Significance
Insulin and glucagon are key hormones controlling blood glucose levels. Insulin binding to its receptor promotes glucose disposal in peripheral tissues and suppresses hepatic glucose output. Patients with inactivating mutations in their insulin receptors experience severe insulin resistance and uncontrolled diabetes. No effective therapy is available. Here we demonstrate that glucagon receptor (GCGR) blockade with monoclonal antibody normalized blood glucose in a mouse model of extreme insulin resistance and hyperglycemia. A surprising finding was that compensatory expansions of α- and β-cell masses in settings of inhibited glucagon and insulin signaling occurred at normal glucose levels. The data show that GCGR antibody inhibition represents a potential therapeutic option for patients with extreme insulin-resistance syndromes.
Keywords: glucagon receptor, antibody, insulin receptor antagonist, α-cell mass, β-cell mass
Abstract
Inactivating mutations in the insulin receptor results in extreme insulin resistance. The resulting hyperglycemia is very difficult to treat, and patients are at risk for early morbidity and mortality from complications of diabetes. We used the insulin receptor antagonist S961 to induce severe insulin resistance, hyperglycemia, and ketonemia in mice. Using this model, we show that glucagon receptor (GCGR) inhibition with a monoclonal antibody normalized blood glucose and β-hydroxybutyrate levels. Insulin receptor antagonism increased pancreatic β-cell mass threefold. Normalization of blood glucose levels with GCGR-blocking antibody unexpectedly doubled β-cell mass relative to that observed with S961 alone and 5.8-fold over control. GCGR antibody blockage expanded α-cell mass 5.7-fold, and S961 had no additional effects. Collectively, these data show that GCGR antibody inhibition represents a potential therapeutic option for treatment of patients with extreme insulin-resistance syndromes.
Inactivating mutations in the insulin receptor are found in patients with Donohue (also called “leprechaunism”), Rabson–Mendenhall, and type A insulin-resistance syndromes (1–9). Patients who survive the first years of life develop persistent hyperglycemia, which is very difficult to treat (10). High doses of insulin, insulin-like growth factor 1 (IGF-1), and the leptin analog metreleptin have been used with limited success to achieve the hemoglobin A1c target (11–13). Therefore, these patients are in need of an efficacious therapy to normalize their blood glucose levels and reduce the risk of early morbidity and mortality from diabetic complications (14).
In normal individuals, plasma insulin levels increase during hyperglycemia to promote glucose disposal in peripheral tissues and reduce hepatic glucose output. On the contrary, glucagon is secreted during periods of fasting to increase hepatic glucose output and thereby restore normal glucose levels. In patients with extreme insulin-resistance syndromes, the lack of insulin receptor signaling results in glucose underutilization and loss of suppression of hepatic glucose output, resulting in severe hyperglycemia (14, 15). Plasma glucagon levels are normally suppressed during hyperglycemia but, unexpectedly, are not repressed and might even be slightly increased in some patients with severe insulin resistance (13, 16, 17). The excess of glucagon and lack of insulin signaling leads to the overproduction of hepatic glucose, contributing to the diabetic state.
It is well established that glucagon receptor (GCGR) inhibition decreases hyperglycemia in animal models of type 1 (18, 19) and type 2 diabetes (20–23) and in patients with type 2 diabetes (24). The improvement in glycemic control results primarily from reduced hepatic glucose output as shown in mice (23) and humans (25). In this study, we extend these data to show that GCGR antibody blockade reduces blood glucose levels to normal levels in a mouse model of extreme insulin resistance. Our findings suggest that GCGR inhibition represents a potential therapeutic option for patients with extreme insulin-resistance syndromes.
Results
REGN1193 Prevents Insulin Receptor Antagonist-Induced Hyperglycemia in Mice.
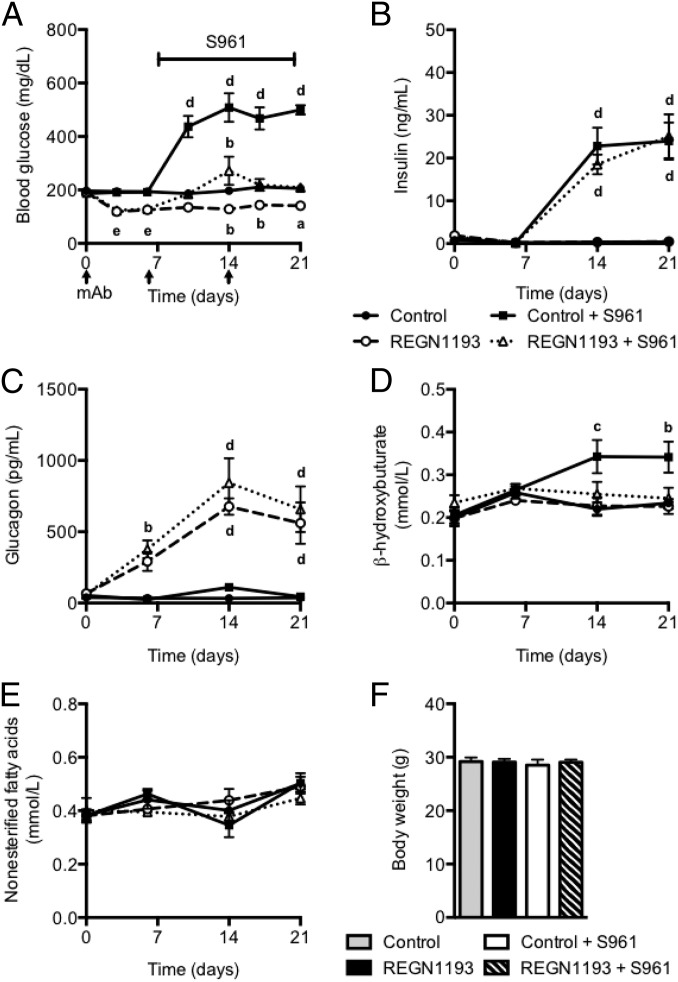
To test if GCGR inhibition prevents insulin receptor antagonist-induced hyperglycemia, we used a recently described fully human GCGR-blocking antibody, REGN1193 (20), derived using VelocImmune technology (26, 27). In particular, we wanted to test if application of a maximal dose of REGN1193 (10 mg/kg) (20) before administration of the insulin receptor antagonist S961 would be sufficient to prevent an increase in blood glucose levels. We divided mice into four groups. One group received control antibody; in this group blood glucose remained unchanged at 200 mg/dL (Fig. 1A). The second group of mice received REGN1193 (10 mg/kg) at days 0, 6, and 14; this administration lowered blood glucose to 120 mg/dL for the duration of the study. The third group of mice received control antibody and was infused at day 7 with a maximal dose of insulin receptor antagonist S961 (20 nmol/wk) for the duration of the study. Consistent with previous reports (28, 29), S961 caused severe insulin resistance associated with hyperglycemia and hyperinsulinemia (Fig. 1 A and B). The fourth group of mice was dosed with REGN1193 (10 mg/kg) at day 0, which lowered blood glucose to 125 mg/dL. At day 7, these mice were infused with S961 (20 nmol/wk), which increased blood glucose levels to 200 mg/dL (Fig. 1A). Although the blood glucose levels in the mice infused with S961 and dosed with REGN1193 were in the normal range, we observed marked increases in plasma insulin levels similar to those in mice receiving control antibody and S961 (Fig. 1B). Consistent with our previous study (20), we found that REGN1193 increased plasma glucagon levels, an effect that was independent of S961 administration (Fig. 1C). Importantly, circulating β-hydroxybutyrate levels were elevated in mice infused with S961 (Fig. 1D). The increased plasma β-hydroxybutyrate returned to normal levels in mice simultaneously treated with S691 and REGN1193 (Fig. 1D). REGN1193 had no effect on plasma β-hydroxybutyrate levels in insulin-sensitive mice. No differences in plasma nonesterified fatty acid levels or body weight were observed between the treatment groups (Fig. 1 E and F).
Fig. 1.
GCGR-blocking antibody prevents hyperglycemia in severely insulin-resistant mice. (A) Fed blood glucose from chow-fed C57BL/6 male mice before and at multiple time points after s.c. injections (10 mg/kg) of REGN1193 or isotype control antibody (n = 6–8 mice per group). Two groups of mice received either REGN1193 or control antibody and were infused with S961 (20 nmol/wk) starting at day 7 and for the duration of the study. The other two groups of mice were infused with saline under the same conditions. (B–F) Plasma levels of insulin (B), glucagon (C), β-hydroxybutyrate (D), and nonesterified fatty acids (E) and body weight (F) from mice dosed as described in A. Values are shown as mean ± SEM. Statistical analysis was conducted by one- or two-way ANOVA with Bonferroni posttest. P values are comparisons to the Control group. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001; eP < 0.01 for REGN1193 and P < 0.05 for REGN1193 + S961.
GCGR Inhibition Reverses Insulin Receptor Antagonist-Induced Expression of Phosphoenolpyruvate Carboxykinase in the Liver.
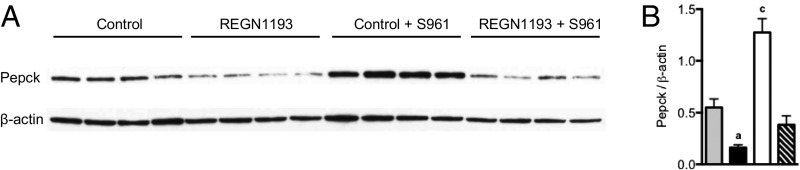
Western blot analysis revealed that levels of the rate-limiting gluconeogenic enzyme phosphoenolpyruvate carboxykinase (Pepck) were reduced by 70% in livers of mice treated with REGN1193 (Fig. 2). On the contrary, Pepck levels increased 2.3-fold in livers of mice infused with S961, an effect that was reversed to 30% below baseline by REGN1193 (Fig. 2 A and B). Thus, the relative levels of glucagon and insulin signaling regulate Pepck expression, as demonstrated previously (30–32). These data suggest that GCGR blockade with REGN1193 prevents severe insulin resistance-induced hyperglycemia in mice, in part by suppressing gluconeogenesis and hepatic glucose output.
Fig. 2.
Glucagon and insulin receptor antagonism regulates liver Pepck expression. (A) Western blot of Pepck and β-actin loading control for liver from the four treatment groups described in Fig. 1. (B) Densiometric analysis of A (n = 4 mice per group). Values are shown as mean ± SEM. Statistical analysis was conducted by one-way ANOVA with Bonferroni posttest. P values are comparisons to the Control group. aP < 0.05; cP < 0.001.
REGN1193 Reverses Insulin Receptor Antagonist-Induced Hyperglycemia in Mice.
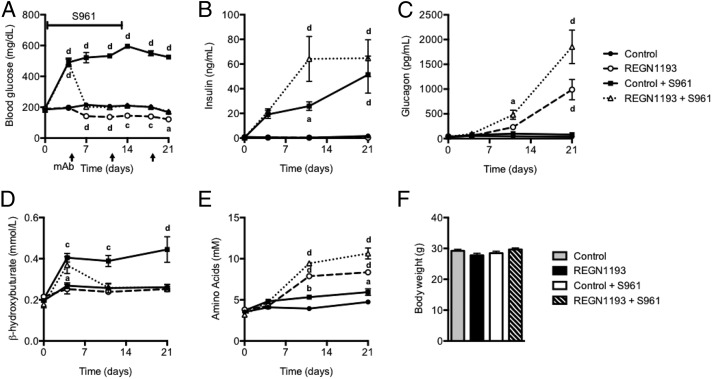
Next, as an important first step in understanding if GCGR blockage with REGN1193 could potentially be used to manage blood glucose levels in patients with severe insulin resistance, we tested if GCGR antibody inhibition could reverse insulin resistance-induced hyperglycemia in mice. To this end, we infused mice with S961 (20 nmol/wk) for 14 d, a treatment that resulted in persistent hyperglycemia and hyperinsulinemia (Fig. 3 A and B). Administration of REGN1193 (10 mg/kg) at day 4, after hyperglycemia and insulin resistance was already established, rapidly normalized the hyperglycemia, but did not lower blood glucose to below-normal levels as observed in mice treated with REGN1193 alone (Fig. 3A). The hyperinsulinemia and hyperglucagonemia were more pronounced in mice that received both receptor antagonists (Fig. 3 B and C). The insulin resistance-induced increase in plasma β-hydroxybutyrate levels was reversed in mice receiving REGN1193 (Fig. 3D). In agreement with our previous report (20), we found that REGN1193 increased circulating amino acid levels (Fig. 3E). Interestingly, S961 also increased plasma amino acid levels but to a lesser extent than REGN1193. Inhibition of both insulin and glucagon receptors caused an additive increase in plasma amino acid levels (Fig. 3E). We did not observe changes in body weight (Fig. 3F).
Fig. 3.
GCGR-blocking antibody reverses hyperglycemia in severely insulin-resistant mice. (A) Fed blood glucose from chow-fed male C57BL/6 mice before and at multiple time points after s.c. administration of REGN1193 or control antibody (10 mg/kg; n = 8 mice per group). Two groups of mice received either REGN1193 or control antibody and were infused with S961 (20 nmol/wk) for 14 d starting at day 0. The other two groups of mice were infused with saline under the same conditions. (B–F) Plasma levels of insulin (B), glucagon (C), β-hydroxybutyrate (D), and amino acids (E) and body weight (F) from mice dosed as described in A were quantified. Values are shown as mean ± SEM. Statistical analysis was conducted by one- or two-way ANOVA with Bonferroni posttest. P values are comparisons to the Control group. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001.
GCGR and Insulin Receptor Antagonism Increase α- and β-Cell Masses.
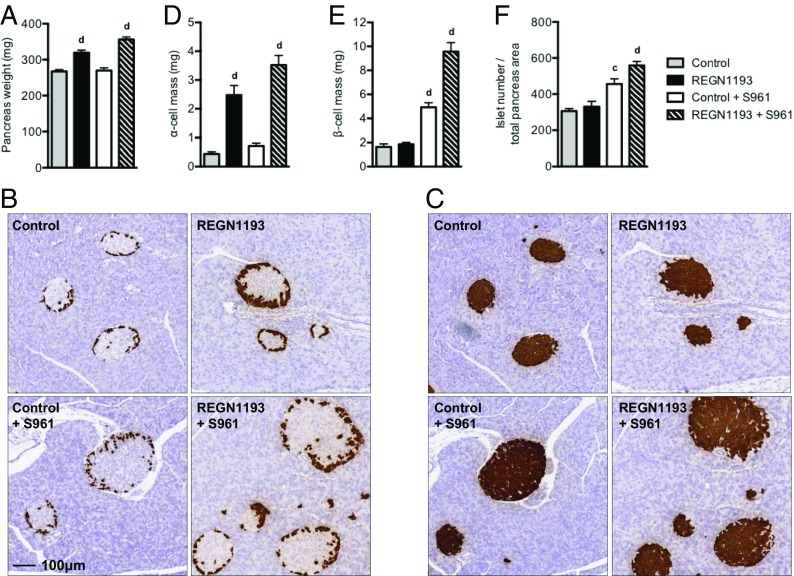
We found that REGN1193 increased pancreas weight by 19%, an effect that was larger (33%) in the presence of both REGN1193 and S961 (Fig. 4A). RNA in situ hybridization (RNA ISH) using probes to glucagon (Gcg) and insulin 2 (Ins2) was used for morphometric analysis of pancreas sections. REGN1193 increased α-cell mass 5.7-fold (Fig. 4 B and D). In agreement with our previous study (28), we found that S961 administration increased β-cell mass threefold (Fig. 4 C and E). REGN1193 did not affect β-cell mass (Fig. 4 C and E). Unexpectedly, β-cell mass doubled in the simultaneous presence of S961 and REGN1193 compared with S961 alone and increased 5.8-fold over control mice (Fig. 4 C and E). It is important to note that the further expansion of the β-cell mass took place in settings of blood glucose levels in the normal range (compare with Fig. 3A). The α-cell mass was increased slightly (1.6-fold) by S961 and in the simultaneous presence of REGN1193 (1.4-fold over REGN1193 alone) (Fig. 4 B and D). S961 increased the islet number per total pancreas area by 49%, whereas the combined treatment with S961 and REGN1193 increased islet number per area by 82% (Fig. 4F). The additional increase in β-cell mass in the simultaneous presence of REGN1193 and S961 is unlikely to result from transdifferentiation of α cells into β cells, because we did not observe a single glucagon-insulin double-positive cell in more than 100 examined islets (Fig. S1). In summary, we observed compensatory increases in α- and β-cell masses when glucagon and insulin signaling was inhibited: The β-cell mass doubled in insulin-resistant mice when glucagon signaling was blocked, and this effect took place at blood glucose levels in the normal range.
Fig. 4.
Glucagon and insulin receptor antagonists increase α- and β-cell masses in mice. (A) Pancreas weights from chow-fed male C57BL/6 mice treated with REGN1193 and S961, as described in the legend of Fig. 3. (B and C) Representative RNA ISH images of a pancreas section from one mouse from each of the four treatment groups stained for glucagon (B) or insulin (C). Images in B and C are taken at the same magnification (10× objective lens). (D–F) α-Cell mass (D), β-cell mass (E), and islet number per total pancreas area (F) for the four treatment groups (n = 8 mice per group). Values are shown as mean ± SEM. Statistical analysis was conducted by one-way ANOVA with Bonferroni posttest. P values are comparisons to the Control group. cP < 0.001; dP < 0.0001.
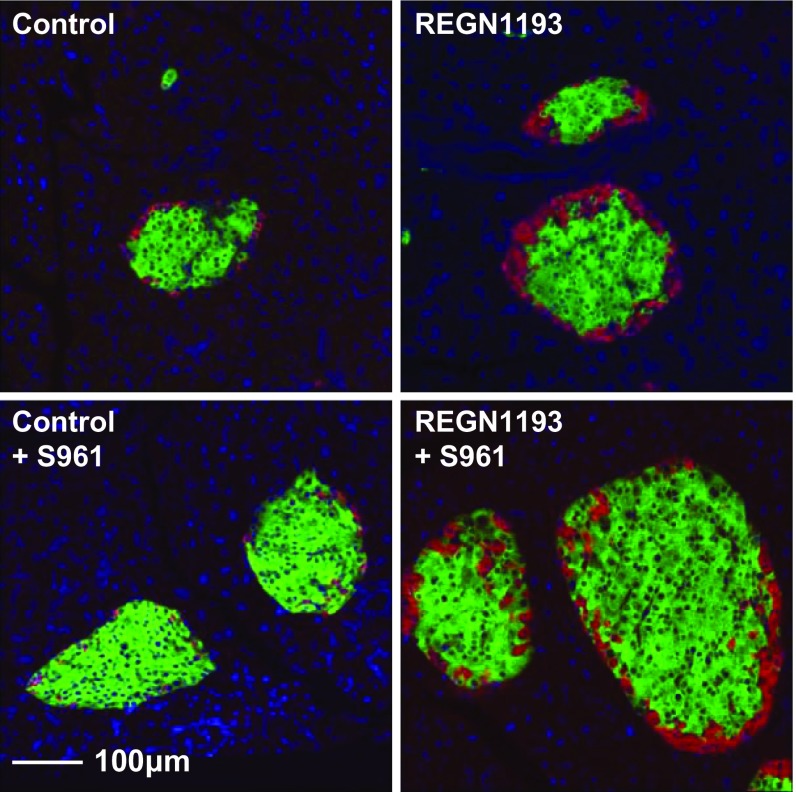
Fig. S1.
Glucagon-insulin double-positive cells were not detected in pancreata from mice treated with glucagon and insulin receptor antagonists. Shown are representative RNA ISH images of pancreas sections from mice treated as described in the legend of Fig. 3. Glucagon is stained red, and insulin is stained green.
Discussion
We report here the use of a severe insulin-resistance mouse model to explore if antibody blockade of GCGR signaling could potentially improve glycemic control in patients with Donohue, Rabson–Mendenhall, and type A insulin-resistance syndromes. We show that the fully human monoclonal anti-GCGR antibody REGN1193 reversed the hyperglycemia and the increase in plasma β-hydroxybutyrate induced by the insulin receptor antagonist S961. The hyperglucagonemia and expansion of α-cell mass in the presence of REGN1193 was comparable in insulin-sensitive and -resistant mice. Furthermore, the S961-induced hyperinsulinemia and the increase in β-cell mass occurred in both hyperglycemic mice and mice with blood glucose levels in the normal range. Unexpectedly, the expansion of β-cell mass was greater in mice with inhibited rather than normal GCGR signaling. Collectively, these data suggest that GCGR blockade with REGN1193 represents a potential treatment option to improve blood glucose levels and reduce the risk for early morbidity and mortality from complications of diabetes in patients with extreme insulin resistance. A potential risk associated with this approach is expansion of α-cell and β-cell mass.
Inactivating mutations in the insulin receptor gene are found in patients with syndromes of severe insulin resistance. It has been established that the degree of impairment of insulin binding by the cells of patients with severe insulin resistance is inversely correlated with the duration of the patient’s survival (33). The Donohue syndrome or leprechaunism is the most extreme form. Patients with Rabson–Mendenhall syndrome and type A insulin resistance have slightly milder insulin resistance because their inactivating mutations do not lead to complete loss of insulin receptor function (33). Despite residual insulin receptor function, hyperglycemia is extremely difficult to treat in patients with Rabson–Mendenhall syndrome (10). High concentrations of insulin, IGF-1, and metreleptin have only limited efficacy in patients with even the mildest forms of the syndrome (11–13). There are case reports that patients with type A insulin resistance can be controlled with insulin and metformin combined (34). Therefore, additional therapies are needed to help normalize blood glucose levels. Our data also suggest that REGN1193 can be used to prevent medically induced insulin resistance and hyperglycemia. For example, cancer patients treated with oncology products that inhibit components of the insulin-signaling cascade (e.g., PI3K inhibitors) can experience acute insulin resistance and hyperglycemia.
We used the insulin receptor antagonist S961 to generate a mouse model of severe insulin resistance to test if GCGR inhibition with REGN1193 would reduce hepatic glucose production and hyperglycemia. It has been shown previously in mice and humans that GCGR inhibition lowers blood glucose primarily by reducing hepatic glucose production (23, 25). The rationale for the study was that patients with severe insulin-resistance syndromes have normal or even slightly elevated plasma glucagon levels despite hyperglycemia (16, 17). The hyperglycemia results from enhanced hepatic glucose output caused by the lack of insulin suppression and abnormally high glucagon signaling. This etiology is supported by the observation that levels of the key gluconeogenic protein Pepck were elevated in livers of mice infused with the insulin receptor antagonist. Importantly, Pepck levels were reduced to below normal by REGN1193 in insulin-resistant mice. Consistent with this result, we found that REGN1193 prevented or reversed hyperglycemia in the S961-treated mice. These data suggest that REGN1193 might be an effective mechanism for lowering blood glucose levels in patients with severe insulin-resistance syndromes.
Our data confirm previous findings that GCGR antibody inhibition causes hyperglucagonemia and α-cell hyperplasia (20, 35). Amino acids mediate these effects via an mTOR-dependent mechanism (36). Elevated circulating amino acid levels arise from reduced uptake and conversion of amino acids into gluconeogenic precursors in the livers of mice with inhibited GCGR signaling (22, 36). We also observed an increase in pancreas weight, which previously has been shown to be secondary to increased circulating amino acid levels (36). The hyperglucagonemia, α-cell hyperplasia, and increase in pancreas weight following GCGR inhibition were induced in both insulin-sensitive and -resistant mice. Our data suggest a potential risk for α-cell hyperplasia following chronic REGN1193 treatment of patients with severe insulin-resistance syndromes. This suggestion is supported by the observation that marked hyperglucagonemia and α-cell hyperplasia was reported in carriers of inactivating mutations in GCGR (37, 38).
Therapeutic antibodies to GCGR normalize blood glucose in preclinical models of type 2 diabetes (20, 23, 35). However, GCGR inhibition improves but does not normalize blood glucose levels in diabetic and insulin-deficient mice (18, 39, 40), indicating that residual β-cell mass is required to normalize blood glucose in settings of reduced GCGR signaling. We now demonstrate that GCGR inhibition can normalize blood glucose levels in mice with extreme insulin resistance. These mice have increased β-cell mass and insulin secretion to help compensate for the insulin resistance. The effects of insulin on IGF receptors are unlikely to account for the ability of GCGR-blocking antibody to normalize blood glucose levels because S961 binds and blocks these receptors with high affinity (41). Leptin could mediate this effect because it has been shown to normalize blood glucose levels in mice with no β cells (42, 43). An alternative explanation is that β cells secrete a factor that reduces glucose absorption in the gut or promotes insulin-independent glucose uptake in peripheral tissues to help normalize blood glucose levels. This factor is not present in diabetic mice with no β cells and might explain why GCGR inhibition could only improve, but not normalize, blood glucose levels (18, 39, 40). Further studies are required to determine the mechanism by which extreme insulin resistant-induced hyperglycemia is reversed following inhibition of glucagon signaling.
Our study shows that the expansion of β-cell mass induced by insulin receptor inhibition does not require concomitant hyperglycemia. This finding is surprising, because glucose has been shown to be an important β-cell mitogen (44, 45). We have excluded a role for ANGPTL8 as the long-sought betatrophin to mediate insulin resistance-induced expansion of the β-cell mass (28), as first proposed by Yi et al. (29). Inhibition of insulin receptors on β cells is unlikely to account for the effects, because mice with specific deletion of insulin receptors in β cells have normal β-cell mass (46). One potential candidate is serpinB1, a liver-derived secreted protein that regulates β-cell mass (47). However, whether sepinB1 is secreted from the liver and promotes expansion of β-cell mass in normoglycemic mice remains to be determined. An unexpected finding was that the β-cell mass doubled in insulin-resistant mice treated with the GCGR-blocking antibody. We speculate that the elevated plasma amino acid levels in mice with both glucagon and insulin receptor inhibitors could be responsible for the enhancement in the β-cell mass.
Uncontrolled diabetes can be associated with ketoacidosis. The plasma β-hydroxybutyrate level is raised in ketosis. This level is easily measured and often serves as a marker for ketoacidosis. We found that β-hydroxybutyrate plasma levels were strongly elevated in mice treated with S961. Ketoacidosis is normally treated with insulin to promote glucose utilization and reduce fatty acid and amino acid metabolism. Unexpectedly, we demonstrate that the administration of the GCGR antibody effectively prevented or reversed the insulin resistance-induced increase in circulating β-hydroxybutyrate levels. This result is unlikely to be secondary to increased glucose utilization caused by the severe insulin resistance or reduced fatty acid uptake, because plasma levels did not differ among the treatment groups. Because amino acids serve as important substrates for ketogenesis in the liver (48, 49), we propose that reduced amino acid uptake and metabolism can account for the normalization of β-hydroxybutyrate levels. These data suggest that GCGR-blocking antibody may be used to treat ketoacidosis in highly insulin-resistant diabetics and in patients with severe insulin resistance caused by inactivating mutations in the insulin receptor.
In this study, we provide evidence that the inhibition of GCGR signaling is a potential therapeutic option for the management of severe hyperglycemia in patients with extreme insulin resistance caused by inactivating mutations in their insulin receptors. Patients with syndromes of severe insulin resistance are in high need for better medications to manage their severe hyperglycemia, which often results in early morbidity and mortality from complications of diabetes.
Materials and Methods
In Vivo Studies.
We previously have described the in vitro and in vivo characteristics of REGN1193, a potent monoclonal GCGR-blocking antibody (20). This antibody was used in all studies in this report. The GCGR antibody and isotype control antibody were diluted with sterile PBS. C57BL/6 mice (10-wk-old males; Taconic) were housed (five mice per cage) in a controlled environment (12-h light/dark cycle, 22 ± 1 °C, 60–70% humidity) and were fed ad libitum with standard chow (PicoLab Rodent Diet 20 EXT IRR 5R53; LabDiet). The insulin receptor antagonist S961 (41) was administrated at 20 nmol/wk by osmotic minipumps (Alzet 2002) as described earlier (28). In the treatment study, 32 mice were equally divided into four groups. The first group was infused s.c. with PBS by osmotic minipumps from day 0 and was injected s.c. with 10 mg/kg of hIgG4 isotype control antibody (control antibody) on days 4, 11, and 18. The second group was infused s.c. with PBS from day 0 and was injected s.c. with 10 mg/kg of REGN1193 on days 4, 11, and 18. The third group was infused s.c. with S961 from day 0 and was injected s.c. with 10 mg/kg of control antibody on days 4, 11, and 18. The fourth group was infused s.c. with S961 from day 0 and was injected s.c. with 10 mg/kg of REGN1193 on days 4, 11, and 18. Mice were bled on days 0, 4, 7, 11, 14, 18, and 21 for blood glucose measurements. Plasma was collected at baseline and days 4, 11, and 21 to determine insulin, glucagon, β-hydroxybutyrate, and amino acid levels. For the prevention study, 29 mice were divided into four groups of six to eight mice per group. The study was conducted as described above, except that the mice were dosed s.c. with 10 mg/kg of control antibody or REGN1193 on days 0, 6, and 14 and were infused s.c. with PBS or S961 by osmotic minipumps from day 7. Mice were bled on days 0, 3, 6, 10, 14, 17, and 21 for blood glucose measurements. Plasma was collected at baseline and days 6, 14, and 21 to determine insulin, glucagon, β-hydroxybutyrate, and nonesterified fatty acid levels. All procedures were conducted in compliance with protocols approved by the Regeneron Pharmaceuticals Institutional Animal Care and Use Committee.
Blood Chemistry.
Blood glucose was determined using ACCU-CHEK Compact Plus (Roche Diagnostics). Plasma glucagon and insulin levels were determined using Mercodia glucagon and insulin ELISA. Plasma β-hydroxybutyrate and nonesterified fatty acids were assayed in a Beckman Coulter UniCel DxC 800 Synchron Clinical System (Beckman Coulter). Plasma amino acid was quantified using an l-amino acid quantification kit (Sigma).
Histology.
Pancreata were fixed in 10% (vol/vol) neutral-buffered formalin solution for 48 h, embedded in paraffin, and sectioned onto slides. Pancreas tissue and cells were permeabilized and hybridized with combinations of mRNA probes for mouse Gcg and Ins2 according to the manufacturer’s instructions (Advanced Cell Diagnostics). A chromogenic or florescence kit was used to amplify mRNA signal (Advanced Cell Diagnostics). Areas of glucagon- and insulin-positive cells were measured using Halo digital imaging analysis software (Indica Labs). The percent of glucagon- and insulin-positive areas relative to the whole pancreas area were calculated. We calculated α- and β-cell mass by multiplying the α- and β-cell area for each animal by the weight of the animal’s pancreas. Islet number was measured by counting the number of insulin-positive islets on a section with the use of Halo digital imaging analysis software and was normalized by the entire pancreas area of the section.
Western Blotting.
Liver samples were lysed with ice-cold RIPA buffer (50 mM Tris, 150 mM NaCl, 1 mM of EDTA, 50 mM NaF, 10 mM β-glycerophosphate, 5 mM sodium pyrophosphate dibasic, and 1% Nonidet P-40) in the presence of protease and phosphatase inhibitor mixtures (Thermo-Fisher), 1 mM DTT, and 2 mM Na3VO4. Total sample lysates were mixed with 6× SDS loading buffer (Alfa-Aesar) and were boiled for 5 min. Protein samples (10–100 μg) were loaded and separated on 4–20% (wt/vol) gradient SDS/PAGE gels (Bio-Rad) and transferred to PVDF membranes. The membranes were blocked for 1 h with 5% (wt/vol) BSA in 1× TBS supplemented with 0.1% Tween20 (Bio-Rad) and were incubated with antibody against Pepck (1:250; Abcam). Bound antibody was detected using HRP-conjugated anti-rabbit antibody (1:10,000; Jackson ImmunoResearch) and enhanced chemiluminescence reagent (Thermo-Fisher). Band intensities were quantified using Image J software.
Data Analyses.
Data are expressed as mean ± SEM. Statistical analyses were performed using Prism 6.0 software (GraphPad). All parameters were analyzed by one-way or two-way ANOVA; a threshold of P < 0.05 was considered statistically significant. If a significant F ratio was obtained, post hoc analysis was conducted with Bonferroni posttests.
Acknowledgments
We thank Angelos Papatheodorou for help with the plasma β-hydroxybutyrate and nonesterified fatty acid assays and Samantha Intriligator for manuscript editing.
Footnotes
Conflict of interest statement: All authors are employees and shareholders of Regeneron Pharmaceuticals.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621069114/-/DCSupplemental.
References
- 1.Kadowaki T, et al. Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science. 1988;240(4853):787–790. doi: 10.1126/science.2834824. [DOI] [PubMed] [Google Scholar]
- 2.Klinkhamer MP, et al. A leucine-to-proline mutation in the insulin receptor in a family with insulin resistance. EMBO J. 1989;8(9):2503–2507. doi: 10.1002/j.1460-2075.1989.tb08387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadowaki T, et al. Five mutant alleles of the insulin receptor gene in patients with genetic forms of insulin resistance. J Clin Invest. 1990;86(1):254–264. doi: 10.1172/JCI114693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimasa Y, et al. Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science. 1988;240(4853):784–787. doi: 10.1126/science.3283938. [DOI] [PubMed] [Google Scholar]
- 5.Moller DE, Flier JS. Detection of an alteration in the insulin-receptor gene in a patient with insulin resistance, acanthosis nigricans, and the polycystic ovary syndrome (type A insulin resistance) N Engl J Med. 1988;319(23):1526–1529. doi: 10.1056/NEJM198812083192306. [DOI] [PubMed] [Google Scholar]
- 6.Accili D, et al. A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J. 1989;8(9):2509–2517. doi: 10.1002/j.1460-2075.1989.tb08388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taira M, et al. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989;245(4913):63–66. doi: 10.1126/science.2544997. [DOI] [PubMed] [Google Scholar]
- 8.Odawara M, et al. Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science. 1989;245(4913):66–68. doi: 10.1126/science.2544998. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki T, Kadowaki H, Taylor SI. A nonsense mutation causing decreased levels of insulin receptor mRNA: Detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci USA. 1990;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semple RK, Williams RM, Dunger DB. What is the best management strategy for patients with severe insulin resistance? Clin Endocrinol (Oxf) 2010;73(3):286–290. doi: 10.1111/j.1365-2265.2010.03810.x. [DOI] [PubMed] [Google Scholar]
- 11.Cochran E, et al. Efficacy of recombinant methionyl human leptin therapy for the extreme insulin resistance of the Rabson-Mendenhall syndrome. J Clin Endocrinol Metab. 2004;89(4):1548–1554. doi: 10.1210/jc.2003-031952. [DOI] [PubMed] [Google Scholar]
- 12.McDonald A, Williams RM, Regan FM, Semple RK, Dunger DB. IGF-I treatment of insulin resistance. Eur J Endocrinol. 2007;157(Suppl 1):S51–S56. doi: 10.1530/EJE-07-0271. [DOI] [PubMed] [Google Scholar]
- 13.Brown RJ, Cochran E, Gorden P. Metreleptin improves blood glucose in patients with insulin receptor mutations. J Clin Endocrinol Metab. 2013;98(11):E1749–E1756. doi: 10.1210/jc.2013-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso C, et al. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): A 30-year prospective. Medicine (Baltimore) 2004;83(4):209–222. doi: 10.1097/01.md.0000133625.73570.54. [DOI] [PubMed] [Google Scholar]
- 15.Longo N, Wang Y, Pasquali M. Progressive decline in insulin levels in Rabson-Mendenhall syndrome. J Clin Endocrinol Metab. 1999;84(8):2623–2629. doi: 10.1210/jcem.84.8.5902. [DOI] [PubMed] [Google Scholar]
- 16.West RJ, Lloyd JK, Turner WM. Familial insulin-resistant diabetes, multiple somatic anomalies, and pineal hyperplasia. Arch Dis Child. 1975;50(9):703–708. doi: 10.1136/adc.50.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desbois-Mouthon C, et al. Major circadian variations of glucose homeostasis in a patient with Rabson-Mendenhall syndrome and primary insulin resistance due to a mutation (Cys284-->Tyr) in the insulin receptor alpha-subunit. Pediatr Res. 1997;42(1):72–77. doi: 10.1203/00006450-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Damond N, et al. Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. eLife. 2016;5:e13828. doi: 10.7554/eLife.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang MY, et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc Natl Acad Sci USA. 2015;112(8):2503–2508. doi: 10.1073/pnas.1424934112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto H, et al. Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology. 2015;156(8):2781–2794. doi: 10.1210/en.2015-1011. [DOI] [PubMed] [Google Scholar]
- 21.Yan H, et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther. 2009;329(1):102–111. doi: 10.1124/jpet.108.147009. [DOI] [PubMed] [Google Scholar]
- 22.Mu J, et al. Anti-diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small-molecule glucagon receptor antagonist. PLoS One. 2012;7(11):e49572. doi: 10.1371/journal.pone.0049572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim WD, et al. Human monoclonal antibodies against glucagon receptor improve glucose homeostasis by suppression of hepatic glucose output in diet-induced obese mice. PLoS One. 2012;7(12):e50954. doi: 10.1371/journal.pone.0050954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly RP, et al. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes Metab. 2015;17(4):414–422. doi: 10.1111/dom.12446. [DOI] [PubMed] [Google Scholar]
- 25.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia. 2001;44(11):2018–2024. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 26.Macdonald LE, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111(14):5147–5152. doi: 10.1073/pnas.1323896111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy AJ, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111(14):5153–5158. doi: 10.1073/pnas.1324022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gusarova V, et al. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell. 2014;159(3):691–696. doi: 10.1016/j.cell.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi P, Park JS, Melton DA. Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell. 2013;153(4):747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Iynedjian PB, et al. Glucokinase and cytosolic phosphoenolpyruvate carboxykinase (GTP) in the human liver. Regulation of gene expression in cultured hepatocytes. J Clin Invest. 1995;95(5):1966–1973. doi: 10.1172/JCI117880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rucktäschel AK, Granner DK, Christ B. Regulation by glucagon (cAMP) and insulin of the promoter of the human phosphoenolpyruvate carboxykinase gene (cytosolic) in cultured rat hepatocytes and in human hepatoblastoma cells. Biochem J. 2000;352(Pt 1):211–217. [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol. 2005;40(3):129–154. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- 33.Longo N, et al. Genotype-phenotype correlation in inherited severe insulin resistance. Hum Mol Genet. 2002;11(12):1465–1475. doi: 10.1093/hmg/11.12.1465. [DOI] [PubMed] [Google Scholar]
- 34.Enkhtuvshin B, et al. Successful pregnancy outcomes in a patient with type A insulin resistance syndrome. Diabet Med. 2015;32(6):e16–e19. doi: 10.1111/dme.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu W, et al. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther. 2009;331(3):871–881. doi: 10.1124/jpet.109.157685. [DOI] [PubMed] [Google Scholar]
- 36.Solloway MJ, et al. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Reports. 2015;12(3):495–510. doi: 10.1016/j.celrep.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 37.Zhou C, Dhall D, Nissen NN, Chen CR, Yu R. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, alpha cell hyperplasia, and islet cell tumor. Pancreas. 2009;38(8):941–946. doi: 10.1097/MPA.0b013e3181b2bb03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sipos B, et al. Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J Clin Endocrinol Metab. 2015;100(5):E783–E788. doi: 10.1210/jc.2014-4405. [DOI] [PubMed] [Google Scholar]
- 39.Neumann UH, et al. Glucagon receptor gene deletion in insulin knockout mice modestly reduces blood glucose and ketones but does not promote survival. Mol Metab. 2016;5(8):731–736. doi: 10.1016/j.molmet.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steenberg VR, et al. Acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes. Diabetologia. 2016;59(2):363–370. doi: 10.1007/s00125-015-3794-2. [DOI] [PubMed] [Google Scholar]
- 41.Schäffer L, et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun. 2008;376(2):380–383. doi: 10.1016/j.bbrc.2008.08.151. [DOI] [PubMed] [Google Scholar]
- 42.Fujikawa T, et al. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18(3):431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.German JP, et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152(2):394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alonso LC, et al. Glucose infusion in mice: A new model to induce beta-cell replication. Diabetes. 2007;56(7):1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porat S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 2011;13(4):440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96(3):329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 47.El Ouaamari A, et al. SerpinB1 promotes pancreatic β cell proliferation. Cell Metab. 2016;23(1):194–205. doi: 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keller U, Schnell H, Sonnenberg GE, Gerber PP, Stauffacher W. Role of glucagon in enhancing ketone body production in ketotic diabetic man. Diabetes. 1983;32(5):387–391. doi: 10.2337/diab.32.5.387. [DOI] [PubMed] [Google Scholar]
- 49.Kirsch JR, D’Alecy LG. Glucagon stimulates ketone utilization by rat brain slices. Stroke. 1984;15(2):324–328. doi: 10.1161/01.str.15.2.324. [DOI] [PubMed] [Google Scholar]