-SLN mutant expression constructs, NF-SLN 1-27 was the shortest construct that induced defined ER staining in transfected cells, although residual plasma membrane staining was still observed (Fig. 4). All other SLN expression constructs, including SLN 1-28, 1-29, Q30A, Y29A, Y29E, Y31A, and Y31E showed immunofluorescence patterns that were similar to those observed in NF-SLN-transfected cells (Fig. 2).
Fig. 2.
Presence of NF-SLN proteins in the ER of intact cells and the microsomal fraction from these cells. (A) Subcellular distribution of NF-SLN expression constructs. (B) Transfected cultures were harvested, and broken cell pellet and microsomal fractions were isolated, solubilized, subjected to SDS/PAGE, and probed by using the anti-FLAG antibody. For the constructs SLN 1-22 and SLN 1-23, protein loading was increased to visualize bands. Note that the NF-SLN Y29E/Y31E mutant and those constructs with long C-terminal deletions were virtually absent from the microsomal fraction, but were present in the cell pellet. (C) Ethidium bromide-stained agarose gel of NF-SLN RT-PCR products obtained from RNA isolated from cells transfected with SLN expression constructs, along with a negative control (Neg.).
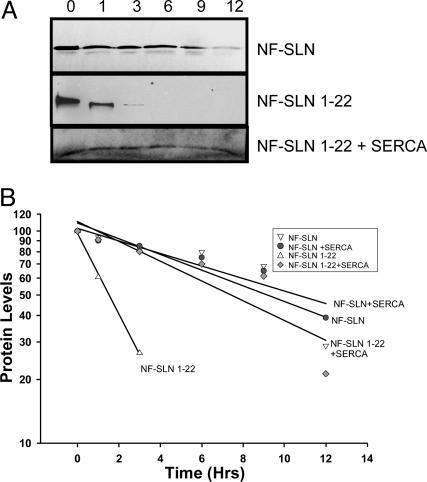
Fig. 4.
Stability of NF-SLN protein in HEK-293 cells. Cells were transfected with NF-SLN constructs, radiolabeled with [35S]methionine for 1 h, and followed by a chase for up to 12 h in medium containing an excess of unlabeled methionine. (A) Anti-FLAG IPs were performed and the protein half-life was determined. (B) The t1/2 values for NF-SLN and NF-SLN with SERCA were 10.1 and 10.8 h, respectively. The t1/2 for NF-SLN 1-22 was 1.9 h and the NF-SLN with SERCA was 8.6 h. Results shown represent the data from three pooled independent experiments.
Cells were also classified qualitatively according to whether they demonstrated normal ER immunofluorescence, similar to NF-SLN staining patterns, or abnormal/no ER staining (Table 1). Only our first two series of four quantitative measurements were carried out blindly, but all series of results yielded similar findings. We observed that ≈85% of transfected cells for NF-PLN (200 of 242 fluorescent cells; 83.2 ± 5.0%) or NF-SLN (130 of 152 cells) showed normal ER immunostaining (Table 1). Similar results were determined for SLN 1-28 (126 of 139 cells); SLN1-29 (89 of 104 cells); and the SLN single-point mutants Q30A (211 of 258 cells, 83.0 ± 4.0%); Y29A (165 of 195 cells, 86.0 ± 5.0%); Y29E (182 of 213 cells, 87.0 ± 7.0%); Y31A (143 of 158 cells, 88.0 ± 8.0%); and Y31E (221 of 243 cells, 89.0 ± 4.0%).
The smaller truncation deletion mutants showed significantly lower numbers of fluorescent cells with normal ER staining patterns: SLN 1-24 (109 of 274 cells); SLN 1-25 (67 of 278 cells); SLN 1-26 (30 of 213 cells); and SLN 1-27 (101 of 238 cells). Finally, the SLN double-point mutant Y29E/Y31E showed 80 of 287 cells with fluorescence distribution patterns that were similar to those of NF-SLN. Data for the NF-SLN deletion constructs, NF-SLN 1-22 and 1-23, are not shown because of the very low number of immunofluorescent cells in these cultures.
Immunoblot analysis of fractions by using the FLAG antibody showed that the longer SLN constructs (NF-SLN, NF-SLN 1-27, 1-28, and 1-29), as well as the NF-SLN point mutants (Q30A, Y29A, Y29E, Y31A, and Y31E) were abundant in the ER-enriched microsomal fraction (Fig. 2B), but were virtually absent in the residual pellet (Fig. 2B). By contrast, NF-SLN 1-22, 1-23, 1-24, and SLN Y29E/Y31E were absent from the microsomal fraction, but significant amounts were found in the residual pellet (Fig. 2). Samples transfected with NF-SLN 1-25 or 1-26 showed detectable protein in the microsomal fractions, but a significant proportion of these proteins was contained in the residual pellet. Finally, SLN 1-27 was preferentially located in the microsomal fraction, but with detectable levels in the residual pellet (Fig. 2B).
RT-PCR analyses were performed to verify that cells were transfected and expressed our SLN constructs. SLN 1-22 and SLN 1-23 transcript levels were similar to NF-SLN, indicating that equivalent amounts of plasmid DNAs were transfected into the cells and that each construct was transcribed to a similar level. These results indicate that the significantly lower levels of FLAG-expression seen with SLN 1-22, and SLN 1-23 is the result of an unstable protein and is not due to low transcript expression.
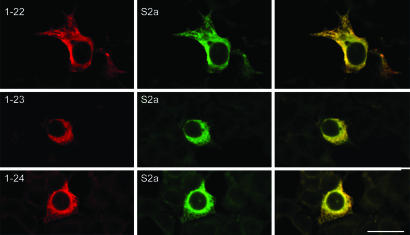
Coexpression of NF-SLN and SERCA2a. We coexpressed SLN and SERCA2a in a 1:1 molar ratio and examined their codistribution. As shown in Fig. 4, there is nearly 100% colocalization between the NF-SLN expression constructs and SERCA2a (Fig. 3). In fact, NF-SLN 1-27, 1-28, 1-29, as well as NF-SLN 1-22, 1-23, 1-24, 1-25, and 1-26, and SLN Y29E/Y31E, were all colocalized with SERCA2a (Fig. 3 and Fig. 7, which is published as supporting information on the PNAS web site). For NF-SLN 1-22 and 1-23, there was not only colocalization but also a significantly increased number of FLAG-expressing cells. In the absence of SERCA2a, we found ≈50 FLAG-expressing cells per coverslip, but, in the presence of SERCA2a, this number increased to ≈800–900 cells per coverslip (Table 1). These values are comparable with those obtained by expression of NF-SLN in the absence of SERCA2a expression. For all constructs, ≈90% of all cotransfected cells demonstrated colocalization between FLAG staining and SERCA2a expression.
Fig. 3.
Immunofluorescence analysis of cells cotransfected with NF-SLN together with SERCA2a. Cells were cotransfected with SLN expression constructs together with SERCA2a in a 1:1 ratio and examined after 48 h by using the anti-FLAG and anti-SERCA antibodies. Shown are representative examples of overlay staining of FLAG and SERCA2a in cells cotransfected with SERCA2a and one of NF-SLN1-22, 1-23, or 1-24, showing significant overlap between SERCA2a and SLN expression. (Bar, 40 μm.)
We compared the effects of these mutant proteins on the Ca2+ affinity (KCa) of SERCA2a in an assay of the Ca2+ dependence of Ca2+ transport in microsomes from HEK-293 cells transfected with SERCA2a alone, or together with the NF-SLN mutants (Table 1). SERCA2a alone had an apparent KCa of 6.39 ± 0.04 pCa units. A significant (P < 0.05) decrease in Ca2+ affinity was observed in the presence of NFSLN, NF-SLN 1-27, NF-SLN 1-28, and NF-SLN 1–29 (P < 0.05). NF-SLN 1-22, 1-23, 1-24, 1-25, 1-26, and the double-point mutant NF-SLN Y29E/Y31E all resulted in Ca2+ affinities that were significantly different from NF-SLN (P < 0.05). For NF-SLN 1-22 and 1-23, values were not significantly different from SERCA2 alone (P > 0.05). Previous experiments (5) using the NF-SLN point mutants all showed equal (Y29E, Q30A, Y31A, and Y31E) or greater (Y29A) reductions in SERCA2a Ca2+ affinity than NF-SLN.
These results clearly show that the interaction of NF-SLN with SERCA2a is one of the most important factors in the localization of NF-SLN in the ER. It also appears that such interactions mediate the stability of NF-SLN in the ER membrane. To test this hypothesis, we performed [35S]methionine pulse–chase assays. They showed that the half-life of NF-SLN protein in cultured cells is 10.1 and 10.8 h in the absence and presence of SERCA, respectively (Fig. 4). The NF-SLN 1-22-truncated mutant has a half-life of 1.9 h in the absence of SERCA, but this half-life was prolonged significantly to 8.6 h in the presence of SERCA.
Identification of Cellular Proteins Associating with NF-SLN. Radiolabeled lysates from NF-SLN- and NF-PLN-transfected cells were subjected to IPs by using the M2 antibody, separated by SDS/PAGE, and exposed to autoradiography (Fig. 5A). Proteins distinct to the NF-SLN Co-IP were observed at ≈64, ≈74, ≈78, and ≈85 kDa, as well as at ≈10 kDa (representing NF-SLN) and ≈100 kDa (representing SERCA2). These larger proteins were not seen in the NF-PLN-transfected culture, although we detect the 100-kDa SERCA protein and the ≈11-kDa NF-PLN protein.
Fig. 5.
Identification of NF-SLN-interacting proteins. (A) HEK-293 cells were transfected with NF-SLN or NF-PLN and labeled with [35S]methionine for 6 h. IPs were performed with anti-FLAG antibody, and samples were separated by SDS/PAGE and subjected to autoradiography. (B) IPs were performed with lysates from NF-SLN- and NF-SLN 1-26-transfected cells and antibodies to FLAG (M2), followed by immunoblots against GRP78/BiP, GRP94, and GRP75. Input Control (CTL) is HEK cell lysate not subjected to IP with M2. (C) Cardiac lysates obtained from wild-type and transgenic mice overexpressing NF-SLN were subjected to IPs with anti-FLAG (M2), GRP78/BiP, GRP94, and GRP75 and subsequently immunoblotted. α-actinin immunoblots were performed to ensure equal loading. I.P., immunoprecipitation; I.B., immunoblot.
NF-SLN-binding proteins were identified through MS by applying our recently developed multidimensional protein identification technology (20, 21). Co-IPs with the anti-FLAG antibody were carried out with lysates from cardiac ventricular tissue from wild-type mice and a transgenic mouse overexpressing NF-SLN in the heart (22). This tissue was used because it was most likely to provide a significantly greater enrichment of endogenous NF-SLN-interacting proteins than transfected HEK cells, which do not express PLN or SLN endogenously. These experiments yielded a tentatively filtered list of 85 proteins. Refinement of candidate proteins to those identified with >95% confidence reduced this list to 41 proteins. Further refinement to exclude high abundance, contaminating proteins also found in wild-type IP experiments, such as contractile proteins, myoglobin, or hemoglobin, or IgGs present due to the IP assay, resulted in the identification of 12 proteins including: 3-ketoacyl-CoA thiolase, 60S ribosomal protein, ATP-dependent RNA helicase, gap-junction membrane-channel protein α-1, glutamate receptor 4, a hypothetical 28.1-kDa protein, microsomal dipeptidase, SERCA2, serine/threonine protein kinase 16, similar to LRP16 protein, stress-70 protein (a GRP), and transient receptor protein 2.
The discovery of the interaction of NF-SLN with the ER luminal chaperone protein, Stress-70/GRP75, a member of the heat shock protein 70 family of proteins, led us to examine the interaction further using conventional IPs with antibodies against GRP75. For this set of experiments, we used full-length NF-SLN and the NF-SLN 1-26 construct because shorter NF-SLN constructs were unstable and likely to give a false-negative result. In our IP assays, we were unable to visualize specific interactions of GRP75 with NF-SLN in HEK cell lysates (Fig. 5B) or cardiac lysates from wild-type and NF-SLN-transgenic hearts (Fig. 5C). However, upon examination of the ability of GRP75-related proteins to interact with NF-SLN, we did determine that NF-SLN interacts with the GRP75-related proteins, GRP94 and GRP78/BiP (Fig. 5B). Both binding interactions were reduced significantly in the NF-SLN truncation mutant 1-26 (Fig. 5B).
Discussion
In this study, we show that two features of SLN affect its retention in the ER. The first is the C-terminal sequence Arg-Ser-Tyr-Gln-Tyr-31, which appears to act as an ER retention signal. If NF-SLN is overexpressed, it is retained in the ER, despite the progressive removal of the sequence Ser-Tyr-Gln-Tyr-31. When Arg-27 is removed, however, NF-SLN is misrouted to other membrane compartments. As truncation progresses upstream, NF-SLN continues to be stably expressed until the sequence Leu-Leu-Val-Arg-Ser-Tyr-Gln-Tyr-31 is deleted. At this point, NF-SLN is degraded. The second important feature of NF-SLN retention in the ER is its association with SERCA. When SERCA2a is also overexpressed with NF-SLN, misrouting ceases and even those constructs missing Leu-Leu-Val-Arg-Ser-Tyr-Gln-Tyr-31 are stably expressed and colocalize with SERCA2a. Thus, in the absence of stoichiometric levels of SERCA, NF-SLN finds a docking site in the ER that uses all or part of the C-terminal sequence Arg-Ser-Tyr-Gln-Tyr-31. In the presence of SERCA, much more of the transmembrane sequence of NF-SLN is used to dock the protein to SERCA and retain it in the ER. In the presence of SERCA, even NF-SLN, which is missing 8 C-terminal amino acids and contains only 16 instead of 19 hydrophobic amino acids in a transmembrane helix is stabilized and retained in the ER. Finally, we identified the cellular sorting proteins to be the luminal proteins, GRp78/BiP and GRP94, and showed that they interact with the C-terminal portion of NF-SLN.
Recently, NMR spectroscopy has provided a high-resolution structure of SLN (23), verifying earlier predictions that SLN possess an α-helical transmembrane domain, an N-terminal cytosolic domain, and a C-terminal luminal domain (3). In this structure, the C-terminal amino acids are exposed to the lumen and appear to be unstructured, making them candidate ER retention signals (12). However, previously characterized C-terminal ER retention signals, such as KDEL or dilysine KKxx, or KxKx motifs (10), are not found in SLN. Nonetheless, it is noteworthy that there is 100% conservation of the RSYQY sequence in the C terminus of SLN in six diverse species (Fig. 1). Such a high degree of conservation suggests a critical role for this sequence in SLN function that has survived evolutionary divergence.
Our observation that the two smallest SLN truncation–deletion constructs, SLN 1-22 and 1-23, had a short half-life, were poorly expressed, difficult to visualize, and did not display normal ER distribution, indicates to us that these expressed proteins were unstable and subject to degradation. These observations are similar to those obtained in our recent analysis of a naturally occurring truncation mutation in human PLN (Leu39stop) that is causal of dilated cardiomyopathy. This protein would contain only 9 of 22 hydrophobic amino acids that make up the PLN transmembrane sequence. The expression of recombinant Leu39stop cDNA in HEK-293 cells showed the absence of stable PLN protein expression, even with coexpression of stoichiometric amounts of SERCA2a, and no PLN inhibition of SERCA2a activity (24). As well, PLN protein was not detected in immunoblots of a biopsy from the failed heart of a Leu39stop patient (24), consistent with the view that truncated forms of both PLN and SLN are unstable and subject to intracellular degradation.
Strikingly, SLN retention in the presence of SERCA2a overexpression does not appear to rely on the RSYQY sequence. When SLN and SERCA2a cDNAs were cotransfected they were colocalized in the ER. This finding was also true for a truncated NF-SLN variant that had 9 aa removed from its C terminus and retained only 15 of 19 hydrophobic amino acids in its transmembrane sequence. Thus, the presence of abundant SERCA can stabilize truncated SLN and restore its ER localization. This interaction is clearly not a simple retention mechanism, dependent on the RSYQY sequence, but rather, a direct, hydrophobic, transmembrane interaction that stabilizes and protects the remnant of the SLN transmembrane helix from degradation. The stabilization of SLN by SERCA is likely to explain the presence of SLN in the ER. Structural modeling, for example, predicts that amino acid Ile-14 in SLN binds to Ala-100 and Val-104 of SERCA, and the luminal amino acids in SLN interact with aromatic residues in the loop connecting M1 and M2 of SERCA: SLN Tyr-29 interacts with Phe-73, Trp-77, and Phe-88 of SERCA, whereas SLN Tyr-31 reacts with Phe-88 and Ile-85 of SERCA. On the basis of this model, the interaction of SLN Ile-14 with SERCA Ala-100 and Va104 appears sufficiently strong to retain SLN in the ER, because the SLN constructs missing luminal amino acids are retained in the ER, when coexpressed with SERCA2a.
When SERCA and SLN are coexpressed, ER retention of SERCA becomes the primary mechanism for SLN retention. Amino acids 1–211, which encompass helices M1 and M2 in SERCA1, have been shown to be critical for ER localization (25). The mechanism of ER retention appears to be the retrieval of SERCA1 from the cis–Golgi network. It remains to be determined whether SLN is bound to SERCA during this retrieval process. Because the SLN-SERCA1a interaction survives purification in detergent and concentrated salt (4), it seems likely that SLN and SERCA would be associated under the milder conditions of the cell, and retrieved simultaneously.
Our IP and multidimensional protein identification technology experiments identified GRP75, a member of the heat shock 70 family of proteins, as an NF-SLN-interacting protein. Interestingly, GRP75 has many assigned functions, including intracellular trafficking (26, 27). Although the identification of this protein as an NF-SLN-interacting candidate appeared particularly attractive to investigate further, we were unable to verify these findings by conventional IPs. One possible reason for such a discrepancy may be that GRP75 does not interact with NF-SLN, and the MS data are an artifact because of the high sensitivity of MS. On the other hand, it is possible that GRP75 interacts transiently with NF-SLN and the sensitivity of conventional IPs is not high enough to detect these interactions by IPs, whereas the MS is extremely sensitive. However, these findings led us to the further investigation of the ability of other GRP-related protein family members to interact with NF-SLN. Specifically GRP94 and GRP78/BiP were shown by IP to interact with NF-SLN, and, importantly, required the C-terminal sequences of NF-SLN. The fact that these proteins were not identified in our MS experiments can occur for several reasons, including masking of low-abundant spectra by saturation of high-abundant proteins and/or poor tryptic digestion of proteins into smaller fragments (20). Nonetheless, the appearance of these proteins in IPs from both transfected cells and cardiac tissue, together with their dependence on the C-terminal sequence of NF-SLN, strongly indicates their role in NF-SLN intracellular targeting. These results are also consistent with a previous observation that GRP75 binds GRP94 (28), a protein that we have shown to interact with NF-SLN. Because GRP75, GRP78/BiP, and GRP94 transiently interact with proteins during ER processing, it appears likely that they interact with NF-SLN transiently during the shuttling of NF-SLN to its proper subcellular compartment.
Multidimensional protein identification technology analyses also identified 11 other potential NF-SLN-interacting proteins. Of these proteins, interesting candidates were: SERCA, known to interact with NF-SLN (3, 6, 7); a hypothetical 28-kDa protein with no assigned function; protein LRP16, also with unknown function; TRP2 (a store-operated Ca2+ channel), a plasma membrane channel postulated to refill the ER when emptied of Ca2+ (29); and serine/threonine protein kinase 16 and microsomal dipeptidase. The role of these proteins in regulating either SLN or SERCA is not known and needs additional investigation.
Our results raise an intriguing issue regarding the ER localization of PLN because PLN does not contain any C-terminal sequence that is comparable with that found in SLN. Even though PLN, like SLN, should be retained in the ER primarily by a direct interaction with SERCA, the problem with such an extrapolation is that a large fraction of PLN exists in homopentamers that are not bound to SERCA. Thus, if PLN–SERCA interactions were the primary retention mechanism, a substantial amount of PLN would be expected to exit the ER. It is, therefore, likely that different mechanisms of ER retention for the PLN pentamers are required, that do not demand a direct interaction with SERCA.
Supplementary Material
Acknowledgments
We thank Adrian Yen for expert technical assistance. This work was supported by Heart and Stroke Foundation Ontario Grant T-5042 and Canadian Institutes of Health Research Grant MT-12545 (to D.H.M.). A.O.G. is a Research Fellow of the Heart and Stroke Foundation of Canada.
Author contributions: A.O.G. designed research; A.O.G., T.K., M.A., and W.L. performed research; T.K., M.A., and A.E. contributed new reagents/analytic tools; A.O.G. and T.K. analyzed data; and A.O.G. and D.H.M. wrote the paper.
Abbreviations: SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase; PLN, phospholamban; SLN, sarcolipin; NF-SLN, SLN tagged N-terminally with a FLAG epitope; NF-PLN, PLN tagged N-terminally with a FLAG epitope; ER, endoplasmic reticulum; SR, sarcoplasmic reticulum; HEK, human embryonic kidney; IP, immunoprecipitation.
References
- 1.Simmerman, H. K. & Jones, L. R. (1998) Physiol. Rev. 78, 921–947. [DOI] [PubMed] [Google Scholar]
- 2.Wawrzynow, A., Theibert, J. L., Murphy, C., Jona, I., Martonosi, A. & Collins, J. H. (1992) Arch. Biochem. Biophys. 298, 620–623. [DOI] [PubMed] [Google Scholar]
- 3.Odermatt, A., Taschner, P. E., Scherer, S. W., Beatty, B., Khanna, V. K., Cornblath, D. R., Chaudhry, V., Yee, W. C., Schrank, B., Karpati, G., et al. (1997) Genomics 45, 541–553. [DOI] [PubMed] [Google Scholar]
- 4.MacLennan, D. H., Yip, C. C., Iles, G. H. & Seeman, P. (1972) in The Mechanism of Muscle Contraction (Cold Spring Harbor Lab. Press, Plainview, NY), Vol. 37, pp. 469–478. [Google Scholar]
- 5.Odermatt, A., Becker, S., Khanna, V. K., Kurzydlowski, K., Leisner, E., Pette, D. & MacLennan, D. H. (1998) J. Biol. Chem. 273, 12360–12369. [DOI] [PubMed] [Google Scholar]
- 6.Asahi, M., Kurzydlowski, K., Tada, M. & MacLennan, D. H. (2002) J. Biol. Chem. 277, 26725–26728. [DOI] [PubMed] [Google Scholar]
- 7.Asahi, M., Sugita, Y., Kurzydlowski, K., De Leon, S., Tada, M., Toyoshima, C. & MacLennan, D. H. (2003) Proc. Natl. Acad. Sci. USA 100, 5040–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura, Y., Kurzydlowski, K., Tada, M. & MacLennan, D. H. (1997) J. Biol. Chem. 272, 15061–15064. [DOI] [PubMed] [Google Scholar]
- 9.Toyoshima, C., Asahi, M., Sugita, Y., Khanna, R., Tsuda, T. & MacLennan, D. H. (2003) Proc. Natl. Acad. Sci. USA 100, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teasdale, R. D. & Jackson, M. R. (1996) Annu. Rev. Cell Dev. Biol. 12, 27–54. [DOI] [PubMed] [Google Scholar]
- 11.Gomord, V., Wee, E. & Faye, L. (1999) Biochimie 81, 607–618. [DOI] [PubMed] [Google Scholar]
- 12.Pelham, H. R. (1990) Trends Biochem. Sci. 15, 483–486. [DOI] [PubMed] [Google Scholar]
- 13.Townsley, F. M. & Pelham, H. R. (1994) Eur. J. Cell Biol. 64, 211–216. [PubMed] [Google Scholar]
- 14.Pelham, H. R. (1996) Cell Struct. Funct. 21, 413–419. [DOI] [PubMed] [Google Scholar]
- 15.Asahi, M., Kimura, Y., Kurzydlowski, K., Tada, M. & MacLennan, D. H. (1999) J. Biol. Chem. 274, 32855–32862. [DOI] [PubMed] [Google Scholar]
- 16.Tupling, A. R., Gramolini, A. O., Duhamel, T. A., Kondo, H., Asahi, M., Tsuchiya, S. C., Borrelli, M. J., Lepock, J. R., Otsu, K., Hori, M., et al. (2004) J. Biol. Chem. 279, 52382–52389. [DOI] [PubMed] [Google Scholar]
- 17.Gramolini, A. O., Angus, L. M., Schaeffer, L., Burton, E. A., Tinsley, J. M., Davies, K. E., Changeux, J. P. & Jasmin, B. J. (1999) Proc. Natl. Acad. Sci. USA 96, 3223–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gramolini, A. O. & Jasmin, B. J. (1999) Nucleic Acids Res. 27, 3603–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gramolini, A. O., Burton, E. A., Tinsley, J. M., Ferns, M. J., Cartaud, A., Cartaud, J., Davies, K. E., Lunde, J. A. & Jasmin, B. J. (1998) J. Biol. Chem. 273, 736–743. [DOI] [PubMed] [Google Scholar]
- 20.Kislinger, T., Rahman, K., Radulovic, D., Cox, B., Rossant, J. & Emili, A. (2003) Mol. Cell. Proteomics 2, 96–106. [DOI] [PubMed] [Google Scholar]
- 21.Pan, Y., Kislinger, T., Gramolini, A. O., Zvaritch, E., Kranias, E. G., MacLennan, D. H. & Emili, A. (2004) Proc. Natl. Acad. Sci. USA 101, 2241–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asahi, M., Otsu, K., Nakayama, H., Hikoso, S., Takeda, T., Gramolini, A. O., Trivieri, M. G., Oudit, G. Y., Morita, T., Kusakari, Y., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 9199–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascioni, A., Karim, C., Barany, G., Thomas, D. D. & Veglia, G. (2002) Biochemistry 41, 475–482. [DOI] [PubMed] [Google Scholar]
- 24.Haghighi, K., Kolokathis, F., Pater, L., Adamopoulos, S., Linch, R. A., Asahi, M., Gramolini, A. O., Fan, G., Liggett, S. B., Dorn, G., II, et al. (2003) J. Clin. Invest. 111, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newton, T., Black, J. P., Butler, J., Lee, A. G., Chad, J. & East, J. M. (2003) Biochem. J. 371, 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizukoshi, E., Suzuki, M., Misono, T., Loupatov, A., Munekata, E., Kaul, S. C., Wadhwa, R. & Imamura, T. (2001) Biochem. Biophys. Res. Commun. 280, 1203–1209. [DOI] [PubMed] [Google Scholar]
- 27.Mizukoshi, E., Suzuki, M., Loupatov, A., Uruno, T., Hayashi, H., Misono, T., Kaul, S. C., Wadhwa, R. & Imamura, T. (1999) Biochem. J. 343, 461–466. [PMC free article] [PubMed] [Google Scholar]
- 28.Takano, S., Wadhwa, R., Mitsui, Y. & Kaul, S. C. (2001) Biochem. J. 357, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clapham, D. E. (2003) Nature 426, 517–524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.