Significance
Contact-dependent growth inhibition (CDI) systems enable cells to bind competing bacteria and deliver toxins that cleave nucleic acids or form membrane pores. Here, we characterize a CDI toxin that specifically cleaves transfer RNA (tRNA), thereby blocking protein synthesis and inhibiting bacterial growth. Remarkably, two highly conserved and essential translation factors, EF-Ts and EF-Tu, are critical for this toxic nuclease activity. The toxin binds EF-Tu with high affinity and only cleaves tRNA in complex with the translation factor. EF-Ts appears to increase the rate of tRNA turnover. The activities of two other distinct CDI toxins are also regulated by EF-Ts. We propose that the regulation of toxin activity by the protein synthesis apparatus may play a role in intercellular communication.
Keywords: cell communication, protein synthesis, toxin-immunity proteins, ribonuclease, bacterial competition
Abstract
Contact-dependent growth inhibition (CDI) is a mechanism by which bacteria exchange toxins via direct cell-to-cell contact. CDI systems are distributed widely among Gram-negative pathogens and are thought to mediate interstrain competition. Here, we describe tsf mutations that alter the coiled-coil domain of elongation factor Ts (EF-Ts) and confer resistance to the CdiA-CTEC869 tRNase toxin from enterohemorrhagic Escherichia coli EC869. Although EF-Ts is required for toxicity in vivo, our results indicate that it is dispensable for tRNase activity in vitro. We find that CdiA-CTEC869 binds to elongation factor Tu (EF-Tu) with high affinity and this interaction is critical for nuclease activity. Moreover, in vitro tRNase activity is GTP-dependent, suggesting that CdiA-CTEC869 only cleaves tRNA in the context of translationally active GTP·EF-Tu·tRNA ternary complexes. We propose that EF-Ts promotes the formation of GTP·EF-Tu·tRNA ternary complexes, thereby accelerating substrate turnover for rapid depletion of target-cell tRNA.
Bacteria use several strategies to compete and cooperate with neighboring microorganisms in the environment. Contact-dependent growth inhibition (CDI) represents one important form of interbacterial competition that is common among Gram-negative pathogens (1–3). CDI is mediated by the CdiB/CdiA family of two-partner secretion proteins, which assemble as a complex on the surface of CDI+ bacteria. CdiB is an Omp85 β-barrel protein embedded in the outer membrane, where it functions to export long filamentous CdiA effector proteins. CdiA effectors project from the inhibitor-cell surface and bind to receptors on susceptible neighboring bacteria. Upon binding receptor, CdiA transfers its C-terminal toxin domain (CdiA-CT) into the target bacterium through an incompletely understood translocation mechanism (4, 5). Genome and protein database surveys show that CdiA effectors carry a wide variety of distinct toxins (1, 6–8). CDI+ cells protect themselves from self-intoxication by producing CdiI immunity proteins, which bind specifically to cognate CdiA-CT domains and neutralize their toxic activities. Because cdi loci encode an elaborate network of toxin/immunity protein pairs, the systems are hypothesized to mediate interstrain competition and self-/non–self-recognition.
Our previous studies have shown that CDI toxins inhibit cell growth using different mechanisms. The CdiA-CTEC93 domain deployed by Escherichia coli isolate EC93 increases target-cell permeability to protons (9, 10), suggesting that this toxin forms pores in the inner membrane. Many other CdiA-CT toxins are nucleases that must be delivered into the target-cell cytoplasm to inhibit growth. CdiA-CT3937 from Dickeya dadantii 3937 has potent DNase activity that destroys the target-cell chromosome (1, 11), whereas the CdiA-CTECL toxin from Enterobacter cloacae ATCC 13047 cleaves 16S rRNA to block protein synthesis (12). tRNA molecules are particularly common substrates for CDI nuclease toxins. Burkholderia pseudomallei isolates K96243, 1026b, and E479 deploy tRNase toxins with distinct specificities. CdiA-CTK96243 has anticodon nuclease activity on tRNAHis, tRNAAsp, tRNAAsn, and tRNATyr isoacceptors, and CdiA-CTE479 cleaves the T-loop of tRNA molecules between conserved residues Ψ54 and T55 (13, 14). CdiA-CTIIBp1026b preferentially cleaves within the aminoacyl acceptor stem of tRNAAla to block translation (15). Other unrelated CdiA-CT toxins from E. coli isolates EC869 and 3006 also cleave tRNA acceptor stems but are specific for tRNAGln and tRNAIle, respectively (5, 16). Thus, interbacterial competition has exerted a selective pressure to evolve diverse tRNase toxins with distinct specificities.
Most CDI nuclease domains efficiently cleave their substrates in vitro, but the CdiA-CTEC536 toxin deployed by uropathogenic E. coli 536 requires an additional factor to promote its tRNA anticodon nuclease activity (17). Using biochemical approaches, we discovered that the biosynthetic enzyme O-acetylserine sulfhydrylase A (CysK) binds the toxin with high affinity and stimulates its nuclease activity. This interaction is critical for toxin activity, and target bacteria deleted for cysK are fully resistant to CdiA-CTEC536 toxin (17). Because cysK mutations confer CDI-resistance (CDIR) to target bacteria, the advantage of an additional toxin-activation step is not clear. Recent work indicates that CysK stabilizes the CdiA-CTEC536 fold and promotes toxin interaction with tRNA (18). It is also possible that CdiA-CTEC536 modulates CysK activity in immune sibling cells, perhaps serving a role in intercellular signaling. To explore whether other CDI toxins are also subject to extrinsic activation, we used a genetic approach to identify target-cell factors required for growth inhibition by the CdiA-CTEC869 tRNase from enterohemorrhagic E. coli EC869. We isolated two CDI-resistant (CDIR) mutants with Ala202Glu and Arg219Pro missense substitutions in tsf, which encodes the essential translation factor EF-Ts. Both mutations alter the EF-Ts coiled-coil domain and significantly diminish CdiA-CTEC869 tRNase activity in target bacteria. We find that the CdiA-CTEC869 toxin binds to EF-Tu with high affinity and only cleaves tRNA in the context of GTP·EF-Tu·tRNA ternary complexes. Although wild-type EF-Ts is required for CdiA-CTEC869 toxicity in target bacteria, it appears to be dispensable for in vitro tRNase activity. We propose that EF-Ts promotes tRNase activity by accelerating the delivery of tRNAs to EF-Tu·toxin complexes, thereby increasing the rate of substrate cleavage.
Results
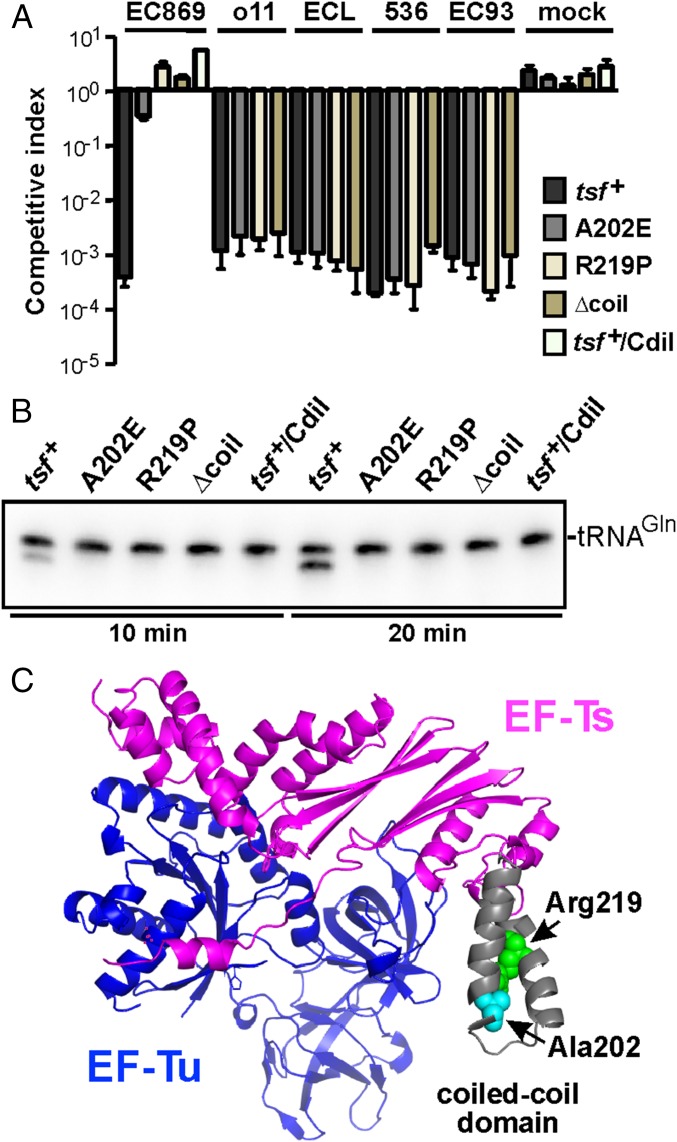
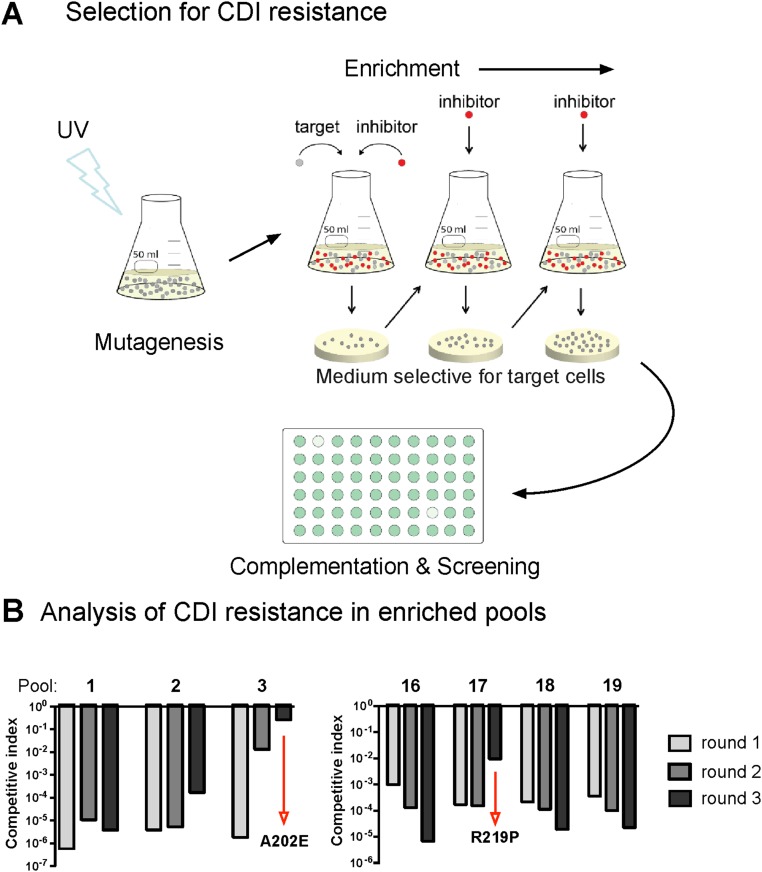
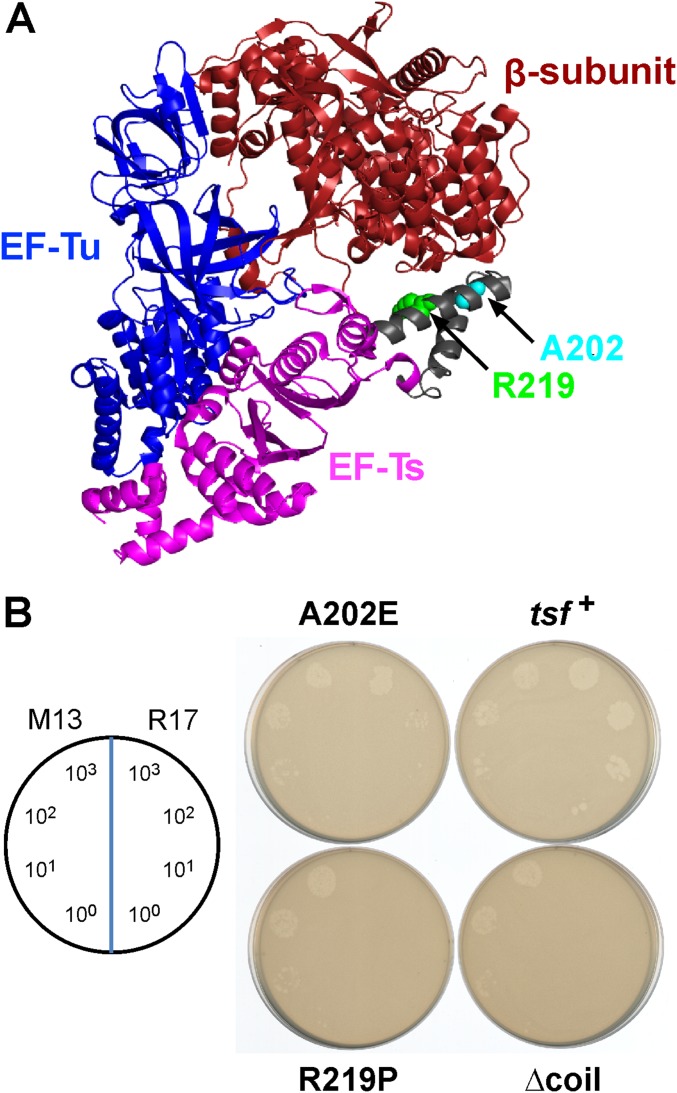
We used a genetic approach to identify target-cell factor(s) required for CdiA-CTEC869–mediated growth inhibition. Cells that deploy CdiA-CTEC869 significantly inhibit the growth of wild-type E. coli, but target bacteria are fully protected when they express the CdiIEC869 immunity protein (Fig. 1A, compare EC869 to mock). We then selected for CDIEC869-resistant (CDIR) target-cell mutants, reasoning that the protective mutations would disrupt genes required for toxin import and/or activation. Independent pools of CDI-sensitive (CDIS) E. coli target cells were subjected to mutagenesis with UV light. To avoid isolating mutations that disrupt the CdiA receptor BamA, the mutagenized target cells were also provided with the bamA gene on a multicopy plasmid. The target-cell pools were then cocultured with CdiA-CTEC869–expressing inhibitors to enrich for resistant mutants (Fig. S1A). Most of the UV-irradiated pools (22/24) failed to yield CDIR mutants, but resistant populations were obtained from pools 3 and 17 after three rounds of selection (Fig. S1B). We then used complementation analysis to map the CDIR mutations. The CDIR mutant isolated from pool 3 was first labeled with GFP, then transduced with a cosmid library of E. coli chromosomal DNA. The resulting clones were cocultured individually with CDIEC869 inhibitors in a microtiter plate and target-cell growth monitored by GFP fluorescence (Fig. S1A). We identified one cosmid that rendered the mutant sensitive to CDI. Given the very low frequency of CDIR mutations induced by UV irradiation, we reasoned that the affected gene was likely essential and therefore sequenced candidate genes within 40 kb of one end of the cosmid insert, located at 193,800 bp on the E. coli chromosome (19). We identified an Ala202Glu missense mutation in the tsf gene, which encodes the essential translation elongation factor EF-Ts. Analysis of the second CDIR mutant from pool 17 revealed an Arg219Pro substitution in tsf. Introduction of tsf(A202E) and tsf(R219P) alleles into wild-type E. coli MG1655 conferred CDIR phenotypes, demonstrating that the mutations are sufficient for resistance to the CDIEC869 toxin (Fig. 1A). This resistance is specific, because tsf(A202E) and tsf(R219P) cells are sensitive to the CdiA-CTo11EC869 DNase toxin from E. coli EC869, the CdiA-CTECL 16S rRNase toxin from E. cloacae, the CdiA-CTEC536 anticodon nuclease from E. coli 536, and the CdiA-CTEC93 pore-forming toxin from E. coli EC93 (Fig. 1A). Both tsf mutations alter residues in the coiled-coil domain of EF-Ts (Fig. 1C), suggesting that this region of the translation factor is important for toxin activity. The coiled-coil domain is not present in eukaryotic or mitochondrial orthologs and can be deleted from E. coli without loss of cell viability (20). Therefore, we generated a tsf(∆coil) target strain and found that these cells are also resistant to CdiA-CTEC869 activity (Fig. 1A). Together, these results indicate that the coiled-coil domain of EF-Ts plays a critical and specific role in the CDIEC869 growth inhibition pathway.
Fig. 1.
E. coli tsf mutants are resistant to CdiA-CTEC869. (A) E. coli MG1655 target strains carrying the indicated tsf alleles were cocultured with E. coli EPI100 inhibitor cells that deploy CdiA-CTEC869 (EC869), CdiA-CTo11EC869 (o11), CdiA-CTECL (ECL), CdiA-CTEC536 (536), or CdiA-CTEC93 (EC93) toxins. After 3 h, viable cell counts for each population were determined and used to calculate the competitive index as described in Materials and Methods. Mock inhibitors do not express a CDI system. Data are presented as averages ± SEM for three independent experiments. (B) E. coli EPI100 inhibitor cells expressing CdiA-CTEC869 were cultured at a 1:1 ratio with E. coli MG1655 target strains carrying the indicated tsf alleles. Total RNA was isolated after 10 and 20 min and subjected to Northern blot hybridization for tRNACUGGln. Because CDIEC869 inhibitor cells are immune to toxin activity, 50% substrate conversion is indicative of complete cleavage in target cells. (C) Structure of the E. coli EF-Tu·EF-Ts complex [Protein Data Bank (PDB) ID code 1EFU]. The locations of EF-Ts Ala202, Arg219, and the coiled-coil domain are indicated.
Fig. S1.
Selection of CdiA-CTEC869–resistant mutants. (A) Cultures of logarithmic growth-phase E. coli cells were UV-irradiated and cocultured at a 1:1 ratio with CDIEC869 inhibitor cells for 3 h. After three cycles of selection, target-cell clones from independent experiments were isolated and tested for CDI resistance. Complementation analysis was used to identify the gene(s) responsible for the CDIR phenotype. The CDIR mutant from pool 3 was labeled with GFP and transduced with a cosmid library of E. coli genomic DNA. Individual transductants were screened for CDIS in competition cocultures with CDIEC869 inhibitor cells in microtiter plates. Complementation to the CDIS phenotype results in low GFP fluorescence in competition well. (B) Pools of UV-irradiated target cells were mixed 1:1 with CDIEC869 inhibitor cells and cocultured for 3 h. CDIR mutants were selected with iterative cycles of competition coculture. Mutant pools containing CDIR target cells were identified by determining the competitive index for round of enrichment.
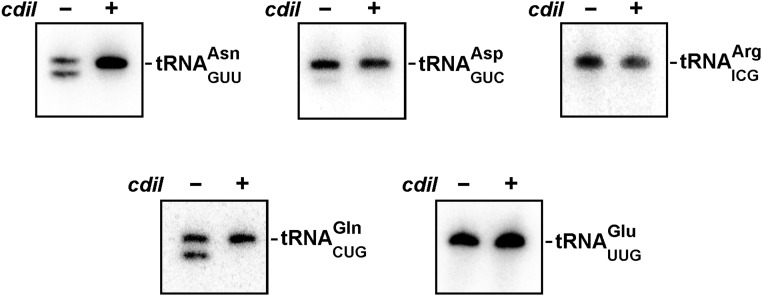
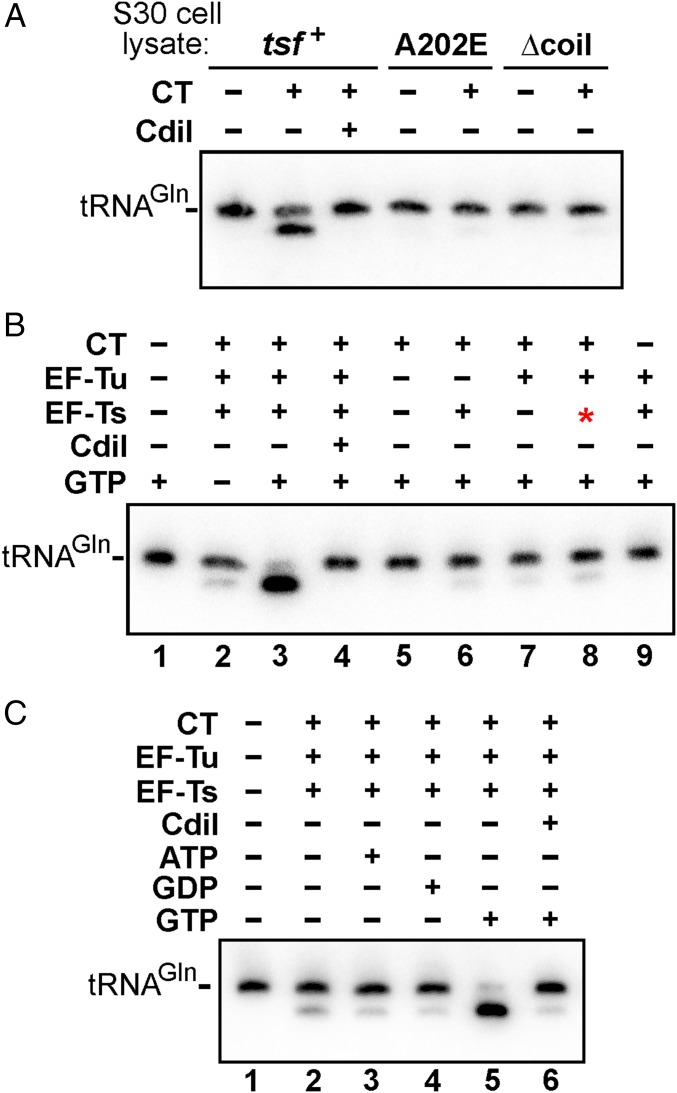
CdiA-CTEC869 is an RNase that preferentially cleaves tRNAGln and tRNAAsn, and this tRNase activity can be detected in CDI competition cocultures (Fig. S2) (16). Therefore, we asked whether CdiA-CTEC869 tRNase activity is detectable in the tsf mutants. Northern blot analysis revealed cleaved tRNACUGGln in total RNA isolated from cocultures with tsf+ target cells, but no tRNase activity was detected when tsf+ target cells express cdiIEC869 from a plasmid (Fig. 1B). There was no evidence of tRNase activity in tsf(A202E), tsf(R219P), or tsf(∆coil) target cells (Fig. 1B), suggesting that wild-type EF-Ts is required for toxin activity. Alternatively, it is possible that EF-Ts is required for toxin entry into the target bacteria. We addressed this latter possibility using a biochemical approach. We overexpressed His6-tagged CdiA-CTEC869 in complex with CdiIEC869, then purified the toxin by Ni2+-affinity chromatography under denaturing conditions to remove the immunity protein. The isolated toxin was refolded and its nuclease activity assayed in S30 extracts prepared from tsf+, tsf(A202E), and tsf(∆coil) cells. tRNACUGGln cleavage was observed upon toxin addition to wild-type cell extract, but no activity was detected in tsf(A202E) and tsf(∆coil) extracts (Fig. 2A). Again, the nuclease activity was specific to the CdiA-CTEC869 toxin, because tRNACUGGln cleavage was blocked in reactions supplemented with purified CdiIEC869. Together, these results indicate that EF-Ts promotes CdiA-CTEC869 tRNase activity.
Fig. S2.
CdiA-CTEC869 tRNase activity. CDIEC869 inhibitor cells were incubated with nonimmune (cdiI–) or immune (cdiI+) target cells at a 1:1 ratio for 20 min. Total RNA was isolated from the cocultures and analyzed by Northern blotting using probes to the indicated tRNAs. Because CDIEC869 inhibitor cells are immune to toxin activity, 50% substrate conversion is indicative of complete cleavage in target cells.
Fig. 2.
Efficient CdiA-CTEC869 tRNase activity requires EF-Tu, EF-Ts, and GTP. (A) E. coli S30 cell lysates were supplemented with purified CdiA-CTEC869 (0.1 µM) and incubated at ambient temperature for 1 h. Total RNA was isolated and analyzed by Northern blot hybridization for E. coli tRNACUGGln. (B) Total E. coli RNA (0.1 mg/mL) was treated with purified CdiA-CTEC869 (0.1 µM), EF-Tu (0.25 µM), and EF-Ts (0.25 µM) and incubated at ambient temperature for 1 h. Where indicated, CdiA-CTEC869 was preincubated with CdiIEC869-His6 (0.3 µM) for 30 min before addition to the reaction. The red asterisk in lane 8 indicates addition of EF-Ts (Ala202Glu). Reactions were supplemented with 1 mM GTP where indicated and analyzed by Northern blot hybridization for E. coli tRNACUGGln. (C) Purified CdiA-CTEC869 (0.1 µM), EF-Tu (0.25 µM), and EF-Ts (0.25 µM) were incubated with total E. coli RNA (0.1 mg/mL) for 1 h at room temperature. Where indicated, CdiA-CTEC869 was preincubated with CdiI-His6 (0.3 µM) for 30 min before RNA addition. Reactions were supplemented with ATP, GDP, or GTP (1 µM) where indicated. Reactions were analyzed by denaturing urea polyacrylamide gel electrophoresis and Northern blot hybridization for E. coli tRNACUGGln.
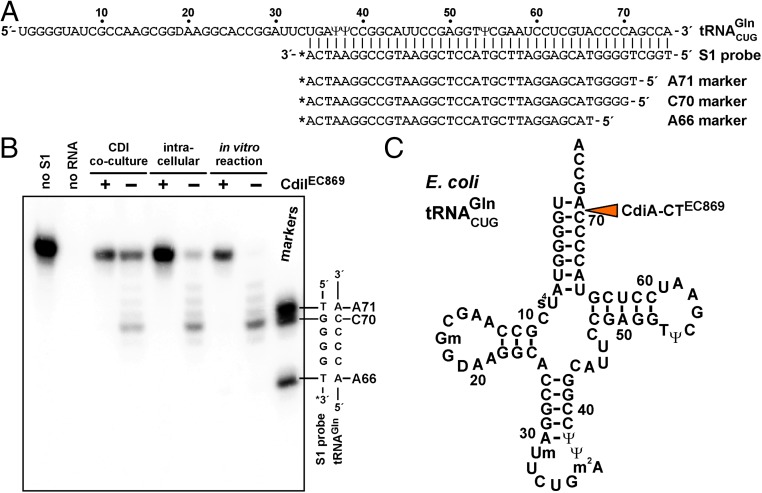
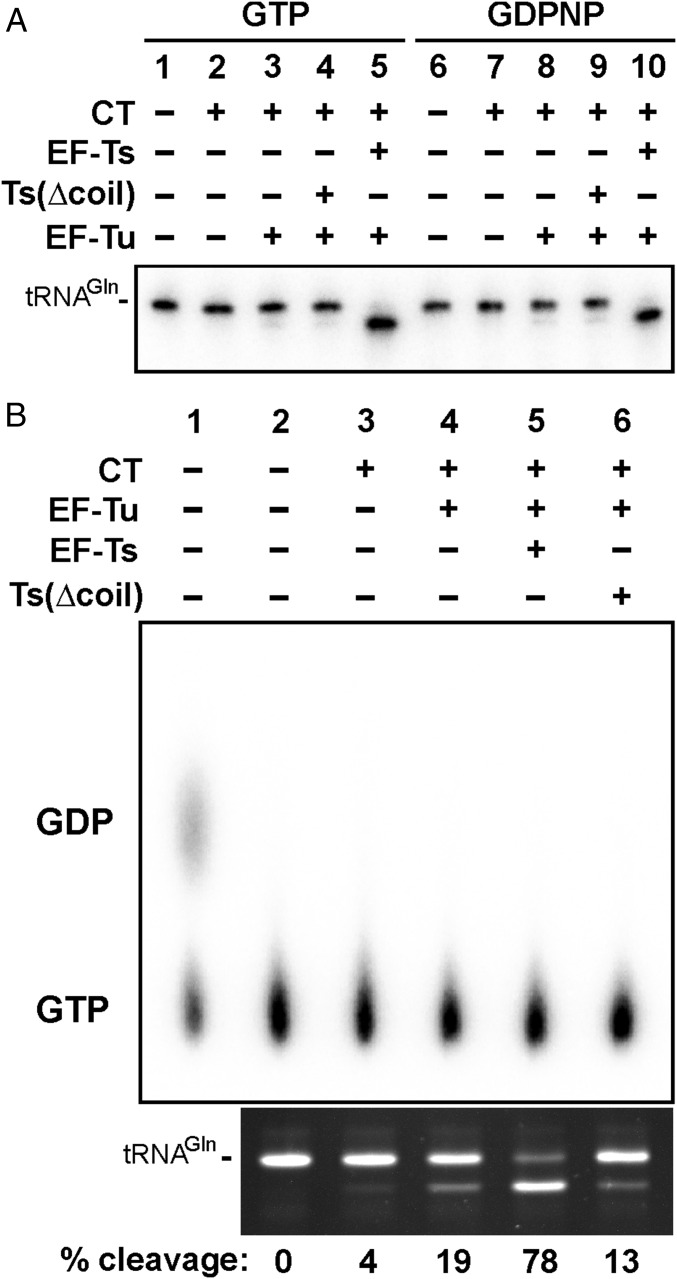
The critical role of EF-Ts in CdiA-CTEC869 toxicity appears to be similar to CdiA-CTEC536, which only exhibits tRNA anticodon nuclease activity when bound to CysK (17, 18). To determine whether EF-Ts activates CdiA-CTEC869 in the same manner, we tested CdiA-CTEC869 activity in defined in vitro reactions. No tRNase activity was observed when E. coli tRNA was treated with purified CdiA-CTEC869 (Fig. 2B, lane 5), suggesting that the toxin requires an additional factor for activity. However, the inclusion of purified EF-Ts led to only a modest increase in nuclease activity (Fig. 2B, lane 6). This latter result led us to consider whether another factor may be required in conjunction with EF-Ts. Notably, we found that elongation factor EF-Tu copurifies with the CdiA-CT·CdiIEC869 complex under nondenaturing conditions (Fig. S3), suggesting that this translation factor may contribute to toxin activity. Markedly, a combination of purified CdiA-CTEC869, EF-Tu, and wild-type EF-Ts was sufficient to cleave most of the tRNACUGGln molecules in vitro, provided the reaction was supplemented with GTP (Fig. 2B, compare lanes 2 and 3). However, purified EF-Ts carrying the Ala202Glu substitution did not support robust tRNase activity under the same conditions (Fig. 2B, compare lanes 3 and 8). Further, we found that the nucleotide requirement is specific, as neither ATP nor GDP supported full nuclease activity in vitro (Fig. 2C, compare lanes 3 and 4–5). Because GTP is required for high-affinity binding of tRNA to EF-Tu, this latter result strongly suggests that the toxin cleaves substrate in the context of GTP·EF-Tu·tRNA complexes. To address the structural feasibility of this model, we used S1 nuclease protection to map the cleavage site on tRNACUGGln (Fig. 3A) and found that CdiA-CTEC869 cleaves between C70 and A71, near the 3′-end of the acceptor stem (Fig. 3 B and C). Crystal structures of GTP·EF-Tu·tRNA ternary complexes show that the scissile phosphodiester is close to EF-Tu but is solvent-exposed to allow nuclease access (21, 22). Taken together, these data demonstrate that efficient toxin activity requires EF-Ts, EF-Tu, and GTP.
Fig. S3.
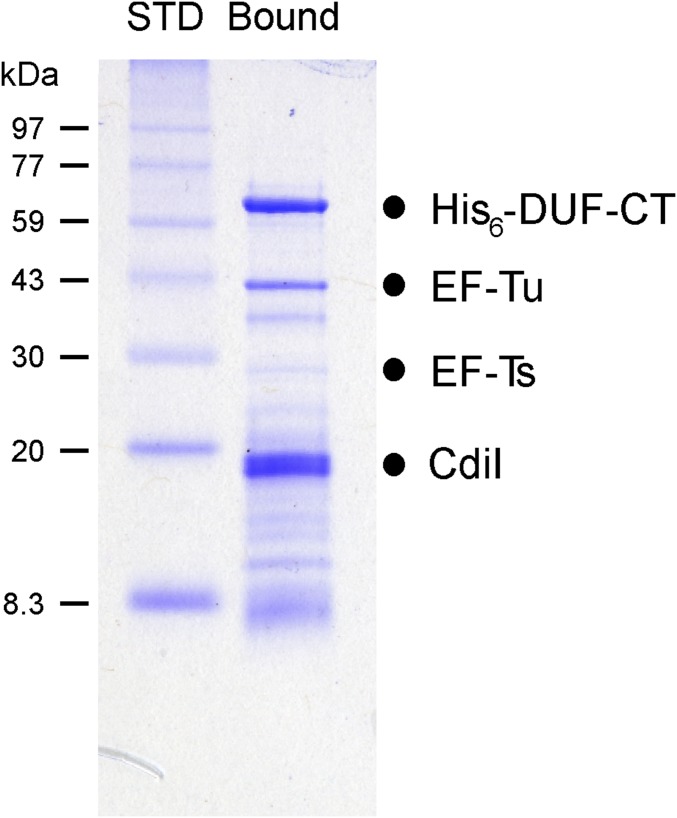
EF-Tu copurifies with the CdiA-CT·CdiIEC869 complex. The His6-DUF-CdiA-CT·CdiIEC869 complex was purified from E. coli lysates by Ni2+ affinity chromatography under nondenaturing conditions. SDS/PAGE analysis reveals that endogenous EF-Tu copurifies with the complex under these conditions. Electrophoretic migration positions are indicated for each protein.
Fig. 3.
CdiA-CTEC869 cleaves at the tRNAGln acceptor stem. (A) tRNACUGGln sequence showing the hybridized S1 probe and oligonucleotide standards used to map the toxin cleavage site. (B) S1 nuclease protection assays. RNA was isolated from CDIEC869 competition cocultures and cells intoxicated by intracellular CdiA-CTEC869 expression. Samples from in vitro nuclease reactions were also analyzed. Where indicated, the neutralizing effect of CdiIEC869 immunity protein was examined. RNA samples were incubated with the 3′-radiolabeled S1 probe and treated with S1 nuclease as described in Materials and Methods. A portion of the S1 probe-tRNAGln heteroduplex sequence is shown to the right of the autoradiogram. (C) Secondary structure diagram of tRNACUGGln. The orange arrow indicates cleavage site within the acceptor stem.
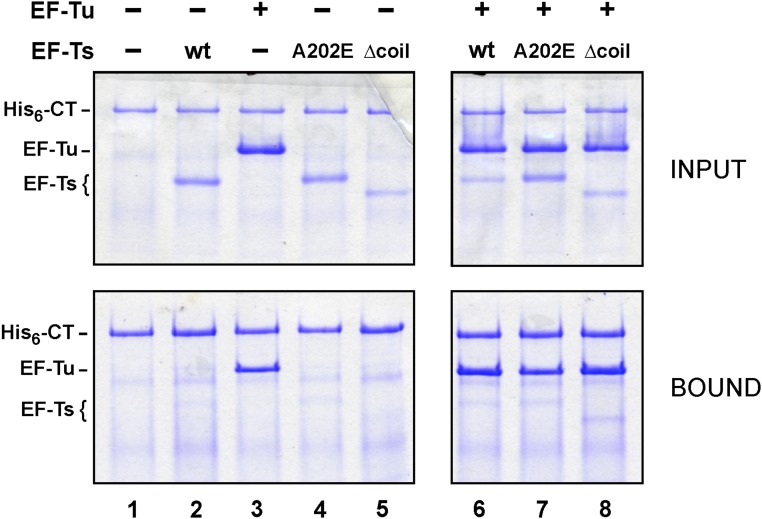
The collaboration between CdiA-CTEC869, EF-Tu, and EF-Ts is reminiscent of the bacteriophage Qβ replicase complex (23, 24). Qβ is a member of the Leviviridae family of small RNA phages, which use RNA-dependent RNA polymerases to replicate their genomes. The phage-encoded β-subunit requires host-cell factors EF-Ts, EF-Tu, and ribosomal protein S1 to form the functional replicase complex. Intriguingly, the coiled-coil domain of EF-Ts interacts with the phage β-subunit (Fig. S4A) and is critical for replicase activity (25–27). These observations raise the possibility that the tsf mutations disrupt formation of a higher order complex with EF-Tu and CdiA-CTEC869. We explored this hypothesis by first examining the plating efficiency of phage R17, which is another levivirus that requires EF-Ts and EF-Tu for replication, on the tsf mutants. We found that tsf(R219P) and tsf(∆coil) mutants are completely resistant to phage R17, whereas tsf(A202E) cells are partially resistant (Fig. S4B). To determine if the toxin forms a stable ternary complex with the elongation factors, we tested whether EF-Tu and EF-Ts copurify with His6-tagged CdiA-CTEC869 during Ni2+ affinity chromatography. EF-Tu bound to the toxin, but substantially less EF-Ts was copurified, even when the binding reactions included EF-Tu and GTP (Fig. S5, lanes 2 and 6). Thus, CdiA-CTEC869 binds to EF-Tu with high affinity, but EF-Ts does not appear to associate stably with the complex.
Fig. S4.
R17 phage replication is disrupted in tsf mutants. (A) The locations of EF-Ts Ala202 and Arg219 are shown in the context of the Qβ replicase complex (PDB ID code 3MMP). (B) M13 and R17 phage lysates were serial diluted and spotted onto lawns of E. coli MG1655 F´ cells that carry tsf+, tsf(A202E), tsf(R219P), and tsf(∆coil) alleles. The numbers of phage delivered per spot are indicated.
Fig. S5.
Binding of the CdiA-CTEC869 toxin to EF-Tu. Purified His6–DUF–CdiA-CTEC869 was mixed with EF-Tu and EF-Ts variants as indicated and then subjected to Ni2+ affinity chromatography under nondenaturing conditions. The Upper panels (INPUT) show the protein mixtures before chromatography, and the Lower panels (BOUND) show the proteins that eluted with imidazole.
We next considered the possibility that the guanine nucleotide exchange (GEF) function of EF-Ts contributes to toxin activity. If EF-Tu GTPase activity is stimulated by CdiA-CTEC869, then EF-Ts could be required to displace GDP from EF-Tu, thereby accelerating the formation of new GTP·EF-Tu·tRNA substrate complexes. To probe whether GTP hydrolysis accompanies toxin activity, we first tested nuclease activity in reactions supplemented with nonhydrolyzable GDPNP, which supports the same level of tRNACUGGln cleavage as GTP (Fig. 4A, lanes 5 and 10). We next used TLC to monitor the hydrolysis of radiolabeled GTP in tRNase reactions. In vitro-transcribed tRNACUGGln (rather than total E. coli tRNA) was used in these experiments to ensure the availability of sufficient substrate to drive detectable GTPase activity. Notably, we did not observe GDP production even in the presence of 0.5 µM wild-type EF-Ts, which supported efficient cleavage of 10 µM tRNACUGGln substrate (Fig. 4B, lane 5). Because GTPase activity was not detected, we performed a positive-control reaction using polynucleotide kinase and radiolabeled GTP to phosphorylate 5 µM of an oligonucleotide substrate. We observed GDP production in the latter reaction (Fig. 4B, lane 1), indicating that GTPase activity is detectable under these assay conditions. Together, these data show that GTP hydrolysis is not concomitant with tRNA cleavage, suggesting that GEF function per se does not promote toxin activity.
Fig. 4.
GTPase activity is not stimulated by CdiA-CTEC869. (A) Purified CdiA-CTEC869 (0.1 µM), EF-Tu (0.25 µM), and EF-Ts (0.25 µM) were added to total E. coli RNA (0.1 mg/mL) as indicated. Reactions were supplemented with GTP or nonhydrolyzable GDPNP (1 mM) and analyzed by Northern blot hybridization for E. coli tRNACUGGln. (B) GTPase assays. tRNACUGGln transcript (10 µM), radiolabeled GTP (15 µM), CdiA-CTEC869 (0.5 µM), EF-Tu, and/or EF-Ts (0.5 µM) were incubated at ambient temperature for 10 min. Lane 1 corresponds to a polynucleotide kinase reaction using radiolabeled GTP as the phosphoryl donor. Reactions were analyzed by TLC (Upper) and denaturing polyacrylamide gel analysis (Lower) as described in Materials and Methods. The migration positions of GDP and GTP are indicated.
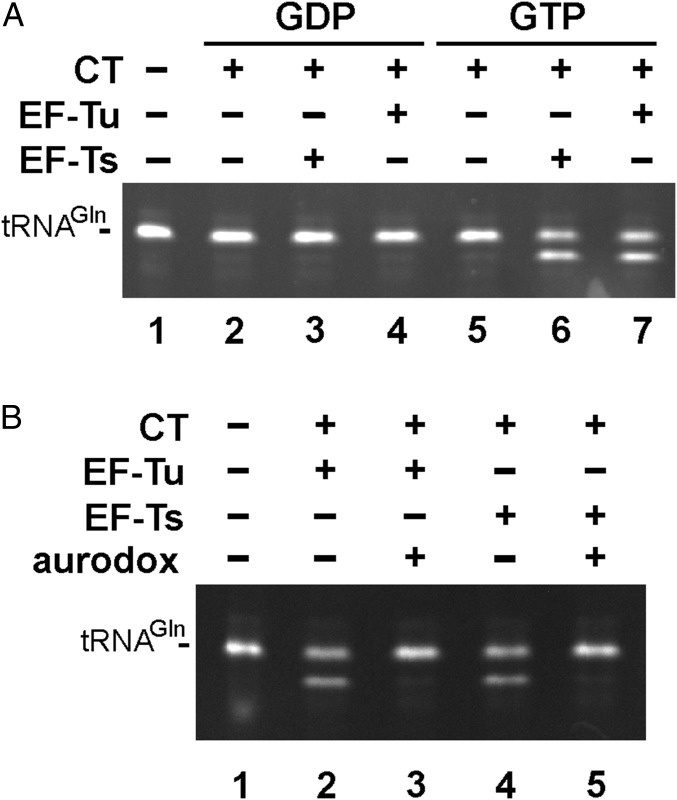
We noted that EF-Tu alone supported significant nuclease activity at the high tRNACUGGln concentrations (10 µM) used in the GTPase assays (Fig. 4B, lane 4). Because CdiA-CTEC869 and EF-Tu were purified from tsf(Δcoil) cells for this experiment, and additional EF-Ts(Δcoil) did not enhance activity (Fig. 4B, lane 6), we reasoned that EF-Tu alone could be sufficient for toxin activity. Indeed, we obtained efficient GTP-dependent nuclease activity in reactions containing 1 µM tRNACUGGln transcript and 0.5 µM EF-Tu (Fig. 5A, lanes 4 and 7). However, similar activity was observed in reactions containing toxin and EF-Ts (Fig. 5A, lanes 3 and 6). Because EF-Ts does not bind GTP, this latter observation suggests that trace EF-Tu contamination in toxin preparations (Fig. S5, lane 1) may support tRNase activity in conjunction with EF-Ts. To test this possibility, we examined the effect of the antibiotic aurodox on in vitro nuclease reactions. Aurodox binds specifically to EF-Tu and significantly reduces its affinity for tRNA (28). We found that aurodox blocks EF-Tu–dependent tRNase activity (Fig. 5B, lanes 2 and 3), consistent with the hypothesis that the toxin acts on GTP⋅EF-Tu⋅tRNA complexes. Aurodox also abrogated nuclease activity in the EF-Ts reactions (Fig. 5B, lanes 4 and 5), indicating that contaminating EF-Tu contributes to this activity. Together, these results indicate that EF-Tu is required for CdiA-CTEC869 nuclease activity, whereas EF-Ts promotes activity at low substrate concentrations.
Fig. 5.
Roles of EF-Ts and EF-Tu in toxin-dependent tRNACUGGln cleavage. (A) tRNACUGGln transcript (1 µM) was incubated with CdiA-CTEC869 (0.5 µM), EF-Tu, and/or EF-Ts (0.5 µM) and GDP or GTP (1 mM) for 10 min at ambient temperature. Reactions were resolved on urea-polyacrylamide gels and visualized by ethidium bromide staining. (B) tRNACUGGln transcripts (1 µM) were treated with purified CdiA-CTEC869 (0.5 µM), EF-Tu, and/or EF-Ts (0.5 µM) and GTP (1 mM) for 10 min at ambient temperature. Aurodox (4 µM) was added to reactions where indicated. Reactions were analyzed as in A.
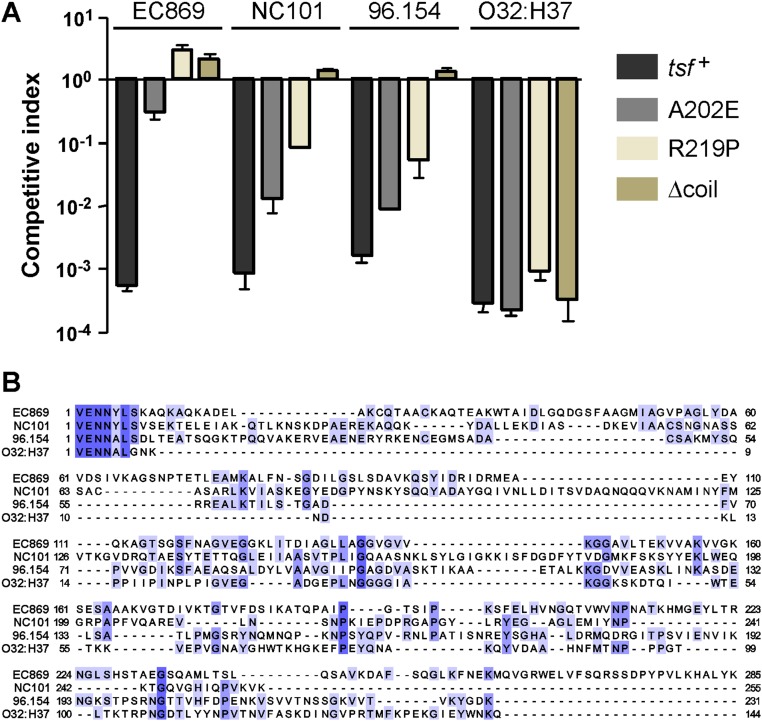
Finally, we identified two additional CDI toxins that require wild-type EF-Ts to promote their toxicity. Competition cocultures showed that E. coli tsf(∆coil) target cells are resistant to growth inhibition by the CDI toxins from E. coli isolates NC101 and 96.154 (Fig. S6A). In addition, the E. coli tsf(A202E) and tsf(R219P) mutants exhibited partial resistance to these toxins (Fig. S6A). Remarkably, CdiA-CTNC101 and CdiA-CT96.154 do not share significant sequence identity with each other, nor with the CdiA-CTEC869 toxin (Fig. S6B). Thus, EF-Ts is critical for the in vivo activities of at least three nonhomologous CDI toxins.
Fig. S6.
Mutations in the coiled-coil domain of EF-Ts confer CDI resistance to other tRNase toxins. (A) E. coli MG1655 target strains carrying the indicated tsf alleles were cocultured at a 1:1 ratio with E. coli EPI100 inhibitors that deploy CdiA-CTEC869 (EC869), CdiA-CTNC101 (NC101), CdiA-CT96.154 (96.154), or CdiA-CTO32:H37 (O32:H37). After 3 h, viable cell counts were determined for each population and used to calculate the competitive index as described in Materials and Methods. Data are presented as averages ± SEM for three independent experiments. (B) Alignment of CdiA-CT sequences from E. coli strains EC869 (NCBI reference WP_001075571.1), NC101 (WP_000554167.1), 96.154 (EIH97650.1), and O32:H37 (EIF16908.1). Sequences (beginning at the conserved VENN motif) were aligned using Clustal Omega on the Uniprot server (www.uniprot.org/align/), and the results were rendered with Jalview (www.jalview.org).
Discussion
These results show that CDIEC869-mediated growth inhibition requires two essential and core components of the translational apparatus. There are at least two models to explain how EF-Tu and EF-Ts promote CdiA-CTEC869 nuclease activity. The first mechanism entails the formation of a EF-Ts·EF-Tu·CdiA-CTEC869 ternary complex, in which the two translation factors form a scaffold that activates the toxin. In this model, the coiled-coil domain of EF-Ts would interact directly with CdiA-CTEC869, analogous to its structural role in the phage Qβ RNA replicase complex (20, 25). However, unlike the Qβ replicase, EF-Ts does not form a stable stoichiometric complex with EF-Tu and CdiA-CTEC869. Together with data showing that in vitro tRNase activity is GTP-dependent and blocked by aurodox, it appears that CdiA-CTEC869 only cleaves tRNA in the context of translationally active GTP⋅EF-Tu⋅tRNA complexes. We note that although deacylated tRNA can be cleaved in vitro, aminoacylated tRNA has greater affinity for EF-Tu and is almost certainly the physiologically relevant toxin substrate. We propose that EF-Ts acts as a critical accessory factor to load tRNA onto EF-Tu, accelerating the production of GTP⋅EF-Tu⋅tRNA substrate for cleavage. This model is supported by recent work from Blanchard and colleagues, who have shown that EF-Ts promotes both the association and dissociation of tRNA from EF-Tu (29). This activity is concomitant to yet distinct from the well-described GEF function of EF-Ts (30). Knudsen and colleagues recently suggested that the coiled-coil domain of EF-Ts, and in particular its conserved basic patch (including Arg219), could promote tRNA loading onto EF-Tu (31). This model is also supported by the observation that the EF-Ts coiled-coil resembles the tRNA-binding region of some aminoacyl tRNA synthetases (32).
Given that most characterized CDI nuclease toxins are autonomous, it is unclear why some systems have evolved to exploit target-cell factors for toxin activation. Because CdiA-CTEC869 cleaves near the 3′-end of tRNACUGGln, we hypothesize that EF-Tu positions the toxin active site near the scissile bond. However, EF-Tu is not necessary for such activity, because other CDI toxins cleave tRNA acceptor stems independently of the translation factor (5, 15). One intriguing explanation is that toxin·EF-Tu interactions serve additional roles in intercellular signaling. In this model, toxins delivered into immune sibling cells would form EF-Tu·CdiA-CT·CdiI ternary complexes that modulate physiology in response to cell density. EF-Tu function could be modulated directly by CdiA-CT·CdiIEC869, analogous to the posttranslational modifications that regulate the translation factor in response to environmental cues (33, 34). Alternatively, it is possible that tRNA fragments produced from sublethal nuclease activity could regulate gene expression in a manner similar to that described for eukaryotes (35). This general model is supported by work from Cotter and colleagues showing that intersibling CDI toxin exchange influences biofilm gene expression in Burkholderia thailandensis (36).
Recently Mougous and colleagues reported an interaction between EF-Tu and the Tse6 toxin of Pseudomonas aeruginosa PAO1 (37). Tse6 is a type VI secretion system (T6SS) effector with novel NADase activity. The crystal structure of the EF-Tu·Tse6 complex reveals that the toxin makes contact with nucleotide-binding domain 1 of EF-Tu, exploiting a surface that binds directly to EF-Ts. Unlike the CdiA-CTEC869 toxin, EF-Tu is not required for the enzymatic activity of Tse6. However, Tse6 mutations that block EF-Tu binding also abrogate the ability to intoxicate target bacteria, suggesting that the interaction with EF-Tu could be required for T6SS-mediated secretion of Tse6 or entry of the effector into the target-cell cytoplasm (37). These observations, together with the data presented herein, show that diverse bacterial toxin domains exploit EF-Tu to promote interbacterial competition.
Materials and Methods
Bacteria and CDI Competition Cocultures.
Bacterial strains are listed in Table S1. Bacteria were grown at 37 °C in LB medium or on LB agar unless otherwise noted. Media were supplemented with antibiotics at the following concentrations: ampicillin (Amp) 150 μg⋅mL−1, kanamycin (Kan) 40 μg⋅mL−1, chloramphenicol (Cm) 12.5 or 34 μg⋅mL−1, and spectinomycin (Spc) 50 μg⋅mL−1. For competition cocultures, inhibitor and target cells were first grown to midlog phase, then mixed at a 10:1 ratio in medium without antibiotics. Cocultures were incubated with shaking at 225 rpm in baffled flasks. After 3 h, viable inhibitor and target cells were enumerated as colony-forming units on selective LB agar. The competitive index was calculated as the ratio of target to inhibitor cells at 3 h divided by the initial ratio.
Table S1.
Bacterial strains
| Strain | Description | Source |
| XL-1 | F´::Tn10 proA+B+ lacIq ∆(lacZ)M15/recA1 endA1 gyrA96 thi hsdR17 (rk– rk+) glnV44 relA1 lac. TetR NalR | Stratagene |
| EPI100 | F– mcrA ∆(mrr-hsdRMS-mcrBC) φ80dlacZ∆M15 ∆lacXcZ∆M15 ∆lacX recA1 endA1 araD139 ∆(ara, leu)7697 galU galK λ– rpsL nupG, StrR | Epicentre |
| DY378 | W3110 λcI857 ∆(cro-bioA) | (44) |
| DA5438 | MG1655 (F− λ− ilvG− rbf-50 rph-1) | Dan Andersson |
| DL5562 | JCM158 ∆wzb::kan, KanR | (45) |
| DL5850 | MG1655 ∆araBAD::spec, SpcR | (1) |
| CH2016 | X90 (DE3) ∆rna ∆slyD::kan, RifR, KanR | (40) |
| DL8818 | MG1655 F´::Tn10 ∆wzb ara::spec, TetR SpcR | This study |
| DL8819 | MG1655 F´::Tn10 ∆wzb ara::spec tsf(A202E), TetR SpcR | This study |
| DL8820 | MG1655 F´::Tn10 ∆wzb ara::spec tsf(R219P), TetR SpcR | This study |
| DL8821 | MG1655 F´::Tn10 ∆wzb ara::spec tsf(∆coil), TetR SpcR | This study |
| DL8530 | CH2016 tsf(∆coil), RifR, KanR | This study |
| DL8546 | CH2016 tsf(A202E), RifR, KanR | This study |
| DL8698 | MG1655 ∆wzb | This study |
| DL8705 | MG1655 ∆wzb ara::spec, SpcR | This study |
| DL8730 | MG1655 ∆wzb ara::spec tsf(A202E), SpcR | This study |
| DL8731 | MG1655 ∆wzb ara::spec tsf(∆coil), SpcR | This study |
| DL8783 | MG1655 ∆wzb ara::spec tsf(R219P), SpcR | This study |
KanR, kanamycin resistant; NalR, nalidixic acid resistant; RifR, rifampicin resistant; SpcR, spectinomycin resistant; StrR, streptomycin resistant; TetR, tetracycline resistant.
Plasmid Constructions.
Plasmids and oligonucleotides are listed in Tables S2 and S3, respectively. The tufA, tsf, and cdiA-CT/cdiIEC869 genes were amplified with primer pairs CH3671/CH367, CH3673/CH2188, and 3875/3834 (respectively) and ligated to plasmid pCH10068 using BamHI/XhoI restriction sites. The cdiIEC869 gene was amplified with primers 3890/3781 and CH220/CH221 and ligated to pET21b (NdeI/XhoI) and pTrc99KX (KpnI/XhoI), respectively. The duf-cdiA-CT/cdiIEC869 fragment was amplified from pCH10525 with CH2324/CH3834 and ligated to plasmid pCH6243 using SpeI/XhoI sites. The cdiA-CT/cdiI modules from E. coli 96.154 and O32:H37 were amplified with primers CH3170/CH3171 and CH3567/CH3568 (respectively) and combined with cdiAEC93 fragments amplified with 1527/2470 and 1663/2368 by overlapping-end PCR (15). The final products were electroporated together with plasmid pCH10163 into E. coli strain DY378 and recombinant plasmids selected as described (15).
Table S2.
Plasmids
| Plasmid | Description | Source |
| pRK6 | pBAD33::gfp, AmpR CmR | (46) |
| pRK793 | Expresses His6-tagged TEV protease, AmpR | Addgene |
| pWEB::TNC | Cosmid cloning vector, AmpR CmR | Epicentre |
| pRE112 | Allelic exchange vector, CmR | (39) |
| pZS21-bamA+ | pZS21 derivative that expresses E. coli bamA, KanR | (47) |
| pCH2262 | Constitutive expression of chimeric cdiAEC93-CTO32:H37 and cdiIO32:H37, CmR | This study |
| pTrc99KX | Derivative of pTrc99a that contains a 5′-KpnI restriction site and 3′-SpeI and XhoI sites, AmpR | (12) |
| pCH6243 | pET21::his6-cdiA-CT/cdiIUPEC536, AmpR | (17) |
| pCH9305 | Constitutive expression of chimeric cdiAEC93-CTo11EC869 and cdiIo11EC869, CmR | (15) |
| pCH10163 | Cosmid pCdiA-CT/pheS* that carries a kan-pheS* cassette in place of the E. coli EC93 cdiA-CT/cdiI coding sequence. Used for allelic exchange and counter selection, CmR KanR | (15) |
| pCH10068 | pSH21P::trxA-TEV-rhs-CT(H208A)orphan-rhsIorphan, orphan rhs-CT/rhsI module fused to His6-tagged thioredoxin gene through TEV cleavage site, AmpR | (48) |
| pCH10256 | pTrc99KX::cdiIEC869, AmpR | This study |
| pCH10445 | Constitutive expression of chimeric cdiAEC93-CTECL and cdiIECL, CmR | (12) |
| pCH10525 | Constitutive expression of chimeric cdiAEC93-CTEC869 and cdiIEC869, CmR | (16) |
| pCH10673 | Constitutive expression of chimeric cdiAEC93-CTEC536 and cdiIEC536, CmR | (16) |
| pCH11434 | Constitutive expression of chimeric cdiAEC93-CTNC101 and cdiINC101, CmR | (5) |
| pCH11435 | Constitutive expression of chimeric cdiAEC93-CT96.154 and cdiI96.154, CmR | This study |
| pDAL660Δ1–39 | Constitutive expression of cdiAEC93 and cdiIEC93, CmR | (9) |
| pDAL1028 | pREG153 carrying sfsA – dxr of the E. coli MG1655 chromosome, AmpR | This study |
| pDAL1029 | pRE112::tsf(A202E), CmR | This study |
| pDAL1030 | pRE112::tsf(R219P), CmR | This study |
| pDAL1031 | pRE112::tsf(∆coil), CmR | This study |
| pDAL1032 | pRE112::∆wzb, CmR | This study |
| pDAL1033 | pSH21::trxA-TEV-CTEC869-cdiIEC869, AmpR | This study |
| pDAL1034 | pSH21::trxA-TEV-tsf, AmpR | This study |
| pDAL1035 | pSH21::trxA-TEV-tufA, AmpR | This study |
| pDAL1036 | pSH21::trxA-TEV-tsf(A202E), AmpR | This study |
| pDAL1037 | pSH21::trxA-TEV-tsf(∆coil), AmpR | This study |
| pDAL1038 | pET21b::cdiIEC869, AmpR | This study |
| pDAL1039 | pET21::his6-DUF-cdiA-CTEC869-cdiIEC869, AmpR | This study |
AmpR, ampicillin resistance; CmR, chloramphenicol resistance; KanR, kanamycin resistance.
Table S3.
Oligonucleotides
| Identifier | Descriptive name | Sequence |
| 1527 | EC93-up-for | 5′-GAA CAT CCT GGC ATG AGC G |
| 1663 | EC93-down-for | 5′-CCC AAA GGT TAG ACA CCA GAC C |
| 2368 | EC93-down-rev | 5′-GTT GGT AGT GGT GGT GCT G |
| 2470 | EC93-up-rev | 5′-ATT ATT CTC AAC CGA GTT CCT ACC TG |
| 3513 | cosmid sequencing | 5′-AGC CAA GCC GAG AAG AAA GC |
| 3617 | tsf-for | 5′-CGA AGA TAA CGC ATC TGC GG |
| 3618 | tsf-rev | 5′-CCT GAG ACT GTC AAC GAT TG |
| 3677 | tsf-mut-SacI-for | 5′-GGC GAG CTC TGA AGC TAA CGG CGA CAT CG |
| 3678 | tsf-mut-XbaI-rev | 5′-GGC TCT AGA TCC TGA GCC ATA CGA TCC AG |
| 3781 | cdiI-nostop-XhoI-rev | 5′-GCG CTC GAG ACC TAC TGC CTC AAA AAA AC |
| 3818 | tsf-cc-Sac-for | 5′-GCC GAG CTC TAC TGG CGC AGG CAT GAT GG |
| 3819 | tsf-cc-OE-Bam-rev | 5′-AGC TTC ACC CCC GGG TTC GTC TTC CGG TTT GAT GAA TTC |
| 3820 | tsf-cc-OE-Bam-for | 5′-GAA CCC GGG GGT GAA GCT GAA GTT TCT CTG ACC GGT CAG |
| 3821 | tsf-cc-Xba-rev | 5′-GCC TCT AGA GCG CCA CGG AAC AGG TTA CC |
| 3834 | cdiI-stop-Xho-rev | 5′-CGG CTC GAG CTA ACC TAC TGC CTC AAA AAA AC |
| 3836 | tsf-seq-for | 5′-ACC AGG TAG TAC ACG TTT GG |
| 3837 | tsf-seq-rev | 5′-AAT ACT AGC GAC ATT GTA CG |
| 3875 | CTEC869-Bam-for | 5′-GGC GGG ATC CAC TTC TGA GAA CCT GTA TTT TCA GTC TGA ATT CGT TGA GAA TAA CTA TCT G |
| 3890 | cdiI-Nde-for | 5′-GCA ACT ACC ATA TGA AAT TAA CTG TAG ATA GC |
| 3894 | Gln2 probe | 5′-TCG GAA TGC CGG AAT CAG A |
| 3900 | wzb-up-Sac-for | 5′-GGA ACG AGC TCG AGC GCA TTT CTT TGC AGG C |
| 3901 | wzb-up-Bam-for | 5′-CGT AGG GAT CCC ATG ACG ATT ACC AGT TAT G |
| 3902 | wzb-down-Bam-for | 5′-GAT CGG GAT CCC AGG TAT AAG AAT GAC AG |
| 3903 | wzb-down-Xba-for | 5′-GGA ACT CTA GAG CAC ATT AAG CGT AAA CAC C |
| 3918 | wzb-KO-for | 5′-GAC CGA CAC CGC TGA CTG GC |
| 3919 | wzb-KO-rev | 5′-CGT CGC TGC TCA GGG TGT AG |
| CH220 | EC869-cdiI-Kpn-for | 5′-TTT GGT ACC ATG AAA TTA ACT GTA G |
| CH221 | EC869-cdiI-Xho-rev | 5′-GGA CTC GAG CTA AAC TAC TGC CTC AAA A |
| CH452 | argQZYV probe | 5′-CCT CCG ACC GCT CGG TTC G |
| CH800 | aspTUV probe | 5′-TCG AAC CCG CGA CCC CCT GCG |
| CH801 | asnTUVW probe | 5′-CTC GAA CCA GTG ACA TAC GG |
| CH1417 | gltT probe | 5′-CCC CTG TTA CCG CCG TG |
| CH2188 | tufA-Xho-rev | 5′-ATT CTC GAG TAA TTA GCC CAG AAC |
| CH2324 | DUF-CT-Spe-for | 5′-TTT GAG CTC ACT AGT AAT TAC GAT TCT GTC CGG GAA C |
| CH3170 | 96.154-OE-for | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATG CGC TCA GTG ATT TAA CCG AAG CTA C |
| CH3171 | 96.154-OE-rev | 5′-GGT CTG GTG TCT AAC CTT TGG GTT ATT GCA GTG TAT TGA GTA ACT CAG CC |
| CH3567 | O32:H37-OE-for | 5′-CAG GTA GGA ACT CGG TTG AGA ATA ATG CGC TGG GTA AC |
| CH3568 | O32:H37-OE-rev | 5′-GGT CTG GTG TCT AAC CTT TGG TTA CTG TTC GTT AAA TC TCG TTT C |
| CH3671 | TEV-tsf-Bam-for | 5′-TTG GAT CCA CTT CTG AGA ACC TGT ATT TTC AGG CTG AAA TTA CCG CAT CCC TGG |
| CH3672 | tsf-Xho-rev | 5′-CTT CTC GAG AAT TAA GAC TGC TTG G |
| CH3673 | TEV-tufA-Bam-for | 5′-TTG GAT CCA CTT CTG AGA ACC TGT ATT TTC AGT CTA AAG AAA AAT TTG AAC GTA C |
| CH3850 | glnX S1 probe | 5′-TGG CTG GGG TAC GAG GAT TCG AAC CTC GGA ATG CCG GAA TC |
| CH3851 | glnX A66 marker | 5′-TAC GAG GAT TCG AAC CTC GGA ATG CCG GAA TC |
| CH3852 | glnX C70 marker | 5′-GGG GTA CGA GGA TTC GAA CCT CGG AAT GCC GGA ATC |
| CH3853 | glnX A71 marker | 5′-TGG GGT ACG AGG ATT CGA ACC TCG GAA TGC CGG AAT C |
UV Mutagenesis and Complementation Analysis.
E. coli MG1655 cells carrying pZS21-bamA+ were grown to midlog phase, harvested by centrifugation, and resuspended in 0.1 M MgSO4 at an optical density at 600 nm (OD600) of 0.4. The suspension was irradiated at 32 J/m2 in a Stratalinker 1800. Irradiated cells were collected by centrifugation and resuspended in LB medium and maintained in the dark for all subsequent steps. Irradiated cells were cultured at a 1:1 ratio with E. coli EPI100 inhibitors carrying pCH10525 to select for CDIR mutants. Surviving target cells were isolated on Kan-supplemented LB agar, pooled, and subjected to two additional rounds of CDIEC869 selection. Individual colonies were isolated from the final selection and CDIR phenotypes confirmed in competition cocultures. CDIR mutations were mapped by complementation analysis. Mutants were transformed with plasmid pRK6, then transduced with a low-copy pREG153 cosmid library of E. coli genomic DNA (38). Transductants were individually cocultured with CDIEC869 inhibitors in microtiter plates and target-cell growth monitored by fluorimetry. Wells showing low GFP fluorescence indicate growth inhibition, suggesting complementation to the CDIS phenotype. Results were compared with control cocultures containing mock (CDI—) inhibitor cells. The complementing cosmid was sequenced using primer 3513.
Construction and Genetic Manipulation of MG1655 Target Cells.
The ∆wzb mutation was introduced into E. coli MG1655 by allelic exchange. Sequences flanking wzb were amplified with primers 3900/3901 and 3902/3903. The upstream fragment was digested with SacI/BamHI and the downstream fragment with BamHI/XbaI. The two fragments were then ligated to pRE112 using SacI/XbaI restriction sites. The resulting plasmid was introduced into E. coli DL8705 by conjugation, and exconjugants were selected on LB agar supplemented with Cm. Counterselection was performed on LB agar supplemented with 5% (wt/vol) sucrose (39). Deletion of wzb in DL8698 was confirmed by PCR using 3918/3919. The ∆araBAD::spec allele from DL5850 was introduced into DL8698 by phage P1 transduction. The tsf(A202E) and tsf(R219P) mutations were introduced into E. coli MG1655 using allelic exchange. The alleles were amplified with 3677/3678 and ligated to pRE112 using SacI/XbaI restrictions sites. The tsf(∆coil) allele (in which Val186–Gly225 is replaced by the EPGGEA peptide) (20) was constructed from fragments amplified with 3818/3819 and 3820/3821. The products were combined by OE-PCR with 3818/3821, then ligated to plasmid pRE112 using SacI/XbaI restriction sites. Recombinants were screened by PCR using 3836/3837, and all alleles were confirmed by DNA sequencing. The F´::Tn10 episome from E. coli XL-1 was mated into E. coli MG1655 derivatives to generate strains for phage-plating assays.
Protein Purification and Analysis.
All proteins were overproduced in E. coli CH2016 by addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) to 1 mM. After incubation for 2 h, cells were harvested and frozen at –80 °C. Cell pellets were resuspended in buffer A [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 10 mM β-mercaptoethanol (β-ME), 0.05% Triton X-100, 20 mM imidazole] and broken by two passages through a French press at 20,000 psi. Cell debris was removed by two rounds of centrifugation at 16,000 × g at 4 °C. His6-tagged proteins were purified by Ni2+ affinity chromatography in buffer A. For the purification of CdiA-CTEC869 fusions, affinity resins were washed with 20 mM Tris⋅HCl (pH 7.5) and 6 M guanidine–HCl to release CdiIEC869 before elution. His6-tagged proteins were eluted with 20 mM Tris⋅HCl (pH 7.5) and 250 mM imidazole and dialyzed against buffer B [20 mM sodium phosphate (pH 7.8), 150 mM NaCl, 10 mM β-ME]. After dialysis, His6-TrxA was cleaved using TEV protease and removed by Ni2+ affinity chromatography. Purified proteins were stored at –20 °C in buffer B plus 50% glycerol. Protein–protein interactions were assessed in buffer C [10 mM sodium phosphate (pH 7.5), 150 mM NaCl, 20 mM MgCl2, 1 mM DTT] using 5 µM of each protein in 500 µL reaction at 4 °C as previously described (1).
RNA Isolation and Analysis.
Competition cocultures were harvested into an equal volume of ice-cold methanol, and the cells were collected by centrifugation and frozen at –80 °C. Total RNA was extracted with guanidinium isothiocyanate (GITC)-phenol as described (40, 41) and resuspended in 10 mM sodium acetate (pH 5.2), which stabilizes the aminoacyl linkage (42). Total RNA (5 µg) was analyzed by Northern blot hybridization using [32P]-labeled oligonucleotides 3894, CH452, CH800, CH801, and CH1417 as probes (16). S30 extracts were prepared from E. coli CH2016, DL8530, and DL8546 cells grown to OD600 ∼ 0.5. Cells were resuspended in buffer B and broken by passage through a French press at 20,000 psi. Lysates were clarified by two cycles of centrifugation at 14,000 × g for 10 min. Purified CdiA-CTEC869 (0.1 µM) was added to cell supernatants and incubated ambient temperature for 1 h. RNA was then isolated by GITC-phenol extraction and analyzed by Northern blot hybridization. For analysis of toxin activity using purified components, total E. coli RNA was incubated with purified CdiA-CTEC869, EF-Tu, EF-Ts, and/or CdiIEC869 in buffer C or buffer D [20 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1 mM DTT]. tRNACUGGln was produced by in vitro transcription as described previously (43). S1 nuclease protection analysis was performed as described (41) using oligonucleotide CH3850 to map the 3′-end of tRNACUGGln. Migration standard oligonucleotides (CH3851, CH3852, and CH3853) were 3′-labeled with [α-32P]-cordycepin triphosphate and terminal transferase and desalted using a G-25 spin column and 5′-phosphorylated with polynucleotide kinase and unlabeled ATP.
GTPase Assays.
In vitro-transcribed tRNACUGGln (10 µM) was incubated with CdiA-CTEC869, EF-Tu, and EF-Ts variants (0.5 µM each) in buffer D. Reactions were supplemented with 15 µM GTP (0.033 µM [α-32P]-GTP) and incubated at ambient temperature for 10 min. Positive control reactions were conducted under the same conditions with oligonucleotide 3894 (5 µM) and polynucleotide kinase. Reactions were resolved by TLC on polyethyleneimine cellulose (Polygram cel 300 PEI/UV254) using 0.3 M sodium phosphate (pH 3.5) as the mobile phase. Cellulose sheets were exposed to phosphorimager screens and chromatograms visualized on a Bio-Rad phosphorimager using Quantity One software.
Acknowledgments
We thank Kurt Fredrick, John Perona, and Zachary Ruhe for technical insight and helpful discussions and Julia Willett for plasmid constructions. This work was supported by NIH Grant R01 GM117373 (to C.S.H. and D.A.L.) and National Science Foundation Grant MCB1545720 (to D.A.L. and C.S.H.). A.M.J. received support from the Ralph M. Parsons Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619273114/-/DCSupplemental.
References
- 1.Aoki SK, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468(7322):439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes CS, Koskiniemi S, Ruhe ZC, Poole SJ, Low DA. Mechanisms and biological roles of contact-dependent growth inhibition systems. Cold Spring Harb Perspect Med. 2014;4(2):a010025. doi: 10.1101/cshperspect.a010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruhe ZC, Low DA, Hayes CS. Bacterial contact-dependent growth inhibition. Trends Microbiol. 2013;21(5):230–237. doi: 10.1016/j.tim.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruhe ZC, Wallace AB, Low DA, Hayes CS. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. MBio. 2013;4(4):e00480-13. doi: 10.1128/mBio.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willett JL, Gucinski GC, Fatherree JP, Low DA, Hayes CS. Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. Proc Natl Acad Sci USA. 2015;112(36):11341–11346. doi: 10.1073/pnas.1512124112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Iyer LM, Aravind L. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res. 2011;39(11):4532–4552. doi: 10.1093/nar/gkr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willett JL, Ruhe ZC, Goulding CW, Low DA, Hayes CS. Contact-dependent growth inhibition (CDI) and CdiB/CdiA two-partner secretion proteins. J Mol Biol. 2015;427(23):3754–3765. doi: 10.1016/j.jmb.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki SK, et al. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309(5738):1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- 10.Aoki SK, Webb JS, Braaten BA, Low DA. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J Bacteriol. 2009;191(6):1777–1786. doi: 10.1128/JB.01437-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb JS, et al. Delivery of CdiA nuclease toxins into target cells during contact-dependent growth inhibition. PLoS One. 2013;8(2):e57609. doi: 10.1371/journal.pone.0057609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck CM, et al. CdiA from Enterobacter cloacae delivers a toxic ribosomal RNase into target bacteria. Structure. 2014;22(5):707–718. doi: 10.1016/j.str.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolakakis K, et al. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol Microbiol. 2012;84(3):516–529. doi: 10.1111/j.1365-2958.2012.08039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PM, et al. Functional diversity of cytotoxic tRNase/immunity protein complexes from Burkholderia pseudomallei. J Biol Chem. 2016;291(37):19387–19400. doi: 10.1074/jbc.M116.736074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morse RP, et al. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc Natl Acad Sci USA. 2012;109(52):21480–21485. doi: 10.1073/pnas.1216238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruhe ZC, Nguyen JY, Beck CM, Low DA, Hayes CS. The proton-motive force is required for translocation of CDI toxins across the inner membrane of target bacteria. Mol Microbiol. 2014;94(2):466–481. doi: 10.1111/mmi.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diner EJ, Beck CM, Webb JS, Low DA, Hayes CS. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI) Genes Dev. 2012;26(5):515–525. doi: 10.1101/gad.182345.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson PM, et al. Unraveling the essential role of CysK in CDI toxin activation. Proc Natl Acad Sci USA. 2016;113(35):9792–9797. doi: 10.1073/pnas.1607112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blattner FR, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277(5331):1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 20.Karring H, Björnsson A, Thirup S, Clark BF, Knudsen CR. Functional effects of deleting the coiled-coil motif in Escherichia coli elongation factor Ts. Eur J Biochem. 2003;270(21):4294–4305. doi: 10.1046/j.1432-1033.2003.03822.x. [DOI] [PubMed] [Google Scholar]
- 21.Nissen P, Thirup S, Kjeldgaard M, Nyborg J. The crystal structure of Cys-tRNACys-EF-Tu-GDPNP reveals general and specific features in the ternary complex and in tRNA. Structure. 1999;7(2):143–156. doi: 10.1016/s0969-2126(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 22.Nissen P, et al. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995;270(5241):1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- 23.Blumenthal T, Carmichael GG. RNA replication: Function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- 24.Blumenthal T, Landers TA, Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci USA. 1972;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karring H, et al. Qbeta-phage resistance by deletion of the coiled-coil motif in elongation factor Ts. J Biol Chem. 2004;279(3):1878–1884. doi: 10.1074/jbc.M306605200. [DOI] [PubMed] [Google Scholar]
- 26.Kidmose RT, Vasiliev NN, Chetverin AB, Andersen GR, Knudsen CR. Structure of the Qbeta replicase, an RNA-dependent RNA polymerase consisting of viral and host proteins. Proc Natl Acad Sci USA. 2010;107(24):10884–10889. doi: 10.1073/pnas.1003015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita D, Tomita K. Assembly of Qbeta viral RNA polymerase with host translational elongation factors EF-Tu and -Ts. Proc Natl Acad Sci USA. 2010;107(36):15733–15738. doi: 10.1073/pnas.1006559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahams JP, van Raaij MJ, Ott G, Kraal B, Bosch L. Kirromycin drastically reduces the affinity of Escherichia coli elongation factor Tu for aminoacyl-tRNA. Biochemistry. 1991;30(27):6705–6710. doi: 10.1021/bi00241a010. [DOI] [PubMed] [Google Scholar]
- 29.Burnett BJ, et al. Elongation factor Ts directly facilitates the formation and disassembly of the Escherichia coli elongation factor Tu·GTP·aminoacyl-tRNA ternary complex. J Biol Chem. 2013;288(19):13917–13928. doi: 10.1074/jbc.M113.460014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnett BJ, et al. Direct evidence of an elongation factor-Tu/Ts·GTP·Aminoacyl-tRNA quaternary complex. J Biol Chem. 2014;289(34):23917–23927. doi: 10.1074/jbc.M114.583385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thirup SS, Van LB, Nielsen TK, Knudsen CR. Structural outline of the detailed mechanism for elongation factor Ts-mediated guanine nucleotide exchange on elongation factor Tu. J Struct Biol. 2015;191(1):10–21. doi: 10.1016/j.jsb.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Cahuzac B, Berthonneau E, Birlirakis N, Guittet E, Mirande M. A recurrent RNA-binding domain is appended to eukaryotic aminoacyl-tRNA synthetases. EMBO J. 2000;19(3):445–452. doi: 10.1093/emboj/19.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraal B, Lippmann C, Kleanthous C. Translational regulation by modifications of the elongation factor Tu. Folia Microbiol (Praha) 1999;44(2):131–141. doi: 10.1007/BF02816232. [DOI] [PubMed] [Google Scholar]
- 34.Young CC, Bernlohr RW. Elongation factor Tu is methylated in response to nutrient deprivation in Escherichia coli. J Bacteriol. 1991;173(10):3096–3100. doi: 10.1128/jb.173.10.3096-3100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diebel KW, Zhou K, Clarke AB, Bemis LT. Beyond the ribosome: Extra-translational functions of tRNA fragments. Biomark Insights. 2016;11(Suppl 1):1–8. doi: 10.4137/BMI.S35904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia EC, Perault AI, Marlatt SA, Cotter PA. Interbacterial signaling via Burkholderia contact-dependent growth inhibition system proteins. Proc Natl Acad Sci USA. 2016;113(29):8296–8301. doi: 10.1073/pnas.1606323113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitney JC, et al. An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell. 2015;163(3):607–619. doi: 10.1016/j.cell.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White-Ziegler CA, Low DA. Thermoregulation of the pap operon: Evidence for the involvement of RimJ, the N-terminal acetylase of ribosomal protein S5. J Bacteriol. 1992;174(21):7003–7012. doi: 10.1128/jb.174.21.7003-7012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards RA, Keller LH, Schifferli DM. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene. 1998;207(2):149–157. doi: 10.1016/s0378-1119(97)00619-7. [DOI] [PubMed] [Google Scholar]
- 40.Garza-Sánchez F, Janssen BD, Hayes CS. Prolyl-tRNA(Pro) in the A-site of SecM-arrested ribosomes inhibits the recruitment of transfer-messenger RNA. J Biol Chem. 2006;281(45):34258–34268. doi: 10.1074/jbc.M608052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes CS, Sauer RT. Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol Cell. 2003;12(4):903–911. doi: 10.1016/s1097-2765(03)00385-x. [DOI] [PubMed] [Google Scholar]
- 42.Janssen BD, Diner EJ, Hayes CS. Analysis of aminoacyl- and peptidyl-tRNAs by gel electrophoresis. Methods Mol Biol. 2012;905:291–309. doi: 10.1007/978-1-61779-949-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherlin LD, et al. Chemical and enzymatic synthesis of tRNAs for high-throughput crystallization. RNA. 2001;7(11):1671–1678. [PMC free article] [PubMed] [Google Scholar]
- 44.Datta S, Costantino N, Court DL. A set of recombineering plasmids for gram-negative bacteria. Gene. 2006;379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Aoki SK, et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol. 2008;70(2):323–340. doi: 10.1111/j.1365-2958.2008.06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan-Kiss RM, Wadler C, Cronan JE., Jr Long-term and homogeneous regulation of the Escherichia coli araBAD promoter by use of a lactose transporter of relaxed specificity. Proc Natl Acad Sci USA. 2002;99(11):7373–7377. doi: 10.1073/pnas.122227599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S, et al. Structure and function of an essential component of the outer membrane protein assembly machine. Science. 2007;317(5840):961–964. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- 48.Koskiniemi S, et al. Selection of orphan Rhs toxin expression in evolved Salmonella enterica serovar Typhimurium. PLoS Genet. 2014;10(3):e1004255. doi: 10.1371/journal.pgen.1004255. [DOI] [PMC free article] [PubMed] [Google Scholar]