Significance
Inhaled particulates, such as multiwalled carbon nanotubes, can induce neuroinflammatory outcomes. The present study shows that acute neuroinflammation is dependent on the impairment of blood-brain barrier function. Pharmacologic restoration of blood-brain barrier integrity prevented the neuroinflammatory responses to pulmonary multiwalled carbon nanotube exposure. Circulating factors, including possibly thrombospondin-1, recapitulate inflammatory responses in cultured cerebrovascular endothelial cells, suggesting a mechanism for indirect systemic effects of inhaled nanoparticles.
Keywords: nanoparticle, blood-brain barrier, microglia, thrombospondin-1, multiwalled carbon nanotube
Abstract
Pulmonary exposure to multiwalled carbon nanotubes (MWCNTs) causes indirect systemic inflammation through unknown pathways. MWCNTs translocate only minimally from the lungs into the systemic circulation, suggesting that extrapulmonary toxicity may be caused indirectly by lung-derived factors entering the circulation. To assess a role for MWCNT-induced circulating factors in driving neuroinflammatory outcomes, mice were acutely exposed to MWCNTs (10 or 40 µg/mouse) via oropharyngeal aspiration. At 4 h after MWCNT exposure, broad disruption of the blood-brain barrier (BBB) was observed across the capillary bed with the small molecule fluorescein, concomitant with reactive astrocytosis. However, pronounced BBB permeation was noted, with frank albumin leakage around larger vessels (>10 µm), overlain by a dose-dependent astroglial scar-like formation and recruitment of phagocytic microglia. As affirmed by elevated inflammatory marker transcription, MWCNT-induced BBB disruption and neuroinflammation were abrogated by pretreatment with the rho kinase inhibitor fasudil. Serum from MWCNT-exposed mice induced expression of adhesion molecules in primary murine cerebrovascular endothelial cells and, in a wound-healing in vitro assay, impaired cell motility and cytokinesis. Serum thrombospondin-1 level was significantly increased after MWCNT exposure, and mice lacking the endogenous receptor CD36 were protected from the neuroinflammatory and BBB permeability effects of MWCNTs. In conclusion, acute pulmonary exposure to MWCNTs causes neuroinflammatory responses that are dependent on the disruption of BBB integrity.
Environmental and occupational exposures to respirable toxicants are associated with neurodevelopmental and neurodegenerative outcomes (1–3). An influence of inhaled environmental stressors on the integrity of the blood-brain barrier (BBB) has been identified (4). BBB deficits are common to various pathologies and linked to phenotypic changes in microglial and astrocyte populations, which may detract from normal neurosupportive function essential to learning, memory, and neuroplasticity (5–7). The mechanism by which inhaled particulates may adversely impact the neurovascular unit and ultimately lead to neuroinflammatory outcomes remains unclear.
A subgroup of respirable particulate matter (PM), engineered nanoparticles, includes multiwalled carbon nanotubes (MWCNTs), which are being increasingly used in industry owing to their range of unique properties (8, 9). Inhalation of MWCNTs induces lung inflammation, as indicated by infiltration of inflammatory cells from the periphery, increased lung permeability, and fibrosis (10, 11). Despite their very limited ability to translocate beyond the lung (12), MWCNTs have been shown to induce extrapulmonary toxicity through as-yet unknown pathways (13–15). Pulmonary interactions with inhaled pollutants, including MWCNTs, can generate secondary bioactive factors that spill over into the systemic circulation and are distributed throughout the body (15–17). One consequence of these circulating factors is activation of the endothelium, characterized by the expression of adhesion molecules and recruitment/activation of circulating inflammatory cells (18). The nature and outcome of these effects are dependent on the vascular bed in which the inflammation occurs and can include systemic or cerebrovascular vessels.
Cerebrovascular inflammation increases permeability of the BBB (19), a specialized structure composed of astrocytes, pericytes, and endothelial cells (20). Under normal conditions, the BBB prevents passage of undesired molecules from the bloodstream into the central nervous system; however, under inflammatory conditions, the tight junctions between the endothelial cells can become destabilized, resulting in a leaky BBB and allowing peripheral inflammatory molecules to invade and activate neuroglial cells, specifically microglia (21) and astrocytes. Oppenheim et al. (22) found that inhalation of vehicle emissions increased BBB permeability, and also increased levels of inducible nitric oxide synthase and IL-1β in the parenchyma. Neuroinflammation has been demonstrated in rodent models exposed to PM or gaseous pollutants (23, 24); however, a mechanistic link between neuroinflammation and BBB permeability has not been established, and potential intermediate drivers of such effects remain unclear.
We hypothesized that neuroinflammation arises after MWCNT exposure, owing at least in part to increased BBB permeability caused by circulating thrombospondin-1 (TSP-1). In this study, we demonstrate that MWCNT exposure induces the generation of circulating bioactive factors, leading to activation of the cerebrovasculature and inflammatory cells, as well as increased BBB permeability.
Results
Pulmonary Delivery of MWCNTs Drives Lung and Systemic Transcriptional Responses.
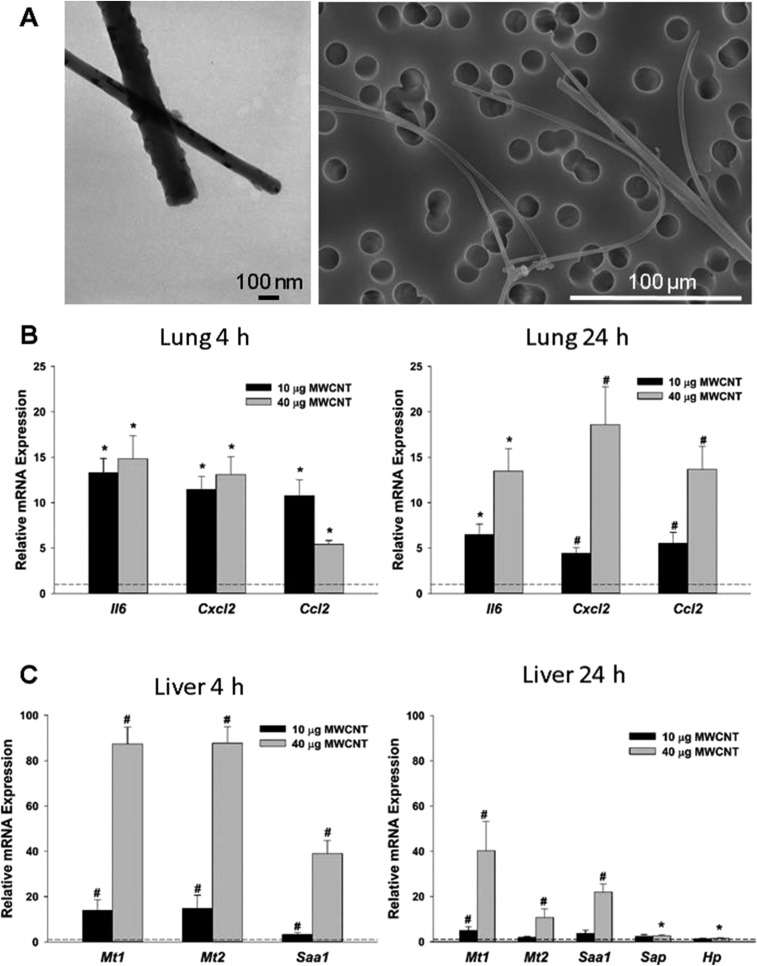
Representative transmission and scanning electron microscopy images of the MWCNTs demonstrate the relative size and adequacy of dispersion (Fig. S1A). More thorough descriptions of the formulation and delivery methodologies for this material have been published previously (25). Following pulmonary exposure to 10 and 40 µg of MWCNTs, multiple markers of inflammation were significantly increased in the lungs, including systemic proinflammatory markers interleukin 6 (Il6) and C-C motif chemokine ligand 2 (Ccl2), as well as C-X-C motif chemokine ligand 2 (Cxcl2) (Fig. S1B). These effects persisted at 24 h in a primarily dose-dependent manner. In addition, MWCNT aspiration significantly induced liver enzyme gene expression, indicative of an acute inflammatory phase response that is systemic in nature (Fig. S1C). All responses were dose-dependent, with the 40 μg dose eliciting a particularly strong response. For remaining studies, we used the lower dose (10 µg) and focused on the 4 h time point to examine the earliest events at the lowest dose.
Fig. S1.
Exposure to MWCNTs induces dose-dependent pulmonary and systemic inflammation. (A) MWCNT characterization by electron microscopy demonstrates the relative size and adequacy of dispersion. (B) Relative mRNA expression of pulmonary inflammatory markers was increased at 4 h and 24 h postexposure. (C) Relative mRNA expression of stress response and acute-phase response genes were dose-dependently increased at 4 h and 24 h postexposure. n = 6 per group. *P < 0.05 vs. DM; #P < 0.05 vs. all groups, one-way ANOVA.
Evidence of Broad Cerebrovascular BBB Disruption and Glial Reactivity Following MWCNT Exposure.
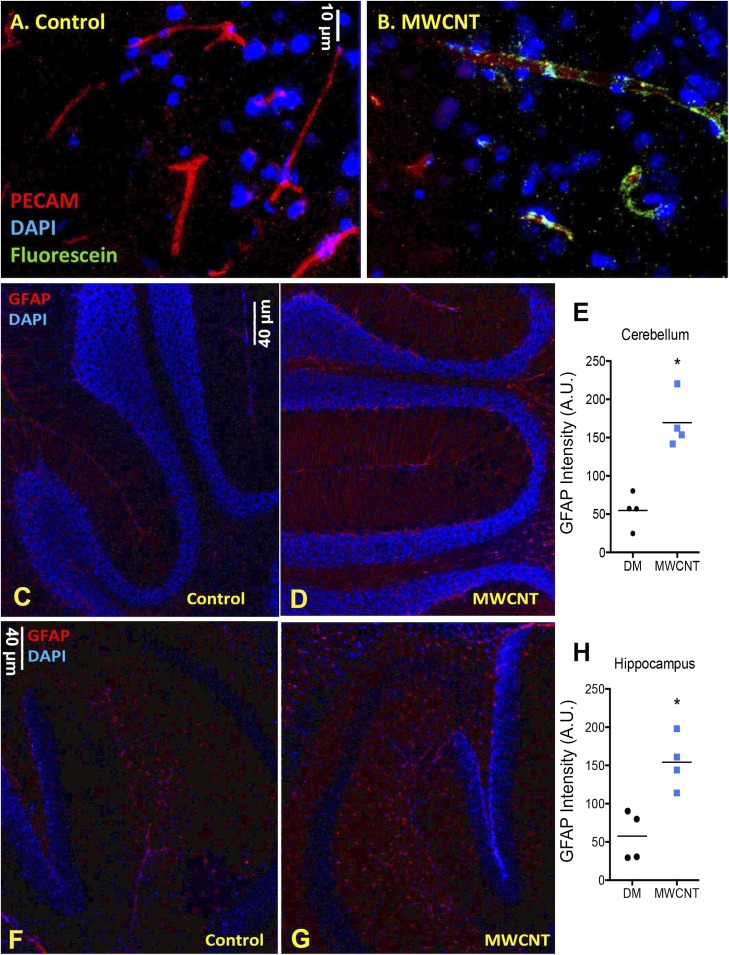
To test BBB integrity, we injected mice systemically with sodium fluorescein at 3 h after MWCNT exposure (i.e., 1 h before euthanasia). In cryosections of frontal cortex from control mice, fluorescein was not readily evident (Fig. 1A); however, in MWCNT-exposed mice, fluorescein was clearly present in close association with platelet endothelial cell adhesion molecule (PECAM)-stained cerebral capillaries, indicative of initial penetration through the cerebral wall (Fig. 1B). Immunohistochemistry analysis of the cerebellum and hippocampus in mice at 4 h after MWCNT exposure also revealed elevated glial fibrillary acidic protein (GFAP) staining density, indicative of reactive astrocytes, compared with dispersion medium (DM)-exposed control mice (Fig. 1 C–H).
Fig. 1.
MWCNT (10 µg) aspiration acutely reduces BBB integrity and induces GFAP expression on astrocytes. (A and B) Fluorescein staining (green) is not apparent in control-treated mouse brains (A), but is clearly adjacent to vascular structures (red; PECAM) in MWCNT-treated mice (B). (C–H) Enhanced GFAP staining (red) in the cerebellum (C–E) and hippocampus (F–H) of control and MWCNT-treated mice, indicative of astrocyte activation. *P <0.05, Student’s t test.
Dose-Dependent MWCNT-Induced BBB Disruption and Neuroinflammation Around Larger Cerebrovasculature.
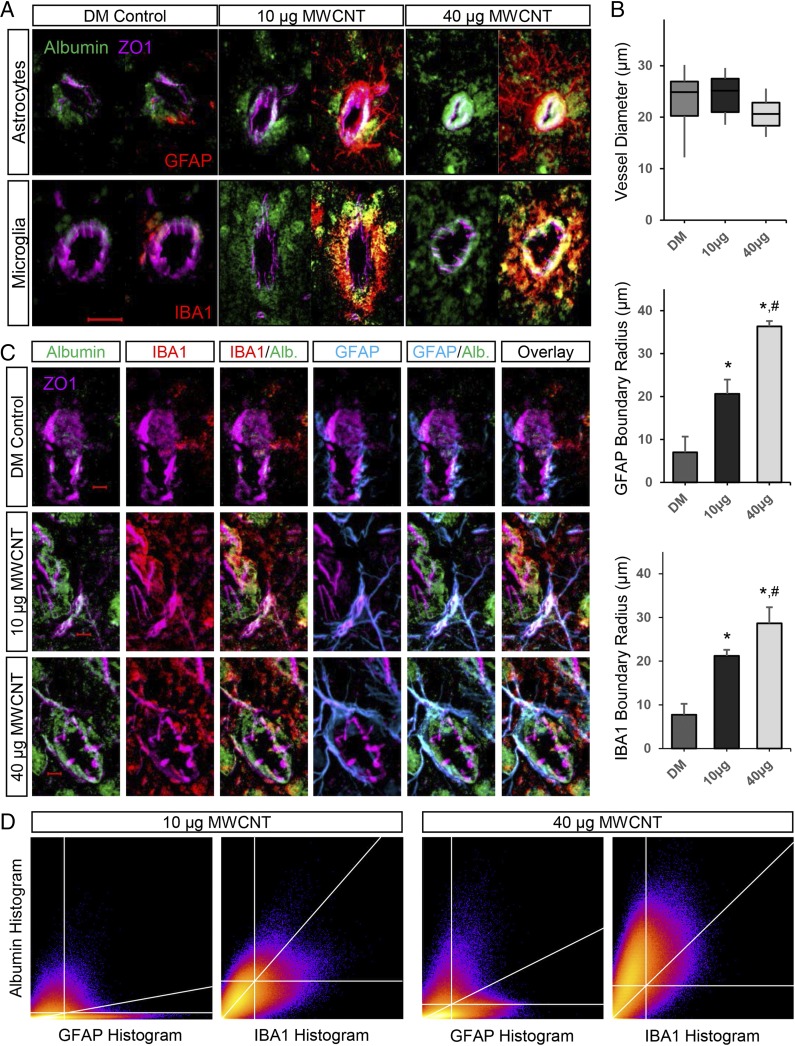
To further assess the extent of MWCNT-induced BBB permeation, mouse brains were stained for extravascular albumin. Using the large molecule albumin to stain for a more pronounced compromise of the BBB, our study of the neurovascular unit became focused on larger-diameter vessels (>10-µm diameter), such as penetrating arterioles, that feed the microvasculature. In DM control mice, albumin-positive staining was not readily evident, with limited amounts found in close association with zonula occludens 1 (ZO1)-stained vessels, consistent with residual albumin following perfusion (Fig. 2A). Vessels in DM control brains exhibited the expected GFAP staining of astrocytic endfeet close to the vascular wall and typical IBA1 staining of a sentinel microglial cell toward one side; however, at 4 h after mice were exposed to MWCNTs at either dose, robust extravascular albumin staining was observed selectively accumulated around larger (>10-µm diameter) cerebrovasculature (Fig. 2A). Furthermore, vessels exhibiting this extravascular albumin halo were also enveloped by a dense accumulation of GFAP-stained astrocytes and ionized calcium-binding adapter molecule 1 (IBA1)-stained microglia, both with morphology indicative of activation following MWCNT exposure. Assessed over a population of similar-sized vessels (mean, 22.7 ± 4.9-µm diameter), the extent of glial ensheathment was dose-dependent, with the boundaries of reactive astrocytes and microglial extending 35.2% and 76.4% further, respectively, into the neuropil from the vessel wall in mice given 40 µg rather than 10 µg of MWCNTs (Fig. 2B). Glial activation was not noted broadly throughout the brain (Fig. S2), highlighting a selective response at the neurovascular unit.
Fig. 2.
MWCNT-induced BBB disruption promotes astrocytic and microglial reactivity selectively around larger cerebrovasculature. (A) Distinct extravascular accumulation of serum albumin (green) is observed following 10 µg and 40 µg MWCNT exposures relative to control-treated mouse brains in larger cerebral vessels (>10-µm diameter) stained for ZO1 (magenta). These vessels are further enveloped by a dense accumulation of astrocytes (GFAP-stained) and microglia (IBA1-stained) that are reactive to MWCNT exposure, distinct from basal glial associations with vessels in DM control mouse brains. (Magnification: 200×.) (Scale bar: 20 µm.) (B) Boxplot depicting the diameter distribution in the assessed vessels. Bar graphs show the boundary thickness for accumulated extravascular reactive glia extending out from the vessel wall (ZO1) in a dose-dependent manner. *P < 0.05 compared with DM control; #P < 0.05 compared with 10 µg MWCNT dose. (C) Optical sectioning (0.5-µm thickness) revealing a predominant colocalization of microglial (IBA1) staining encircling and engulfing leaked serum albumin, but astrocytes (GFAP) costaining with serum albumin only when in tight association with the vessel wall, indicative of the functional difference for the two glial responses. (Magnification: 400×.) (Scale bar: 5 µm.) (D) A 2D heatmap of results from colocalization analysis (Costes method), showing the correlation between albumin and either GFAP or IBA1 histogram pixel data. The upper right quadrant includes pixels demonstrating significant positive correlation between channels, the diagonal calculated linear regression line.
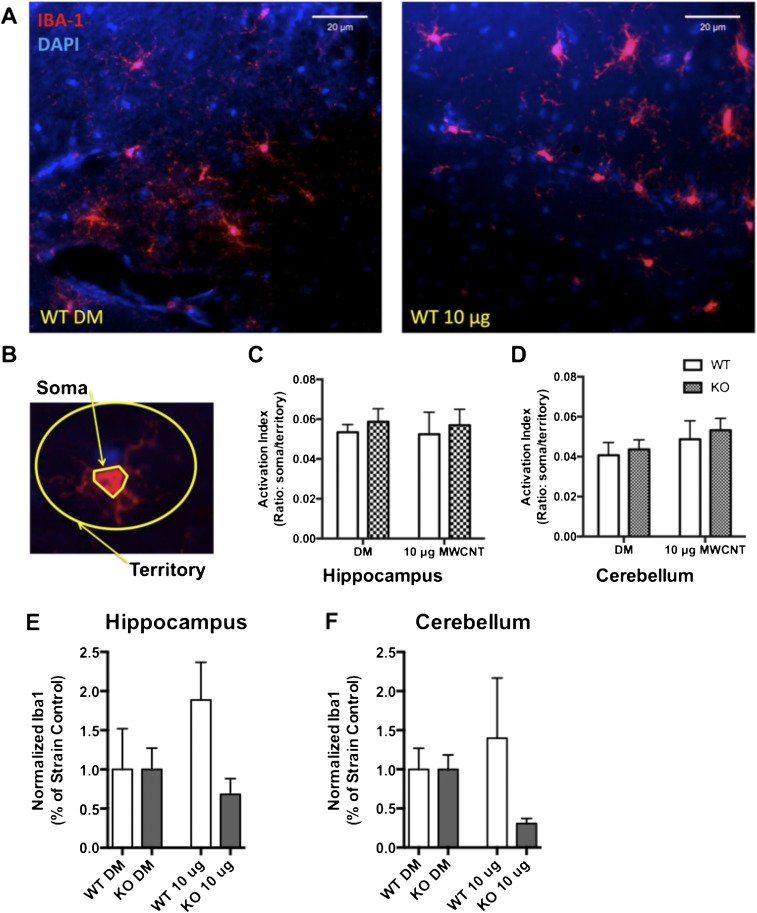
Fig. S2.
Exposure to 10 μg of MWCNTs does not induce microglial activation broadly throughout the brain. Activation of microglia was assessed at 24 h after exposure to 10 μg of MWCNTs in WT and KO animals. (A) Representative images obtained with a 40× objective of the hippocampus for DM WT animals (Left) and WT 10 μg MWCNT-exposed animals (Right). Iba-1 immunohistochemistry (red) indicates microglial cells in this region. (B) Microglial activation was determined by assessing the perimeter of the soma relative to the territory occupied by the ramified processes for each cell and is presented as the ratio of soma to territory. (C and D) Exposure to 10 μg of MWCNTs did not alter the activation index of microglia in the hippocampus (C) or in the cerebellum (D) of animals, as assessed by two-way ANOVA. (E and F) Total Iba-1 immunofluorescence, suggesting that whereas microglia proximal to MWCNT-affected neurovascular units are responding to BBB deficits, the overall population of microglia does not appear to be activated.
Evidence for Differential Glial Functions in Reactivity to MWCNT-Induced BBB Disruption.
The observation of astrocytic and microglial reactivity in close proximity to leaking vessels raised questions as to the respective functions of these cells. Optical sectioning revealed the accumulation of reactive astrocytes in a tight glial scar-like network distinct from the intermittent array of processes surrounding DM control vessels (GFAP staining; Fig. 2C). Furthermore, reactive astrocytic processes were seen to completely envelop capillary branches extending out from the larger vessels, which was not evident across similar-sized vessels in DM control mice. Both the scar-like accumulation around larger vessels and the reactive processes extending along adjoining capillaries were closely associated with morphologically restricted albumin staining in tight proximity with the vasculature. In comparison, the MWCNT-induced dense recruitment of activated microglia appeared to be broadly arrayed around larger vessels, with no specific morphological association to the endothelial wall or capillary branches (IBA1 staining; Fig. 2C). However, arrayed microglial processes were significantly correlated with the extravascular albumin halo found to surround the larger vessels of mice exposed to 10 µg (rp = 0.6; rs = 0.62; P < 0.0001) or 40 µg (rp = 0.58; rs = 0.63; P < 0.0001) MWCNTs, which was otherwise absent in DM control animals. Two-dimensional histograms showing albumin and glial markers emphasize the strong correlation (r ≥0.6) of IBA1 staining selectively with the albumin halo (Fig. 2D), as opposed to GFAP staining, in which the majority of higher-intensity pixels were correlated with low-intensity albumin staining. However, a greater correlation between higher-intensity GFAP-stained pixels and higher-intensity albumin staining is seen in the 40 µg MWCNT dose (rp = 0.32, rs = 0.44; P < 0.0001) relative to the 10 µg MWCNT dose (rp = 0.18, rs = 0.29; P < 0.0001), indicative of the greater amount of albumin confined in close proximity to the vasculature by reactive glial processes. These data support the conclusion of a predominant colocalization between microglia and leaked albumin, consistent with the phagocytic functionality of these cells under neuroinflammatory conditions.
Dependence of MWCNT-Induced Neuroinflammation on BBB Disruption.
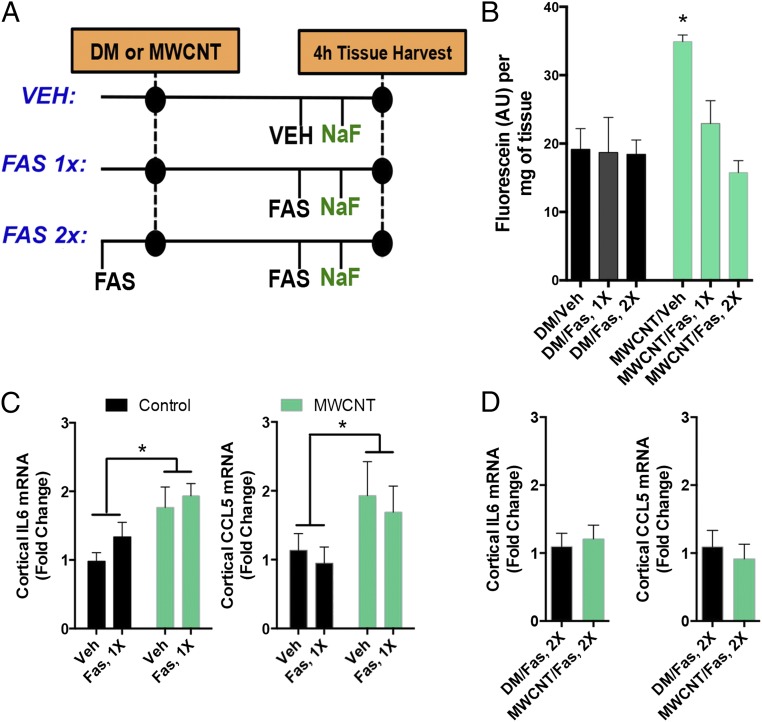
To mechanistically delineate whether neuroinflammation was the result of BBB disruption, mice were administered vehicle or the rho kinase inhibitor fasudil, which has been shown to improve BBB function by relaxing cytoskeletal interactions in endothelial cells, thereby improving outcomes in stroke-related models (26, 27). Mice received fasudil either 2 h after MWCNT aspiration but before injection of the BBB tracer sodium fluorescein or just before MWCNT aspiration and again 2 h later (Fig. 3A). The former permutation was designed to test whether fluorescein uptake in the brain was inhibited after exposure and exposure-induced neuroinflammation had occurred. The latter permutation was implemented to prevent BBB disruption prophylactically and throughout the exposure to test whether neuroinflammatory changes still occurred in the absence of BBB disruption.
Fig. 3.
MWCNT-induced neuroinflammatory responses are dependent on BBB disruption. (A) General study design, incorporating a single (1×) fasudil treatment after MWCNT to reduce brain fluorescein uptake and a preventative (2×) fasudil treatment to prevent MWCNT-induced BBB disruption throughout the 4-h time course. (B) Fluorescein uptake in brains at 4 h following MWCNT aspiration with vehicle, 1× fasudil, or 2× fasudil treatment. * P < 0.05 compared with control, ANOVA. (C) Inflammatory markers Il6 and Ccl5 mRNA in the cortex at 4 h after DM or MWCNT aspiration. *A significant effect of MWCNT compared with control in two-way ANOVA (P < 0.05) with no influence of the single post-MWCNT fasudil treatment. (D) Preventative (2×) fasudil administration abrogated inflammatory marker mRNA expression (Il6, Ccl5) in the cortex.
As was demonstrated microscopically (Fig. 1 A and B), fluorescein uptake in brain homogenates was significantly enhanced at 4 h after pulmonary exposure to MWCNTs (Fig. 3B). Increased Il6 and Ccl5 mRNA expression was induced by MWCNTs in cortical regions (Fig. 3C). Treatment with fasudil at 2 h postexposure completely blocked fluorescein uptake in the brain, but had no effect on neuroinflammatory outcomes (Fig. 3 B and C); however, prophylactically administered fasudil was able to completely abrogate both fluorescein uptake (Fig. 3B) and neuroinflammatory outcomes (Fig. 3D), demonstrating that these neuroinflammatory responses depend on the loss of BBB integrity and penetration of circulating factors.
Bioactivity of MWCNT-Induced, Serum-Borne Factors: Endothelial Inflammatory Responses.
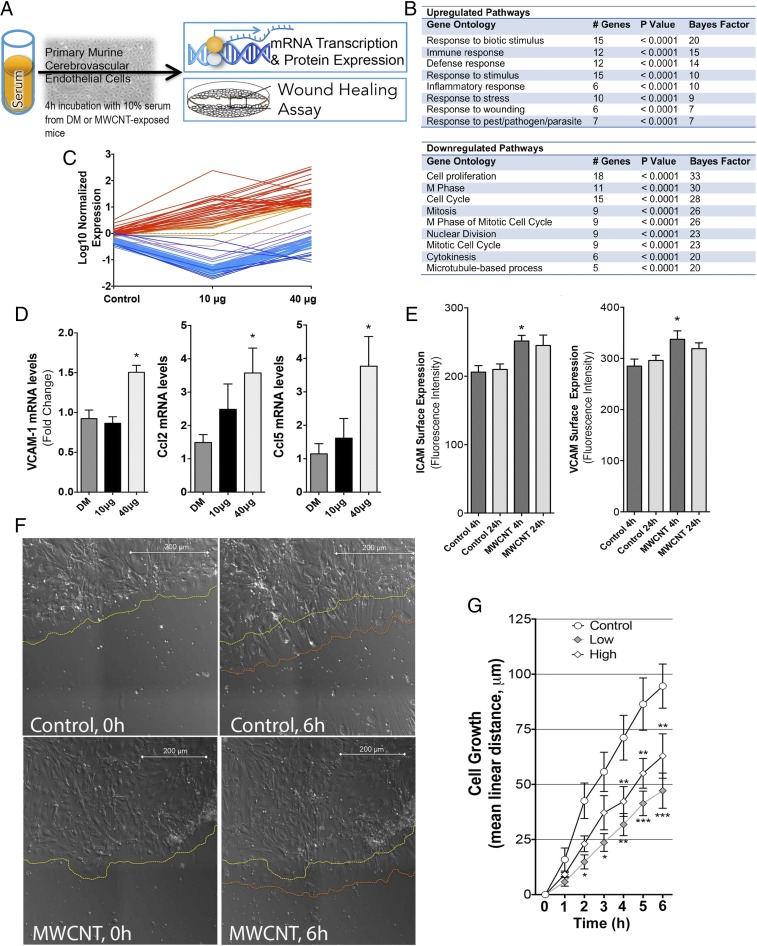
We used cultured mouse cerebrovascular endothelial cells (mCECs) as biosensors of cumulative inflammatory mediators in serum from MWCNT-exposed mice, an approach that has proven valuable in toxicologic and clinical applications (16, 28). Microarray analysis was performed on mRNA isolated from primary mCECs following incubation with serum collected from MWCNT- or DM-exposed mice (at 4 h postexposure) at 10% in medium for 4 h (Fig. 4A). Microarray results revealed up-regulated genes associated with inflammation, immune response, and chemoattraction/recruitment of leukocytes (Figs. 4 B and C and Fig. S3). Importantly, the majority of up-regulated genes behaved in a dose-dependent manner, with more prominent effects seen in the 40 µg dose group (Table S1). Microarray findings were confirmed in subsequent assays, in which application of 10% serum from MWCNT-treated mice on primary mCECs for 4 h resulted in a 50% increase in relative mRNA expression of Vcam1 and approximately threefold increases in relative mRNA expression of Ccl2 and Ccl5 proinflammatory cytokines in the 40 μg dose group at the 4 h timepoint (Fig. 4D).
Fig. 4.
Inflammatory responses of cerebrovascular endothelial cells treated with serum from MWCNT-exposed mice. (A) General depiction of inflammatory potential assay and wound healing protocols, with serum from exposed mice incubated on primary murine cerebrovascular endothelial cells. (B) Microarray results indicating numerous significantly altered transcripts (n = 84) from endothelial cells incubated with serum from MWCNT-exposed mice, compared with cells incubated with serum from control mice (at 4 h after aspiration). (C) Elevated transcripts (in red) were ontologically related to inflammatory and/or cellular defense response, whereas down-regulated transcripts (blue) were related to proliferation and migration pathways, listed in the table. (D) Confirmation of key inflammatory mRNA responses by PCR showing that endothelial Vcam1, Ccl2, and Ccl5 mRNA were significantly up-regulated by serum from MWCNT-treated mice. (E) Endothelial cell surface Icam-1 and Vcam-1 protein expression was elevated by serum from mice exposed to the 40 μg dose of MWCNTs at 4 h compared with controls. *P < 0.05 compared with DM control serum. (F) Live cell images of wound recovery in mCECs treated with serum from control and 40 µg MWCNT-treated mice, showing the initial edge of endothelial cells (yellow dashed line) and the edge after 6 h (orange dashed line). (G) Mean cell regrowth following wounding in primary cerebrovascular endothelial cells incubated with serum from DM- and MWCNT-exposed mice. *P < 0.05; **P < 0.01; ***P < 0.001 compared with DM control serum effects, two-way repeated-measures ANOVA.
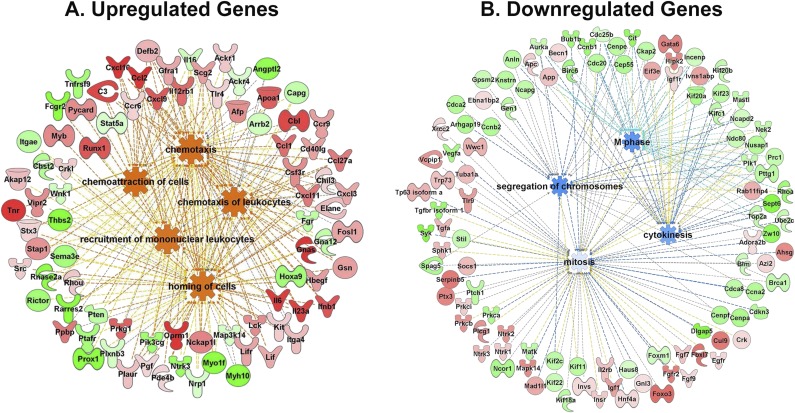
Fig. S3.
Gene ontology graphs showing the relationships between up-regulated, predominantly inflammatory genes (A) and down-regulated pathways (B).
Table S1.
Gene array data for cultured mCECs treated with serum from mice exposed to 10 µg and 40 µg of MWCNTs obtained at 4 h postexposure, compared with DM control serum
| Probe set ID | Gene name | Gene symbol | Log 10 µg v DM | Log 40 µg v DM | Entrez gene ID |
| 1427747_a_at | Lipocalin 2 | Lcn2 | 1.86 | 1.02 | 16819 |
| 1421009_at | Radical S-adenosyl methionine domain containing 2 | Rsad2 | 1.30 | 2.47 | 58185 |
| 1427126_at | Heat shock protein 1B | Hspa1b | 1.21 | 0.94 | 15511 |
| 1427127_x_at | Heat shock protein 1B | Hspa1b | 1.17 | 0.93 | 15511 |
| 1436058_at | Radical S-adenosyl methionine domain containing 2 | Rsad2 | 1.13 | 2.29 | 58185 |
| 1452318_a_at | Heat shock protein 1B | Hspa1b | 1.11 | 0.82 | 15511 |
| 1448239_at | Heme oxygenase (decycling) 1 | Hmox1 | 1.04 | 0.71 | 15368 |
| 1450783_at | IFN-induced protein with tetratricopeptide repeats 1 | Ifit1 | 1.03 | 2.14 | 15957 |
| 1420380_at | Chemokine (C-C motif) ligand 2 | Ccl2 | 1.03 | 2.22 | 20296 |
| 1452388_at | Heat shock protein 1A | Hspa1a | 1.02 | 1.18 | 193740 |
| 1426276_at | IFN induced with helicase C domain 1 | Ifih1 | 0.98 | 1.73 | 71586 |
| 1450297_at | Interleukin 6 | Il6 | 0.96 | 1.81 | 16193 |
| 1417065_at | Early growth response 1 | Egr1 | 0.96 | 1.32 | 13653 |
| 1418930_at | Chemokine (C-X-C motif) ligand 10 | Cxcl10 | 0.95 | 2.15 | 15945 |
| 1421008_at | Radical S-adenosyl methionine domain containing 2 | Rsad2 | 0.93 | 1.96 | 58185 |
| 1423100_at | FBJ osteosarcoma oncogene | Fos | 0.90 | 1.48 | 14281 |
| 1424339_at | 2'-5′ oligoadenylate synthetase-like 1 | Oasl1 | 0.86 | 1.65 | 231655 |
| 1449984_at | Chemokine (C-X-C motif) ligand 2 | Cxcl2 | 0.80 | 1.39 | 20310 |
| 1449025_at | IFN-induced protein with tetratricopeptide repeats 3 | Ifit3 | 0.73 | 2.03 | 15959 |
| 1422134_at | FBJ osteosarcoma oncogene B | Fosb | 0.69 | 1.57 | 14282 |
| 1416756_at | DnaJ (Hsp40) homolog, subfamily B, member 1 | Dnajb1 | 0.57 | 1.16 | 81489 |
| 1449363_at | Activating transcription factor 3 | Atf3 | 0.54 | 1.14 | 11910 |
| 1417263_at | Prostaglandin-endoperoxide synthase 2 | Ptgs2 | 0.52 | 1.42 | 19225 |
| 1431591_s_at | Predicted gene 9706///ISG15 ubiquitin-like modifier | Gm9706 | 0.52 | 1.52 | 677168 |
| 1457035_at | Expressed sequence AI607873 | AI607873 | 0.52 | 1.21 | 226691 |
| 1418936_at | V-maf musculoaponeurotic fibrosarcoma oncogene family, protein F (avian) | Maff | 0.49 | 1.06 | 17133 |
| 1433699_at | Tumor necrosis factor, alpha-induced protein 3 | Tnfaip3 | 0.48 | 1.42 | 21929 |
| 1434350_at | Cysteine-serine–rich nuclear protein 1 | Csrnp1 | 0.47 | 1.25 | 215418 |
| 1421551_s_at | IFN-activated gene 202B | Ifi202b | 0.47 | 1.25 | 26388 |
| 1418126_at | Chemokine (C-C motif) ligand 5 | Ccl5 | 0.45 | 1.30 | 20304 |
| 1418293_at | IFN-induced protein with tetratricopeptide repeats 2 | Ifit2 | 0.43 | 1.59 | 15958 |
| 1457666_s_at | IFN-activated gene 202B/IFN-activable protein 202-like | Ifi202b | 0.42 | 1.19 | 26388 |
| 1425837_a_at | CCR4 carbon catabolite repression 4-like (S. cerevisiae) | Ccrn4l | 0.42 | 1.28 | 12457 |
| 1429029_at | Sphingomyelin synthase 2 | Sgms2 | 0.31 | 1.08 | 74442 |
| 1426181_a_at | Interleukin 24 | Il24 | 0.13 | 1.22 | 93672 |
| 1449133_at | Small proline-rich protein 1A | Sprr1a | 0.09 | 1.21 | 20753 |
| 1418191_at | Ubiquitin specific peptidase 18 | Usp18 | −0.01 | 1.17 | 24110 |
| 1418392_a_at | Guanylate binding protein 3 | Gbp3 | −0.17 | 1.07 | 55932 |
| 1438403_s_at | Metastasis-associated lung adenocarcinoma transcript 1 (noncoding RNA) | Malat1 | −0.18 | −1.04 | 72289 |
| 1438732_at | NHS-like 2 | Nhsl2 | −0.64 | −1.01 | 100042480 |
| 1416258_at | Thymidine kinase 1 | Tk1 | −1.01 | 0.10 | 21877 |
| 1437244_at | Growth arrest-specific 2-like 3 | Gas2l3 | −1.01 | −0.43 | 237436 |
| 1450496_a_at | Spindle and kinetochore-associated complex subunit 1 | Ska1 | −1.01 | 0.05 | 66468 |
| 1416664_at | Cell division cycle 20 | Cdc20 | −1.01 | −0.06 | 107995 |
| 1425815_a_at | Hyaluronan-mediated motility receptor (RHAMM) | Hmmr | −1.02 | −0.14 | 15366 |
| 1453416_at | Growth arrest-specific 2-like 3 | Gas2l3 | −1.02 | −0.14 | 237436 |
| 1447363_s_at | Budding uninhibited by benzimidazoles 1 homolog, beta (S. cerevisiae) | Bub1b | −1.02 | −0.13 | 12236 |
| 1433543_at | Anillin, actin-binding protein | Anln | −1.03 | 0.04 | 68743 |
| 1416558_at | Maternal embryonic leucine zipper kinase | Melk | −1.03 | 0.29 | 17279 |
| 1431358_at | RIKEN cDNA 4930547N16 gene | 4930547N16Rik | −1.03 | −0.23 | 75317 |
| 1455978_a_at | Matrilin 2 | Matn2 | −1.03 | 0.31 | 17181 |
| 1417911_at | Cyclin A2 | Ccna2 | −1.04 | 0.15 | 12428 |
| 1436654_at | Gen homolog 1, endonuclease (Drosophila) | Gen1 | −1.05 | −0.12 | 209334 |
| 1452040_a_at | Cell division cycle-associated 3 | Cdca3 | −1.05 | −0.01 | 14793 |
| 1424649_a_at | Tetraspanin 8 | Tspan8 | −1.06 | −0.15 | 216350 |
| 1452458_s_at | Leucine-rich repeat protein 1 | Lrr1 | −1.06 | 0.19 | 69706 |
| 1417910_at | Cyclin A2 | Ccna2 | −1.06 | 0.04 | 12428 |
| 1424046_at | Budding uninhibited by benzimidazoles 1 homolog (S. cerevisiae) | Bub1 | −1.07 | 0.06 | 12235 |
| 1448205_at | Cyclin B1 predicted gene 5593 | Ccnb1 | −1.07 | −0.09 | 434175 |
| 1416309_at | Nucleolar and spindle-associated protein 1 | Nusap1 | −1.07 | −0.03 | 108907 |
| 1437580_s_at | NIMA (never in mitosis gene a)-related expressed kinase 2 | Nek2 | −1.07 | −0.14 | 18005 |
| 1419943_s_at | Cyclin B1 predicted gene 5593 | Ccnb1 | −1.08 | 0.05 | 434175 |
| 1452073_at | Family with sequence similarity 64, member A | Fam64a | −1.08 | −0.01 | 109212 |
| 1416076_at | Cyclin B1///predicted gene 5593 | Ccnb1 | −1.08 | 0.12 | 434175 |
| 1453748_a_at | Kinesin family member 23 | Kif23 | −1.11 | −0.27 | 71819 |
| 1452242_at | Centrosomal protein 55 | Cep55 | −1.12 | 0.01 | 74107 |
| 1423774_a_at | Protein regulator of cytokinesis 1 | Prc1 | −1.12 | −0.02 | 233406 |
| 1448191_at | Polo-like kinase 1 | Plk1 | −1.14 | −0.15 | 18817 |
| 1435306_a_at | Kinesin family member 11 | Kif11 | −1.14 | 0.00 | 16551 |
| 1450842_a_at | Centromere protein A | Cenpa | −1.15 | −0.26 | 12615 |
| 1437611_x_at | Kinesin family member 2C | Kif2c | −1.16 | −0.15 | 73804 |
| 1455980_a_at | Growth arrest-specific 2 like 3 | Gas2l3 | −1.16 | −0.48 | 237436 |
| 1419513_a_at | ect2 oncogene | Ect2 | −1.16 | −0.01 | 13605 |
| 1427161_at | Centromere protein F | Cenpf | −1.17 | 0.03 | 108000 |
| 1424105_a_at | Pituitary tumor-transforming gene 1 | Pttg1 | −1.17 | −0.18 | 30939 |
| 1450920_at | Cyclin B2 | Ccnb2 | −1.21 | −0.05 | 12442 |
| 1451246_s_at | Aurora kinase B | Aurkb | −1.21 | 0.04 | 20877 |
| 1430574_at | Cyclin-dependent kinase inhibitor 3 | Cdkn3 | −1.24 | −0.16 | 72391 |
| 1428304_at | Establishment of cohesion 1 homolog 2 (S. cerevisiae) | Esco2 | −1.25 | 0.05 | 71988 |
| 1422814_at | Asp (abnormal spindle)-like, microcephaly associated (Drosophila) | Aspm | −1.29 | −0.06 | 12316 |
| 1439040_at | Centromere protein E | Cenpe | −1.29 | −0.56 | 229841 |
| 1424292_at | DEP domain containing 1a | Depdc1a | −1.31 | −0.01 | 76131 |
| 1449207_a_at | Kinesin family member 20A | Kif20a | −1.33 | −0.13 | 19348 |
All are selected at P < 0.01 and reflect mean log difference from control expression (n = 4 per group).
Similarly, treatment of primary mCECs with serum from MWCNT-treated mice elicited a small but significant up-regulation of both vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) protein on the cell surface, as assessed by flow cytometry (Fig. 4E). Serum obtained at 24 h postexposure exhibited lower inflammatory potency. These data, in conjunction with the gene expression findings, suggest that serum-induced increased mRNA expression translates into increased cell surface expression of protein, culminating in activation of the endothelium.
Bioactivity of MWCNT-Induced, Serum-Borne Factors: Endothelial Cell Regrowth and Motility.
Whereas up-regulated genes (Fig. 4B, red) in serum-treated cells trended in a dose-dependent manner, down-regulated genes (Fig. 4B, blue) exhibited a biphasic response, in which effects were most potent at the 10 µg dose. Down-regulated genes aligned with categories of cell proliferation, mitosis, and cytokinesis (Fig. 4B and Fig. S3); thus, wound healing deficits were confirmed functionally by a “scratch” assay. Serum from control and MWCNT-treated mice was applied to confluent primary mCECs that had been “scratched” with a sterile pipette tip and allowed to grow back over 6 h. At the 6 h mark, the control serum-treated cells had achieved a mean distance of cell regrowth of 100 μm, compared with ∼60 μm of growth for the 40 μg dose group and only ∼50 μm of growth for the 10 μg dose group (Fig. 4 F and G). These results are congruent with the biphasic changes seen in the microarray results.
TSP-1 and CD36: Plausible Ligand–Receptor Interaction.
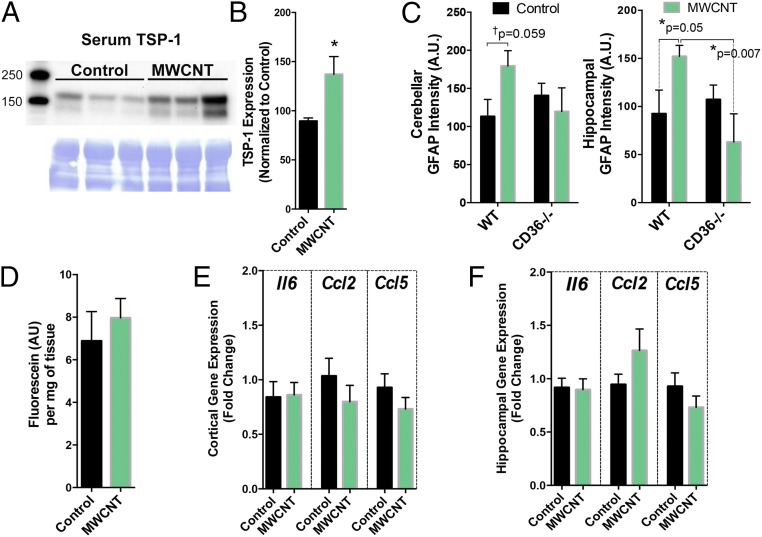
The foregoing findings further suggest that endothelial cells interact with blood-borne factors generated by pulmonary MWCNT exposure. We have previously shown a role for the endothelial cell surface protein cluster of differentiation 36 (CD36) as a mediator of vasodilatory impairment following pulmonary exposure to ozone (17). CD36 is a class II scavenger receptor involved in a wide range of processes, including fatty acid metabolism (29), heart disease, and atherosclerosis (30). The endogenous ligand for CD36, TSP-1 (31), was significantly elevated in serum at 4 h after aspiration of 10 µg of MWCNTs in WT mice (Fig. 5 A and B); however, the MWCNT activation of GFAP observed in WT mice was not observed in CD36−/− mice (Fig. 5C). Furthermore, CD36−/− mice exposed to MWCNTs showed no increases in cortical fluorescein uptake (Fig. 5D) or proinflammatory gene expression in the cortex (Fig. 5E) or hippocampus (Fig. 5F) compared with DM control mice, consistent with a role for scavenger receptor interaction with circulating ligands.
Fig. 5.
Serum TSP-1 levels are increased by MWCNT treatment, and CD36 mice are largely protected from the BBB disruption and neuroinflammatory effects of pulmonary MWCNT exposure. (A) TSP-1 protein levels in serum from control (left three lanes) and MWCNT-treated mice (right three lanes) normalized to Coomassie blue loading controls. (B) Quantification of TSP-1 protein levels from all study subjects. n = 5–6. *P < 0.05, Student’s t test. (C) GFAP staining in WT and CD36−/− mice treated with MWCNTs. Cortex (Left) and hippocampus (Right) were imaged as described for Fig. 1. WT mice displayed similar findings as shown previously, whereas CD36−/− mice showed no impact of MWCNT exposure on GFAP staining intensity. *P < 0.05, two-way ANOVA with Fisher’s least significant difference post hoc comparison test. (D) Fluorescein uptake in the brain of CD36−/− mice at 4 h after treatment with vehicle (DM) or MWCNTs. (E and F) Neuroinflammatory mRNA markers in the cortex (E) and hippocampus (F) of control and MWCNT-treated CD36−/− mice.
Discussion
Neurologic consequences of inhaled PM and gases have been reported in epidemiologic and toxicologic studies, but considerable uncertainty exists regarding the pathway by which toxic effects transfer from the lung to the brain (3, 32–34). The present study establishes that a circulating signal arises after pulmonary exposure to MWCNTs that leads to a BBB-dependent neuroinflammatory response. Serum from MWCNT-exposed mice up-regulated adhesion molecules and proinflammatory cytokines in primary cerebrovascular endothelial cells at both the gene and protein level. Serum from exposed mice also adversely affected the ability of endothelial cells to migrate and recover in a wound-healing assay. TSP-1 was identified as a plausible serum-borne mediator of these effects, via interactions with CD36. Collectively, these outcomes implicate a modified serum composition stemming from pulmonary interactions with MWCNTs as a source of diminished BBB integrity and neural effects.
Inhalation of PM has been associated with BBB integrity deficits and neuroinflammation (23, 35), but this mechanistic study shows an interactive link between the two outcomes. Rats exposed to diesel emissions for 6 mo demonstrated significant increases in cortical TNFα, α-synuclein, and amyloid-β42 peptide expression (36). Oppenheim et al. (37) identified significant increases in brain fluorescein uptake resulting from long-term exposure (50 d) to a mixture of gasoline and diesel emissions in ApoE−/− mice. These findings were associated with increased cerebrovascular gelatinase levels and reduced occludin and claudin-5 expression and, in parallel, increased inducible nitric oxide synthase expression in the brain parenchyma. MWCNT aspiration induced a similar pattern of fluorescein uptake into the brain, whereas rho-kinase inhibition blocked this effect and, when administered prophylactically, also prevented neuroinflammatory responses. Fasudil likely does not alter the pathways leading to occluding and claudin-5 down-regulation, but rather relaxes intracellular endothelial cytosketetal components to shift the force balance from intercellular to intracellular, thereby reducing intercellular gap formation (38).
Our present findings suggest a focused but likely transient reduction in BBB integrity following a single exposure to MWCNTs. Broadly speaking, cerebral capillaries appear to be limited to small-molecule BBB permeation, as demonstrated by fluorescein uptake; however, the larger-diameter vessels and immediate branches that feed the microvasculature sustain greater BBB disruption, as evidenced by dose-dependent albumin protein leakage. Larger arterioles may be more susceptible owing to their higher perfusion pressure, leaving the adjacent neuropils highly vulnerable to MWCNT-induced neuroinflammation. Capillary- and arteriole-associated BBB permeations, along with molecular evidence, were observed across functionally disparate cortical, hippocampal, thalamic, and cerebellar regions, indicating a global rather than a domain-specific cerebrovascular response. However, regional susceptibility remains, likely owing to activity-dependent variations in cerebrovascular density (39), with functional implications to be addressed in follow-up studies. Reactive astrocytes effectively confined capillary leakage in close proximity to the vessel; however, the emergence of an astroglial scar-like formation around larger vessels, likely to limit prolonged leakage around larger vessels, appeared to be transiently ineffective in confining the initial BBB leakage. Permeation of the BBB beyond an astrocytically confined limit also promoted dose-dependent microglial activation and recruitment around larger vessels. Correlated with the halo of leaked serum components, phagocytic microglia provided the means to clean up after acute BBB permeation (40). Future studies are needed to assess the duration of microglial activation in and around larger vessels and to investigate whether chronic MWCNT exposure may promote a worsened proinflammatory phenotype (41).
Disruption of BBB integrity has been documented in major cerebrovascular events or neurologic diseases, and BBB permeability can be tied to neuroinflammation. Mouse models of Alzheimer’s disease have found that BBB permeability precedes the formation of the β-amyloid plaques that are the hallmark of the disease (42). Following MWCNT exposure, BBB disruption led to neuroinflammation, which was effectively blocked with the prophylactic application of the rho-kinase inhibitor fasudil. Rho-kinase inhibition has been shown to reduce neuropathology in scenarios in which BBB is otherwise dysfunctional. Fasudil treatment in a mouse ischemia model was found to prevent BBB permeability, as well as the formation of oxidative stress (27). Rho-kinase inhibition with fasudil also has been shown to accelerate functional recovery in spinal cord injury models (43, 44). Autoimmune demyelinating disorders, such as multiple sclerosis, have been treated with rho-kinase inhibitors with great success; it is thought that rho-kinase inhibition in these disorders prevents leukocyte migration into the central nervous system (45). Fasudil inhibited fluorescein uptake into the brain regardless of the time of injection, but did not inhibit neuroinflammation when given after MWCNT administration, which indicates a potential for circulating factors arising from the lung to trigger a loss of BBB integrity. Moreover, the improvement in BBB integrity with no impact on neuroinflammatory responses seen when fasudil was administered after MWCNTs indicates that the principal target of fasudil is the vascular endothelium.
We have previously demonstrated that serum derived from ozone-exposed mice diminishes vasodilatory responses through the class B scavenger receptor CD36 (17); thus, we tested whether CD36 and its endogenous ligand, TSP-1, may have a role in the BBB alterations caused by MWCNTs. CD36 is expressed in the rodent cerebrovasculature (46) and, interestingly, appears to be present in larger cerebral vessel endothelium (where substantial serum albumin leakage is seen) and in pericytes in humans (47), but may be less relevant in the microvasculature, although still expressed at low levels (48). Vascular CD36 mediates free radical production and brain injury in cerebral ischemia (46) and also has been shown to activate the rho/rho-kinase pathway (49). In the present study, serum TSP-1 level was elevated by MWCNT aspiration, and the absence of CD36 prevented both the increased BBB permeability and neuroinflammation induced by MWCNT exposure. Importantly, previous studies with related pollutants have noted the involvement of related scavenger receptors (including CD36, Toll-like receptor 4, and lectin-like receptor for oxidized low-density lipoprotein 1) and ligands (including oxidized lipids and lipoproteins) (17, 50). Thus, TSP-1 is likely an important facet of a more complex response to inhaled agents, and further studies are needed to identify the origin of TSP-1 and the timing of the response, to mechanistically confirm the interaction with CD36 and/or CD47 on endothelial cells, and to ascertain whether TSP-1 specifically crosses into the brain.
Alternate pathways for the neural effects of inhaled PM, such as direct delivery of inhaled particulates to the brain or other target organs, have been hypothesized, but quantitative evidence demonstrating substantive transference of CNTs across the BBB are limited (12) and, more crucially, no evidence of a direct biological effect has been mechanistically established. Specifically, MWCNTs accumulate in the brain at only small fractions of the original pulmonary dosage (0.00022% of the lung burden following a 12-d exposure), and specific confirmation that the material has penetrated beyond the capillary wall is often lacking in distribution studies; that is, much of the residual mass of MWCNTs may simply reside in the cerebrovascular compartment and not in brain tissue (12). Even when specifically engineering nanoparticles to access the brain, researchers find it exceedingly difficult to overcome the BBB (51, 52). An important factor in the interpretation of our present findings is the short time course (4 h), which is likely too short for significant translocation of MWCNTs beyond the lung; however, longer-term outcomes may arise as a result of either continued pulmonary interactions or direct access and accumulation in the brain.
A broader impact of pulmonary inflammation on neurologic outcomes is implied by our findings, which may explain more generally the comorbid associations between lung disease and cognition deficits and neurodegenerative diseases (53, 54). A loss of BBB integrity caused by circulating factors of pulmonary origin may be central to the neurologic outcomes of pulmonary inflammation, remodeling, or toxicity owing to inhaled toxicants. Given the suggestive adjuvant role for inhaled PM in driving neurodevelopmental and neurodegenerative diseases (1, 2), factors such as TSP-1 may explain in part how indirect pulmonary responses can exacerbate neurologic diseases. A more thorough characterization of circulatory molecular changes after MWCNT exposure is warranted, given the vast number of potential alterations to endogenous components.
Materials and Methods
The study design, assays, and statistical analysis are described in detail in SI Materials and Methods. All procedures involving vertebrate animals were approved by the Animal Care and Use Committees of the University of New Mexico and the National Institute of Occupational Safety and Health.
SI Materials and Methods
Animals and Exposures.
Specific pathogen-free male C57BL/6J mice (Jackson Laboratory) and CD36-deficient (CD36−/−) mice on a C57BL/6 background (a generous gift from Maria Febbraio) (17) were used in this study. All mice were housed in an Association for Assessment and Accreditation of Lab Animal Care International-approved animal facility at the National Institute for Occupational Safety and Health or University of New Mexico, with procedures approved by Institutional Animal Care and Use Committees of the University of New Mexico and the National Institute of Occupational Safety and Health. Animal care and use procedures were conducted in accordance with the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals (https://grants.nih.gov/grants/olaw/references/phspol.htm) and the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf). Food and tap water were provided ad libitum in ventilated cages in a temperature- and humidity-controlled environment with a 12-h light/dark cycle.
The C57BL/6J mice, age 8 wk, were treated via oropharyngeal aspiration with MWCNTs at 0 µg, 10 µg, or 40 µg (n = 12 for each group needed to generate sufficient serum for all tests). The MWCNTs were prepared in DM consisting of mouse serum albumin (0.6 mg/mL) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (10 µg/mL). The MWCNT material used in this study, MWCNT-7, has been extensively characterized (25, 55). The average diameter was 49 nm with a length of 3.86 µm (geometric SD = 1.94). Purity was >99% carbon. Mice were euthanized at 4 h or 24 h following pulmonary exposure. Serum was collected at the time of euthanasia, and livers and left lung lobes were removed and frozen to determine changes in relative mRNA expression.
BBB Permeability Determination with Sodium Fluorescein.
Animals were injected i.p. with 2% fluorescein sodium salt (Sigma-Aldrich; F6377-100G, lot MKBR1855V) at 3 h after MWCNT exposure (1 h before sacrifice). Animals were perfused transcardially with ice-cold saline before brains were removed and dissected. Some animals received fasudil HCl (Selleckchem; S1573) i.p., formulated at 20 mg/kg, either once, 2 h before sacrifice (1 h before fluorescein), or twice, immediately before MWCNT aspiration and again 2 h before euthanasia.
Brains were weighed and homogenized in 500 μL of 50% trichloroacetic acid. Following homogenization, supernatants were neutralized with 400 μL of 5 M sodium hydroxide. Neutralized homogenate was spun down for 10 min at 10,000 × g to pellet the tissue. A 200-μL sample was placed in a 96-well optical bottom plate (Nagle Nunc International) and read at 440/525 nm in a spectrofluorophotometer.
Cell Culture and Serum Treatment.
mCECs were obtained from a commercial vendor (Cell Biologics) and maintained according to the manufacturer’s recommendations at 37 °C and 5% CO2 with complete endothelial cell medium supplemented with 10% (vol/vol) FBS. All experiments were performed between passages 3 and 8. On final plating, ∼2 × 104 cells were added to each well of a 24-well plate (BD Biosciences) and grown to confluence to mimic the cell-cell environment found in the vasculature. Cells were serum-starved for 12 h before treatment with serum. Serum obtained at 4 h and 24 h from control or MWCNT-exposed mice was added to confluent mCECs at a ratio of 1:9 [10% (vol/vol)] with basal endothelial cell medium under FBS-free conditions. All treatments were performed for 4 h, followed by isolation of RNA. Assays were batched by exposure to enhance consistency and comparability across samples.
Immunohistochemistry.
Animals were euthanized and perfused transcardially with saline, followed by ice-cold paraformaldehyde [PFA; 4% (wt/vol) in PBS, pH 7.4]. Brains were removed by decapitation, placed overnight in 4% PFA at 4 °C, and then transferred to a 30% (wt/vol) sucrose solution for ∼48 h at 4 °C. Following cryoprotection, samples were rinsed with PBS and frozen in optimal cutting temperature compound. Then 16-µm sagittal sections were collected with a cryostat, dried, and stored at −20 °C until use.
Sections were blocked and permeabilized with a PBS solution containing 1% BSA, 5% (vol/vol) goat serum, and 0.2% Triton X-100 for 2 h at room temperature. Primary antibodies, either rabbit anti-glial fibrillary acidic protein (GFAP, 1:500; Dako, Z0334) to label astrocytes or rabbit anti-PECAM-1 (1:100; Santa Cruz Biotechnology, sc-1506-R), were applied overnight at 4 °C to visualize cerebral blood vessels. Donkey anti-rabbit Alexa Fluor 555 (1:1,000; Life Technologies, A31572) was applied as a secondary antibody for 2 h at room temperature. All sections were stained with DAPI (1:1,000; Life Technologies, D3571) to label cell nuclei.
Imaging was performed by a blinded researcher using a Nuance multispectral imaging system (PerkinElmer), as described previously (56) and analyzed using ImageJ software. Astrocytes were imaged in the hippocampus and cerebellar regions at 200× magnification, and the mean fluorescent intensity of GFAP was quantified as a marker of astrocyte activation. In the cerebellum and hippocampus, three separate images per animal were obtained at random throughout the lobule regions, and GFAP intensity was averaged. Cerebellar vasculature and fluorescein permeation were visualized at 400× magnification.
Immunohistochemistry and analysis of microglial activation was conducted in a similar manner. Sagittal sections (16 µm) were collected on the cryostat, dried, and stored at −20 °C until use. Sections were washed with 1× PBS and subsequently blocked with 1% BSA, 5% (vol/vol) donkey serum, and 0.2% Triton X-100 for 2 h at room temperature. Incubation with primary rabbit anti-ionized calcium-binding adapter molecule antibody (Iba-1, 1:500; Wako, 019–19741) was performed overnight at 4 °C. Sections were incubated with donkey anti-rabbit Alexa Fluor 555 (1:1,000; Life Technologies) for 2 h at room temperature and with DAPI (1:1,000; Life Technologies) for 20 min at room temperature the next day. Microglia were visualized in the hippocampus (images obtained distal and superior to the dentate gyrus, inferior to cornu ammonis 1, and medial to cornu ammonis 3) and lobules V and VIa of the cerebellar cortex using a Zeiss AxioSkop microscope with a Nuance spectra camera and software. Quantitative morphometric analysis of Iba-1 tissue was performed in two images in three sections per region of interest for six animals in each treatment group. Microglia morphology was assessed as the cell soma size, and the territory occupied by an individual cell was determined as the perimeter created by the ends of the processes. Data are presented as the ratio of the soma size over the territory, which should increase as microglia become activated.
BBB Permeability Determination with Albumin.
Brains were collected for immunofluorescence microscopy as described above for MWCNT-exposed and DM control mice, except without fluorescein injection. PFA-fixed thin parasagittal sections (10 μm) were blocked in 5% (vol/vol) normal goat serum and 0.1% Triton X-100 and probed at 4 °C overnight with primary antibodies in blocking buffer: vascular marker anti-rat ZO1 tight junction protein 1 (Developmental Studies Hybridoma Bank), BBB permeation marker anti-rabbit albumin (Bioss), microglial marker anti-guinea pig IBA1 (Synaptic Systems), and astrocytic marker anti-chicken GFAP (Abcam).
After washing, sections were probed with corresponding Alexa-Fluor 350/488/568/680-conjugated goat secondary antibodies in blocking buffer at room temperature for 2 h. Mounted sections were coverslipped in ProLong Diamond Antifade Mountant (Thermo Fisher Scientific). Section were assessed by multichannel immunofluorescence microscopy with a Zeiss Axio Imager M2 structured illumination microscope for both widefield and optical sectioning (0.25-μm stack thickness). Image acquisition, processing, and morphometric analysis were performed with Zeiss Zen 2.1 software, and colocalization analysis was performed in Fiji (57) using the Costes automated threshold method (58) and the Pearson and Spearman linear regression methods.
Tissue Relative mRNA Expression.
RNA was isolated from frozen lung (left lobe) and liver using the RNeasy Mini Kit (Qiagen). Evaluation of gene expression was determined by standard 96-well technology using a StepOne real-time PCR system (Applied Biosystems) with predesigned Assays-on-Demand TaqMan probes and primers (Applied Biosystems). For lung, Il6 (Mn00446190_m1), Ccl2 (Mn00441242_m1), and Cxcl2 (Mn00436450_m1) were measured. For liver, Mt1 (Mn00496660_g1), Mt2 (Mn00809556_s1), Saa1 (Mn00656927_g1), Hp (Mn00516884_m1), and Apcs (Mn00488099_g1) were measured. Using 96-well plates, 1 µg of total RNA was reverse-transcribed using random hexamers (Applied Biosystems) and SuperScript III (Invitrogen). Then 9 µL of cDNA (1/10) was used for gene expression determination. Hypoxanthine-guanine phosphoribosyltransferase served as an internal reference. Relative gene expression was calculated using the comparative threshold method (2-ΔΔCt), with vehicle-treated mice serving as the reference group (59).
Microarray Hybridization.
Affymetrix GeneChip Mouse 430 2.0 arrays were used for gene expression analysis. All procedures were performed in accordance with the manufacturer’s instructions. In brief, 1 µg of total RNA was used to generate double-stranded cDNA with an oligo(dT) primer containing the T7 RNA polymerase promoter site and a One-Cycle Target Labeling Kit (Affymetrix). cDNA was purified by column purification using the GeneChip Sample Cleanup Module (Affymetrix), and biotinylated cRNA was synthesized by in vitro transcription using the GeneChip IVT Labeling Kit (Affymetrix). Biotin-labeled cRNA was purified, and absorbance was measured at 260 nm to determine yield (NanoDrop). Then 20 µg of the labeled cRNA was fragmented and hybridized to the Affymetrix GeneChip Mouse 430 2.0 arrays for 16 h at 45 °C, following the Affymetrix protocol specific to this array type. Washing and staining were performed on a Affymetrix Fluidics 450 station in accordance with the antibody amplification protocol (the EukGE-Ws2v5 fluidics script). The GeneChips arrays were scanned with an Affymetrix GeneChip Scanner 3000.
Statistical analysis procedures for whole microarray datasets have been extensively described previously (60). Gene lists containing group means of expression, P values, and standard fold changes served as input for subsequent bioinformatics analysis. The downstream effects analysis was generated with Ingenuity Pathway Analysis (Ingenuity Systems; www.ingenuity.com). Whole datasets containing gene identifiers and corresponding expression values were uploaded into the application, and a core analysis was performed. Each identifier was mapped to its corresponding object in Ingenuity's Knowledge Base. In this study, the following analysis criteria were used: log ratio of 0.5 and P < 0.01. We have previously shown that a 1.1- to 1.3-fold change offered a reasonable number of molecules to evaluate responses (60). The log ratio of 0.5 used in this study corresponds to a 1.4-fold change. The differentially expressed genes were evaluated by downstream effects analysis, which enables visualization of biological trends in the dataset and prediction of the effect of gene expression changes on biological processes and disease or on toxicologic functions. The analysis identifies functions that are expected to increase or decrease, given the observed gene expression changes.
RNA Purification and Quantitative PCR.
Following the 4-h serum incubation, mCECs were washed with PBS, lysed, and collected for RNA purification. Total RNA was isolated using the RNeasy Mini Prep Kit (Qiagen), and RNA was reverse-transcribed using the High-Capacity Reverse-Transcriptase Kit (Applied Biosystems) before quantitative real-time PCR assessment of endothelial markers. Amplification of the target message was performed with TaqMan Universal Master Mix (Applied Biosystems) following the manufacturer’s recommendations with TaqMan gene expression assays for TBP (TATA-binding protein) (Applied Biosystems, Mm00446973), VCAM-1 (Applied Biosystems, Mm01320970), and eNOS (Applied Biosystems, Mm00435217).
Flow Cytometry for Cell Surface Markers.
Following serum treatment, mCECs were washed with PBS, trypsonized, and collected in 12 × 75-mm culture tubes (VWR). The mCECs were then incubated with primary antibody for either VCAM-1 (BD Biosciences, lot 2117560, FITC conjugate) or ICAM-1 (BD Biosciences, lot 25775, PE conjugate), washed three times with PBS, and suspended in 3% (wt/vol) BSA. Samples were read on an LSR Fortessa cell analyzer (BD Biosciences).
Cell Migration Assay.
mCECs were plated on eight-well chamber slides and allowed to come to confluence. Cells were then “scratched” with a p200 pipette tip and washed with PBS, followed by application of serum from control or MWCNT-exposed mice to the cells. Chambers were then placed in a live cell imaging system (Olympus), and a digital image was obtained every 15 min for 6 h. Cell area was quantified with ImageJ and plotted over time.
Immunoassay for Serum TSP-1.
Serum collected from WT mice treated with vehicle or 10 µg of MWCNT-7 was assayed for TSP-1 protein levels by Western blot analysis. In brief, serum protein was quantified via spectrophotometric analysis (DS-11 spectrophotometer; DeNovix). Then 100 µg of total protein was separated on 4–15% Mini Protean Precast Gels (Bio-Rad, 456-1085) and transferred to a 0.2-µm Immun-Blot PVDF membrane (Bio-Rad, 162-0177). The membrane was blocked with 5% (wt/vol) nonfat dry milk in Tris-buffered saline containing 0.05% Tween-20 (TBS-T) for 1 h at room temperature. Membranes were then incubated in goat polyclonal primary antibody against TSP-1 in TBS-T containing 5% nonfat dry milk (1:1,000; Santa Cruz Biotechnology, sc-12312) overnight at 4 °C. The membrane was washed three times for 10 min each with TBS-T, and then incubated in an HRP-conjugated rabbit anti-goat IgG antibody (1:5,000; Santa Cruz Biotechnology, sc-2768) diluted in TBS-T containing 5% nonfat dry milk for 1 h at room temperature. The membrane was washed three times for 10 min each in TBS-T. Antigen-antibody complexes were detected using Pierce ECL Plus chemiluminescent substrate (Thermo Fisher Scientific, 32132). Images were obtained using the FluorChem E system (ProteinSimple). The membrane was then washed three times in TBS and stained with a 0.1% Coomassie Brilliant Blue R-250 (Bio-Rad, 161-0400) solution (50% methanol, 10% acetic acid, and 40% H2O by volume) for 5–10 min at room temperature and destained with several changes in 1× Coomassie R-250 Destain Solution (Bio-Rad, 161-0438) until an appropriate contrast between protein bands and background was achieved. The membrane was then washed in TBS, followed by image capture. Protein bands and total protein were quantified using ImageJ version 1.50i. Data were analyzed using GraphPad Prism 6.
Statistics.
Data are expressed as raw values, the percentage of control, or the difference from control. Data for the treatment groups are expressed as mean ± SEM, and statistical significance was assessed with one- or two-way ANOVA followed by Bonferroni’s post hoc analysis or Student’s t test, as indicated in the figure legends. Functional assays were assessed by two-way ANOVA, with time and exposure as the factors of variance. A P value <0.05 was considered to indicate statistical significance.
Acknowledgments
A.E. thanks Diane Schwegler-Berry for the electron microscopy images of the MWCNT. Microarray analyses were conducted in the KUGR Genomics Facility at University of New Mexico with valuable support from Gavin Pickett. Dr. Michael Paffett supported a portion of the immunohistochemical image generation and analysis through the University of New Mexico and Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed at hsc.unm.edu/crtc/microscopy/acknowledgement.shtml. The antibody to ZO1 (R26.4C) was developed by D. A. Goodenough at Harvard Medical School and was obtained from the Developmental Studies Hybridoma Bank, created by the Eunice Kennedy Shriver National Institute of Child Health and Human Development NICHD and maintained at the University of Iowa. This study was funded by grants from the National Institute for Occupational Safety and Health [010828 (to M.J.C.), 010495 (to M.J.C. and A.K.O.), and NTRC 939ZXFL (to A.E.)] and the National Institutes of Health [ES014639 (to M.J.C.), ES023060 (to A.K.O.), GM088021 (to K.Z.), and HL007736 (to M.J.A.)]. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health and the National Institute for Occupational Safety and Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616070114/-/DCSupplemental.
References
- 1.Kioumourtzoglou MA, et al. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect. 2016;124(1):23–29. doi: 10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loane C, Pilinis C, Lekkas TD, Politis M. Ambient particulate matter and its potential neurological consequences. Rev Neurosci. 2013;24(3):323–335. doi: 10.1515/revneuro-2013-0001. [DOI] [PubMed] [Google Scholar]
- 3.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119(6):873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kafa H, et al. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials. 2015;53:437–452. doi: 10.1016/j.biomaterials.2015.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Pittà M, Brunel N, Volterra A. Astrocytes: Orchestrating synaptic plasticity? Neuroscience. 2016;323:43–61. doi: 10.1016/j.neuroscience.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Lou N, et al. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci USA. 2016;113(4):1074–1079. doi: 10.1073/pnas.1520398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salter MW, Beggs S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell. 2014;158(1):15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Ormsby RW, Modreanu M, Mitchell CA, Dunne NJ. Carboxyl functionalised MWCNT/polymethyl methacrylate bone cement for orthopaedic applications. J Biomater Appl. 2014;29(2):209–221. doi: 10.1177/0885328214521252. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton RF, Jr, Wu Z, Mitra S, Shaw PK, Holian A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part Fibre Toxicol. 2013;10(1):57. doi: 10.1186/1743-8977-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercer RR, et al. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryman-Rasmussen JP, et al. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40(3):349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercer RR, et al. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol. 2013;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdely A, et al. Cross-talk between lung and systemic circulation during carbon nanotube respiratory exposure: Potential biomarkers. Nano Lett. 2009;9(1):36–43. doi: 10.1021/nl801828z. [DOI] [PubMed] [Google Scholar]
- 14.Erdely A, et al. Identification of systemic markers from a pulmonary carbon nanotube exposure. J Occup Environ Med. 2011;53(6 Suppl):S80–86. doi: 10.1097/JOM.0b013e31821ad724. [DOI] [PubMed] [Google Scholar]
- 15.Aragon M, et al. MMP-9-dependent serum-borne bioactivity caused by multiwalled carbon nanotube exposure induces vascular dysfunction via the CD36 scavenger receptor. Toxicol Sci. 2016;150(2):488–498. doi: 10.1093/toxsci/kfw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: Evidence from a novel translational in vitro model. Toxicol Sci. 2012;127(1):179–186. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson S, et al. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci. 2013;134(2):304–311. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison DG. Endothelial dysfunction in atherosclerosis. Basic Res Cardiol. 1994;89(Suppl 1):87–102. doi: 10.1007/978-3-642-85660-0_8. [DOI] [PubMed] [Google Scholar]
- 19.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: Relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. doi: 10.3389/fnagi.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat Neurosci. 2016;19(6):771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussy C, et al. Microglia determine brain region-specific neurotoxic responses to chemically functionalized carbon nanotubes. ACS Nano. 2015;9(8):7815–7830. doi: 10.1021/acsnano.5b02358. [DOI] [PubMed] [Google Scholar]
- 22.Lund AK, et al. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1–mediated pathways. Arterioscler Thromb Vasc Biol. 2009;29(4):511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlofs-Nijland ME, et al. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levesque S, et al. Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011;119(8):1149–1155. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter DW, et al. Mouse pulmonary dose and time course responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269(2-3):136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Satoh S, et al. Neuroprotective properties of a protein kinase inhibitor against ischaemia-induced neuronal damage in rats and gerbils. Br J Pharmacol. 1996;118(7):1592–1596. doi: 10.1111/j.1476-5381.1996.tb15579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson CL, Srivastava K, Sprigg N, Bath PM, Bayraktutan U. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J Neurochem. 2014;129(5):816–826. doi: 10.1111/jnc.12681. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal B, et al. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int J Cardiol. 2013;166(1):246–248. doi: 10.1016/j.ijcard.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109(10):1381–1389. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Febbraio M, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105(8):1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell A, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26(1):133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H, et al. Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Perspect. 2016;124(10):1537–1546. doi: 10.1289/EHP134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumaw CL, et al. Microglial priming through the lung-brain axis: The role of air pollution-induced circulating factors. FASEB J. 2016;30(5):1880–1891. doi: 10.1096/fj.201500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell A, Araujo JA, Li H, Sioutas C, Kleinman M. Particulate matter-induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol. 2009;9(8):5099–5104. doi: 10.1166/jnn.2009.gr07. [DOI] [PubMed] [Google Scholar]
- 36.Levesque S, Surace MJ, McDonald J, Block ML. Air pollution and the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oppenheim HA, et al. Exposure to vehicle emissions results in altered blood-brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 2013;10:62. doi: 10.1186/1743-8977-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumer Y, Spindler V, Werthmann RC, Bünemann M, Waschke J. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol. 2009;220(3):716–726. doi: 10.1002/jcp.21819. [DOI] [PubMed] [Google Scholar]
- 39.Klein B, Kuschinsky W, Schröck H, Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol. 1986;251(6 Pt 2):H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- 40.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 41.Block ML. Neuroinflammation: Modulating mighty microglia. Nat Chem Biol. 2014;10(12):988–989. doi: 10.1038/nchembio.1691. [DOI] [PubMed] [Google Scholar]
- 42.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer’s disease model. Microcirculation. 2003;10(6):463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 43.Dergham P, et al. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22(15):6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23(4):1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters CE, et al. Inhibition of Rho GTPases with protein prenyltransferase inhibitors prevents leukocyte recruitment to the central nervous system and attenuates clinical signs of disease in an animal model of multiple sclerosis. J Immunol. 2002;168(8):4087–4094. doi: 10.4049/jimmunol.168.8.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueno M, et al. The expression of CD36 in vessels with blood-brain barrier impairment in a stroke-prone hypertensive model. Neuropathol Appl Neurobiol. 2011;37(7):727–737. doi: 10.1111/j.1365-2990.2011.01172.x. [DOI] [PubMed] [Google Scholar]
- 47.Rustenhoven J, et al. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. doi: 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem. 2011;117(4):735–746. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- 49.Wraith KS, et al. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood. 2013;122(4):580–589. doi: 10.1182/blood-2013-04-491688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kampfrath T, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108(6):716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren J, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33(11):3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 52.Kateb B, et al. Internalization of MWCNTs by microglia: Possible application in immunotherapy of brain tumors. Neuroimage. 2007;37(Suppl 1):S9–S17. doi: 10.1016/j.neuroimage.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 53.Bu XL, et al. Serum amyloid-beta levels are increased in patients with chronic obstructive pulmonary disease. Neurotox Res. 2015;28(4):346–351. doi: 10.1007/s12640-015-9552-x. [DOI] [PubMed] [Google Scholar]
- 54.Habeych ME, Castilla-Puentes R. Comorbid medical conditions in vascular dementia: A matched case-control study. J Nerv Ment Dis. 2015;203(8):604–608. doi: 10.1097/NMD.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 55.Porter DW, et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2013;7(7):1179–1194. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topper LA, Baculis BC, Valenzuela CF. Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflammation. 2015;12:160. doi: 10.1186/s12974-015-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costes SV, et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86(6):3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60.Erdely A, et al. Oxidative stress and reduced responsiveness of challenged circulating leukocytes following pulmonary instillation of metal-rich particulate matter in rats. Part Fibre Toxicol. 2014;11:34. doi: 10.1186/s12989-014-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]