Summary
Background
The aim of the study was to assess if the presence of nasal septal deviation and concha bullosa is connected with the development of sinuses and the incidence of inflammation within them.
Material/Methods
We retrospectively analysed 214 patients who underwent paranasal sinus computed tomography. There were 125 females and 89 males, the mean age being 47.67±16.74 years (range 18–97). Exclusion criteria included: age under 18 years, prior sinonasal surgery and S-shaped septum.
Results
Mean volume of the right maxillary sinus was 17.794 cm3, while for the left one it was 17.713 cm3. Nasal septal deviation was found in 79.9% of computed tomography examinations and concha bullosa was observed in 42.1% of the patients’ examinations. There was an association between the presence of unilateral or dominant concha bullosa and contralateral direction of septal deviation [right-sided (p=0.039), left-sided (p=0.003)]. There was higher incidence of bilateral maxillary sinusitis in patients with septal deviation (p=0.007). Bilateral concha bullosa did not influence the incidence of bilateral maxillary sinusitis (p=0.495). Neither septal deviation (right sided: p=0.962; left-sided: p=0.731), nor unilateral/dominant concha bullosa (right: p=0.512; left: p=0,430) affected the asymmetry in volumes of maxillary sinuses. Bilateral concha bullosa was connected with larger volume of maxillary sinuses (right sinus: p=0.005; left sinus: p=0.048).
Conclusions
Nasal septal deviation, contrary to concha bullosa, has influence on the development of maxillary sinusitis. There is a connection between the presence of concha bullosa and direction of septal deviation. Only bilateral concha bullosa affects maxillary sinus volumes.
MeSH Keywords: Imaging, Three-Dimensional; Maxillary Sinus; Maxillary Sinusitis; Nasal Septum; Sinusitis; Turbinates
Background
Concepts of the anatomy of the paranasal sinuses have been known since the turn of the 19th and 20th century [1]. Nowadays, computed tomography (CT) of the paranasal sinuses is a simple tool for the diagnosis of pathologies. Therefore, it provides a precise and reliable preoperative roadmap for an endoscopic sinus surgeon [2].
Maxillary sinuses are located in the maxillary bones, below the orbits. Their development begins in the third week of gestation, and continues through childhood until early adulthood. The most commonly observed variations in maxillary sinus volume and configuration include posterior extension towards the zygomatic recess and inferior pneumatisation either into the dental alveolus above the roots of the posterior teeth, or between them in toothless areas [3,4].
Numerous mechanisms seem to play an important role in the growth of the sinus cavities. These are, among others: brain growth, muscular traction, as well as molecular adhesion [5]. Airflow through the nasal cavities affects the development of the paranasal sinuses and, in general, craniofacial skeleton [6]. Muscle-induced positive air pressure in the nasopharynx is crucial for the process of sinus shaping, since it lets the air enter them, which is where it can be assimilated into the circulatory system. Thus, the volume of sinuses varies [7]. This is the reason why any obstruction within the nasal respiratory complex may affect the development of pneumatised regions within the skull. It can be caused for example by the presence of concha bullosa (CB), as well as nasal septal deviation (NSD) [8].
Most often, bone pneumatisation is located in the middle nasal turbinate and can occur either unilaterally or bilaterally. Air-filled cavity in the superior turbinate can be present less frequently, whereas the aeration of the inferior turbinate is hardly observed. In most cases such a pneumatic chamber remains asymptomatic. There is a classification of pneumatisation of CB based on its location: lamellar, bulbous and extensive CB [8]. Moreover, the type of epithelium in an air-filled concha bullosa is the same as in the rest of the sinonasal tract [9].
NSD is described as an asymmetry of the nasal septum. Both traumatic deviation and growth-associated abnormalities of the nasal septum may lead to significant airway obstruction and also cosmetic deformity.
The limitation of air flow results in low oxygen pressure and disrupts paranasal sinus growth, decreases ciliar motion motility, and consequently promotes bacterial expansion [10]. It is commonly believed that the difference in volume is related to sinusitis. However, less is known about the role of NSD or CB as potential promoters of the development of sinusitis. It is still debated, since some authors suggest that NSD and CB promote the development of sinusitis, whereas others present contradictory statements [9,11].
The prevalence of chronic rhinosinusitis for a European population ranges from 6.9 to 27.1%, with a mean rate of 10.9% and a rate for the citizens of Cracow – 19.7%. [12]. The reason might be the fact that low air quality can lead to chronic sinusitis [13,14] and the air pollution level in Cracow has been among the worst of all the European cities in the recent years [15]. That is why the population of this city was chosen for the study.
The aim of the study was to find out whether the variants of the nasal septum, as well as the presence of CB in the nasal cavities correlate with the volume of maxillary sinuses and the risk of maxillary sinusitis development. Also, we wanted to investigate if there is a correlation between the presence of CB and direction of NSD.
Material and Methods
We included into the study and retrospectively analysed 214 paranasal sinus CT examinations, acquired from the Department of Diagnostic Imaging of the University Hospital (Szpital Uniwersytecki) in Cracow. All of the examinations were performed using an 80-row helical CT scanner Toshiba Aquilion PRIME 80 (Toshiba America Medical System, Irvine, CA, USA). Scan parameters: 120 kVp, 60 mA; rotation time: 500ms; slice increment: 1 mm (coronal plane). There was no contrast media administration. DICOM data were examined with the use of Impax 6.4 software (Agfa HealthCare, Mortsel, Belgium) in the coronal plane. Images were interpreted in bone windows. Examinations were evaluated by two independent investigators and all differences in opinions were solved through a consensus in the presence of a third independent examiner. The volume of each sinus was assessed by two independent examiners and the mean value from two measurements of volume was considered as the final one.
Patients included into the study were referred to the Department of Diagnostic Imaging due to their clinical symptoms within the sinonasal region. The exclusion criteria were: age under 18 years, previous history of sinonasal surgery, tumours destroying anatomical structures of the sinonasal region and an S-shaped nasal septum.
Maxillary sinusitis was defined as thickening of the mucous membrane in the sinus of more than 3 millimetres. [16] Chronic maxillary sinusitis was characterized as mucosal thickening or opacification of a sinus with coexisting polypoid changes in it, thickening of its bony walls or presence of calcifications in its lumen [17]. Each sinus was assessed separately. The convex part of the nasal septum was identified as the direction of deviation. Convexity was evaluated in relationship to the line between the anterior nasal spine and crista galli observed in the coronal plane [18] (Figure 1). CB was described as pneumatisation of the bulbous part of the middle nasal turbinate [9]. As a consequence, only patients with bulbous and extensive types of concha bullosa were included in the study group. Patients with lamellar type of concha bullosa were classified into the group without concha bullosa [19] (Figures 2, 3). For cases with bilateral concha, the larger one was considered dominant [9] (Figure 4).
Figure 1.
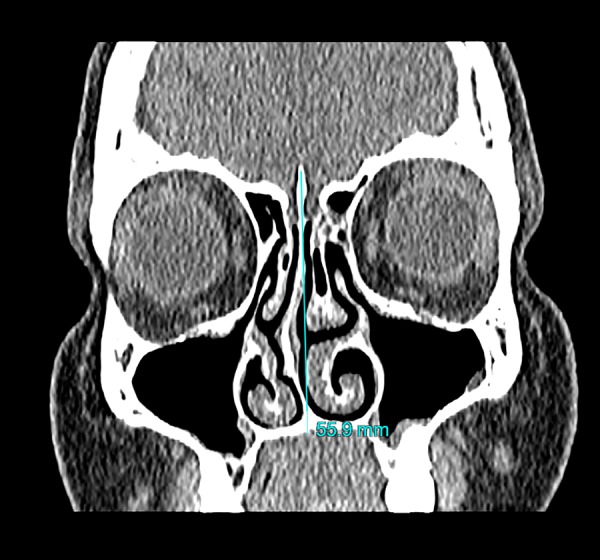
Demonstration of the method used for assessing the direction of nasal septal deviation; blue line – line between anterior nasal spine and crista galli.
Figure 2.
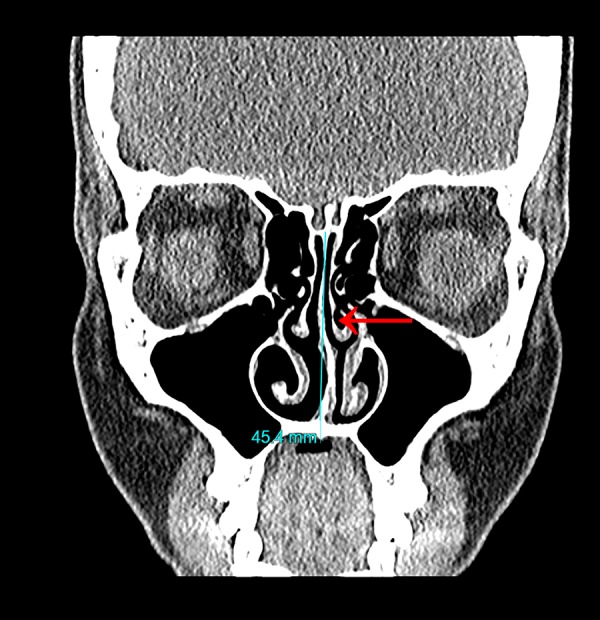
Small left-sided concha bullosa of bulbous type (red arrow) and left-sided nasal septal deviation.
Figure 3.
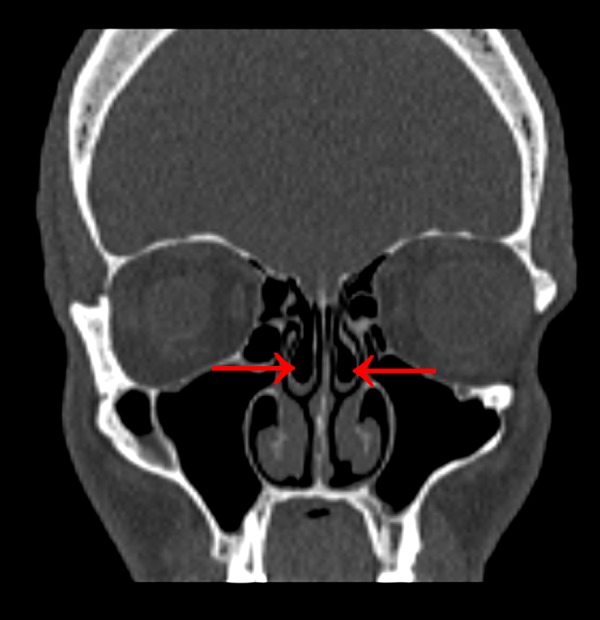
Bilateral and equal in size concha bullosa of extensive type (red arrows).
Figure 4.
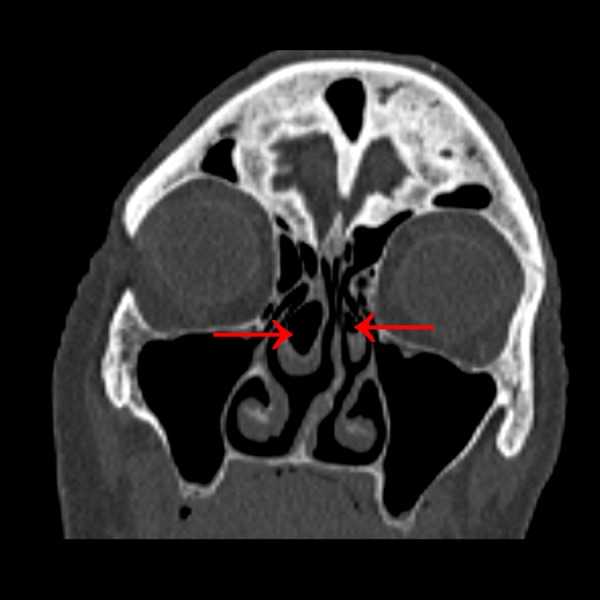
Bilateral concha bullosa with domination of the right concha (red arrows).
Volume of maxillary sinuses was measured using Vitrea Software 6.7.1 (Vital Images Inc., Minnetonka, MN, USA). It was done manually with Volume Measurement Tool in the 2D view of the whole maxillary sinus region creating 3D view of maxillary sinuses (Figure 5). After defining the bony walls of each maxillary sinus on multiple coronal sections, using the adjustments for bone window in CT, the volume was calculated by the software. Thickness of mucosa did not affect the measurement.
Figure 5.
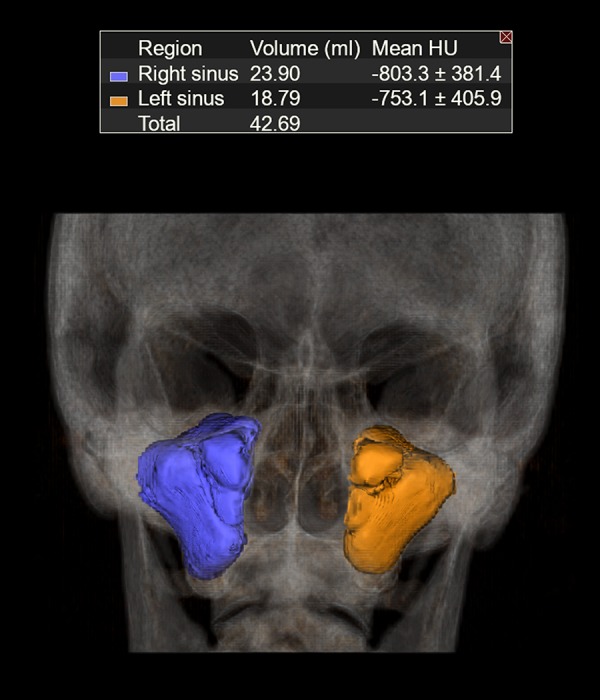
Three-dimensional reconstruction of volumes of both maxillary sinuses.
All volumes were given as mean values ± standard deviation. Statistical analysis was performed using Statistica 12.5 software (StatSoft Inc., Tulsa, OK, USA) with Pearson’s chi-square (χ2) test for categorical variables and independent two-sample Student’s t-test for continuous variables. Results are given as odds ratio (OR), as estimates of the relative risk with 95% confidence interval (CI). Statistical significance was set at p<0.05.
Results
Out of the 214 patients who were included in the study, 58.4% were females (n=125) and 41.6% were males (n=89). The age ranged from 18 to 97 years and the mean age was 47.67±16.74 years.
The mean volumes of the right and left maxillary sinuses were 17.794±6.115 cm3 and 17.713±5.715 cm3, respectively. No statistically significant difference was observed between both sides (p=0.747). Maxillary sinuses of larger size (absolute value) were observed among male patients. There was a statistically significant difference between sexes both for the right side (p<0.001) and the left side (p<0.001) (Table 1).
Table 1.
Gender differences in maxillary sinus volumes.
| Female (mean ±SD) | Male (mean ±SD) | p value | |
|---|---|---|---|
| Right sinus volume [cm3] | 16.186±4.777 | 20.052±7.039 | <0.001 |
| Left sinus volume [cm3] | 15.874±4.265 | 20.296±6.470 | <0.001 |
| p value | 0.586 | 0.810 |
Maxillary sinusitis was discovered in 73.8% (n=158) of CT examinations of the patients, it was bilateral in 62.7% (n=99) of cases and unilateral in 37.3% (n=59) of cases. Chronic maxillary sinusitis was present in 42.1% (n=90) of the total amount of patients and in 57.0% (n=90) in the group with maxillary sinusitis.
NSD was found in 79.9% (n=171) of CT examinations. It was right-sided in 50.3% (n=86) of the cases and left-sided in 49.7% (n=84). CB was observed in 42.1% (n=90) of the patients’ examinations. It was only right-sided in 34.4% (n=31) of the cases, only left-sided in 24.4% (n=22) of the cases and bilateral in 41.1% (n=37) of the cases. In the last group, 40.54% (n=15) of patients had both conchae of approximately equal size. Right-sided and left-sided dominant concha was present in both cases in 29.7% of the patients (for both: n=11).
NSD with coexisting CB was discovered in 35.5% (n=76) of the patients’ examinations. CB was unilateral in 59.2% (n=45) of the cases and bilateral in 40.8% (n=31) of the cases. In the latter group, in 64.5% (n=20) of the cases dominant CB was present and in 35.5% (n=11) of the examinations it was equal in size on both sides.
There was a statistically significant association between the presence of unilateral or dominant CB and the higher incidence of contralateral direction of NSD. It was noticed both for right-sided (p=0.039; OR=2.121) and left-sided (p=0.003; OR=2.817) deviations.
In the group with straight nasal septum there was no statistically significant difference between volumes of maxillary sinuses (p=0.982). The presence of NSD did not have an impact on the size of maxillary sinuses, either for right-sided (p=0.962) or left-sided deviations (p=0.731) (Table 2).
Table 2.
Maxillary sinus volume according to the type of nasal septum.
| Straight nasal septum | Right-sided NSD | Left-sided NSD | |
|---|---|---|---|
| Right sinus volume [cm3] (mean ±SD) | 17.304±5.661 | 16.849±6.008 | 19.216±6.163 |
| Left sinus volume [cm3] (mean ±SD) | 17.277±5.751 | 16.808±5.577 | 18.900±5.720 |
| p value | 0.982 | 0.962 | 0.731 |
Considering the frequency of maxillary sinusitis in patients with NSD, there was a higher incidence of bilateral maxillary sinusitis in patients with septal deviation (p=0.007; OR=2.676) in comparison to the group where no signs of septal curvature were observed. Chronic bilateral maxillary sinusitis was also notably more common among the patients with presence of NSD (p=0.035; OR=3.075). On the other hand, there was a lower risk of unilateral maxillary sinusitis on CT examinations of patients with NSD (p=0.019; OR=0.438) as compared to the individuals with straight nasal septum.
The relationship between the direction of NSD and increased incidence of inflammation in ipsilateral sinus was not statistically significant either for right-sided (p=0.094; OR=1.606) or left-sided (p=0.565; OR=0.845) deviations.
The link between the absence of CB and variations in size of maxillary sinuses was not discovered (p=0.800). No correlation was found between the side of unilateral or dominant CB and the volume of maxillary sinuses, either for the right (p=0.512) and the left (p=0.430) side (Table 3).
Table 3.
Maxillary sinus volumes determined by the presence of concha bullosa.
| Absence of CB or equal bilateral CB | Only right CB or dominant right CB | Only left CB or dominant left CB | |
|---|---|---|---|
| Right sinus volume [cm3] (mean ±SD) | 17.227±6.094 | 20.154±6.204 | 17.471±5.306 |
| Left sinus volume [cm3] (mean ±SD) | 17.048±5.672 | 19.276±6.003 | 18.481±5.185 |
| p value | 0.800 | 0.512 | 0.430 |
Bilateral CB is associated with larger volumes of both maxillary sinuses as compared to cases without the presence of CB or with unilateral type of CB, both for the right (p=0.005) and the left sinus (p=0.048) (Table 4).
Table 4.
Differences in maxillary sinus volumes connected with presence of bilateral concha bullosa.
| Without CB or unilateral CB (mean ±SD) | Bilateral CB (mean ±SD) | p value | |
|---|---|---|---|
| Right sinus volume [cm3] | 17.217±5.971 | 20.154±6.204 | 0.005 |
| Left sinus volume [cm3] | 17.331±5.594 | 19.276±6.003 | 0.048 |
As for the bilateral CB, there was no statistical significance concerning the incidence of bilateral maxillary sinusitis (p=0.495; OR=1.280). Bilateral CB did not have an influence on higher frequency of chronic bilateral maxillary sinusitis either (p=0.180; OR=1.716).
We did not find any correlation between the presence of CB and the increased risk of inflammation in ipsilateral sinus, either for the right (p=0.607; OR=0.849) and the left (p=0.728; OR=0.903) side.
There was an association between coexisting NSD with bilateral CB and increased incidence of chronic bilateral maxillary sinusitis (p=0.040; OR=2.326).
Maxillary sinusitis was significantly more common among patients aged between 45 and 54 years (p=0.028; OR=3.213). Patients in age group between 18 and 24 years are less prone to inflammation of the maxillary sinuses (p=0.004; OR=0.242).
Discussion
To maximize the reliability of our study, we performed an independent assessment of sinusitis signs in CT examinations [20]. It is a worldwide acknowledged method proven to have higher sensitivity and accuracy in diagnostics of inflammation of paranasal sinuses in comparison to X-ray imaging, which is a rather obsolete diagnostic tool for this entity [21,22].
According to some authors, sinus volume estimation is simple and constitutes the most important index of sinus evaluation; it could be also used as abnormality indicator if a normative sinus size scale was available [23]. To estimate the sinus volume precisely, a time-consuming manual measurement on coronal slices was performed, similar to the one suggested by Pirner et al. [24] According to our experience and literature, an automatic measurement often gives an inexact result (for example it does not include the mucosa or exceeds the bony walls of the sinus) [24–26]. Only few other authors used similar measurement techniques [24,27], while others used automatic measurements [5,18,28]. Emirzeouglu used a different method – square grid test systems with six different point densities combined with the Cavalieri principle [23,29]. This method is based on point-counting systems superimposed randomly in the entire area of imaging at all intersections and then the final volume is estimated basing on an appropriate formula. The fact that two independent researchers assessed the volume and the final measurement was the mean value from their calculations additionally improves the accuracy of our study. Furthermore, one of the exclusion criteria in our work was the age under 18 years, since the osseous development of sinuses and osteomeatial complex is already finished at that age [30]. Additionally, there is no nasal cycle in maxillary sinuses (periodic changes of mucosal volume, alternating from side to side) so the study of CT examinations of the sinuses remains objective [31].
The study group in our research is relatively numerous in comparison to other studies, in which samples were rather small (Figure 6). Only two other studies based on larger samples.
Figure 6.
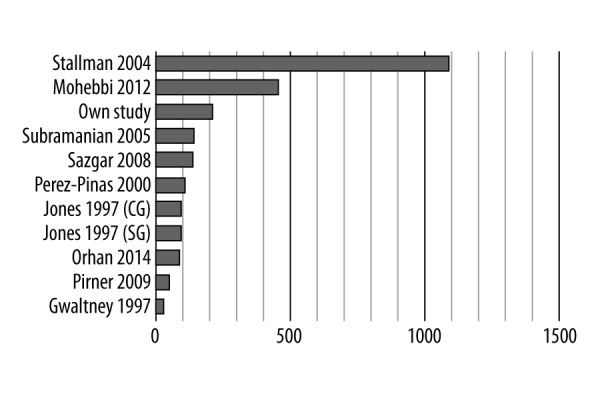
Number of subjects included in similar studies; CG – control group; SG – study group.
Volumes of sinuses estimated in our study are consistent with measurements performed by other authors (Figure 7).
Figure 7.
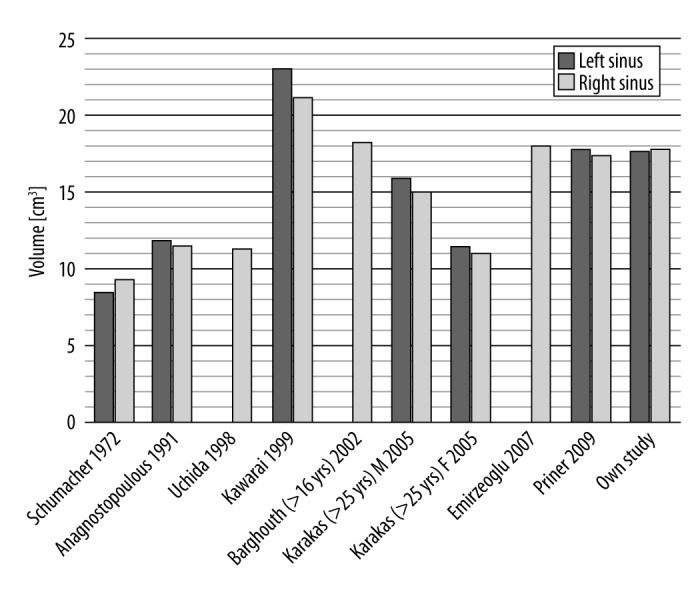
Comparison of volumes of maxillary sinuses between various authors; M – males; F – females.
Our result concerning statistically significant gender differences in size of maxillary sinuses confirms the findings of Ermizeoglu, Uchida and Karakas, who concordantly claim that males develop larger sinuses than females [23,26,32]. In contrary to these results, some studies did not report any important difference [24,33].
NSD is an object of interest of many researchers [5,9,34–36]. One of the most interesting aspects of our study is that almost 80% of the sample had a deviated nasal septum. This provokes a discussion whether NSD is really a pathology or, in fact, a regular anatomical variant of this structure. The incidence of NSD varies among other studies between 9% and 63% of the population (Figure 3). However, all of the imaging data that we evaluated were taken from patients who were previously referred to a CT examination because of some clinical problems connected with the sinonasal region. As a result, the incidence of NSD and CB might be slightly elevated.
Our results concerning CB are within the range of values obtained by other researchers, as shown in Figure 8. However, the definitions of CB were not consistent among the mentioned studies. We used descriptions concordant with the one used in the Stallman’s et al. study [9]. In the past, the presence of any pneumatised structure in the area of osteomeatial complex was called CB [24,37,38], while today, scientists use a more precise definition, as the one used by us [8,9,19].
Figure 8.
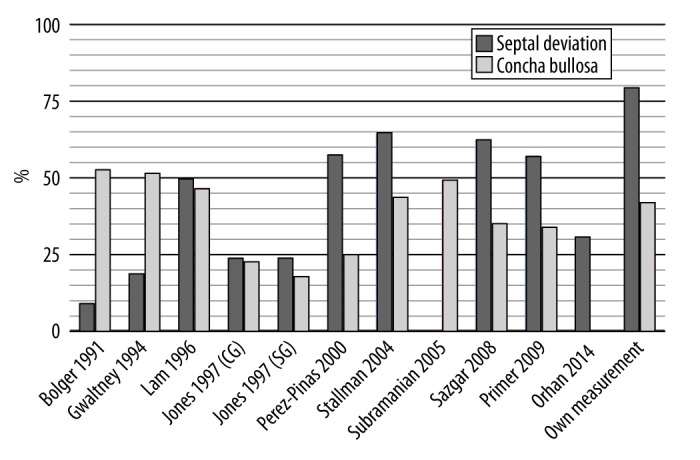
Incidence of nasal septal deviation and concha bullosa in comparison to other authors.
It is being suggested that the space created by the concave part of the nasal septum in cases with its abnormalities provide conditions for pneumatisation of the middle turbinate [37]. There is also a thesis that abgrowing concha bullosa (creating a mass effect) pushes the septum in the opposite direction [9]. Our observations, as well as those by Stallman et al., confirm these statements [9]. However, there are also authors whose results deny this relationship [39,40].
We chose the above mentioned anatomical variants because of their potential great clinical significance [31,39,41]. Similarly to our study, Calhoun suggested a possible relationship between CB or NSD and chronic rhinosinusitis [42]. According to Orlandi [43], NSD as well as other possible factors (for example an intrinsic defect in the mucociliary clearance; anatomic narrowing of the frontal recess or tortuous passageways) may cause rhinosinusitis. On the other hand, Hamdan et al. did not prove any link between NSD and chronic rhinosinusitis [44]. Jones in his work did not find any statistically significant evidence that either anatomical variants narrowing the osteomeatial complex, or NSD influence the prevalence of maxillary sinusitis [45]. Kennedy suggested that such anatomical variations are potentially predisposing, but they are not etiological factors of chronic rhinosinusitis [46].
We revealed a lower risk of unilateral maxillary sinusitis in patients with NSD. In literature, we did not find any similar results. On the other hand, Matsumoto et al. suggested that the most frequent cause of unilateral maxillary sinusitis is odontogenic infection, as 72.6% of unilateral maxillary sinusitis cases in his study were caused by this reason [47]. Contrary to these results, Shin suggested that NSD might have an influence on the development of unilateral sinusitis [36].
In our study, the presence of anatomical variants, such as NSD and CB, did not influence the left-right asymmetry of the maxillary sinuses, similarly to the study by Demir et al. [48]. However, there seems to be a connection between larger volume of the maxillary sinuses and symmetrically disturbed ventilation caused by bilateral CB. A hypothesis can be developed that poor ventilation of both sinuses contributes to their intensified growth. These results are contradictory to the conclusions drawn by other authors, who observed an asymmetry in maxillary sinus volumes – larger sinus was located contralaterally to the direction of NSD [5,18].
A similar study based on the magnetic resonance imaging (MRI) data should be performed, as the MRI examination shows the mucosa perfectly and is better than CT for recognition of the cause of chronic maxillary sinusitis [49]. A study based on a sample of paediatric patients should be also conducted, as rhinosinusitis is a common disease in childhood [50,51].
As well as the anterior ethmoid and frontal sinuses, the maxillary sinus drains through the ostiomeatial unit, which is a relatively narrow drainage pathway. The obstruction of the sinus ostium often occurs in patients with acute and chronic rhinosinusitis [52]. Therefore, further research on ethmoid, sphenoid and frontal sinusitis should be conducted to check whether other sinuses are also influenced by the same factors that we investigated in this study. Nowadays, with the fast development of body imaging techniques, it seems that more accurate high-quality CT scanners might be available in the future. It may help in precise visualisation of complex structures of sinuses and nasal cavity.
It seems that patients with chronic rhinosinusitis should undergo a more complex evaluation of NSD. Additionally, screening for NSD and CB presence should be possibly performed to prevent the development of maxillary sinusitis, especially in patients with cystic fibrosis, immunodeficiency, and immunosuppression, who are particularly prone to severe, life-threatening orbital (e.g. permanent blindness, subperiosteal abscess, orbital abscess) and intracranial (e.g. cavernous sinus thrombosis, subdural empyema, epidural empyema, cerebritis, brain abscess, and meningitis) complications [22,53]. Various screening tools can be used: physical examination, anterior rhinoscopy, nasal endoscopy, or imaging techniques [54]. Probably surgical correction of the septum should be planned more often.
The approach to septoplasty in patients with sinusitis is a disputable issue. Some authors claim the illness does not affect the quality of life [55]. Baumann et al. emphasized that most of the septoplasties are successful but there is a relevant number of patients whose quality of life decreased thereafter [56].
We support the thesis of Bansal et al. that sinus CT before Computer-Assisted Functional Endoscopic Sinus Surgery (C-A FESS) helps the surgeon in preoperative surgical planning and subsequently in avoiding complications during operation [57]. Gunbey also recommended performing preoperative paranasal sinus CTs in patients with chronic sinusitis in whom septoplasty is planned [58].
Conclusions
The main conclusions drawn from our study are:
Neither NSD nor CB has an influence on asymmetric development of maxillary sinuses
Bilateral CB is linked with symmetrically larger volumes of both maxillary sinuses
NSD is connected with the increased incidence of bilateral maxillary sinusitis
CB seems to be irrelevant for the prevalence of maxillary sinusitis.
The side of CB has an impact on contralateral direction of NSD.
Acknowledgements
We would like to express our gratitude to Associate prof. Robert Chrzan, MD, PhD for content-related help. Without his invaluable guidance our work would be uncompleted.
References
- 1.Onodi A, Thomson SC. The anatomy of the nasal cavity and its accessory sinuses: An atlas for practitioners and students. J Anat Physiol. 1895;29( Pt 3):471. [Google Scholar]
- 2.Vaid S, Vaid N. Normal anatomy and anatomic variants of the paranasal sinuses on computed tomography. Neuroimaging Clin N Am. 2015;25(4):527–48. doi: 10.1016/j.nic.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Wang RG, Jiang SC, Gu R. The cartilaginous nasal capsule and embryonic development of human paranasal sinuses. J Otolaryngol. 1994;23(4):239–43. [PubMed] [Google Scholar]
- 4.Marquez S, Lawson W, et al. Anatomy of the nasal accessory sinuses. In: Wackym PA, Rice D, Schaefer SD, editors. Minimally invasive surgery of the head, neck, and cranial base. Lippincott; 2002. pp. 153–93. [Google Scholar]
- 5.Kapusuz Gencer Z, Ozkırış M, Okur A, et al. The effect of nasal septal deviation on maxillary sinus volumes and development of maxillary sinusitis. Eur Arch Otorhinolaryngol. 2013;270(12):3069–73. doi: 10.1007/s00405-013-2435-y. [DOI] [PubMed] [Google Scholar]
- 6.Klein JC. Nasal respiratory function and craniofacial growth. Arch Otolaryngol Head Neck Surg. 1986;112(8):843–49. doi: 10.1001/archotol.1986.03780080043009. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Song SW, Cho JH, et al. Comparative study of the pneumatization of the mastoid air cells and paranasal sinuses using three-dimensional reconstruction of computed tomography scans. Surg Radiol Anat. 2010;32(6):593–99. doi: 10.1007/s00276-009-0618-4. [DOI] [PubMed] [Google Scholar]
- 8.Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope. 1991;101:56–64. doi: 10.1288/00005537-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Stallman JS, Lobo JN, Som PM. The incidence of concha bullosa and its relationship to nasal septal deviation and paranasal sinus disease. Am J Neuroradiol. 2004;25:1613–18. [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons DS, Wald ER. Otitis media and sinusitis: similar diseases. Otolaryngol Clin North Am. 1996;29:11–25. [PubMed] [Google Scholar]
- 11.Subramanian S, Lekhraj Rampal GR, Wong EF, et al. Concha bullosa in chronic sinusitis. Med J Malaysia. 2005;60(5):535–39. [PubMed] [Google Scholar]
- 12.Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe – an underestimated disease. A GA2LEN study. Allergy. 2011;66(9):1216–23. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 13.Campos CA, Dolci EL, Silva Ld, et al. Osteitis and mucosal inflammation in a rabbit model of sinusitis. Braz J Otorhinolaryngol. 2015;81(3):312–20. doi: 10.1016/j.bjorl.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilos DL. Chronic rhinosinusitis: Epidemiology and medical management. J Allergy Clin Immunol. 2011;128(4):693–707. doi: 10.1016/j.jaci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Oudinet JP, Méline J, Chełmicki W, et al. Towards a multidisciplinary and integrated strategy in the assessment of adverse health effects related to air pollution: The case study of Cracow (Poland) and asthma. Environ Pollut. 2006;143(2):278–84. doi: 10.1016/j.envpol.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Rak KM, Newell JD, Yakes WF, et al. Paranasal sinuses on MR images of the brain: Significance of mucosal thickening. Am J Roentgenol. 1991;156(2):381–84. doi: 10.2214/ajr.156.2.1898819. [DOI] [PubMed] [Google Scholar]
- 17.Harnsberger HR, et al. Diagnostic Imaging Head and Neck. 1st ed. Lippincott Amirsys Inc.; 2004. [Google Scholar]
- 18.Orhan I, Ormeci T, Aydin S, et al. Morphometric analysis of the maxillary sinus in patients with nasal septum deviation. Eur Arch Otorhinolaryngol. 2014;271:727–32. doi: 10.1007/s00405-013-2617-7. [DOI] [PubMed] [Google Scholar]
- 19.Hatipoglu HG, Cetin MA, Yuksel E. Concha bullosa types: Their relationship with sinusitis, osteomeatial and frontal recess disease. Diagn Intervent Radiol. 2005;11:145–49. [PubMed] [Google Scholar]
- 20.Boari L, de Castro NP., Júnior Diagnosis of chronic rhinosinusitis in patients with cystic fibrosis: Correlation between anamnesis, nasal endoscopy and computed tomography. Braz J Otorhinolaryngol. 2005;71(6):705–10. doi: 10.1016/S1808-8694(15)31236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharyya N, Fried MP. The accuracy of computed tomography in the diagnosis of chronic rhinosinusitis. Laryngoscope. 2003;113(1):125–29. doi: 10.1097/00005537-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Younis RT, Anand VK, Davidson B. The role of computed tomography and magnetic resonance imaging in patients with sinusitis with complications. Laryngoscope. 2002;112(2):224–29. doi: 10.1097/00005537-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Emirzeoglu M, Sahin B, Bilgic S, et al. Volumetric evaluation of the paranasal sinuses in normal subjects using computer tomography images: A stereological study. Auris Nasus Larynx. 2007;34(2):191–95. doi: 10.1016/j.anl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Pirner S, Tingelhoff K, Wagner I, et al. CT-based manual segmentation and evaluation of paranasal sinuses. Eur Arch Otorhinolaryngol. 2009;266:507–18. doi: 10.1007/s00405-008-0777-7. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher GH, Heyne HJ, Fanghanel R. Anatomy of the human paranasal sinuses. 2. Volumetric measurement. Anat Anz. 1972;130(1):143–57. [PubMed] [Google Scholar]
- 26.Uchida Y, Goto M, Katsuki T, Akiyoshi T. A cadaveric study of maxillary sinus size as an aid in bone grafting of the maxillary sinus floor. J Oral Maxillofac Surg. 1998;56(10):1158–63. doi: 10.1016/s0278-2391(98)90761-3. [DOI] [PubMed] [Google Scholar]
- 27.Strauss G, Hofer M, Grunert R, et al. [Manipulator assisted endoscope guidance in functional endoscopic sinus surgery: Proof of concept]. HNO. 2007;55(3):177–84. doi: 10.1007/s00106-006-1434-3. [in German] [DOI] [PubMed] [Google Scholar]
- 28.Lee DH, Shin JH, Lee DC. Three-dimensional morphometric analysis of paranasal sinuses and mastoid air cell system using computed tomography in pediatric population. Int J Pediatr Otorhinolaryngol. 2012;76(11):1642–46. doi: 10.1016/j.ijporl.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 29.Emirzeoglu M, Sahin B, Selcuk MB, Kaplan S. The effects of section thickness on the estimation of live volume by the Cavalieri principle using computed tomography images. Eur J Radiol. 2005;56:391–97. doi: 10.1016/j.ejrad.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Lorkiewicz-Muszyńska D, Kociemba W, Rewekant A, et al. Development of the maxillary sinus from birth to age 18. Postnatal growth pattern. Int J Pediatr Otorhinolaryngol. 2015;79(9):1393–400. doi: 10.1016/j.ijporl.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Zinreich J. Imaging of inflammatory sinus disease. Otolaryngol Clin North Am. 1993;26(4):535–47. [PubMed] [Google Scholar]
- 32.Karakas S, Kavakli A. Morphometric examination of paranasal sinuses and mastoid air cells using computed tomography. Ann Saudi Med. 2005;25:1–6. doi: 10.5144/0256-4947.2005.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez Fernandez JM, Anta Escuredo JA, Sanchez Del Rey A, Montoya FS. Morphometric study of the paranasal sinuses in normal and pathological conditions. Acta Otolaryngol. 2000;120:273–78. doi: 10.1080/000164800750001080. [DOI] [PubMed] [Google Scholar]
- 34.Elahi MM, Frenkiel S, Fageeh N. Paraseptal structural changes and chronic sinus disease in relation to the deviated septum. J Otolaryngol. 1997;26(4):236–40. [PubMed] [Google Scholar]
- 35.Seltzer AP. A new instrument for correcting the obstructing deviated septum. J Natl Med Assoc. 1963;55:409–10. [PMC free article] [PubMed] [Google Scholar]
- 36.Shin HS. Clinical significance of unilateral sinusitis. J Korean Med Sci. 1986;1:69–74. doi: 10.3346/jkms.1986.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sazgar AA, Massah J, Sadeghi M, et al. The incidence of concha bullosa and the correlation with nasal septal deviation. B-ENT. 2008;4:87–91. [PubMed] [Google Scholar]
- 38.Smith KD, Edwards PC, Saini TS, Norton NS. The prevalence of concha bullosa and nasal septal deviation and their relationship to maxillary sinusitis by volumetric tomography. Int J Dent. 2010;2010 doi: 10.1155/2010/404982. pii: 404982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd GAS. CT of the paranasal sinuses: Study of a control series in relation to endoscopic sinus surgery. J Laryngol Otol. 1990;104(6):477–81. doi: 10.1017/s0022215100112927. [DOI] [PubMed] [Google Scholar]
- 40.Javadrashid R, Naderpour M, Asghari S, et al. Concha bullosa, nasal septal deviation and paranasal sinusitis; A computed tomographic evaluation. B-ENT. 2014;10(4):291–98. [PubMed] [Google Scholar]
- 41.Stammberger H, Wolf G. Headaches and sinus disease: The endoscopic approach. Ann Otol Rhinol Laryngol Suppl. 1988;134:3–23. doi: 10.1177/00034894880970s501. [DOI] [PubMed] [Google Scholar]
- 42.Calhoun KH, Waggenspack GA, Simpson CB, et al. CT evaluation of the paranasal sinuses in symptomatic and asymptomatic populations. Otolaryngol Head Neck Surg. 1991;104(4):480–83. doi: 10.1177/019459989110400409. [DOI] [PubMed] [Google Scholar]
- 43.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 44.Hamdan AL, Bizri AR, Jaber M, et al. Nasoseptal variation in relation to sinusitis: A computerized tomographic evaluation. J Med Liban. 2001;49:2–5. [PubMed] [Google Scholar]
- 45.Jones NS, Strobl A, Holland I. A study of the CT findings in 100 patients with rhinosinusitis and 100 controls. Clin Otolaryngol Allied Sci. 1997;22(1):47–51. doi: 10.1046/j.1365-2273.1997.00862.x. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy DW. Functional endoscopic sinus surgery: Concepts, surgical indications, and instrumentation. In: Kennedy DW, Zinreich J, editors. Diseases of the sinuses Diagnoses and endoscopic management. Elsevier; 2000. pp. 197–210. [Google Scholar]
- 47.Matsumoto Y, Ikeda T, Yokoi H, Kohno N. Association between odontogenic infections and unilateral sinus opacification. Auris Nasus Larynx. 2015;42(4):288–93. doi: 10.1016/j.anl.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Demir UL, Akca ME, Ozpar R, et al. Anatomical correlation between existence of concha bullosa and maxillary sinus volume. Surg Radiol Anat. 2015;37(9):1093–98. doi: 10.1007/s00276-015-1459-y. [DOI] [PubMed] [Google Scholar]
- 49.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wald ER. Sinusitis in children. N Engl J Med. 1992;326(5):319–23. doi: 10.1056/NEJM199201303260507. [DOI] [PubMed] [Google Scholar]
- 51.Steele RW. Rhinosinusitis in children. Curr Allergy Asthma Rep. 2006;6(6):508–12. doi: 10.1007/s11882-006-0029-0. [DOI] [PubMed] [Google Scholar]
- 52.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S103–15. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 53.Dankbaar JW, van Bemme AJM, Pameijer FA. Imaging findings of the orbital and intracranial complications of acute bacterial rhinosinusitis. Insights Imaging. 2015;6:509–18. doi: 10.1007/s13244-015-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mamikoglu B, Houser S, Akbar I, et al. Acoustic rhinometry and computed tomography scans for the diagnosis of nasal septal deviation, with clinical correlation. Otolaryngol Head Neck Surg. 2000;123(1):61–68. doi: 10.1067/mhn.2000.105255. [DOI] [PubMed] [Google Scholar]
- 55.Rudmik L, Mace J, Ferguson BJ, Smith TL. Concurrent septoplasty during endoscopic sinus surgery for chronic rhinosinusitis: Does it confound outcomes assessment? Laryngoscope. 2011;121(12):2679–83. doi: 10.1002/lary.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baumann I. Quality of life before and after septoplasty and rhinoplasty. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2010;9:Doc06. doi: 10.3205/cto000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bansal M. Computer-assisted functional endoscopic sinus surgery (c-a fess) – a review. Indian J Otolaryngol Head Neck Surg. 2000;52(3):311–14. doi: 10.1007/BF03006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Günbey E, Günbey HP, Uygun S, et al. Is preoperative paranasal sinus computed tomography necessary for every patient undergoing septoplasty? Int Forum Allergy Rhinol. 2015;5(9):839–45. doi: 10.1002/alr.21545. [DOI] [PubMed] [Google Scholar]


