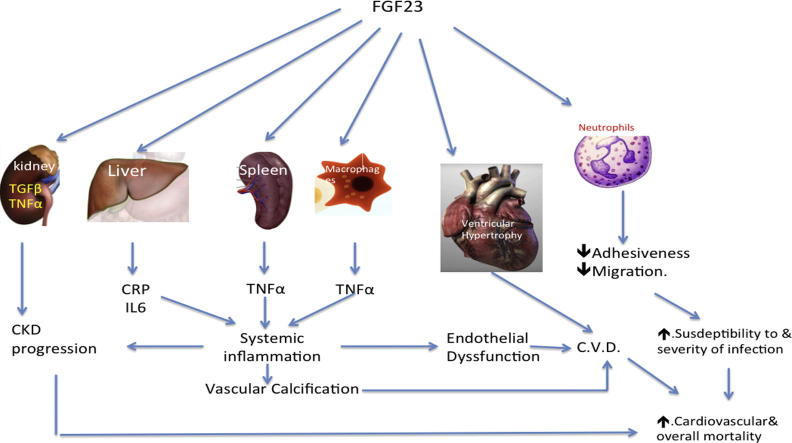
Graphical abstract
Keywords: Chronic kidney disease, Fibroblast growth factor 23, Left ventricular hypertrophy, Inflammation, Vascular calcification, Mortality
Abstract
The death rate among chronic kidney disease patients is the highest compared to other chronic diseases. 60% of these fatalities are cardiovascular. Cardiovascular calcifications and chronic inflammation affect almost all chronic kidney disease patients and are associated with cardiovascular mortality. Fibroblast growth factor 23 is associated with vascular calcification. Systemic inflammation in chronic kidney disease patients is multifactorial. The role of systemic inflammation in the pathogenesis of vascular calcification was recently reappraised. Fibroblast growth factor 23 was accused as a direct stimulus of left ventricular hypertrophy, uremic inflammation, and impaired neutrophil function. This review will discuss the underlying mechanisms that underlie the link between Fibroblast growth factor 23 and increased mortality encountered among chronic kidney disease patients.
Introduction
Fibroblast growth factor 23 (FGF23) is a member of a large family of structurally related polypeptide growth factors found in different species including humans [1]. Its main function is to regulate serum phosphate level [2]. The serum level of FGF23 starts to rise in early chronic kidney disease (CKD) [3]. By the time of starting dialysis, this level reaches hundred to thousand folds the normal level [4]. According to its role in phosphate metabolism, it was initially thought that the rise of FGF23 in serum carries a favorable effect on bone metabolism and cardiovascular welfare. However, subsequent research disclosed a significant association between FGF23 and vascular calcification (VC) [5], [6], left ventricular hypertrophy (LVH) [7], and mortality [8], [9] among CKD patients. To further complicate this puzzle, neutralization of FGF23 in CKD rats using monoclonal antibodies accelerated VC and increased mortality [10]. This raised the question whether FGF23 is a friend or a foe? [11], [12].
The story of FGF23
FGF23 was identified 16 years ago as a member of FGF family [13]. FGFs are a group of polypeptide growth factors that are involved in metabolic, developmental, neoplastic, and neurologic disorders [14]. The human FGF gene has 22 members, namely FGF1 to FGF14 and FGF16 to FGF23. Humans lack FGF15 [15]. FGF23 exerts its hypophosphatemic effect through inhibition of the luminal sodium-phosphate co-transporters in the proximal tubular epithelial cells [2]. The affinity of FGF23 for its ubiquitous FGF receptors (FGFR) is enhanced by α klotho [16]. FGF23 inhibits 1-α-hydroxylase activity and thus decreases 1,25(OH)2 vitamin D level and increases serum parathormone (PTH) level [2], [17].
Vascular calcification in CKD patients
In a prospective observational 3 years follow-up study, the prevalence of VC among predialysis CKD stage G3-5 patients was 79% [18]. It approaches 100% in prevalent dialysis patients [5]. VC affects almost all arteries whether large, medium or small-sized, including the coronary arteries [5], [19]. In vivo molecular imaging techniques has disclosed that VC is preceded by inflammation within arterial wall [20], [21]. A similar finding was confirmed by a longitudinal study using PET/CT scan [22]. VC is one of the predictors of increased cardiovascular mortality among CKD patients [23].
Inflammation in CKD patients
Systemic inflammation is one of the hallmarks of CKD. The exact pathogenesis of inflammation in CKD was not fully understood. Multiple comorbid conditions (like infections and autoimmune systemic diseases) can underlie inflammation in some CKD cases [24]. Blood translocation of bacteria and uremic toxins was recently suggested as an alternative mechanism of uremic chronic inflammation [25]. Lastly, Singh et al., demonstrated that FGF23 stimulates hepatocytes to increase secretion of the inflammatory markers IL6 and C-reactive protein (CRP) [26]. Many of the inflammatory markers and mediators can promote VC in CKD patients. These factors include interleukin 1 (IL-1), IL-6, CRP and tumor necrosis factor α (TNFα) [27], [28]. In addition, inflammation causes structural and functional abnormalities in HDL, an important antioxidant that defends the endothelium against the effects of cytokines [29]. Inflammation is interrelated to oxidative stress in CKD [30]. Systemic inflammation is independently associated with increased mortality among pre-dialysis and dialysis CKD patients [31]. High sensitivity CRP has been shown to independently predict mortality in CKD patients [30].
FGF23 and vascular calcification
The positive correlation between FGF23 and VC was first reported among hemodialysis (HD) patients 6 years ago [5]. A second trial in pre-dialysis CKD patients showed that FGF23 is independently associated with carotid artery calcification [32]. In CKD patients in stage G2-5D, higher aortic and coronary calcification scores were encountered in those having elevated FGF23 levels [6]. Similar results were even reported in healthy older men irrespective of traditional risk factors [33]. Pediatric studies confirmed the same results in children with CKD [34]. FGF23 predicts coronary calcification and poor outcome in patients with CKD stages 3-5D [35]. The same association was recorded in patients kept on HD for more than one year [36]. Four isoforms of FGF23 receptors were identified in male mouse aorta. Direct stimulation of these receptors increased free oxygen radicles release and a distinct decrease of nitric oxide bioavailability [37]. The strong correlation between aortic calcification and FGF23 was mitigated by the inflammatory marker high sensitivity CRP [38]. Finally, the lack of association between FGF23 and arterial calcification in the work done by Scialla et al. [39] is probably related to time gap between blood sample collection and imaging procedure (median time gap 376 days, range 331–420 days). FGF23 serum level varies with time according to serum phosphorus level, phosphate load, type and dose of phosphate binder used, vitamin D status, serum PTH and serum calcium level and more important with GFR. These CKD patients were not yet on dialysis and their GFR decline with time rendering the level of FGF23 in blood samples collected 1 year before imaging inaccurate for correlation analysis.
FGF23 and inflammation
The last 4 years have witnessed a revolution of knowledge about the relation between FGF23 and inflammation. In 2012, Munoz Mendoza et al., discovered that higher FGF23 levels are associated with IL6, CRP, and TNFα in CKD patients [40]. One year later FGF-23 was found to strongly correlate to hsCRP in HD patients [38].
In a mouse model of CKD, FGF23 was able to induce the genes responsible for TNFα and transforming growth factor β (TGFβ) production within the kidney [41]. FGF23 significantly increases TNF-α production within splenic cells [42]. It also stimulates TNFα expression by macrophages [43], [44]. Hepatocytes were found to have membrane receptors to FGF23. These receptors are similar to those previously characterized on the cardiomyocyte membrane, namely, FGFR4 isoform. On binding to these receptors, FGF23 signaling increased synthesis of CRP and IL6 through stimulation of intracellular calcineurin [26].
On the other hand, many studies demonstrated induction of FGF23 by immune reaction or inflammation. Inflammatory cytokines can directly increase production of FGF23 in bone [45], [46], [47]. Immune stimulation following parenteral bacterial inoculation or intra-peritoneal injection of lipopolysaccharides is associated with increased expression of FGF23 by activated dendritic cells and phagocytes [43]. FGF23 is up regulated on stimulation of pro-inflammatory macrophages [44].
In view of the current findings, it is possible that FGF23 and inflammatory cytokines constitute a positive feedback loop in which FGF23 stimulates expression of inflammatory cytokines, which in turn increase expression of FGF23. This vicious cycle can explain the 100- to 1000-fold increase of FGF23 in advanced CKD.
FGF23 and LVH
The first report on the association between FGF23 and LVH was in 2009 [48]. In spite of stable blood pressure and kidney functions, CKD patients in stage 3 develop LVH that is associated to FGF23/klotho ratio [49]. FGF23 stimulates hypertrophy of isolated rat cardiomyocytes. By binding to FGFR4 on the cell membrane, activation of the calcineurin-nuclear factor of activated T-cell (NFAT) signaling pathway ensues [50]. Similar results were encountered in CKD patients [51]. FGF-receptor antagonism ameliorates CKD-induced LVH in rats [52]. In addition, higher FGF-23 was independently associated with graded risk of CHF and to less extent with atherosclerotic events [53].
On the other hand, a recent study failed to find out LVH in patients with FGF23-related hypophosphatemic rickets/osteomalacia [54].
FGF23 and CKD progression
The first study that highlighted an off-target offensive role of FGF23 was in 2007. In this study of non-diabetic CKD patients, FGF23 was an independent predictor of CKD progression after adjustment for age, gender, serum calcium, phosphorus, parathyroid hormone, glomerular filtration rate (GFR), and proteinuria [55]. The cohort investigated in this study was composed of relatively young patients with mild to moderate impairment of kidney function. Similar findings were encountered in a Swedish cohort of patients with chronic IgA nephropathy [56] and in patients suffering diabetic kidney disease [57]. A Japanese group further supported these results. In their prospective study in a cohort of 738 pre-dialysis patients, 213 reached the endpoint (defined as either doubling of serum creatinine or starting regular dialysis) over a median duration of 4.4 years. High serum level of FGF23 and low level of 25-hydroxy vitamin D were the only factors significantly associated with the endpoint. This association was consistent regardless of the baseline GFR [58]. In a later study of African Americans, FGF23 had a dose–response relationship with the risk for end-stage renal disease (ESRD) or death [59]. In a recent study of 419 CKD children, 1–16 years old that were followed for a median of 5.5 years, 32.5% of them reached the progression end point (needed dialysis, underwent kidney transplant or had more than 50% reduction in GFR. FGF23 was independently associated with higher risk to reach the progression end point [60].
The strong relationship between FGF23 and development of progressive CKD was proved by the large study of 13,448 healthy participants at entry. These participants were black and white men and women and were followed for up to 21 years. The mean age of this group at recruitment was 56.9 years and their mean GFR was 97 ml/min per 1.73 m2. During this long-term follow-up, 2.0% of participants developed ESRD. The highest FGF23 quintile was associated with risk of developing ESRD compared with the lowest quintile. This association was independent of the different possible confounders [61]. In patients with advanced CKD (mean GFR = 18 mL/min/1.73 m2), a progressive increase in the risks of death, development of cardiovascular events, and need for dialysis was encountered with each subsequent quartile of FGF23 level compared to the lowest quartile [62].
FGF23 and α-Klotho
α-Klotho is an anti-senescence protein [63]. It exists in 2 forms: the transmembrane and the soluble secreted form [64]. α-Klotho is detected as a soluble protein in body fluids [65]. Membrane α-Klotho functions as the coreceptor for FGF23, which amplifies and confers specificity of FGF23 hypophosphatemic action [66]. In contrast, soluble α-Klotho protein functions independently of FGF23 and plays an important role in antioxidation [67] and anti senescence [68]. FGF23 suppresses α-klotho gene transcription in the kidney [41].
FGF23 and parathyroid gland
FGF23, in vitro, inhibits PTH secretion and mRNA transcription [69]. On the contrary, primary hyperparathyroidism in rodents is associated with increased serum levels of FGF23. This rise is reduced by parathyroidectomy. PTH stimulates secretion of FGF23 by osteocytes [70]. In normal physiological settings of normal Klotho and FGFR expression, FGF23 decreases PTH production, increases expression of both the parathyroid Ca-sensing receptor and the vitamin D receptor, and decreases parathyroid cell proliferation [71]. In CKD, increased FGF23 is associated with secondary hyperparathyroidism [72]. This paradox is likely a consequence of resistance to FGF23, probably due to down-regulation of the FGFR in the parathyroid gland, decreased affinity of FGF23 for its FGFR secondary to α klotho deficiency [16], and decreased serum level of 1,25(OH)2 vitamin D. FGF23 inhibits Cyp27b1 (1-α-hydroxylase) activity and activates Cyp24 (24-hydroxylase) and thus decreases 1,25(OH)2 vitamin D level [73].
FGF23 and renin-angiotensin system (RAS)
The effect of FGF23 on the RAS system is indirect. Through inhibition of vitamin D activation, FGF23 can stimulate RAS [74]. Angiotensin II (AII) activates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, leading to production of the superoxide anion and decreased availability of nitric oxide (NO). Reactive oxygen species (ROS), thus produced, stimulates vascular cell proliferation and hypertrophy [29]. Angiotensin converting enzyme inhibitors (ACEIs) can decrease the systemic and renal expression of FGF23 in a diabetic nephropathy model. In this model, ACEIs increased Klotho expression and normalized phosphate level and excretion [75]. However, a posthoc analysis of the ESCAPE trial in CKD children showed that ACEIs were associated with increased FGF23 [76]. High FGF23 is associated with impaired Reno protective response to RAS blockers [77].
FGF23 and neutrophil function
Increased susceptibility to infections is one of the adverse effects of CKD. In CKD patients treated by HD, high serum level of FGF23 was associated with a higher risk of infectious events [78]. In CKD patients, the leukocyte recruitment into inflamed tissue is impaired but is restored if FGF23 is neutralized. In CKD mice, intra-vital microscopy showed that FGF23 is able to inhibit leukocyte adhesiveness to endothelial surface [79]. Polymorph nuclear leucocytes (PMNLs) carry FGF receptor isoform called FGFR2 [80]. Binding of FGF23 to FGFR2 activates protein kinase A (PKA) within PMNs. PKA activation inhibits integrin activation on PMNs and thus blocks the action of selectins and chemokines on PMNLs adhesion and trans endothelial migration [79].
Discussion
When FGF23 was first characterized and subsequent studies disclosed its hypophosphatemic action, its high level in CKD was appreciated as a protective mechanism against disturbed phosphate handling by the diseased kidneys. This impression was reinforced by the studies on FGF23 knockout mice. In these mice premature aging, soft tissue and arterial calcification were outstanding [81], [82]. However, later observational clinical trials disclosed opposite results. FGF23 was significantly and independently associated with disease progression, increased mortality, LVH, and VC in CKD. The association between FGF23 and increased mortality was further supported by a recent meta analysis of seven studies including 1406 ESRD patients maintained on dialysis [83]. Shalhoub and his colleagues used monoclonal antibodies to block the action of FGF23 in CKD rats. VC and mortality significantly increased in the treated rats in spite of control of hyperparathyroidism. They explained these results as a consequence of the significant rise of serum phosphorus in the treated rats [10]. These results favored the concept that FGF23 is a mere risk marker [11], [12]. By that time, FGF23 was considered a favorable factor and the adverse events associated with its pathologic high level are the consequences of either the phosphate load or the deficient klotho or due to its effect on vitamin D activation. Suppression of 1,25(OH)2 vitamin D synthesis results in secondary hyperparathyroidism [2], [17]. However, many studies in the last five years have disclosed the offensive role of FGF23. The direct effect of FGF23 on cardiac mass was the 1st evidence of this offensive role [50]. It also induces the genes responsible for TNFα and TGFβ production within the renal tissue of CKD mice [41]. This might be the 2nd evidence of the direct damage induced by FGF23. The significant increase of TNF-α production within splenic cells [42], and macrophages [43], [44] and of CRP and IL6 by hepatocytes [26] on stimulation of these cells points to a crucial role of high FGF23 in the pathogenesis of systemic inflammation in CKD. These findings confirmed that FGF23 in CKD patients is a real foe. Being triggered by immune and inflammatory reactions [43], [44], [45], [46], [47] and at the same time to increase secretion of inflammatory mediators, it seems that FGF23 fuels the fire of inflammation and hence confirms its link to vascular inflammation and subsequent calcification and to increased mortality of CKD patients. Further support to the direct adverse effect of FGF23 was achieved by the work done by Rossaint et al., published last March. Binding of FGF23 to its receptors on the surface of neutrophils impaired their response to selectin and chemokine stimulation [79].
These accumulating findings should trigger the energetic control of FGF23 once its serum level starts to rise above normal in almost all CKD patients. FGF23 starts to increase as early as stage G2 [84]. Alimentary phosphate absorption is the most modifiable target to control FGF23 level. Many short-term small studies looked for the control of FGF23 by dietary phosphate restriction [85], non-calcium based phosphate binders [86], [87], [88], [89], phosphate restriction plus non-calcium based phosphate binders [90], cinacalcet [91], [92], or cinacalcet plus low-dose vitamin D [93]. Although appreciable reduction of FGF23 was achieved in these studies, the dual treatment by dietary phosphate restriction and non-calcium based phosphate binders showed the most consistent effect [90]. Calcium-based phosphate binders do not achieve similar results [94] probably because calcium stimulates FGF23 production [95]. Nicotinamide blocks the intestinal sodium phosphate co-transporter “NPT2b” [96]. This active transporter is up regulated by dietary phosphate restriction [97]. It seems that combining nicotinamide to dietary phosphate restriction and non-calcium based phosphate binder would give the most efficient control of intestinal phosphate absorption and hence FGF23 control. This is the rationale of the currently underway CKD Optimal Management with BInders and NicotinamidE (COMBINE) study [98]. Selective blocking of FGF23-FGFR signaling is another hopeful alternative that waits for clinical studies. Finally, we like to emphasize that there is no randomized clinical trials that show that the therapeutic reduction of FGF23 is associated with better outcomes. In the secondary analysis of EVOLVE study, hyperparathyroid patients treated with cinacalcet showed a sustained significant decrease of FGF23 levels after 20 weeks of cinacalcet treatment. Reduced FGF23 levels were associated with lower cardiovascular mortality and major cardiovascular events [99].
Conclusions
It became evident that FGF23 is neither a good friend nor an innocent bystander. Energetic control of FGF23 through control of phosphate load and or using selective receptor blockers should have a significant favorable impact on CKD progression, systemic inflammation, immunity, cardiovascular disease, cardiovascular, and overall mortality. The link between the effect of non-calcium based phosphate binders on FGF23 and their anti-inflammatory actions and their impact on overall mortality would support this view.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Usama A.A. Sharaf El Din in an Emeritus Professor of Internal Medicine and Nephrology at Cairo University. He is an author of more than 70 articles in the field of Internal Medicine, Nephrology and diabetes, many of them are peer reviewed with more than 200 citations. He is a chairman of the Vascular Calcification group and a peer reviewer in 10 international indexed medical journals. He is the Ex-board member of the Egyptian Universities Promotion Committee (EUPC), Ministry of Higher Education and the Editor of Textbook of Internal Medicine, School of Medicine-Cairo University.

Mona M. Salem is a Professor of Internal Medicine and Endocrinology, Cairo University. She is a member of the vascular calcification group and an author of more than 10 peer reviewed scientific manuscripts in the fields of Internal Medicine and Endocrinology.

Dina O. Abdulazeem Resident of Rheumatology and Rehabilitation starting in May 2013 Till May 2016. M.Sc. Rheumatology and Rehabilitation May, 2016. Assistant Lecturer of Rheumatology, School of Medicine, Cairo University. Co-author of 4 peer reviewed literature reviews.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2(3): REVIEWS3005, 2001. [DOI] [PMC free article] [PubMed]
- 2.Liu S., Quarles L.D. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez O., Isakova T., Rhee E., Shah A., Holmes J., Collerone G. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 4.Viaene L., Bammens B., Meijers B.K., Vanrenterghem Y., Vanderschueren D., Evenepoel P. Residual renal function is an independent determinant of serum fgf-23 levels in dialysis patients. Nephrol Dial Transplant. 2012;27:2017–2022. doi: 10.1093/ndt/gfr596. [DOI] [PubMed] [Google Scholar]
- 5.Nasrallah M.M., El-Shehaby A.R., Salem M.M., Osman N.A., El Sheikh E., Sharaf El Din U.A. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679–2685. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 6.Desjardins L., Liabeuf S., Renard C., Lenglet A., Lemke H.D., Choukroun G. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23:2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 7.Mirza M.A., Larsson A., Melhus H., Lind L., Larsson T.E. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Olauson H., Qureshi A.R., Miyamoto T., Barany P., Heimburger O., Lindholm B. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant. 2010;25(9):3033–3038. doi: 10.1093/ndt/gfq191. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez O.M., Mannstadt M., Isakova T., Rauh-Hain J.A., Tamez H., Shah A. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shalhoub V., Shatzen E.M., Ward S.C., Davis J., Stevens J., Bi V. FGF23 neutralization improves secondary hyperparathyroidism and osteodystrophy parameters yet exacerbates vascular calcification in chronic kidney disease rats. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson T.E. The role of FGF-23 in CKD–MBD and cardiovascular disease: friend or foe? Nephrol Dial Transplant. 2010;25:1376–1381. doi: 10.1093/ndt/gfp784. [DOI] [PubMed] [Google Scholar]
- 12.Moe O.W. Fibroblast growth factor 23: friend or foe in uremia? J Clin Invest. 2012;122(7):2354–2356. doi: 10.1172/JCI64184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita T., Yoshioka M., Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 14.Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh N., Ornitz D.M. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149(2):121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 17.Saito H., Kusano K., Kinosaki M., Ito H., Hirata M., Segawa H. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxy-vitamin D3 production. J Biol Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 18.Górriz J.L., Molina P., Cerverón M.J., Vila R., Bover J., Nieto J. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10(4):654–666. doi: 10.2215/CJN.07450714. Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeseun G.A., Xie D., Wang X., Joffe M.M., Joffe M.M., Mohler E.R., 3rd Carotid plaque, carotid intima-media thickness, and coronary calcification equally discriminate prevalent cardiovascular disease in kidney disease. Am J Nephrol. 2012;36(4):342–347. doi: 10.1159/000342794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New S.E., Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381–1391. doi: 10.1161/CIRCRESAHA.110.234146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demer L.L., Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelbaky A., Corsini E., Figueroa A.L., Fontanez S., Subramanian S., Ferencik M. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imag. 2013;6(5):747–754. doi: 10.1161/CIRCIMAGING.113.000382. Sep. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S., Bogle R., Banerjee D. Why do young people with chronic kidney disease die early? World J Nephrol. 2014;3(4):143–155. doi: 10.5527/wjn.v3.i4.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenvinkel P., Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial. 2002;15:329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 25.Lau W.L., Kalantar-Zadeh K., Vaziri N.D. The gut as a source of inflammation in chronic kidney disease. Nephron. 2015;130:92–98. doi: 10.1159/000381990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S., Grabner A., Yanucil C., Schramm K., Czaya B., Krick S. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90:985–996. doi: 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stompór T., Pasowicz M., Sulłowicz W., Dembińska-Kieć A., Janda K., Wójcik K. An association between coronary artery calcification score, lipid profile, and selected markers of chronic inflammation in ESRD patients treated with peritoneal dialysis. Am J Kidney Dis. 2003;41:203–211. doi: 10.1053/ajkd.2003.50005. [DOI] [PubMed] [Google Scholar]
- 28.Jung H.H., Kim S.W., Han H. Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant. 2006;21:1915–1920. doi: 10.1093/ndt/gfl118. [DOI] [PubMed] [Google Scholar]
- 29.Kaysen G.A., Eiserich J.P. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol. 2004;15(3):538–548. doi: 10.1097/01.asn.0000111744.00916.e6. [DOI] [PubMed] [Google Scholar]
- 30.Stenvinkel P. New insights on inflammation in chronic kidney disease-genetic and non-genetic factors. Nephrol Ther. 2006 Jul;2(3):111–119. doi: 10.1016/j.nephro.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Barreto D.V., Barreto F.C., Liabeuf S., Temmar M., Lemke H.D., Tribouilloy C., for the European Uremic Toxin Work Group (EUTox) Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama M., Kaizu Y., Nagata M., Ura Y., Ikeda H., Shimamoto S. Fibroblast growth factor 23 is associated with carotid artery calcification in chronic kidney disease patients not undergoing dialysis: a cross-sectional study. BMC Nephrol. 2013;14:22. doi: 10.1186/1471-2369-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoppet M., Hofbauer L.C., Brinskelle-Schmal N., Varennes A., Goudable J., Richard M. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab. 2012;97(4):E575–E583. doi: 10.1210/jc.2011-2836. [DOI] [PubMed] [Google Scholar]
- 34.Paoli S., Mitsnefes M.M. Coronary artery calcification and cardiovascular disease in children with chronic kidney disease. Curr Opin Pediatr. 2014;26(2):193–197. doi: 10.1097/MOP.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M., Yan J., Zhu M., Ni Z. Fibroblast growth factor 23 predicts coronary calcification and poor prognosis in patients with chronic kidney disease stages 3–5D. Ann Clin Lab Sci Winter. 2015;45(1):17–22. [PubMed] [Google Scholar]
- 36.Zayed BE, El-Fishawy H, Al-Shihaby AR, Salem MA, Sharaf El Din UA, Salem MM. Sevelamer hydrochloride and coronary artery calcification in chronic hemodialysis patients: a new mechanism of action. Egypt J Intern Med [serial online] [cited 2016 October 21]; 27:133-8. Available from: http://www.esim.eg.net/text.asp?2015/27/4/133/174928, 2015.
- 37.Silswal N., Touchberry C.D., Daniel D.R., McCarthy D.L., Zhang S., Andresen J. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab. 2014;307(5):E426–E436. doi: 10.1152/ajpendo.00264.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasrallah M.M., El-Shehaby A.R., Osman N.A., Fayad T., Nassef A., Salem M.M. The association between fibroblast growth factor-23 and vascular calcification is mitigated by inflammation markers. Nephron Extra. 2013;3:106–112. doi: 10.1159/000356118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scialla J.J., Lau W.L., Reilly M.P., Isakova T., Yang H.Y., Crouthamel M.H. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83(6):1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz Mendoza J., Isakova T., Ricardo A.C., Xie H., Navaneethan S.D., Anderson A.H. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7(7):1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai B., David V., Martin A., Huang J., Li H., Jiao Y. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS ONE. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamauchi M., Hirohashi Y., Torigoe T., Matsumoto Y., Yamashita K., Kayama M. Wound healing delays in alpha-Klotho-deficient mice that have skin appearance similar to that in aged humans: study of delayed wound healing mechanism. Biochem Biophys Res Commun. 2016;473:845–852. doi: 10.1016/j.bbrc.2016.03.138. [DOI] [PubMed] [Google Scholar]
- 43.Masuda Y., Ohta H., Morita Y., Nakayama Y., Miyake A., Itoh N. Expression of Fgf23 in activated dendritic cells and macrophages in response to immunological stimuli in mice. Biol Pharm Bull. 2015;38:687–693. doi: 10.1248/bpb.b14-00276. [DOI] [PubMed] [Google Scholar]
- 44.Han X., Li L., Yang J., King G., Xiao Z., Quarles L.D. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett. 2016;590:53–67. doi: 10.1002/1873-3468.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David V., Martin A., Isakova T., Spaulding C., Qi L., Ramirez V. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89:135–146. doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito N., Wijenayaka A.R., Prideaux M., Kogawa M., Ormsby R.T., Evdokiou A. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015;399:208–218. doi: 10.1016/j.mce.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Pathak J.L., Bakker A.D., Luyten F.P., Verschueren P., Lems W.F., Klein-Nulend J. Systemic inflammation affects human osteocyte-specific protein and cytokine expression. Calcif Tissue Int. 2016;98:596–608. doi: 10.1007/s00223-016-0116-8. [DOI] [PubMed] [Google Scholar]
- 48.Hsu H.J., Wu M.S. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337(2):116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 49.Seifert M.E., de Las Fuentes L., Ginsberg C., Rothstein M., Dietzen D.J., Cheng S.C. Left ventricular mass progression despite stable blood pressure and kidney function in stage 3 chronic kidney disease. Am J Nephrol. 2014;39(5):392–399. doi: 10.1159/000362251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faul C., Amaral A.P., Oskouei B., Hu M.C., Sloan A., Isakova T. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leifheit-Nestler M., Große Siemer R., Flasbart K., Richter B., Kirchhoff F., Ziegler W.H. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant. 2016;31(7):1088–1099. doi: 10.1093/ndt/gfv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ketteler M., Biggar P.H., Liangos O. FGF23 antagonism: the thin line between adaptation and maladaptation in chronic kidney disease. Nephrol Dial Transplant. 2013;28(4):821–825. doi: 10.1093/ndt/gfs557. [DOI] [PubMed] [Google Scholar]
- 53.Scialla J.J., Xie H., Rahman M., Anderson A.H., Isakova T., Ojo A. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25(2):349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takashi Y., Kinoshita Y., Hori M., Ito N., Taguchi M., Fukumoto S. Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res. 2016;18:1–6. doi: 10.1080/07435800.2016.1242604. [DOI] [PubMed] [Google Scholar]
- 55.Fliser D., Kollerits B., Neyer U., Ankerst D.P., Lhotta K., Lingenhel A. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 56.Lundberg S., Qureshi A.R., Olivecrona S., Gunnarsson I., Jacobson S.H., Larsson T.E. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol. 2012;7(5):727–734. doi: 10.2215/CJN.10331011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Titan S.M., Zatz R., Graciolli F.G., dos Reis L.M., Barros R.T., Jorgetti V. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011;6:241–247. doi: 10.2215/CJN.04250510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakano C., Hamano T., Fujii N., Matsui I., Tomida K., Mikami S. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7(5):810–819. doi: 10.2215/CJN.08680811. [DOI] [PubMed] [Google Scholar]
- 59.Scialla J.J., Astor B.C., Isakova T., Xie H., Appel L.J., Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. 2013;24:125–135. doi: 10.1681/ASN.2012070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Portale A.A., Wolf M.S., Messinger S., Perwad F., Jüppner H., Warady B.A. Fibroblast growth Factor 23 and risk of CKD progression in children. Clin J Am Soc Nephrol. 2016;25 doi: 10.2215/CJN.02110216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rebholz C.M., Grams M.E., Coresh J., Selvin E., Coresh J. Serum fibroblast growth factor-23 is associated with incident kidney disease. J Am Soc Nephrol. 2015;26(1):192–200. doi: 10.1681/ASN.2014020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kendrick J., Cheung A.K., Kaufman J.S., Greene T., Roberts W.L., Smits G. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22(10):1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 64.Shiraki-Iida T., Aizawa H., Matsumura Y., Sekine S., Iida A., Anazawa H. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 65.Imura A., Iwano A., Tohyama O., Tsuji Y., Nozaki K., Hashimoto N. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 66.Goetz R., Nakada Y., Hu M.C., Kurosu H., Wang L., Nakatani T. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rakugi H., Matsukawa N., Ishikawa K., Yang J., Imai M., Ikushima M. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–87. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- 68.Ikushima M., Rakugi H., Ishikawa K., Maekawa Y., Yamamoto K., Ohta J. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 69.Krajisnik T., Björklund P., Marsell R., Ljunggren O., Akerström G., Jonsson K.B. Fibroblast growth factor-23 regulates parathyroid hormone and 1-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 70.Kawata T., Imanishi Y., Kobayashi K., Miki T., Arnold A., Inaba M. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Dov I.Z., Galitzer H., Lavi-Moshayoff V., Goetz R., Kuro-o M., Mohammadi M. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakanishi S., Kazama J.J., Nii-Kono T., Omori K., Yamashita T., Fukumoto S. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 73.Quarles L.D. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Borst M.H., Vervloet M.G., ter Wee P.M., Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603–1609. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zanchi C., Locatelli M., Benigni A., Corna D., Tomasoni S., Rottoli D. Renal expression of FGF23 in progressive renal disease of diabetes and the effect of ACE inhibitor. PLoS ONE. 2013;8:e70775. doi: 10.1371/journal.pone.0070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shroff R., Aitkenhead H., Costa N., Trivelli A., Litwin M., Picca S. Normal 25-hydroxyvitamin D levels are associated with less proteinuria and attenuate renal failure progression in children with CKD. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014090947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Jong M.A., Mirkovic K., Mencke R., Hoenderop J.G., Bindels R.J., Vervloet M.G. Fibroblast growth factor 23 modifies the pharmacological effects of angiotensin receptor blockade in experimental renal fibrosis. Nephrol Dial Transplant. 2016;24(May) doi: 10.1093/ndt/gfw105. [DOI] [PubMed] [Google Scholar]
- 78.Chonchol M., Greene T., Zhang Y., Hoofnagle A.N., Cheung A.K. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol. 2016;27(1):227–237. doi: 10.1681/ASN.2014101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossaint J., Oehmichen J., Van Aken H., Reuter S., Pavenstädt H.J., Meersch M. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126(3):962–974. doi: 10.1172/JCI83470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haddad L.E., Khzam L.B., Hajjar F., Merhi Y., Sirois M.G. Characterization of FGF receptor expression in human neutrophils and their contribution to chemotaxis. Am J Physiol Cell Physiol. 2011;301(5):C1036–C1045. doi: 10.1152/ajpcell.00215.2011. [DOI] [PubMed] [Google Scholar]
- 81.Razzaque M.S., Sitara D., Taguchi T., St-Arnaud R., Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang H., Luo H., Tang X., Zeng X., Yu Y., Ma L. Prognostic value of FGF23 among patients with end-stage renal disease: a systematic review and meta-analysis. Biomark Med. 2016;10(5):547–556. doi: 10.2217/bmm.16.11. [DOI] [PubMed] [Google Scholar]
- 84.Isakova T., Wolf M.S. FGF23 or PTH: which comes first in CKD? Kidney Int. 2010;78:947–949. doi: 10.1038/ki.2010.281. [DOI] [PubMed] [Google Scholar]
- 85.Goto S., Nakai K., Kono K., Yonekura Y., Ito J., Fujii H. Dietary phosphorus restriction by a standard low-protein diet decreased serum fibroblast growth factor 23 levels in patients with early and advanced stage chronic kidney disease. Clin Exp Nephrol. 2014;18(6):925–931. doi: 10.1007/s10157-014-0947-4. Dec. [DOI] [PubMed] [Google Scholar]
- 86.Lin H.H., Liou H.H., Wu M.S., Lin C.Y., Huang C.C. Long-term sevelamer treatment lowers serum fibroblast growth factor 23 accompanied with increasing serum Klotho levels in chronic haemodialysis patients. Nephrology (Carlton) 2014;19:672–678. doi: 10.1111/nep.12319. [DOI] [PubMed] [Google Scholar]
- 87.Rao M., Steffes M., Bostom A., Ix J.H. Effect of niacin on FGF23 concentration in chronic kidney disease. Am J Nephrol. 2014;39(6):484–490. doi: 10.1159/000362424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soriano S., Ojeda R., Rodríguez M., Almadén Y., Rodríguez M., Martín-Malo A. The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol. 2013;80(1):17–22. doi: 10.5414/CN107764. [DOI] [PubMed] [Google Scholar]
- 89.Iguchi A., Kazama J.J., Yamamoto S., Yoshita K., Watanabe Y., Iino N. Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron. 2015;131(3):161–166. doi: 10.1159/000440968. [DOI] [PubMed] [Google Scholar]
- 90.Smith E.R. The use of fibroblast growth factor 23 testing in patients with kidney disease. Clin J Am Soc Nephrol. 2014;9(7):1283–1303. doi: 10.2215/CJN.10941013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moe S.M., Chertow G.M., Parfrey P.S., Kubo Y., Block G.A., Correa-Rotter R. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation. 2015;132(1):27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. [DOI] [PubMed] [Google Scholar]
- 92.Koizumi M., Komaba H., Nakanishi S., Fujimori A., Fukagawa M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27(2):784–790. doi: 10.1093/ndt/gfr384. [DOI] [PubMed] [Google Scholar]
- 93.Wetmore J.B., Liu S., Krebill R., Menard R., Quarles L.D. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5(1):110–116. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radanovic T., Wagner C.A., Murer H., Biber J. Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P(i) diet of the type IIb Na(+)-P(i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G496–G500. doi: 10.1152/ajpgi.00167.2004. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez-Ortiz M.E., Lopez I., Muñoz-Castañeda J.R., Muñoz-Castañeda J.R., Martinez-Moreno J.M., Ramírez A.P. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012;23(7):1190–1197. doi: 10.1681/ASN.2011101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katai K., Tanaka H., Tatsumi S., Fukunaga Y., Genjida K., Morita K. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant. 1999;14(5):1195–1201. doi: 10.1093/ndt/14.5.1195. [DOI] [PubMed] [Google Scholar]
- 97.Giral H., Caldas Y., Sutherland E., Wilson P., Breusegem S., Barry N. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol. 2009;297(5):F1466–F1475. doi: 10.1152/ajprenal.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Isakova T., Ix J.H., Sprague S.M., Raphael K.L., Fried L., Gassman J.J. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol. 2015;26:2328–2339. doi: 10.1681/ASN.2015020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moe S.M., Chertow G.M., Parfrey P.S., Kubo Y., Block G.A. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation. 2015;132(1):27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. [DOI] [PubMed] [Google Scholar]