Abstract
Current observed as well as projected changes in biodiversity are the result of multiple interacting factors, with land use and climate change often marked as most important drivers. We aimed to disentangle the separate impacts of these two for sets of vascular plant, bird, butterfly and dragonfly species listed as characteristic for European dry grasslands and wetlands, two habitats of high and threatened biodiversity. We combined articulations of the four frequently used SRES climate scenarios and associated land use change projections for 2030, and assessed their impact on population trends in species (i.e. whether they would probably be declining, stable or increasing). We used the BIOSCORE database tool, which allows assessment of the effects of a range of environmental pressures including climate change as well as land use change. We updated the species lists included in this tool for our two habitat types. We projected species change for two spatial scales: the EU27 covering most of Europe, and the more restricted biogeographic region of ‘Continental Europe’. Other environmental pressures modelled for the four scenarios than land use and climate change generally did not explain a significant part of the variance in species richness change. Changes in characteristic bird and dragonfly species were least pronounced. Land use change was the most important driver for vascular plants in both habitats and spatial scales, leading to a decline in 50–100% of the species included, whereas climate change was more important for wetland dragonflies and birds (40–50 %). Patterns of species decline were similar in continental Europe and the EU27 for wetlands but differed for dry grasslands, where a substantially lower proportion of butterflies and birds declined in continental Europe, and 50 % of bird species increased, probably linked to a projected increase in semi-natural vegetation. In line with the literature using climate envelope models, we found little divergence among the four scenarios. Our findings suggest targeted policies depending on habitat and species group. These are, for dry grasslands, to reduce land use change or its effects and to enhance connectivity, and for wetlands to mitigate climate change effects.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-016-0840-3) contains supplementary material, which is available to authorized users.
Keywords: Climate envelope modelling, Dry grasslands, Habitat connectivity, Land use change, Species sensitivity database, SRES scenario articulation, Wetlands
Introduction
The effects of ongoing and anticipated climate change on European biodiversity are well studied (e.g. Harrison et al. 2006; Paterson et al. 2008; Huntley et al. 2008, 2010; Araújo et al. 2011; Fronzek et al. 2012; Jaeschke et al. 2013). A growing consensus converges on the following points: (a) Within distribution ranges, currently observed phenological changes are already substantial (Menzel et al. 2006). (b) Current distribution ranges of many species are observed to move northwards (up to several kilometres per year, e.g. Hickling et al. 2006; Campbell et al. 2009), although many species lag behind the moving isotherms (Devictor et al. 2012). European biodiversity conservation policy recognizes the importance of climate change (EEA 2012). Specific adaptation measures are beginning to be designed and evaluated (Van Teeffelen et al. 2015). This is a pressing issue, since bioclimatic envelope modelling (cf. Araújo and Peterson 2012) suggests that in the current network of conservation areas in Europe about two-thirds of the angiosperm and terrestrial vertebrate species concerned would lose suitable habitat by 2080 (Araújo et al. 2011). Similar dramatic changes were projected by Thuiller et al. (2005: 27–43 % of all European angiosperm species would be lost by 2080) and Settele et al. (2008: 70 % of butterflies lose more than half of their climatologically suitable range by 2080). Thus, protecting key ‘retention areas’ for conservation, and enhancing connectivity among protected habitats are important policy challenges (Cliquet et al. 2009; Dodd et al. 2010; Van Teeffelen et al. 2015).
However, Beale et al. (2008) suggest that land use change and biotic interactions exceed the effects of climate change as projected by climate envelope models (i.e. since these models did not perform better than properly designed random null models with current spatial autocorrelation; see also Suttle et al. (2007) and BISE (2016). Projected trajectories of future land use change, however, are highly divergent, depending on the articulation of world economic development as well as changing socio-cultural constellations (Lorenzoni et al. 2000; Busch 2006). This divergence is generally grasped in scenarios, and the Special Report on Emission Scenarios (SRES) scenarios have become a benchmark set of scenarios for global change modelling (Lorenzoni et al. 2000; Berkhout et al. 2002; Busch 2006), and are the fundament for the next generation of climate change scenarios (Moss et al. 2010; Van Vuuren and Carter 2014).
Where species distribution modelling studies included socio-economic aspects, this has generally been restricted to the climatic consequences of socio-economic developments, such as differences in temperature increase and net water availability (Araújo et al. 2011; Hickler et al. 2012). The parallel changes in land use and human occupation that go along with such divergent scenarios (e.g. Busch 2006; Verboom et al. 2007; Verburg et al. 2008; Spangenberg et al. 2012), or the potential of successfully implemented near-future mitigation measures (e.g. reforestation, Fletcher et al. 2010; Hellmann and Verburg 2010; Dale et al. 2011; Pawson et al. 2013), have generally been ignored in biodiversity modelling (but see Verboom et al. 2007; Titeux et al. 2016). Both Olivier and Morecroft (2014) and De Chazal and Rounsevell (2009) argue that understanding the mechanisms underlying the interactive effects of climate change and land use change would overcome attribution errors in interpretation and help in a more robust design of adaptive conservation measures. All this suggests that the potentially interacting effects of climate and land use change should be studied in concert.
Quantifying the magnitude of this climate versus land use change interaction in Europe is hampered by the high geographical variability in both biodiversity (Anderson and Ferree 2010) and land use patterns (Verburg et al. 2008; Kleijn et al. 2009). Also, foreseen climate change differs greatly in intensity across Europe (Christensen et al. 2007; Rajczak et al. 2013); hence, biodiversity responses will not be uniform (Barbet-Massin et al. 2012). We chose to address the issue of high geographic variability in biodiversity by focusing on specific, comparatively homogeneous habitats: dry grasslands and wetlands. The issue of highly variable land use patterns was covered by using the highest resolution land use projection data available for the SRES scenarios (i.e. 1 km2, from Verburg et al. 2008). Martin et al. (2013) argue for a finer spatial resolution than the 5 km they used to be able to track habitat suitability for a wetland specialist butterfly. We addressed geographic variation in the projected intensity of climate change by comparing responses across the whole of Europe with those from a more homogeneous biogeographic region, Continental Europe (Metzger et al. 2005; Verboom et al. 2007). Barbet-Massin et al. (2012) similarly coupled land use and three SRES scenarios to study their effects on European birds, but did not separate the effects of climate and land use. They concluded that for 70 % of European birds the range would decrease due to a projected northward shift (median 335 km by 2050).
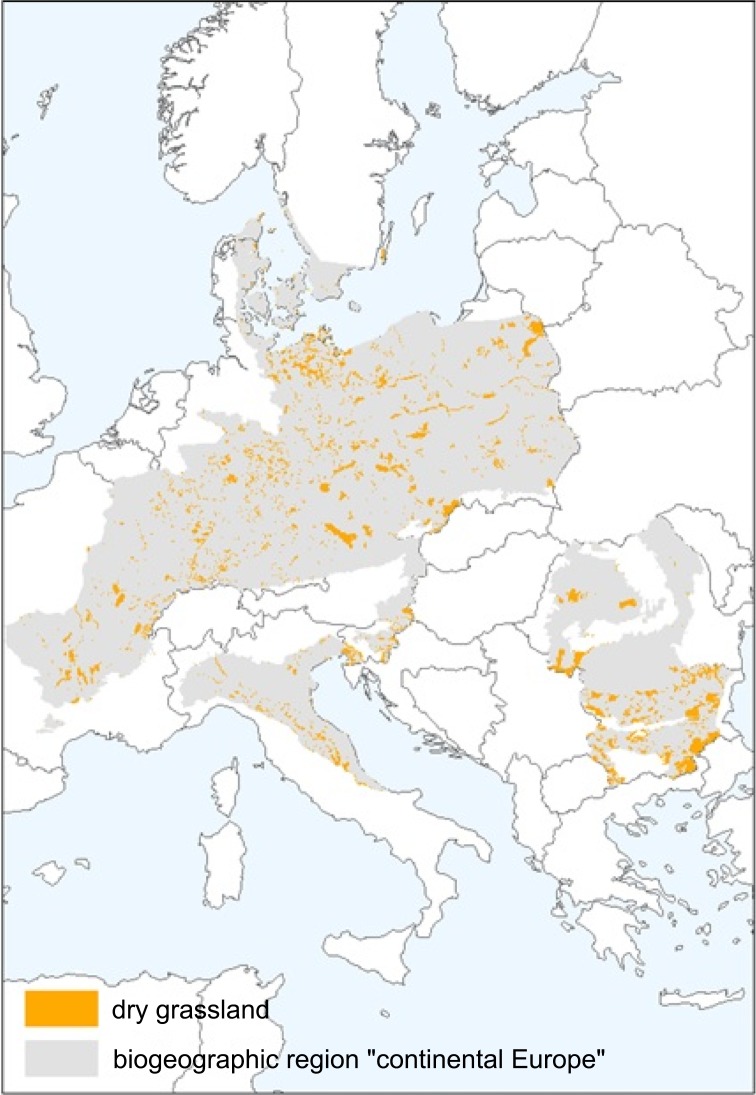
We focused on dry grasslands and wetlands, since these habitats are both well studied and a European conservation target. They represent increasingly threatened habitats that once were widespread and common across Europe. Both habitat types are subject to pronounced decline and fragmentation (cf. Fig. 1). They are considered particularly rich in angiosperms, insects and small vertebrates of which currently many are red-listed (Poschlod and WallisDeVries 2002; Veen et al. 2009; Čížková et al. 2013; Heubes et al. 2011). Despite comparable physiognomy, these habitat types differ in species composition and taxonomic richness (Walker et al. 2004; Dengler 2005).
Fig. 1.
Distribution of dry grasslands across continental Europe. Data derived from the NATURA2000 database of the EEA from which “Dry grassland, steppes” was selected in, and, in the case of Poland and Romania the habitat classes 6 110, 6120 and 6210 (i.e. calcareous grasslands)
We used the BIOSCORE tool, a database of species sensitivity, to a range of environmental pressures (including climate change) and habitat suitability for a wide range of European species (Delbaere et al. 2009; Eggers et al. 2009; Louette et al. 2010; see below).
Specifically, we asked the following questions:
What are projected responses in species richness to climate change and land use change for the period up to 2030, and can the separate effects be disentangled?
To what degree are species responses similar across the two studied habitat types of high conservation value?
Does the regional restriction to Continental Europe lead to marked differences in species responses, compared to an analysis covering the whole of Europe (here represented by 27 European countries, the so-called EU27, because of data availability)?
Materials and methods
The BIOSCORE tool
BIOSCORE is a European biodiversity impact assessment tool (full presentation in Delbaere et al. 2009; applications in Eggers et al. 2009; Louette et al. 2010; www.bioscore.eu). It combines a database on species’ sensitivities to a range of environmental pressures with habitat suitability using CORINE 2000 level 3 land cover types (Davies et al. 2004). It has a user interface that allows changing the impact of these pressures with a five-point Likert scale, and has the possibility to generate outcomes for different biogeographical breakdowns of Europe. User defined combinations of changes in (policy-related) environmental pressures are translated into impacts on a large number of species in nine species groups (birds, mammals, amphibians, reptiles, fish, butterflies, dragonflies, aquatic macro-invertebrates and vascular plants).
BIOSCORE includes expert-based sensitivity scores for each species and environmental pressure. These environmental pressures are labelled here ‘input variable categories’, and are grouped by the BIOSCORE expert group (Delbaere et al. 2009) into pollution, water related changes, climate change, disturbance regimes, direct pressures, species interaction and management.
The BIOSCORE sensitivity scores characterize a species’ response to a relative increase or decrease of the environmental pressure and are thus representing a simplified species’ response curve. The impact of a change in an environmental pressure category on a species is derived from a combination of the species’ sensitivity score and the (projected) magnitude of change in that environmental pressure. Sensitivity is linked to the magnitude and direction of change. Species can respond positively (=population increase), negatively (=population decrease) or show no response (=stable).
The environmental pressures considered differ between species groups (cf. Delbaere et al. 2009). Land use serves as a practical indicator for habitat suitability by giving each CORINE land cover class a score expressing the probability of occurrence in this land cover type. Species respond to area changes of one land cover type according to this habitat’s suitability score, and the effects of land use change can thus be traced. The simplified approach to sensitivity allows coverage of large numbers of species for which comparatively little detailed information is available (Delbaere et al. 2009).The BIOSCORE tool provides output such as tables or maps listing the number of species in a taxonomic group that will probably decline, remain stable or increase under the specified regime under focus. Next to the full effect of a combination it also tracks the separate effect of seven major input variable categories and of land use change if that is specified before the model run. It does not project extinction but indicates a probable trend.
Species groups used
Our analysis has been limited to three species groups in each habitat type: vascular plants, birds, dragonflies (wetlands) and butterflies (dry grasslands). In BIOSCORE, these groups contain a sufficient number of species characteristic for the two selected habitat types. These species are well studied, and their distribution is well known. We used two individual databases: one for dry grassland species and the other one for wetland species.
Characteristic dry grassland species were taken to be those for which the BIOSCORE database indicated a medium-to-high association with the CORINE land cover classes 3.2.1 (“Natural grasslands”) or 3.2.3 (“Sclerophyllous vegetation”). Wetland species were those with a medium or high association with CORINE classes 4.1.1 (“Inland marshes”), 4.1.2 (“Peat bogs”), 5.1.1 (“Watercourses”) and 5.1.2 (“Water bodies”). Preliminary analyses revealed gaps in the BIOSCORE database for species lists as well as habitat suitability scores and pressure sensitivity scores for particular species groups and regions. Therefore, Hellmann, Vermaat and Alkemade revised and extended species lists of characteristic birds, butterflies, dragonflies and angiosperms for wetlands and dry grasslands, using expert judgment and published literature. Our revision is based on data in Van Swaay et al. (2006) and LaFranchis (2004) for butterflies, Svensson and Grant (2013) for birds, Dijkstra and Lewington (2006) for dragonflies, and Van der Meijden (2005) for plants. For dry grasslands, this filtering procedure retained 41 vascular plant species, 28 butterfly species and 24 and 12 bird species for Europe and continental Europe, respectively. For wetlands, we retained 53 and 49 species of vascular plants, 102 and 51 species of dragonflies and 50 and 12 species of birds for Europe and continental Europe, respectively. Only four species of butterfly were associated to wetlands in the database; hence, we decided to exclude these from the analysis. Occurrence in continental Europe is contained in the BIOSCORE database, as it is one of Europe’s biogeographical regions. The revised species lists are obtainable as excel files from the authors (FAH or JEV).
Climate change sensitivity of species as implemented in BIOSCORE
The BIOSCORE database was adjusted in two ways to better reflect the current state of understanding on how species respond to climate change. First, we adjusted the translation of species’ climate sensitivity into population responses (Table 1). Species were allocated to one of four responses: species categorized as ‘not vulnerable’ to climate change are not expected to respond to any (reasonable) magnitude of climatic change because their (European) distributions are not primarily determined by climatic factors. Species categorized as having ‘Low’ climate sensitivity have a negative response (i.e. decrease) to only severe climatic changes. Species categorized with a ‘Medium’ or ‘High’ climate sensitivity also respond to moderate or limited climatic changes. Second, individual species’ sensitivity to climate change was reviewed, and adjusted following expert knowledge and latest research insights. This procedure is documented in Supplementary material S1. Since positive climate sensitivity is uncertain, we lumped the categories ‘stable’ and ‘increase’ into ‘stable’.
Table 1.
Modelled population responses of species with different sensitivities for climate change to different levels of climate change in the BIOSCORE database. Left rows show species’ climate sensitivity, and top columns show the degree of climate change
| Climate change: species’ climate sensitivity: | No | limited | moderate | severe |
|---|---|---|---|---|
| Not sensitive | Stable | Stable | Stable | Stable |
| Low | Stable | Stable | Stable | Decline |
| Medium | Stable | Stable | Decline | Decline |
| High | Stable | Decline | Decline | Decline |
Scenarios
We applied the four SRES scenarios (A1, A2, B1 and B2), which describe four divergent outlooks on global socio-economic development and their climate change impacts (Lorenzoni et al. 2000). They provide broad storylines, in which each scenario corresponds to an anticipated set of mutually consistent societal changes with corresponding climate change. Following Berkhout et al. (2002), Westhoek et al. (2006) and Spangenberg et al. (2012), we articulated the four SRES scenarios into separate qualitative storylines (Supplementary material S2). These scenario storylines offer a framework allowing us to make assumptions on socio-economic developments and land use change and make specific articulations of their consequences for regional land use and the pressure indicators available in the BIOSCORE tool (Supplementary material S2). For each scenario, the environmental pressures in BIOSCORE were set according to these assumptions (Table 2). We did a partial sensitivity analysis by successively setting the effects of continentality, eutrophication and soil moisture to zero, whilst all other settings remained as for the A1 scenario (cf. Table 2).
Table 2.
Articulation of SRES scenarios from Supplementary material S2 in terms of BIOSCORE variables for 2030. The “+” indicate an improvement, and the “−” indicate a deterioration of the driving variable or pressure with respect to biodiversity (BIOSCORE uses a five point Likert-type scale). As an example, water temperature is thought to increase most under A2, and it is also thought to lead to the highest species decline. The zero sign means input variable not adjusted
| Bioscore input variables | Scenario | |||
|---|---|---|---|---|
| A1 | A2 | B1 | B2 | |
| Pollution: | ||||
| Eutrophication | − | −− | + | + |
| Acidification | 0 | 0 | 0 | 0 |
| Salinization | 0 | 0 | 0 | 0 |
| Terrestrial pollution | − | −− | + | + |
| Water eutrophication & organic pollution | − | −− | + | + |
| Water pollution | − | − | + | + |
| Water siltation | 0 | 0 | + | + |
| Water related changes: | ||||
| Soil moisture | − | − | 0 | 0 |
| Permanent water surface | − | − | − | − |
| Temporary water availability | − | − | − | − |
| Water quantity/flow (reduced) | 0 | 0 | 0 | 0 |
| Water transparency | − | −− | 0 | 0 |
| Climate change: | ||||
| Climate change | − | − | − | − |
| Continentality | − | − | − | − |
| Temperature | − | −− | − | − |
| Water temperature | − | −− | − | − |
| Disturbance: | ||||
| Disturbance | − | − | 0 | 0 |
| Powerlines | − | 0 | − | − |
| Trampling | + | 0 | 0 | 0 |
| Direct pressures: | ||||
| Harvesting of crops | − | − | 0 | 0 |
| Hunting | 0 | 0 | 0 | 0 |
| Harvesting of fish | 0 | 0 | 0 | 0 |
| Species interaction: | ||||
| Introduction of non-native species | − | − | − | + |
| Disease organisms or parasites | 0 | 0 | 0 | 0 |
| Management: | ||||
| Amount of dead wood | + | − | + | 0 |
| Even aged forest | + | − | + | 0 |
| Young felling age of forest | + | − | + | 0 |
Land use change projections from 2000 to 2030 are available from the EURURALIS project (Verburg et al. 2008) at 1 km2 resolution for Europe (EU27 = EU25 + Norway and Switzerland, from 2007 to 2013) for each of the four SRES scenarios. Maps of these land use changes for each SRES scenario were used as input for BIOSCORE, alongside the other scenario assumptions (Table 2). Since the land use types defined in BIOSCORE do not exactly match those modelled by Verburg et al. (2008), a match-up operation was carried out (Supplementary material S3). Species distribution data in the BIOSCORE tool reflect those in ‘the late 1990s’ (Delbaere et al, 2009), and hence can be considered to correspond sufficiently with the initial year of the EURURALIS project.
Analysis of model outcomes
Our first question was addressed by comparing our BIOSCORE outcomes for the EU27 with the findings of Araújo et al. (2011). The contrast between climate change and land use change was addressed by firstly running BIOSCORE with the full scenario articulation for all seven input variable categories (Table 2), which has the full interaction, then secondly identifying the separate ‘climate change’ (one of the seven input variables) effect and thirdly ‘land use change’ effects. Question 2 was addressed by running the BIOSCORE tool with the two different species databases we had created for these two habitats, wetlands and dry grasslands. The effect of the high geographic heterogeneity of Europe (question 3) was assessed with a comparison to the more restricted biogeographical region continental Europe. Outcomes are presented in stacked bar charts as percentages of each species group that decrease, are stable or increase, and analysed with separate General Linear Model analyses of variance for each combination of 2 geographic extents × 2 habitat types times the 3 fractions (decline, stable, increase). This allowed us to test the effects of climate, land use and species group as well as the interactions between the climate versus land use contrast with species groups.
Results
Upon first visual inspection, the overall similarity in pattern among the four scenarios within each of the four geographic scale/habitat combinations is striking (Figs. 2, 3). Out of the 16 cases, only three show a distinctly different pattern. Generally, the fraction of species declining due to climate and land use together added up to the total (Figs. 2, 3). This was not the case in (a) dry grassland plants in continental Europe under the A1 scenario (Fig. 2), (b) wetland plant species in the EU27 under A2 and (c) continental wetland plants under A1 (Fig. 3). Here also increased continentality and eutrophication (environmental input variables in Table 2) were responsible for substantial species decline. Across habitats, extents and scenarios, the estimated proportion of declining species was only substantial (50–100 % when climate and land use taken together) for vascular plants. For the other species groups, the patterns were more variable: often at least half of the species will remain stable until 2030 (Figs. 2, 3). In the dry grasslands of continental Europe in contrast, characteristic birds are estimated to increase towards 2030, which may well be linked to a substantial increase in semi-natural vegetation (Supplementary material S1).
Fig. 2.
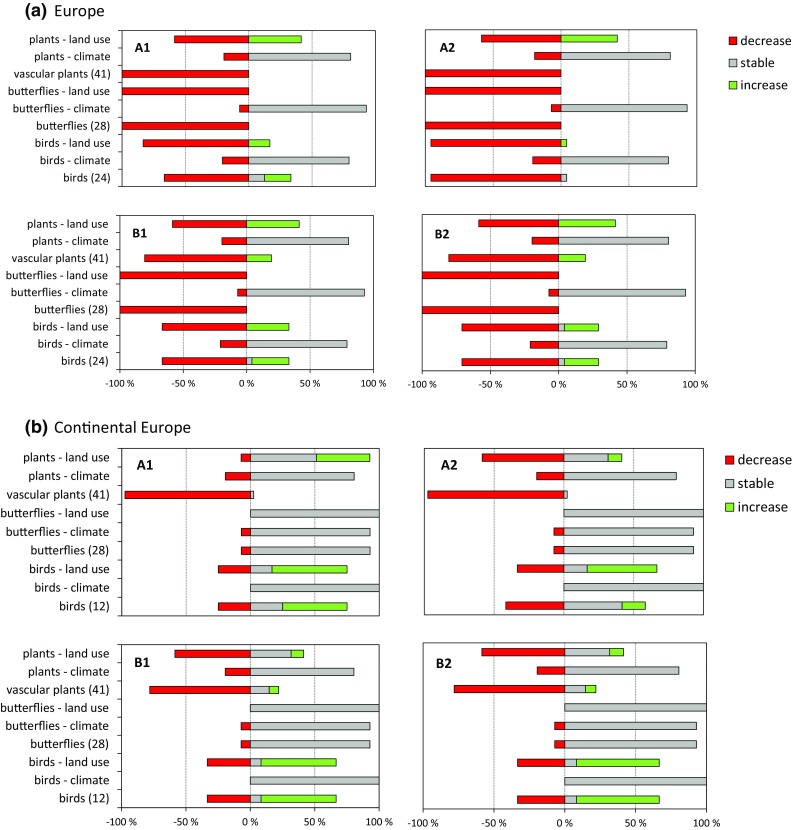
BIOSCORE outcome for dry grasslands in the EU27 (Europe) (a) and continental Europe (b). The percentage of species in a taxonomic group that is projected to decrease, remain stable or increase in occurrence; this is plotted bottom-to-top for the simultaneous effect of the full scenario articulation, for the separate effect of climate change and for the separate effect of land use change, respectively. The first label has the number of species in the species group in parentheses. The full scenario articulation for BIOSCORE is presented in Table 1. Note that we use ‘plants’ in the chart labels only for brevity’s sake, these are vascular plants. Note that in b continental Europe for vascular plants under A1, the percent declining species due to climate and land use do not add up to the total. Here increasing continentality and eutrophication also lead to substantial numbers of declining species
Fig. 3.
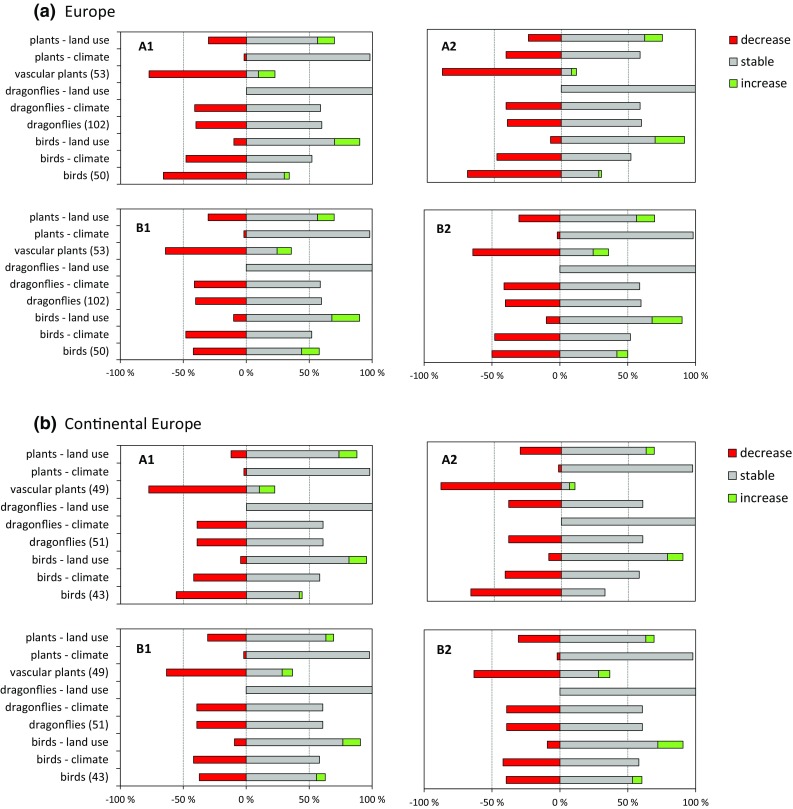
BIOSCORE outcome for wetlands. Further as Fig. 2. Note that where the percentage decline due to climate and land use does not add up to the total decline, this is due to additional effects of continentality and eutrophication
In accordance, the scenarios did not explain a significant part of the variance in our overall GLM in addition to their influence through land use and climate change (Table 3). The contrast climate versus land use explained most of the variance for all species groups in dry grasslands of the EU27, but not in the other three scale–habitat-type combinations, where the different responses among species groups, or the interaction caused most of the variation (Table 3a). This interaction and difference among species groups is clearly reflected in the estimated marginal means (Table 3b): the fraction of declining vascular plant species is mainly due to land use in all four combinations, whereas decline in wetland birds and dragonflies is coupled to climate change and continental birds and butterflies do hardly decline (Table 3; Figs. 2, 3).
Table 3.
Relative contribution of land use and climate change to variance in the fraction of species declining, remaining stable and increasing in each of the four cases modelled in BIOSCORE, respectively, dry grasslands and wetlands in the whole of Europe and continental Europe. Presented are (a) type 3 sums of squares for 4x3 (4 cases x fraction species declining, stable and increasing*) separate GLM analyses, and (b) marginal means of the fraction of species declining in a species group due to climate and land use (these correspond to the numbers presented in Figs. 2 and 3). Sums of squares are only presented when significant (mostly p < 0.001, always p < 0.05), otherwise NS is used. Degrees of freedom were 1 (climate versus land use), 2 (species groups), 2 (interaction), 18 (error) and 23 (corrected total). Bold printed are the sums of squares of factors contributing distinctly most to the total variance, and the major marginal means of proportionate species decline in a species group
| Case | (a) Type 3 sums of squares | fraction of species group | (b) Marginal means in the fraction declining due to: | ||||
|---|---|---|---|---|---|---|---|
| Declining | Stable | Increasing | Climate | Land use | |||
| Europe, dry grasslands | Climate vs Land use | 2.41 | 4.21 | 0.25 | Birds | 0.20 | 0.79 |
| Species groups | 0.09 | 0.02 | 0.17 | Butterflies | 0.07 | 1.00 | |
| Interaction | 0.30 | 0.03 | 0.17 | Vascular Plants | 0.20 | 0.59 | |
| Error | 0.05 | 0.01 | 0.05 | ||||
| Corrected total | 2.90 | 4.26 | 0.64 | ||||
| Continental, dry grasslands | Climate vs land use | 0.17 | 1.03 | 0.36 | Birds | 0.01 | 0.31 |
| Species groups | 0.34 | 0.82 | 0.33 | Butterflies | 0.07 | 0.01 | |
| Interaction | 0.17 | 0.90 | 0.33 | Vascular plants | 0.20 | 0.46 | |
| Error | 0.20 | 0.04 | 0.08 | ||||
| Corrected total | 0.89 | 2.78 | 1.11 | ||||
| Europe, wetlands | Climate vs Land use | 0.19 | 0.02 | 0.08 | Birds | 0.48 | 0.10 |
| Species groups | 0.07 | 0.18 | 0.05 | Dragonflies | 0.41 | 0.01 | |
| Interaction | 0.59 | 0.70 | 0.05 | Vascular plants | 0.02 | 0.29 | |
| Error | 0.03 | 0.03 | 0.01 | ||||
| Corrected total | 0.86 | 0.90 | 0.18 | ||||
| Continental, wetlands | Climate vs land use | NS | NS | 0.03 | Birds | 0.42 | 0.08 |
| Species groups | NS | 0.10 | 0.02 | Dragonflies | 0.29 | 0.10 | |
| Interaction | 0.36 | 0.35 | 0.02 | Vascular plants | 0.02 | 0.26 | |
| Error | 0.26 | 0.24 | 0.01 | ||||
| Corrected total | 0.73 | 0.70 | 0.08 | ||||
*The contribution of the four SRES scenarios was not estimated separately over and above ‘climate versus land use’ because of insufficient remaining degrees of freedom. In an overall, GLM with the four cases pooled the scenarios did not explain a significant part of the variance over and above ‘climate versus land use’ and species groups
The magnitude of the response of the characteristic species groups differed greatly, also between the four geographic scale–habitat combinations (Table 3b). Overall (Figs. 2, 3), most characteristic vascular plants were found to decline, and this was mainly due to land use change. Dry grassland birds and butterflies were estimated to decline at the scale of the whole EU27, but this was much less pronounced in continental Europe. Wetland birds and dragonflies declined much less, and mainly due to climate change.
A partial sensitivity analysis for dry grasslands under the A1 scenario (Table 4) suggests that the BIOSCORE variables continentality, eutrophication and soil moisture do not have any additional effect on vascular plants. In contrast, butterflies were found to be quite responsive to changes in eutrophication and soil moisture in BIOSCORE (Table 4).
Table 4.
Partial sensitivity analysis of the BIOSCORE tool. Using the A1 scenario and the continental European dry grasslands subset, the effect of three BIOSCORE environmental switches was successively set to zero, and the outcome for all three species groups is compared with the run depicted in Fig. 2b and with BIOSCORE settings described in Table 2
| Run | Effect on vascular plants | Effects on butterflies | Effects on birds |
|---|---|---|---|
| (a) Continentality from ‘−‘ to zero | 1 Species moved from decline to stable | 28 Species moved from stable to increase due to a land use effect, but this was overshadowed by a negative climate effect so it is not reflected in the overall change | None |
| (b) Eutrophication from ‘−‘ to zero | 3 Species moved from decline to stable, and 1 to increase | 28 Species moved from stable to increase due to land use change, and for 26 this remained the case after incorporating the climate effect | None |
| (c) Soil moisture from ‘−‘ to zero | Same as (b) | Same as (b) | None |
Discussion
Projected responses in species richness to climate change and land use change
Our modelling exercise suggests that by 2030, given land use change and climate change projections, notably many characteristic vascular plant species of dry grasslands and wetlands will have declined substantially (50–100 % of them, Figs. 2, 3), and this decline appears to be mainly due to land use change (cf. Titeux et al. 2016). For birds, butterflies and dragonflies, the pattern was more variable: substantial numbers of species appear stable. Particularly in wetlands (Figs. 2, 3), the (limited) decline in birds and dragonflies was largely driven by climate change (Table 3b). Given our articulation of the scenarios (Table 2), this may be aggravated by both reduced water availability and water quality. Many grassland bird species were found to increase in number, notably in continental Europe (Fig. 2). This may be due to the increase in semi-natural vegetation due to land abandonment (Westhoek et al. 2006). The latter may also imply that further forest expansion may ultimately lead to declines over longer time scales. These aggregate outcomes appear plausible given the overall ecology of the taxonomic groups and results of previous studies (e.g. Huntley et al. 2007; Settele et al. 2008). It should be noted, however, that we have not included specialist dependencies between butterflies and angiosperms: so this analysis cannot have fully grasped the secondary effect of plant decline on specialist insect fauna. The observed sensitivity of butterflies to eutrophication and soil moisture agrees with species trait analyses for this species group (WallisDeVries 2014). It also parallels recent findings of Habel et al. (2016), who demonstrated a century-long decline in specialist butterflies of dry calcareous grasslands in Southern Germany coupled to habitat fragmentation and a decline in host plants due to land use intensification.
Within the four combinations of geographic scale (EU27 vs continental Europe) and habitat type, the response in the different species groups to the scenarios was highly similar: only 3 out of the 4 × 4 combinations of scale x habitat stood out visibly from the rest. This consistency among scenarios suggests that socio-economic development grasped by the SRES scenarios and its consequences for biodiversity has not yet diverged so much yet over the 3 decades covered by our modelling. Similarly, Araújo et al. (2011) found little contrast among the same four SRES scenarios, but estimated a much more pronounced decline across all species groups when modelling survival in conservation areas in Europe until 2080. Interestingly, our findings differ from those of Pompe et al. (2008), who used a detailed niche-based model projection of angiosperm species richness change across Germany by 2080, and found considerable difference among scenarios (corresponding to A1, A2 and B1), but only when dispersal was set to zero. However, when dispersal was included, the differences among scenarios remained but were less outspoken, hence in closer agreement with our results. This underpins the significance of dispersal, firstly for the survival of fragmented meta-populations (as reflected by many dry grassland vascular plants and butterflies, Pompe et al. 2008; Settele et al. 2008; Veen et al. 2009; Habel et al. 2016), and secondly for the design of viable biodiversity policy. Martin et al. (2013) found that climate was more important than land use in explaining the future distribution of a wetland specialist butterfly, but argued that this was because of insufficient spatial and thematic land use resolution. Geographical resolution of available species distribution and environmental data will be important in contributing uncertainty to the width and depth of our conclusions: this is obvious but not trivial and it should lead to caution in interpretation of model projections.
Overall, to answer our first question, our analysis suggests that the different species groups respond differently to land use and climate change, and that we can clearly separate their effects. Over the modelled time span of 30 years, vascular plants mainly decline due to land use, so plant diversity will probably decline, irrespective of habitat type or scenario. For birds and insects, however, the pattern is less straightforward, with winners and losers and a considerable contrast between dry grasslands and wetlands in the main driver responsible for this. For example, in continental Europe under the B1 and B2 scenarios, a substantial proportion of birds were estimated to increase, particularly due to land use change (Fig. 2), which is probably related to projected land abandonment.
Differences between habitat types
Our second question was whether the species of those two types of habitat would differ in their response, and they clearly did, but not in all aspects. In both habitats, vascular plant species declined more strongly than the other species groups. For birds and insects, however, land use was a stronger driver of species decline in dry grasslands of the EU27 (not in continental Europe), whereas in wetlands climate caused stronger declines for these species groups.
Differences between the larger and more restricted spatial extent (EU27 versus continental Europe)
For continental Europe, we found a considerable difference in dry grassland species’ responses compared to the whole EU27 (Fig. 2), but the wetland species groups responded quite similarly. This implies that we have no single answer to our third question. Here, the importance of a homogeneous biogeographic region is overruled by that of the habitat: wetland or grassland is more important than biogeography. In continental Europe, grassland species may well have been estimated to increase due to the increase in semi-natural vegetation following considerable land abandonment. Subsequent forest development is probable (Delbaere et al. 2009) over longer time scales than modelled here and this suggests that this effect will be transient. It is tempting to speculate that wetlands are less fragmented than dry grasslands. To explain the more moderate decline of birds and butterflies in continental grasslands compared to the EU27, a relation with land use intensity appears plausible. Continental Europe excludes the intensively used agricultural areas of Denmark, Germany, The Netherlands, Belgium and Western France, where cattle density and nitrogen surpluses are high (Kleijn et al. 2009). Support can be found in the natural connectedness through river networks, and the importance of migratory wetland birds as dispersal vectors for plants (Amezaga et al. 2002; Santamaria 2002; Beltman et al. 2011), where once widespread transhumance has disappeared across most of Europe (Bruun and Fritzbøger 2002; Ozinga et al. 2009), thus greatly reducing the dispersal of dry grassland species.
Methodological constraints
As already outlined in the methods section, our approach has limitations. Firstly, the BIOSCORE database has been compiled using comparatively crude niche specifications and climate sensitivities (see also Supplementary material S1), introducing uncertainty in species responses. Given the geographic extent, the large number of species included, the wide range of environmental pressures that could play a role, the variation in each species’ responses to pressures and limited knowledge of these, this uncertainty is compounded and will not allow conclusions and generalizations at fine spatial or taxonomic resolution. For this reason, we have selected only those species groups that are well studied and are comparatively rich in species to maximize eco-geographic articulation. Secondly, the database presumes fixed species preferences, similar to climate envelope models, and ignores possibilities for acclimation or selection of new genotypes within species (adaptation). This ignores the potential of evolutionary-driven change. Thirdly, we use climate change projections and land use change deductions from EURURALIS as inputs that in themselves have considerable uncertainty—scenarios are plausible projections and confidence intervals are not straightforwardly derived. Fourthly, indirect effects through food web and competitive interactions among species have not been modelled. Notably for dry grasslands, highly specialized insects have co-evolved with rare, vascular plants into tight host specificity under a probably extensive but age-old ruminant grazing regime (Bruun and Fritzbøger 2002, Suttle et al. 2007; Habel et al. 2016). Loss of these plants will lead to loss of the associated fauna, and this is not reflected in our outcome. Finally, our time horizon was constrained to 2030 by the land use projections done in EURURALIS. Other projective studies of biodiversity consequences of climate change have typically used a longer time horizon. IPCC (Kirtman et al. 2013) accordingly foresees that near-term (2016–2035) global temperature increase ranges between 0.3 and 0.7 °C, and witnesses a modest sensitivity to differences among scenarios. Thus, for the coming decades, this appears consistent with our findings, and it lends plausibility to our observed importance of land use change for species survival and local or regional biodiversity compared to climate change, despite the currently observed northward range extensions.
Implications for biodiversity policy and conservation practice
The implications of our scenario analyses for European biodiversity policy may appear sobering: by 2030 the difference between the four scenarios is fairly limited. Hence, also when climate policy will be effectively implemented and emissions are greatly reduced (the B1 and B2 scenarios used here, or similar RCPs, Moss et al. 2010), many characteristic plant species inhabiting these target habitats are projected to decline strongly, and this is mainly due to land use change. For insects and birds, the pattern is less straightforward and their decline is comparatively limited in wetlands, and in the continental dry grasslands.
Our findings suggest that until 2030 scenarios do not show substantial divergence in line with a.o. Araújo et al. (2011), but also that targeted policies for different habitat types and species groups are to be considered. These are for wetlands to reduce climate change effects, and for dry grasslands to reduce habitat loss due to land use change and to enhance connectivity, e.g. through the EU Green Infrastructure strategy. Hence, conservation of dry grasslands would benefit from simulating seasonal movements of herbivore flocks between different habitat fragments, a practice that is argued for in the literature (Fischer et al. 1996; Poschlod and WallisDeVries 2002; Manzano and Malo 2006) and is applied in Flanders with positive consequences (Couvreur et al. 2004). Fischer et al. (1996) demonstrated that sheep moving from grassland to grassland also disperse insects, such as grasshoppers in their fleece. In a review, Auffret (2011) argued that any measure inspired by traditional agricultural practice can be very effective. This author includes humans and their pets as a modern dispersal analogue which, when allowed to move freely as in the Scandinavian countryside where the freedom to roam is a lawful right, may also contribute to longer distance dispersal. A rejuvenation of a market for mutton and wool through focus on local and ecological production may contribute an economic incentive, notably under the B2 scenario, but this will not likely lead to cattle stocks of the size reported for the mid-nineteenth century (Bruun and Fritzbøger 2002; Poschlod and WallisDeVries 2002).
For wetlands, measures that reduce climate change effects can only be implemented through a careful consideration of the seasonal availability of water at or near the land surface, including flooding regimes, and the sustained connectivity of current river networks. Considerable practical guidance can be obtained from desiccation abatement programmes where groundwater has been overexploited (for example Hinsby et al. 2008), from eutrophication abatement programmes where external loading has been diverted and reduced, as well as from migration assistance programmes for anadromous fish such as salmon.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This paper is based on the outcome of an expert workshop organized in March 2012 in the hamlet Ehrenberg-Seiferts, located in the UNESCO Biosphere Reserve Rhön, Hessen, Germany (www.biosphaerenreservat-rhoen.de). It was supported financially by the European Commission as part of the EU-funded FP7 project RESPONSES, Grant Agreement number 244092.
Biographies
Jan E. Vermaat
heads the department of Environmental Sciences at the Norwegian University of Life Sciences, in Ås, Norway. He is an aquatic and landscape ecologist with an interest in cross-disciplinary approaches to environmental problems.
Fritz A. Hellmann
is a researcher at the Netherlands Environmental Assessment Agency (PBL). His current research interests focus on the land–water–energy nexus.
Astrid J. A. van Teeffelen
is a senior researcher at the Vrije Universiteit Amsterdam. Her research interests focus on conservation of biodiversity and ecosystem services in a context of land use change and climatic change.
Jelle van Minnen
is a senior policy researcher at the Netherlands Environmental Assessment Agency (PBL). One of his research topics is modelling climate change impacts on natural ecosystems and assess adaptation options.
Rob Alkemade
is a senior researcher at the Netherlands Environmental Assessment Agency (PBL). He has a wide experience in biodiversity assessment and scenario analysis at the global level and contributed to the Millennium Ecosystem Assessment, Global Environmental Outlooks and Global Biodiversity Outlooks. For this purpose, he developed the GLOBIO3 model.
Regula Billeter
is a senior researcher trained in environmental sciences and plant ecology. She is interested in effects of climate change on plant biodiversity and distribution as well as in urban ecology and restoration ecology.
Carl Beierkuhnlein
holds the chair of biogeography at the University of Bayreuth. His major research interest is in landscape ecology, island biogeography, and in climate change impact research. He is speaker of the international study programme “Global Change Ecology”. As member of the Biodiversity Council, he gives advice to the Bavarian Environmental Ministry.
Luigi Boitani
is a professor of Animal Ecology and Conservation Biology at the University of Roma Sapienza, Italy. His research interests range from the ecology of wolves and bear to the theory and practice of designing protected areas, to the analysis of macro-ecological patterns of species distribution.
Mar Cabeza
is a senior researcher at the Department of Biosciences of the University of Helsinki, leading the “Global Change and Conservation” team. Her research is focused on conservation, including theoretical and applied projects both in the developed and developing worlds.
Christian K. Feld
is a senior researcher trained in biology, aquatic ecology and biodiversity. He is interested in freshwater bioindicators of environmental change and how they can help ecosystem managers diagnose deterioration and improve ecological status and biodiversity.
Brian Huntley
holds a personal chair in ‘Plant Ecology and Palaeoecology’ at Durham University. His research spans the fields of ecology, biogeography and quaternary paleoecology, using insights from the past as a key to predicting the potential future. He investigates the relationships between environmental change and changes in the distributions of organisms, as well as in the composition, structure and dynamics of ecosystems, and focuses especially upon the potential consequences of anthropogenic climatic changes.
James Paterson
is a researcher at the University of Edinburgh. His main research interests include modelling and developing scenarios for land use change as well as developing research protocols for ecosystem service assessments.
Michiel F. WallisDeVries
is a senior scientist at Dutch Butterfly Conservation and Special Professor in Insect Ecology and Conservation at Wageningen University. His research focusses on the impacts of global change—climate, nitrogen, land use and their interactions—on insect communities, in particular European butterflies.
References
- Amezaga JM, Santamaria L, Green AJ. Biotic wetland connectivity—Supporting a new approach for wetland policy. Acta Oecologica. 2002;23:213–222. doi: 10.1016/S1146-609X(02)01152-9. [DOI] [Google Scholar]
- Anderson M, Ferree C. Conserving the stage: Climate change and the geophysical underpinnings of species diversity. PLoS ONE. 2010;5:e11554. doi: 10.1371/journal.pone.0011554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo MB, Alagador D, Cabeza M, Nogués-Bravo D, Thuiller W. Climate change threatens European conservation areas. Ecology Letters. 2011;14:484–492. doi: 10.1111/j.1461-0248.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo MB, Peterson AT. Uses and misuses of bioclimatic envelope modeling. Ecology. 2012;93:1527–1539. doi: 10.1890/11-1930.1. [DOI] [PubMed] [Google Scholar]
- Auffret AG. Can seed dispersal by human activity play a useful role for the conservation of European grasslands? Applied Vegetation Science. 2011;14:291–303. doi: 10.1111/j.1654-109X.2011.01124.x. [DOI] [Google Scholar]
- Barbet-Massin M, Thuiller W, Jiguet F. The fate of European breeding birds under climate, land-use and dispersal scenarios. Global Change Biology. 2012;18:881–890. doi: 10.1111/j.1365-2486.2011.02552.x. [DOI] [Google Scholar]
- Beale CM, Lennon JJ, Gimona A. Opening the climate envelope reveals no macroscale associations with climate in European birds. Proceedings of the National Academy of Sciences. 2008;105:14908–14912. doi: 10.1073/pnas.0803506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltman BGHJ, Omtzigt N, Vermaat JE. Turbary restoration meets variable success: Does landscape structure force colonization success of wetland plants? Restoration Ecology. 2011;19:185–193. doi: 10.1111/j.1526-100X.2010.00711.x. [DOI] [Google Scholar]
- Berkhout F, Hertin J, Jordan A. Socio-economic futures in climate change impact assessment: Using scenarios as ‘learning machines’. Global Environmental Change. 2002;12:83–95. doi: 10.1016/S0959-3780(02)00006-7. [DOI] [Google Scholar]
- BISE. 2016. Biodiversity information system for Europe. http://biodiversity.europa.eu/topics/land-use-change. Accessed 2 Feb 2016.
- Bruun HH, Fritzbøger B. The past impact of livestock husbandry on dispersal of plant seeds in the landscape of Denmark. Ambio. 2002;31:425–431. doi: 10.1579/0044-7447-31.5.425. [DOI] [PubMed] [Google Scholar]
- Busch G. Future European agricultural landscapes—What can we learn from existing quantitative land use scenario studies. Agriculture, Ecosystems & Environment. 2006;114:121–140. doi: 10.1016/j.agee.2005.11.007. [DOI] [Google Scholar]
- Campbell, A., V. Kapos, J.P.W. Scharlemann, P. Bubb, A. Chenery, L. Coad, B. Dickson, N. Doswald, et al. 2009. Review of the literature on the links between biodiversity and climate change: impacts, adaptation and mitigation. Secretariat of the Convention on Biological Diversity, Montreal. Technical Series No. 42, 124 pages.
- Christensen, J., B. Hewitson, A. Busuioc, A. Chen, X. Gao, I. Held, R. Jones, R. Kolli, et al. 2007. Regional climate projections. In: Solomon S, D.Qin, M.Manning et al. (eds) Climate Change 2007: The Physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 847–940. Cambridge, UK, New York, NY: Cambridge University Press.
- Čížková H, Květ J, Comín FA, Laiho R, Pokorný J, Pithart D. Actual state of European wetlands and their possible future in the context of global climate change. Aquatic Sciences. 2013;75:3–26. doi: 10.1007/s00027-011-0233-4. [DOI] [Google Scholar]
- Cliquet A, Backes C, Harris J, Howsam P. Adaptation to climate change: Legal challenges for protected areas. Utrecht Law Rev. 2009;5:158–175. doi: 10.18352/ulr.100. [DOI] [Google Scholar]
- Couvreur M, Christiaen B, Verheyen K, Hermy M. Large herbivores as mobile links between isolated nature reserves through adhesive seed dispersal. Applied Vegetation Science. 2004;7:229–236. doi: 10.1111/j.1654-109X.2004.tb00614.x. [DOI] [Google Scholar]
- Dale V, Efroymson R, Kline K. The land use–climate change–energy nexus. Landscape Ecology. 2011;26:755–773. doi: 10.1007/s10980-011-9606-2. [DOI] [Google Scholar]
- Davies, C.E., D. Moss, and M.O. Hill. 2004. EUNIS habitat classification revised 2004. Report to the European Environment Agency and the European Topic Centre on Nature Protection and Biodiversity. Centre for Ecology and Hydrology, Dorchester, UK, 307 pp. See also: http://eunis.eea.eu.int/index.jsp.
- Delbaere B, Nieto Serradilla A, Sethlage M, editors. BIOSCORE: A tool to assess the impacts of European Community policies on Europe’s biodiversity. Tilburg: ECNC; 2009. [Google Scholar]
- Dengler J. Zwischen Estland und Portugal—Gemeinsamkeiten und Unterschiede der Phytodiversitätsmuster europäischer Trockenrasen. Tuexenia. 2005;25:387–405. [Google Scholar]
- Devictor V, Van Swaay C, Brereton T, Brotons L, Chamberlain D, Heliölä J, Herrando S, Julliard R, et al. Differences in the climatic debts of birds and butterflies at a continental scale. Nature Climate Change. 2012;1347:121–124. doi: 10.1038/nclimate1347. [DOI] [Google Scholar]
- de Chazal J, Rounsevell MDA. Land-use and climate change within assessments of biodiversity change: A review. Global Environmental Change. 2009;19:306–315. doi: 10.1016/j.gloenvcha.2008.09.007. [DOI] [Google Scholar]
- Dijkstra, K.D.B. and R. Lewington. 2006. Field guide to the dragonflies of Britain and Europe. Totnes: British Wildlife Publishers.
- Dodd A, Hardiman A, Jennings K, Williams G. Protected areas and climate change: Reflections from a practitioner’s perspective. Utrecht Law Rev. 2010;6:141–150. doi: 10.18352/ulr.119. [DOI] [Google Scholar]
- EEA. 2012. Climate change, impacts and vulnerability in Europe 2012. European Environment Agency. Technical report No 12/2012. http://www.eea.europa.eu/pressroom/newsreleases/climate-change-evident-across-europe, Copenhagen.
- Eggers J, Tröltzsch K, Falcucci A, Maiorano L, Verburg PH, Framstad E, Louette G, Maes D. Is biofuel policy harming biodiversity in Europe? Global Change Biology Bioenergy. 2009;1:18–34. doi: 10.1111/j.1757-1707.2009.01002.x. [DOI] [Google Scholar]
- Fischer S, Poschlod P, Beinlich B. Experimental studies on the dispersal of plants and animals by sheep in calcareous grasslands. Journal of Applied Ecology. 1996;33:1206–1222. doi: 10.2307/2404699. [DOI] [Google Scholar]
- Fletcher RJ, Robertson BA, Evans J, Doran PJ, Alavalapati JRR, Schemske DW. Biodiversity conservation in the era of biofuels: Risks and opportunities. Frontiers in Ecology and the Environment. 2010;9:161–168. doi: 10.1890/090091. [DOI] [Google Scholar]
- Fronzek S, Carter TR, Jylha K. Representing two centuries of past and future climate for assessing risks to biodiversity in Europe. Global Ecology and Biogeography. 2012;21:19–35. doi: 10.1111/j.1466-8238.2011.00695.x. [DOI] [Google Scholar]
- Habel JC, Segerer A, Ulrich W, Torchyk O, Weisser WW, Schmitt T. Butterfly community shifts over 2 centuries. Conservation Biology. 2016 doi: 10.1111/cobi.12656. [DOI] [PubMed] [Google Scholar]
- Harrison PA, Berry PM, Butt N, New M. Modelling climate change impacts on species’ distributions at the European scale: Implications for conservation policy. Environmental Science & Policy. 2006;9:116–128. doi: 10.1016/j.envsci.2005.11.003. [DOI] [Google Scholar]
- Hellmann F, Verburg PH. Impact assessment of the European biofuel directive on land use and biodiversity. J Environ Manag. 2010;91:1389–1396. doi: 10.1016/j.jenvman.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Heubes JV, Retzer V, Schmidtlein S, Beierkuhnlein C. Historical land use explains current distribution of calcareous grassland species. Folia Geobotanica. 2011;46:1–16. doi: 10.1007/s12224-010-9090-5. [DOI] [Google Scholar]
- Hickler T, Vohland K, Feehan J, Miller PA, Smith B, Costa L, Giesecke T, Fronzek S, et al. Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Global Ecology and Biogeography. 2012;21:50–63. doi: 10.1111/j.1466-8238.2010.00613.x. [DOI] [Google Scholar]
- Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biology. 2006;12:450–455. doi: 10.1111/j.1365-2486.2006.01116.x. [DOI] [Google Scholar]
- Hinsby K, Condeso de Melo MT, Dahl M. European case studies supporting the derivation of natural background levels and groundwater threshold values for the protection of dependent ecosystems and human health. Sci Tot Env. 2008;401:1–20. doi: 10.1016/j.scitotenv.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Huntley B, Green RE, Collingham Y, Willis SG. A climatic atlas of European breeding birds. Durham: Durham University, RSPB and Lynx Edicions; 2007. [Google Scholar]
- Huntley B, Collingham YC, Willis SG, Green RE. Potential impacts of climatic change on European breeding birds. PLoS ONE. 2008;3:e1439. doi: 10.1371/journal.pone.0001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley B, Barnard P, Altwegg R, Chambers L, Coetzee BWT, Gibson L, Hockey PAR, Hole DG, et al. Beyond bioclimatic envelopes: Dynamic species’ range and abundance modelling in the context of climatic change. Ecography. 2010;33:621–626. [Google Scholar]
- Jaeschke A, Bittner T, Reineking B, Beierkuhnlein C. Can they keep up with climate change? Integrating specific dispersal abilities of protected Odonata in species distribution modelling. Insect Conserv Div. 2013;6:93–103. doi: 10.1111/j.1752-4598.2012.00194.x. [DOI] [Google Scholar]
- Kirtman, B., S.B. Power, J.A. Adedoyin, G.J. Boer, R. Bojariu, I. Camilloni, F.J. Doblas-Reyes, A.M. Fiore, et al. 2013. Near-term climate change: projections and predictability. In Stocker et al. (eds), Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, UK.
- Kleijn D, Kohler F, Báldi A, Batary P, Concepcion ED, Clough Y, Diaz M, Gabriel D, et al. On the relationship between farmland biodiversity and land-use intensity in Europe. Proceedings of the Royal Society B. 2009;276:903–909. doi: 10.1098/rspb.2008.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFranchis, T. 2004. Butterflies of Europe, new field guide and key. Paris: Diatheo.
- Lorenzoni I, Jordan A, Hulme M, Turner RK, O’Riordan T. A co-evolutionary approach to climate impact assessment: Part I. Integrating socio-economic and climate change scenarios. Global Environmental Change. 2000;10:57–68. doi: 10.1016/S0959-3780(00)00012-1. [DOI] [Google Scholar]
- Louette GD, Maes J, Alkemade RM, Boitani L, De Knegt B, Eggers J, Falcucci A, Framstad E, et al. BIOSCORE–cost-effective assessment of policy impact on biodiversity using species sensitivity scores. Journal for Nature Conservation. 2010;18:142–148. doi: 10.1016/j.jnc.2009.08.002. [DOI] [Google Scholar]
- Manzano P, Malo JE. Extreme long-distance seed dispersal via sheep. Frontiers in Ecology and Evolution. 2006;4:244–248. doi: 10.1890/1540-9295(2006)004[0244:ELSDVS]2.0.CO;2. [DOI] [Google Scholar]
- Martin Y, Van Dyck H, Dendoncker N, Titeux N. Testing instead of assuming the importance of land use change scenarios to model species distributions under climate change. Global Ecology and Biogeography. 2013;22:1204–1216. doi: 10.1111/geb.12087. [DOI] [Google Scholar]
- Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, et al. European phenological response to climate change matches the warming pattern. Global Change Biology. 2006;12:1969–1976. doi: 10.1111/j.1365-2486.2006.01193.x. [DOI] [Google Scholar]
- Metzger MJ, Bunce RGH, Jongman RHG, Mücher CA, Watkins JW, et al. A climatic stratification of the environment of Europe. Global Ecology and Biogeography. 2005;14:549–563. doi: 10.1111/j.1466-822X.2005.00190.x. [DOI] [Google Scholar]
- Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, Van Vuuren DP, Carter TR, Emori S, et al. The next generation of scenarios for climate change research and assessment. Nature. 2010;463:747–756. doi: 10.1038/nature08823. [DOI] [PubMed] [Google Scholar]
- Oliver TH, Morecroft MD. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. WIREs Climate Change. 2014;5:317–335. doi: 10.1002/wcc.271. [DOI] [Google Scholar]
- Ozinga WA, Römermann C, Bekker RM, Prinzing A, Tamis WLM, Schaminee JHJ, Hennekens SM, Thompson K, et al. Dispersal failure contributes to plant losses in NW Europe. Ecology Letters. 2009;12:66–74. doi: 10.1111/j.1461-0248.2008.01261.x. [DOI] [PubMed] [Google Scholar]
- Paterson JS, Araújo MB, Berry PM, Piper JM, Rounsevell MDA. Mitigation, adaptation and the threat to biodiversity. Conservation Biology. 2008;22:1352–1355. doi: 10.1111/j.1523-1739.2008.01042.x. [DOI] [PubMed] [Google Scholar]
- Pawson SM, Brin A, Brockerhoff EG, Lamb D, Payn TW, Paquette A, Parrotta JA. Plantation forests, climate change and biodiversity. Biodiversity and Conservation. 2013;22:1203–1227. doi: 10.1007/s10531-013-0458-8. [DOI] [Google Scholar]
- Pompe S, Hanspach J, Badeck F, Klotz S, Thuiller W, Kühn I. Climate and land use change impacts on plant distributions in Germany. Biology Letters. 2008;4:564–567. doi: 10.1098/rsbl.2008.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschlod P, WallisDeVries MF. The historical and socioeconomic perspective of calcareous grasslands—Lessons from the distant and recent past. Biological Conservation. 2002;104:361–376. doi: 10.1016/S0006-3207(01)00201-4. [DOI] [Google Scholar]
- Rajczak J, Pall P, Schär C. Projections of extreme precipitation events in regional climate simulations for Europe and the Alpine region. Journal of Geophysical Research: Atmospheres. 2013;118:3610–3626. [Google Scholar]
- Santamaria L. Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecologica. 2002;23:137–154. doi: 10.1016/S1146-609X(02)01146-3. [DOI] [Google Scholar]
- Settele, J., O. Kudrna, A. Harpke, I. Kühn, C. Van Swaay, R. Verovnik, M. Warren, M. Wiemers, et al. 2008. Climatic risk atlas of European butterflies, vol 1. Biorisk 1. Sofia: Pensoft Publishers.
- Spangenberg JH, Bondeau A, Carter TR, Fronzek S, Jaeger J, Jylha K, Kuhn I, Omann I. Scenarios for investigating risks to biodiversity. Global Ecology and Biogeography. 2012;21:5–18. doi: 10.1111/j.1466-8238.2010.00620.x. [DOI] [Google Scholar]
- Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315:640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- Svensson, L. and P.J. Grant. 2013. Vogelgids van Europa. Voorschoten: ANWB.
- Thuiller W, Lavorel S, Araujo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proc Nat Acad Sci. 2005;102:8245–8250. doi: 10.1073/pnas.0409902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeux N, Henle K, Mihoub JB, Regos A, Geijzendorffer IR, Cramer W, Verburg PH, Brotons L. Biodiversity scenarios neglect future land use changes. Global Change Biology. 2016 doi: 10.1111/gcb.13272. [DOI] [PubMed] [Google Scholar]
- Van der Meijden, R. 2005. Heukel’s Flora van Nederland, 23rd ed. Groningen: Noordhoff Publishers.
- Van Swaay C, Warren M, Loıs G. Biotope use and trends of European butterflies. Journal of Insect Conservation. 2006;10:189–209. doi: 10.1007/s10841-006-6293-4. [DOI] [Google Scholar]
- Van Teeffelen AJA, Meller L, Van Minnen J, Vermaat JE, Cabeza M. How climate proof is the European Union’s biodiversity policy? Reg Env Change. 2015;15:997–1010. doi: 10.1007/s10113-014-0647-3. [DOI] [Google Scholar]
- Van Vuuren D, Carter TR. Climate and socio-economic scenarios for climate change research and assessment: Reconciling the new with the old. Climate Change. 2014;122:415–429. doi: 10.1007/s10584-013-0974-2. [DOI] [Google Scholar]
- Veen P, Jefferson R, de Smidt J, van der Straaten J. Grasslands in Europe of high nature value. Zeist: KNNV; 2009. [Google Scholar]
- Verboom J, Alkemade R, Klijn J, Metzger MJ, Reijnen R. Combining biodiversity modeling with political and economic development scenarios for 25 EU countries. Ecological Economics. 2007;62:267–276. doi: 10.1016/j.ecolecon.2006.04.009. [DOI] [Google Scholar]
- Verburg P, Eickhout B, Van Meijl H. A multi-scale, multi-model approach for analysing the future dynamics of European land use. The Annals of Regional Science. 2008;42:57–77. doi: 10.1007/s00168-007-0136-4. [DOI] [Google Scholar]
- Walker KJ, Stevens PA, Stevens DP, Mountford JO, Manchester SJ, Pywell RF. The restoration and re-creation of species-rich lowland grassland on land formerly managed for intensive agriculture in the UK. Biological Conservation. 2004;119:1–18. doi: 10.1016/j.biocon.2003.10.020. [DOI] [Google Scholar]
- WallisDeVries MF. Linking species assemblages to environmental change: Moving beyond the specialist-generalist dichotomy. Basic and Applied Ecology. 2014;15:279–287. doi: 10.1016/j.baae.2014.05.001. [DOI] [Google Scholar]
- Westhoek H, Van den Berg M, Bakker J. Development of land use scenarios for European land use. Agriculture, Ecosystems & Environment. 2006;114:7–20. doi: 10.1016/j.agee.2005.11.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.