Significance
β-Arrestins (βarrs) interact with G protein-coupled receptors (GPCRs) to desensitize G protein signaling, initiate signaling on their own, and mediate receptor endocytosis. Using a panel of GPCRs believed to couple differently to βarrs, we demonstrate how distinct conformations of GPCR–βarr complexes are specialized to perform different subsets of these cellular functions. Our results thus provide a new signaling paradigm for the understanding of GPCRs, whereby a specific GPCR–βarr conformation mediates receptor desensitization, and another drives internalization and some forms of signaling.
Keywords: GPCR, arrestin, endocytosis, signaling, desensitization
Abstract
β-Arrestins (βarrs) interact with G protein-coupled receptors (GPCRs) to desensitize G protein signaling, to initiate signaling on their own, and to mediate receptor endocytosis. Prior structural studies have revealed two unique conformations of GPCR–βarr complexes: the “tail” conformation, with βarr primarily coupled to the phosphorylated GPCR C-terminal tail, and the “core” conformation, where, in addition to the phosphorylated C-terminal tail, βarr is further engaged with the receptor transmembrane core. However, the relationship of these distinct conformations to the various functions of βarrs is unknown. Here, we created a mutant form of βarr lacking the “finger-loop” region, which is unable to form the core conformation but retains the ability to form the tail conformation. We find that the tail conformation preserves the ability to mediate receptor internalization and βarr signaling but not desensitization of G protein signaling. Thus, the two GPCR–βarr conformations can carry out distinct functions.
Over the past decade, significant efforts have been made to understand the molecular properties and regulatory mechanisms that control the function of β-arrestin (βarr) interactions with G protein-coupled receptors (GPCRs) (1, 2). Once activated, GPCRs initiate a highly conserved signaling and regulatory cascade marked by interactions with: (i) heterotrimeric G proteins, which mediate their actions largely by promoting second-messenger generation (3); (ii) GPCR kinases (GRKs), which phosphorylate activated conformations of receptors (4); and (iii) βarrs, which bind to the phosphorylated receptors to mediate desensitization of G protein signaling and receptor internalization (5, 6). In addition to their canonical function of desensitization and internalization, βarrs have been appreciated as independent signaling units by virtue of their crucial role as both adaptors and scaffolds for an increasing number of signaling pathways (7–11).
There are two driving forces that mediate βarr interactions with an activated GPCR: phosphorylation of the C-terminal tail of the receptor by GRKs and/or binding to the transmembrane core of the receptor. How each of these interactions contributes to βarr functionality remains unclear. Moreover, GPCRs tend to either interact with βarr transiently, termed “class A” GPCRs [e.g., β2-adrenergic receptor (β2AR)], or tightly, known as “class B” GPCRs [e.g., vasopressin type 2 receptor (V2R)]. For the current study, we use a previously described chimeric β2V2R construct, which comprises the β2AR with its C-terminal tail exchanged with the V2R C-terminal tail (12–14). The β2V2R construct provides an ideal system for studying a GPCR–βarr complex in vitro, because it maintains identical pharmacological properties to the WT β2AR and has a robustly increased class B affinity for βarr1, which allows stable β2V2R–βarr complexes to be formed and purified.
Structural insights have shed some light onto the complexity of the interaction between GPCRs and βarrs. A recent structural study of a constitutively active rhodopsin–arrestin fusion protein revealed high-resolution information about a single conformation of the complex in which the arrestin engaged via the transmembrane core of the receptor (12). However, negative-stain electron microscopy (EM) analysis of an antigen-binding fragment 30 (Fab30)–stabilized β2V2R–βarr1–Fab30 complex demonstrated that the β2V2R–βarr1 complex assumes two unique conformations: one in which ∼63% of the βarr1 in the complex is bound only to the phosphorylated receptor C-terminal tail and appears to hang from the receptor (“tail” conformation) and a second more fully engaged conformation representing ∼37%, in which, in addition to the tail interaction, the finger-loop region (FLR) of βarr1 inserts into the transmembrane core of the receptor (“core” conformation) (13).
It is not known whether different GPCR–βarr conformations mediate distinct functional outputs. Thus, we sought to identify βarr1 mutants that predominantly form complexes with β2V2R in one or the other conformation, and then to test their ability to promote βarr-mediated internalization, signaling, and desensitization of G protein signaling.
Results
We focused our mutagenesis approach on the FLR of βarr1 because this region mediates an essential interaction with the receptor transmembrane core (13, 15) that stabilizes the GPCR–βarr complex core conformation (16). Disrupting this interaction through βarr1 mutagenesis, we reasoned, would allow us to obtain a βarr1 that predominantly forms GPCR–βarr tail conformation complexes, and not any core-conformation complexes, when bound to GPCRs.
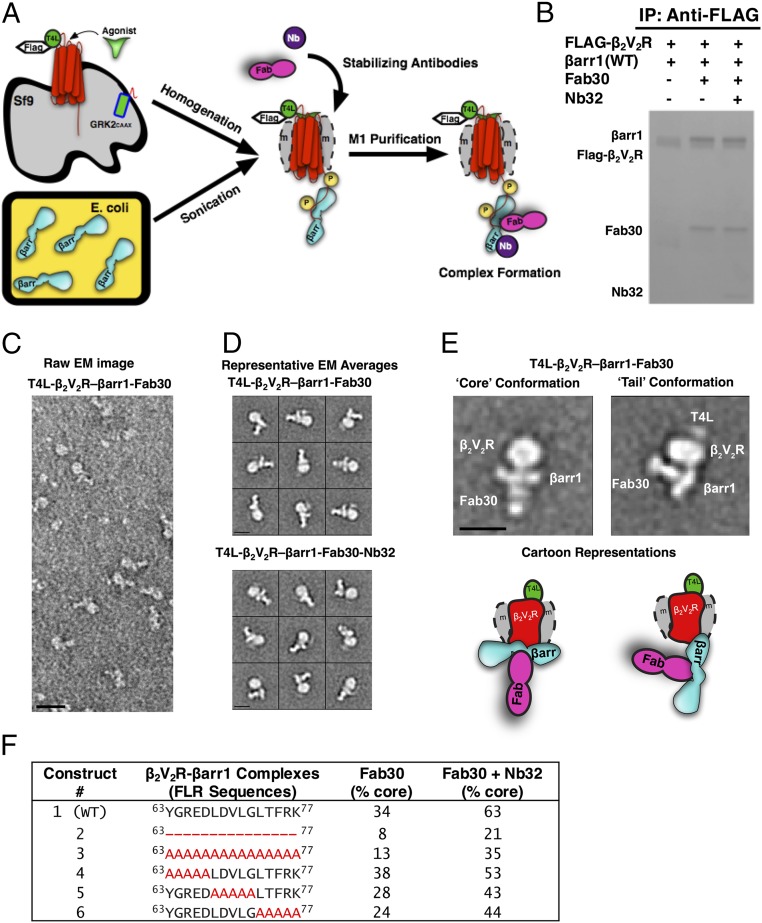
To identify βarr1 mutants that primarily form β2V2R–βarr1 complexes in the tail conformation, we devised a method to form (and purify) these complexes on a small scale (Fig. 1A), and then applied single-particle classification analysis using negative-stain EM to assess their structural features (Fig. 1 B–E). Furthermore, we developed a camelid nanobody, Nb32, which binds to and stabilizes active βarr1 that predominantly complexes with β2V2R in the core conformation (Fig. 1 B–F and Figs. S1 and S2). Using our method, the addition of Nb32 to the β2V2R–βarr1–Fab30 complex increased the percentage of β2V2R–βarr1 complexes in the core conformation from 34 to 63% (Fig. 1F and Figs. S1 and S2), thus allowing a more precise assessment of βarr1 mutants defective in their ability to form β2V2R–βarr1 core-conformation complexes.
Fig. 1.
T4 lysozyme (T4L)β2V2R–βarr1 complexes formed and analyzed via EM with a newly developed functional purification method. (A) Schematic representation of the purification method to generate (T4L)β2V2R–βarr1 complexes. (B) Coomassie gel showing WT βarr1 interaction with (T4L)β2V2R in the absence or presence of conformation-stabilizing antibodies (Fab30, Nb32). IP, immunoprecipitation. (C) Representative negative-stain raw EM image of (T4L)β2V2R–βarr1–Fab30 complexes. (D) Class averages of the (T4L)β2V2R–βarr1–Fab30 complexes (Top) and (T4L)β2V2R–βarr1–Fab30–Nb32 complexes (Bottom) from negative-stain EM classification analysis. (E) Representative class averages (with cartoon representations) of the (T4L)β2V2R–βarr1–Fab30 complex in the tail and core conformations. (Scale bars: C, 20 nm; D and E, 10 nm.) (F) Summarized results of the different βarr1 FLR constructs tested for their ability to form the (T4L)β2V2R–βarr1–Fab30 core conformation in the presence or absence of Nb32. Note that the tail conformation encompasses all those (T4L)β2V2R–βarr1 complexes that are not in the core conformation.
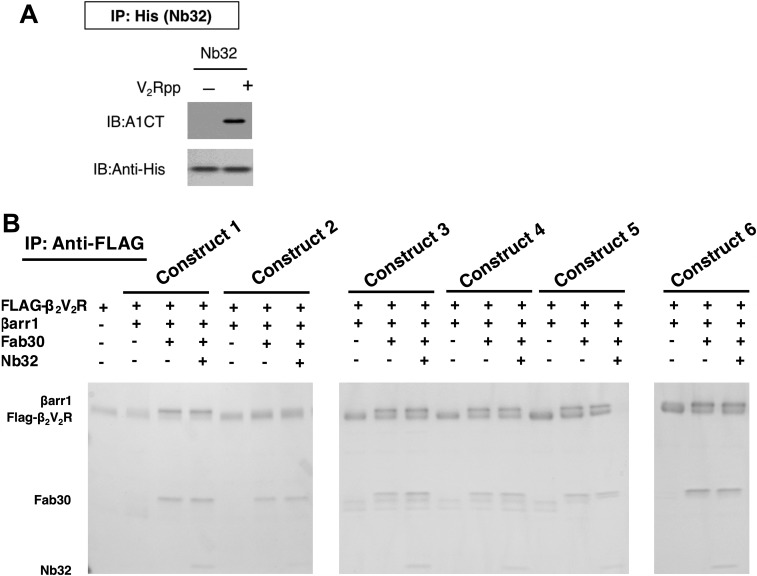
Fig. S1.
(A) Immunoblot (IB) verifying the ability of nanobody (Nb32) to interact with the active V2Rpp-bound βarr1 in vitro. IP, immunoprecipitation. (B) Coomassie gel showing different βarr1 constructs and their interaction with (T4L)β2V2R in the absence or presence of conformation-stabilizing antibodies (Fab30, Nb32).
Fig. S2.
Class averages of the different preparations of (T4L)β2V2R–βarr1–Fab30 complexes and (T4L)β2V2R–βarr1–Fab30–Nb32 complexes, using different βarr1 constructs, from negative-stain EM analysis.
None of the mutations in the FLR of βarr1 that were tested prevented βarr1 from forming complexes with β2V2R as analyzed by pull-down assays and EM (Fig. 1 and Figs. S1 and S2). However, several mutants severely reduced the ability of βarr1 to bind to receptor via the core conformation in the β2V2R–βarr1–Fab30 complex, even in the presence of Nb32 (Fig. 1F). Most notable is the βarr1 (ΔFLR) mutant (Fig. 1F, construct 2), with the entire FLR removed, which led to a substantial decrease in the core conformation of the β2V2R–βarr1–Fab30 complex even in the presence of Nb32. Together, these results demonstrate that the βarr1 (ΔFLR) mutant is strongly impaired in its ability to interact with the receptor transmembrane core, and thus serves as a model for βarr1 that forms a complex with the β2V2R predominantly in the tail conformation.
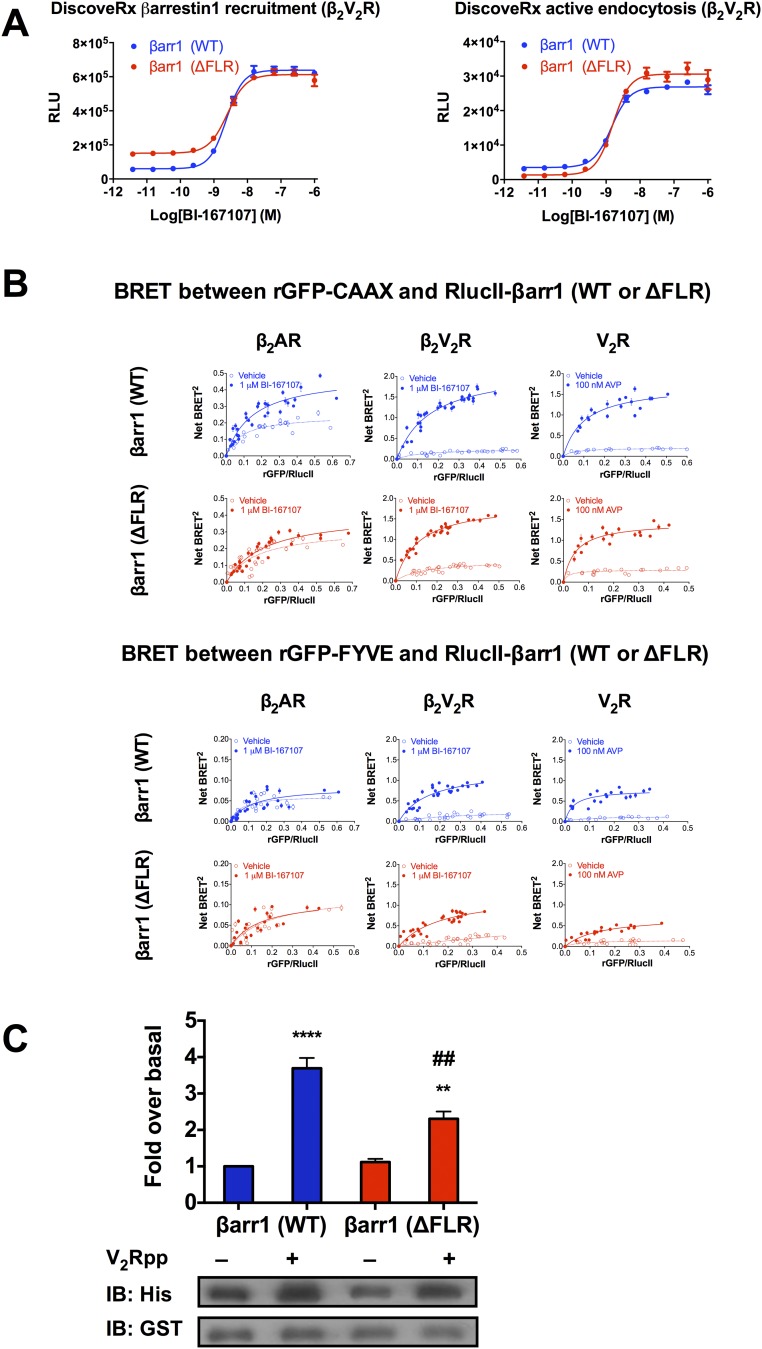
Next, using the β2V2R, the cellular functionality of βarr1 (ΔFLR) was confirmed using well-established βarr1 recruitment and internalization assays (Fig. S3A). Removal of the FLR did not impair agonist-mediated recruitment of βarr1 or βarr1-mediated receptor internalization, indicating that βarr1 (ΔFLR) can perform these functions for the β2V2R (Fig. S3A). We then set out to test whether distinct conformations of GPCR–βarr1 complexes determine differential functional outcomes by using an array of well-established biochemical, cellular, and biophysical assays. In addition to the chimeric β2V2R, its more physiological relatives, β2AR and V2R, were studied in parallel.
Fig. S3.
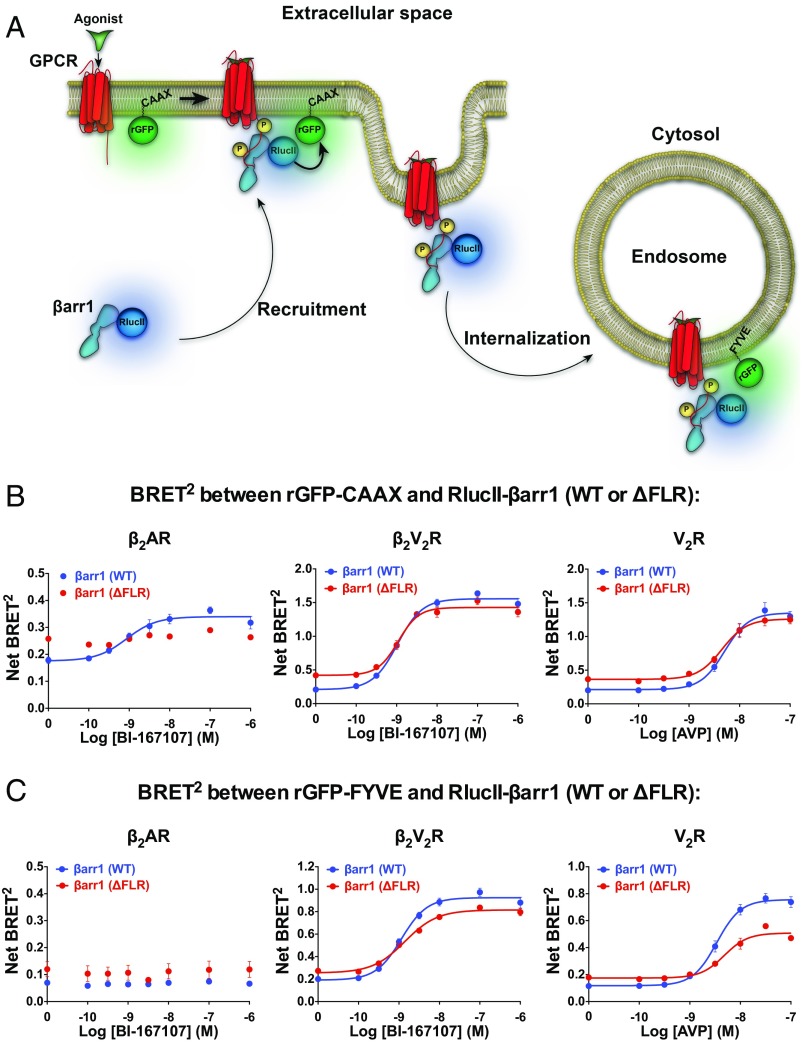
(A) Concentration–response curves of BI-167107–stimulated βarr1 (WT or ΔFLR) recruitment to the β2V2R and βarr1 (WT or ΔFLR)-mediated β2V2R internalization. RLU, relative luminescence units. (B) Interaction between βarr1 (WT or ΔFLR) and either rGFP-CAAX (Top, plasma membrane marker) or rGFP-FYVE (Bottom, early endosomal marker) upon agonist stimulation of β2AR, β2V2R, or V2R. BRET titration curves were obtained using a constant amount of rGFP-CAAX and with increasing amounts of RlucII-βarr1 (WT or ΔFLR). BRET titration curves were obtained using a constant amount of rGFP-FYVE and with increasing amounts of RlucII-βarr1 (WT or ΔFLR). BRET was measured 35 min following addition of agonist or vehicle. To stimulate the GPCRs, 1 μM BI-167107 was applied for the SNAP-β2AR and SNAP-β2V2R, and 100 nM AVP was applied for the SNAP-V2R. Data are expressed as net BRET absolute values, represent the mean ± SE, and are pooled from six experiments. (C) Two hundred nanomolar 6×His-βarr1 (WT or ΔFLR) was incubated with equal concentrations of GST-Src-3D and with either control buffer or V2Rpp. The complexes were pulled down using Glutathione Sepharose beads, and the amount of 6×His-βarr1 (WT or ΔFLR) bound to GST-Src-3D was determined by immunoblotting using an anti-His antibody. Data represent the mean ± SE of three experiments. One-way ANOVA was performed to determine statistical differences between basal and V2Rpp-stimulated states (**P < 0.01, ****P < 0.0001) or V2Rpp-stimulated states of βarr1 (WT) and βarr1 (ΔFLR) (##P < 0.01).
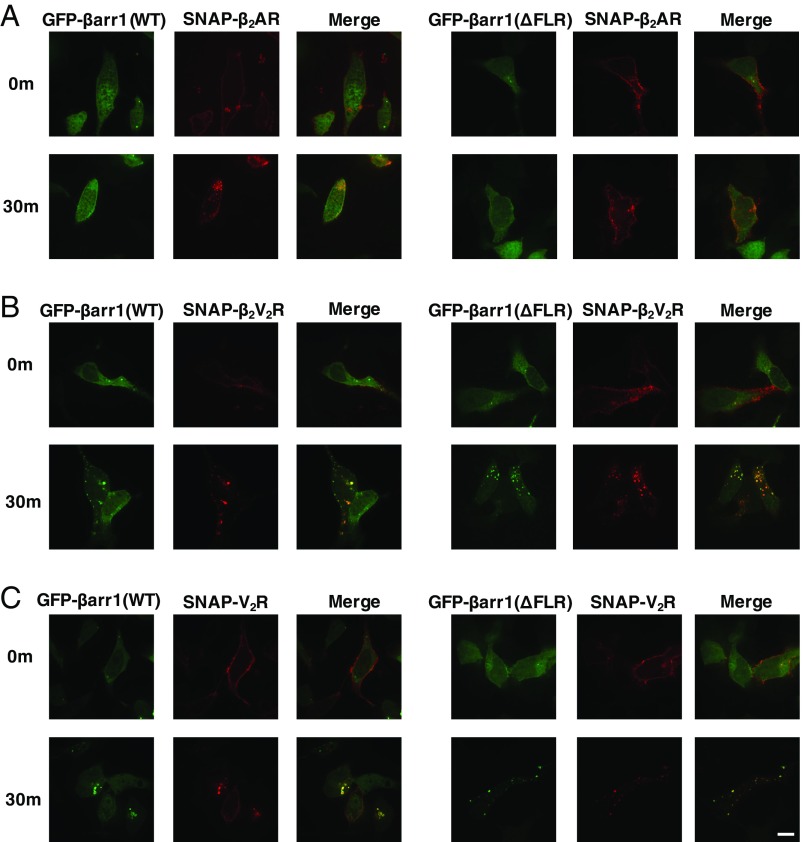
Classical GPCR activation promotes translocation of βarr1 from the cytosol to the GPCRs in the plasma membrane, and subsequently facilitates intracellular trafficking of GPCRs to endosomes (14). Thus, to ascertain the impact of the βarr1 (ΔFLR) mutant on recruitment to the β2AR, β2V2R, and V2R, as well as subsequent trafficking, confocal microscopy imaging was applied. Using this approach, we tracked the cellular localization of N-terminal SNAP-tagged GPCRs (SNAP-β2AR, SNAP-β2V2R, or SNAP-V2R) prelabeled with SNAP-Surface 649 fluorescence substrate and GFP-βarr1 (WT) or GFP-βarr1 (ΔFLR) in βarr1/βarr2 double-knockout (DKO) HEK293 cells following agonist treatment (16). The experiments demonstrate that βarr1 (WT or ΔFLR) is recruited to both the β2V2R and V2R, and that both mediate receptor internalization to endosomes, 30 min poststimulation, to a similar extent (Fig. 2). In contrast, only the βarr1 (WT), but not the βarr1 (ΔFLR), is recruited to the β2AR upon agonist stimulation followed by receptor internalization.
Fig. 2.
Cellular localization of SNAP-β2AR (A), SNAP-β2V2R (B), or SNAP-V2R (C), prelabeled with SNAP-surface 649 fluorescent substrate (red) and GFP-βarr1 (WT or ΔFLR) (green), visualized by confocal microscopy. Cellular localization of fluorescently tagged proteins is shown before agonist addition (0 min) or 30 min after agonist stimulation. To stimulate the GPCRs, 1 μM BI-167107 was applied for the SNAP-β2AR and SNAP-β2V2R, and 100 nM arginine vasopressin was applied for the SNAP-V2R (100× objective, n = 3 independent experiments, n = 20–50 cells per experiment). (Scale bar: 10 μm.)
The cellular trafficking pattern of βarr1 (WT or ΔFLR) was further quantified using bioluminescence resonance energy transfer (BRET) biosensors to monitor recruitment to the plasma membrane [Renilla reniformis green fluorescent protein (rGFP)-CAAX as a plasma membrane marker)] and early endosome (rGFP-FYVE as an early endosomal marker) upon agonist stimulation of the three GPCRs in DKO HEK293 cells (17) (Fig. 3A). Agonist stimulation of β2AR, β2V2R, or V2R caused an increase in the BRET signal between RlucII-βarr1 (WT) and the plasma-membrane rGFP-CAAX biosensor (Fig. 3B and Fig. S3B). With the βarr1 (ΔFLR), agonist stimulation of either β2V2R or V2R also increased the BRET signal between RlucII-βarr1 (ΔFLR) and rGFP-CAAX, but to a slightly reduced extent for the β2V2R compared with RlucII-βarr1 (WT) (Fig. 3B and Fig. S3B). These findings indicate that both β2V2R and V2R are not dependent, to any large extent, on the core interaction to form a stable complex with βarr1. However, for the β2AR, there was no increased BRET signal between RlucII-βarr1 (ΔFLR) and rGFP-CAAX upon agonist stimulation, suggesting that the βarr1 (ΔFLR) is unable to be recruited to this GPCR (Fig. 3B and Fig. S3B).
Fig. 3.
Interaction between βarr1 (WT or ΔFLR) and either rGFP-CAAX (plasma membrane marker) or rGFP-FYVE (early endosomal marker) upon agonist stimulation of β2AR, β2V2R, or V2R. (A) Schematic representation of the experimental design used to monitor agonist-promoted BRET between RlucII-βarr1 (WT or ΔFLR) and rGFP-CAAX or rGFP-FYVE. (B) BRET concentration–response experiments assessing the agonist-stimulated RlucII-βarr1 (WT or ΔFLR) recruitment to plasma membrane-located rGFP-CAAX. Upon agonist addition, a difference in BRET was detected between βarr1 (WT) and β2AR (P = 0.0022), but not between βarr1 (ΔFLR) and β2AR (P = 0.4306). Agonist-mediated changes in net BRET between βarr1 (WT) and βarr1 (ΔFLR) were detected for both the β2AR (P = 0.0015) and β2V2R (P < 0.0001), but not for V2R (P = 0.0820). (C) BRET concentration–response experiments assessing the agonist-stimulated RlucII-βarr1 (WT or ΔFLR) localization to early endosomal-located rGFP-FYVE. Upon agonist addition, no BRET difference was detected between either βarr1 (WT) or βarr1 (ΔFLR) and β2AR (P = 0.4188 or P = 0.9016, respectively). Agonist-mediated changes in net BRET between βarr1 (WT) and βarr1 (ΔFLR) were detected for β2V2R (P = 0.0034) and V2R (P = 0.0014), but not for β2AR (P = 0.9057). In all experiments, BRET was measured 30 min following addition of agonist or vehicle. To stimulate the GPCRs, 1 μM BI-167107 was applied for the β2AR and β2V2R, and 100 nM arginine vasopressin (AVP) was applied for the V2R. Data are expressed as net BRET absolute values, represent the mean ± SE, are pooled from four to six experiments, and are analyzed using either a paired t test (two conditions) or one-way ANOVA with Tukey’s multiple comparisons post hoc test (three or more conditions).
A significant, but slightly reduced, agonist-promoted BRET increase between RlucII-βarr1 (ΔFLR) and the early endosomal marker, rGFP-FYVE, biosensor was detected compared with βarr1 (WT) for the β2V2R or V2R. These results suggest that βarr1 (ΔFLR) is capable of mediating internalization of the β2V2R or V2R to early endosomes, although to a lesser extent than βarr1 (WT) (Fig. 3C and Fig. S3B). In agreement with previous work (16) on the β2AR and its interaction with βarr1 showing that this class A GPCR recycles quickly and that βarr1 is not present in endosomes, no change in the BRET signal was detected between RlucII-βarr1 (WT or ΔFLR) and rGFP-FYVE following agonist treatment of β2AR-transfected DKO HEK293 cells (Fig. 3C and Fig. S3B).
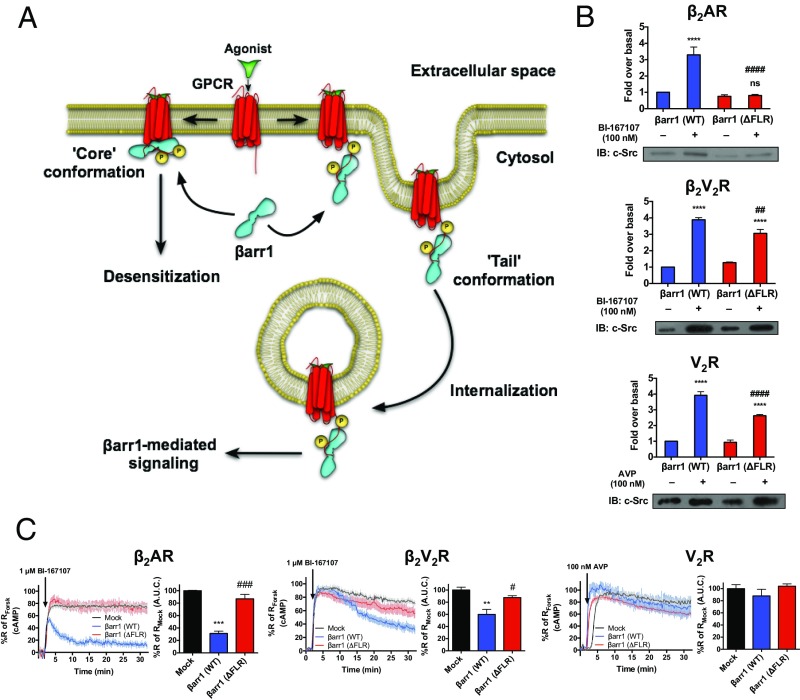
The scaffolding function of βarrs, as signal transducers, has been characterized for multiple signaling proteins, including c-Src (18, 19). Formation of GPCR–βarr1–c-Src ternary complexes has been demonstrated to regulate multiple cellular functions downstream of various GPCRs (20). Thus, to investigate the capacity of βarr1 in the GPCR–βarr1 tail conformation to scaffold c-Src, we evaluated the ability of βarr1 (WT or ΔFLR) to interact with c-Src upon activation of β2AR, β2V2R, or V2R in DKO HEK293 cells by coimmunoprecipitation. As expected, βarr1 (WT) effectively binds c-Src upon stimulation of all three GPCRs (Fig. 4 A and B). We also observed that the ability of the βarr1 (ΔFLR) to scaffold c-Src, upon stimulation of the β2V2R and V2R, was slightly reduced relative to βarr1 (WT) (Fig. 4 A and B). In contrast, βarr1 (ΔFLR) does not interact with c-Src upon β2AR stimulation, as might be expected, because βarr1 (ΔFLR) is not recruited to β2AR. The scaffolding function of βarr1 (ΔFLR) was further explored by Glutathione Sepharose (GST) pull-down assays using purified 6×His-βarr1 (WT or ΔFLR) and GST–c-Src either in the absence or presence of the phosphorylated V2R C-terminal peptide (V2Rpp). In the presence of V2Rpp, an increased interaction was observed between βarr1 (WT or ΔFLR) and GST–c-Src (Fig. S3C). The βarr1 (ΔFLR) mutant is slightly impaired relative to βarr1 (WT) with respect to scaffolding c-Src in vitro, a trend also observed in our aforementioned cellular studies of both βarr1–c-Src scaffolding and βarr1-mediated GPCR internalization to endosomes (Figs. 3C and 4A).
Fig. 4.
Functional outcomes of different GPCR–βarr1 complex conformations. (A) Schematic representation of the functional outcomes mediated by GPCR–βarr1 complex tail conformation and GPCR–βarr1 complex core conformation. (B) βarr1-mediated scaffolding of c-Src upon activation of β2AR, β2V2R, or V2R. HEK293 DKO cells were transfected with plasmids for β2AR, β2V2R, or V2R, c-Src, and HA-βarr1 (WT or ΔFLR). Serum-starved cells were stimulated with or without agonist BI-167107 (1 μM) or AVP (100 nM) for 10 min and then cross-linked using dithiobis(succinimidyl propionate); finally, anti-HA beads were used to pull down βarr1 (WT or ΔFLR). The amount of total c-Src bound to HA-βarr1 (WT or ΔFLR) was determined by immunoblotting (IB). Data represent the mean ± SE of four to five experiments. One-way ANOVA was performed to determine statistical differences between basal and agonist-stimulated states (****P < 0.0001), or agonist-stimulated states in βarr1 (WT)- and βarr1 (ΔFLR)-transfected cells (##P < 0.01, ####P < 0.0001). (C) βarr1-mediated desensitization of Gs-promoted cAMP generation by the β2AR, β2V2R, or V2R. Real-time cAMP measurements, using ICUE2-expressing HEK293 cells, in response to agonist stimulation of β2AR, β2V2R, and V2R are shown. For the β2AR and β2V2R, 1 μM BI-167107 was used to stimulate cells. For V2R, 100 nM AVP was used to stimulate cells. For each GPCR, control plasmid (Mock, black), βarr1 (WT) (blue), or βarr1 (ΔFLR) (red) was transfected. Surface expression of each GPCR was matched within each βarr1 transfection condition. Data represent the mean ± SE of three to four experiments and n ≥ 44 cells. Area under the curve (A.U.C.) from 2 min after agonist stimulation to the end of the experiment was used to calculate desensitization of the cAMP response for each GPCR, and one-way ANOVA was performed to determine statistical differences relative to Mock (**P < 0.01, ***P < 0.001) and βarr1 (WT) (#P < 0.05, ###P < 0.001) responses. Forsk, forskolin.
βarr1 is known to promote desensitization of GPCR-stimulated G protein-mediated signaling. The mechanism underlying βarr1-mediated desensitization is thought to involve the interaction between βarr and the receptor core; this core conformation, presumably, sterically blocks the G protein-binding site in the receptor core (21). To assess the importance of the FLR of βarr1 for receptor desensitization directly, we monitored the attenuation of agonist-stimulated heterotrimeric Gs protein signaling, measured here as cAMP accumulation, in either the DKO (for the β2AR) or a βarr1/βarr2/β2AR triple-knockout (for the β2V2R and V2R) HEK293 cell line expressing ICUE2, a fluorescence resonance energy transfer biosensor-detecting cytoplasmic cAMP (22). This ICUE2 biosensor measures cAMP concentration in real time, and thus represents equilibrium between production and degradation of cAMP. β2AR, β2V2R, and V2R were all expressed at near-endogenous levels (∼100–400 fmol/mg), together with GRK2-CAAX, to ensure effective receptor phosphorylation and βarr1 recruitment upon agonist challenge. For all three GPCRs, agonist stimulation led to a rapid onset of cAMP generation, and this signal was only minimally reduced throughout the 30-min duration of the experiment (Fig. 4C).
We next coexpressed βarr1 (WT or ΔFLR) to test its ability to desensitize G protein signaling. Within the first 2 min of agonist challenge, β2AR, β2V2R, and V2R all stimulated cAMP production to a similar extent. Beyond 2 min, βarr1 (WT) attenuated the cAMP responses differently among these receptors (Fig. 4C), and most prominently for the WT β2AR, where the addition of βarr1 (WT) led to rapid, but incomplete, desensitization. In contrast, βarr1 (ΔFLR) did not mediate any desensitization of the β2AR-stimulated cAMP response because it is not recruited to this receptor. βarr1 (WT)-mediated desensitization was also observed at the β2V2R-stimulated cAMP response (Fig. 4C). βarr1 (WT) did not have a significant effect on V2R-stimulated cAMP signaling, which agrees with previous work (23). Most strikingly, expression of βarr1 (ΔFLR) did not lead to any significant desensitization of G protein signaling for any of the GPCRs tested (Fig. 4C). These results (Fig. 4 A and C) demonstrate that the FLR domain of βarr1, presumably through its role in forming the core interaction, is crucial for βarr1-mediated desensitization of G protein signaling.
Discussion
Our results can be interpreted in the context of the classification of GPCRs according to the strength of their interaction with βarrs. Class A GPCRs, such as the β2AR, bind βarrs relatively weakly and dissociate from them in the course of internalization. They thus recycle rapidly to the plasma membrane. Class B GPCRs, such as the V2R or the β2V2R chimera, bind βarrs much more tightly and, once internalized, remain bound to βarrs and resident in endosomes for significant periods of time. They recycle only slowly to the plasma membrane. For class B GPCRs, the GPCR–βarr complex, in the tail conformation, appears to be capable of promoting βarr-mediated receptor internalization and some forms of signaling, but not desensitization of G protein signaling, which appears to be the exclusive purview of the core-conformation complex (Fig. 4A). A recent study showed that some βarr-mediated functions are maintained when recruited to a potential core-deficient GPCR mutant, which supports our conclusions with respect to the function of the tail conformation complex (24). However, the study did not experimentally demonstrate any biological role of the core conformation. Our finding that the core-conformation complex appears to be crucial for mediating desensitization is in agreement with the classical notion that G proteins and βarrs compete for overlapping binding sites in the receptor transmembrane core (21). Interestingly, for the class A β2AR, which binds βarr more weakly, the tail conformation complex appears to be too unstable to lead to effective recruitment of the βarr1 (ΔFLR). Our data thus suggest that for such GPCRs, the tail conformation complex might not exist in a stable enough form to participate in βarr-mediated activities.
In addition, we have recently demonstrated that some GPCRs, such as the β2V2R and V2R but not the β2AR, can form GPCR–Gs–βarr “megaplexes,” and thus activate G protein from internalized compartments (16). In these megaplexes, the receptor binds βarr in the tail conformation complex. Interestingly, in the current study, we find a clear correlation between the GPCRs that form GPCR–βarr1 tail conformation complexes and GPCRs that can activate G protein from internalized compartments. In contrast, GPCRs that rely more heavily on the core conformation do not seem to activate G protein after being internalized by βarr.
Materials and Methods
Constructs.
Constructs expressing FLAG-β2V2R (13), SNAP-β2AR, SNAP-β2V2R, and SNAP-V2R (16) have been previously reported and functionally verified. The plasmid encoding rat HA-tagged βarr1 was previously described (18). The plasmid encoding human c-Src was reported previously (18). The BRET biosensor constructs rGFP-CAAX and rGFP-FYVE were previously described and functionally verified (17). βarr1 with GFP fused to its C terminus was described previously (25). The plasmid-encoding chicken GST–c-Src for use in the in vitro GST–c-Src pull-down assay was described previously (19). All βarr1 FLR mutants, including ΔFLR, were generated using the Quikchange II Site-Directed Mutagenesis Kit (Stratagene). Mutations to the FLR region of βarr1 did not impact the ability of βarr1 to form complexes with β2V2R, as assessed by M1 pull-down assays and negative-stain EM. The RlucII-βarr1 (ΔFLR) construct was obtained by removing the FLR from RlucII-βarr1 using PCR-driven overlap extension (26).
Identification of Nanobody 32.
β2V2R–βarr1–Fab30 complex-specific camelid nanobodies were selected using phage display technology (27). Briefly, one llama was immunized during 6 wk with 100 μg of the cross-linked β2V2R–βarr1–single-chain fragment variable complex. Peripheral blood lymphocytes were collected, and the nanobody immune repertoire was cloned into the phage display vector pMESy4 as described previously (27). β2V2R–βarr1–Fab30 complex binders were identified by performing a first selection round on solid phase-coated, cross-linked β2V2R–βarr1–Fab30 complex, and a second selection round was performed on anti-Flag captured T4 lysozyme (T4L)β2V2R–βarr1–Fab30 complex. After selections, 188 clones were sequenced and periplasmic extracts were screened in an ELISA. Of the 11 enriched sequence families, nanobody 32 (Nb32) was found to be specific for the native and cross-linked β2V2R–βarr1–Fab30 complex (solid phase-coated and anti-Flag captured) and did not recognize the individual complex partner β2V2R, βarr1, or Fab30 alone.
The ability of Nb32 to interact with the active V2Rpp-bound βarr1 was further verified in vitro. βarr1 was incubated with V2Rpp (Tufts University Analytical Core Facility) in a 1:2 molar ratio for 30 min at 25 °C. Subsequently, purified Nb32 was added at a 1:2 molar ratio with βarr1 and incubated for an additional 30 min at 25 °C. Then, prewashed TALON His-Tag purification resin (Clontech) was added to the reactions and incubated for another 30 min at 25 °C. The final concentration of βarr1 in the binding reaction was 10 nM. Beads were washed four times with 1 mL of buffer [20 mM Hepes (pH 7.5), 100 mM NaCl], and proteins were eluted with SDS/PAGE gel-loading buffer. The eluted proteins were run on a 4–20% SDS/PAGE gel and then stained with SimplyBlue SafeStain (Novex). Nb32 displayed its ability to pull down active V2Rpp-bound βarr1, and was therefore chosen for further experimentation.
Protein Purification.
The plasmid pET22b-βarr1 (WT or ΔFLR) was transformed into Escherichia coli BL21 (DE3) cells. The transformed BL21 cells were cultured in lysogeny broth medium at 37 °C to an OD600 of 0.8 and induced with 0.3 mM isopropyl-β-d-thiogalactoside at 25 °C. After growing overnight, the cells were harvested by centrifugation at 3,200 × g and resuspended in buffer containing 20 mM Tris⋅HCl (pH 8.0) with 150 mM NaCl. The cells were subsequently lysed by high-pressure homogenization, and the cell lysate was then centrifuged at 13,000 × g for 30 min at 4 °C. Protein in supernatant was purified by nickel-nitrilotriacetic acid affinity chromatography and eluted with a gradient from 20 to 500 mM imidazole. The protein was further purified by size exclusion chromatography using a Superdex 200 column (GE Healthcare).
Expression and purification of Fab30 were performed as previously described (28). In short, Fab30 was expressed in the periplasm of E. coli strain M55244, extracted, and purified on protein A/G agarose (Pierce). Fab30 was eluted with 10 mM sodium acetate (pH 3.0) with 40 mM NaCl and neutralized with 0.1 M sodium acetate (pH 5.0). Fab30 was subsequently purified on a prepacked Resource S column followed by dialysis in 20 mM Hepes (pH 7.4) with 100 mM NaCl. Nb32 bearing a C-terminal His6 tag was expressed in the periplasm of E. coli strain WK6 and purified as previously described (29).
Functional Purification of β2V2R–βarr1 Complexes.
βarr1 residues were mutated using the Quikchange II Site-Directed Mutagenesis Kit. All mutant constructs were sequence-verified and tested for expression in small-scale E. coli cultures. Next, sf9 cell lysates containing BI-occupied, phosphorylated Flag-β2V2R (13) were dounce- homogenized in lysis buffer [20 mM Hepes (pH 7.4), 150 mM NaCl, 100 nM BI, 0.01% lauryl maltose neopentyl glycol (LMNG), 100 μM TCEP (tris(2-carboxyethyl)phosphine)], mixed with sonicated E. coli lysates expressing βarr1 mutants in lysis buffer to form β2V2R–βarr1 complexes for 1 h, solubilized in the detergent LMNG for 1 h, and then purified in the presence of Fab30 and/or Nb32 by M1 anti-FLAG immunoprecipitation. This small-scale purification scheme provided enough β2V2R–βarr1 complex to assess the structural features by EM as illustrated in Fig. 1.
EM.
(T4L)β2V2R–βarr1 complexes were prepared for EM using a conventional negative staining protocol (30). Each βarr1 FLR mutant was subjected to two different formats for complex formation: (T4L)β2V2R–βarr1–Fab30 and (T4L)β2V2R–βarr1–Fab30–Nb32. The negative-stained sample was imaged at room temperature with a Tecnai T12 electron microscope operated at 120 kV using low-dose procedures. Images were recorded at a magnification of 71,138× and a defocus value of ∼1.5 μm on a Gatan US4000 CCD camera. All images were binned (2 × 2 pixels) to obtain a pixel size of 4.16 Å on the specimen level. Particles were excised using Boxer (part of the EMAN 2.1 software suite) (31). Two-dimensional reference-free alignment and classification of particle projections were performed using iterative stable alignment and classification (ISAC) (32). For each β2V2R–βarr1 complex, ∼5,000 particle projections (0°) of native complexes were subjected to ISAC, producing classes accounting for the particle projections and conformation assignments shown in Fig. 1 and Fig. S2.
DiscoveRx Cell Lines and Assays
Medium.
Stable cell pools engineered in a U-2 OS cell host (American Type Culture Collection) were maintained in MEM, supplemented with 10% heat-inactivated FBS, 1× penicillin/streptomycin/glutamine plus 250 μg/mL hygromycin [βarr1-enzyme acceptor (EA) selection marker], and 500 μg/mL G418 [selection marker for the β2-adrenergic receptor (ADRB2)-vasopressin type 2 receptor (AVPR2) hybrid receptor]. Growth media for the internalization stable pools was also supplemented with 0.25 μg/mL puromycin (selection marker for the ProLink tag anchored to early endosomes).
βarr1 (ΔFLR)-EA Construct Generation.
A cDNA of an engineered mutant of human βarr1 (NM_004041.3), with the 15 amino acid residues of the FLR deleted (amino acids 63–77), was synthesized as a DNA string (GeneArt Strings; ThermoFisher). The resulting βarr1 (ΔFLR) mutant construct was subsequently engineered into a retroviral vector with the β-galactosidase EA fragment fused in-frame to the C terminus. Sequence analysis of the resulting βarr1 (ΔFLR)–EA fusion construct confirmed that the insert in the retroviral vector contained the expected sequence.
ADRB2-AVPR2 Receptor Chimera Construct Generation.
A hybrid receptor was designed by fusing the intracellular tail of human AVPR2 (NM_000054.4; amino acids 343–371) to the C terminus of the extracellular domain and seven transmembrane domains of human ADRB2 (NM_000024.4; amino acids 1–329) downstream of an amino terminal FLAG tag. A cDNA of the ADRB2-AVPR2 hybrid receptor was synthesized as a DNA string (GeneArt Strings). The resulting receptor chimera was subsequently engineered into two retroviral vectors: one vector with the receptor fused in-frame to a ProLink (PK) β-galactosidase fragment at the C terminus (ADRB2-AVPR2-PK) and the second vector without the PK tag present (ADRB2-AVPR2). Sequence analysis of the two constructs confirmed the expected sequence of the hybrid receptor.
Cell Pool Generation and Validation.
Arrestin-EA parental cell pool.
Native U-2 OS parental cells were retrovirally transduced with vectors encoding the WT or ΔFLR mutant of human βarr1-EA, and then placed under hygromycin selection for 10 d to generate stable pools. Expression of the appropriate EA-fusion construct in each stable pool was evaluated using PathHunter ProLink Detection Reagent (DiscoverX), per the manufacturer’s instructions, but with the following protocol modification: Exogenous EA was substituted with 50 μM exogenous enzyme donor (the complement to EA) or PBS (no enzyme donor control).
Arrestin recruitment assay cell pool.
U-2 OS cells stably expressing the WT or ΔFLR mutant of human βarr1-EA were retrovirally transduced with a construct encoding the ADRB2-AVPR2-PK hybrid receptor and placed under G418 selection for 10 d to generate stable pools. Expression of the ADRB2-AVPR2-PK construct in both stable pools was evaluated with PathHunter ProLink Detection Reagent in the presence or absence of exogenous EA. Functional activity of the hybrid receptor in the stable pools was evaluated by assaying arrestin recruitment in response to the β2AR agonist isoproterenol.
Internalization assay cell pool.
A fusion construct encoding a motif-associated with early endosomes (from EEA1) fused to PK (previously generated and hereafter referred to as Endo-PK) was used to anchor the PK β-galactosidase fragment to early endosomes. Cells from the WT and ΔFLR mutant βarr1-EA stable pools were sequentially transduced with Endo-PK (puromycin) and the ADRB2-AVPR2 hybrid receptor (G418) under appropriate antibiotic selection to produce triple-stable pools. Function of the hybrid receptor was confirmed in the WT and mutant U-2 OS βarr1-EA/Endo-PK/ADRB2-AVPR2 triple-stable pools by arrestin-mediated internalization of the hybrid receptor in response to isoproterenol.
EFC Assays.
To assess WT βarr1 signaling (U-2 OS βarr1-EA ADRB2-AVPR2-PK), mutant βarr1 signaling [U-2 OS βarr1 (mutant)-EA ADRB2-AVPR2-PK], WT βarr1-mediated internalization (βarr1-EA/Endo-PK ADRB2-AVPR2), or mutant βarr1-mediated internalization (βarr1-EA/Endo-PK ADRB2-AVPR2) cells from the respective stable pools were seeded at 5,000 cells per well in 384-well plates in culture medium without selection antibiotics and allowed to adhere and grow overnight in a humidified incubator at 37 °C with 5% CO2. Cells were stimulated with a dose–response of BI for 1.5 h (arrestin recruitment assay) or 3 h (internalization assay) at 37 °C and then lysed for 1 h in the dark in the presence of PathHunter Detection Reagent per the manufacturer’s recommendations (33). Signal was read on an Envision (PerkinElmer) instrument, and data-plotted using GraphPad Prism (GraphPad Software, Inc.).
Generation of HEK293 Cell Lines Deficient for βarr1/2 and β2AR/βarr1/2.
Generation of the Barr1/2-DKO HEK293 cells was previously described (34). Using a similar strategy, functional β2AR product was eliminated by introducing a null mutation into the ADRB2 gene using CRISPR/Cas9 technology. The single-guide (sg)RNA-encoding sequence targeting the ADRB2 gene (5′-CATTCAGATGCACTGGTACC-3′) was inserted into the BbsI site of the pSpCas9(BB)-2A-GFP (PX458) vector (a gift from Feng Zhang, Broad Institute, Cambridge, MA; Addgene plasmid no. 48138) using two synthesized oligonucleotides [5′-caccGCATTCAGATGCACTGGTACC-3′ and 5′-aaacGGTACCAGTGCATCTGAATGC-3′; a guanine nucleotide (G) was introduced at the −21 position of the sgRNA (underlined), which enhances transcription of the sgRNA; nucleotides that annealed to the BbsI-digested PX458 vector are shown as lowercase letters]. The plasmid was purified using a PureYield Plasmid Miniprep System (Promega). Correctly inserted sgRNA-encoding sequences were verified by sequencing using the Sanger method (FASMAC).
The βarr1/2-DKO HEK293 cells were seeded into a six-well plate at cell numbers of 1 × 104 cells (2 mL) per well and incubated for 24 h before transfection. The ADRB2-targeting vector plasmid (1 μg) was transfected into the cells using Lipofectamine 2000 (2.5 μL; Life Technologies) according to the manufacturer’s instructions. Seventy-two hours later, cells were harvested and GFP-positive cells were isolated using a cell sorter (SH800; Sony). The GFP-positive cells were diluted with culture media and seeded in 96-well plates for limiting dilution. After approximately 2 wk of incubation with routine addition of fresh media, wells containing an apparent single clone were treated with trypsin and EDTA. One-half of harvested cells were passaged into a six-well plate, and the other half were analyzed for mutations in the targeted site by a restriction enzyme method. The targeted ADRB2 locus was PCR-amplified using primers (5′- CAGTGGATCGCTACTTTGCC-3′ and 5′-TGGCATAGGCTTGGTTCGTG-3′) with an initial denaturation cycle of 95 °C for 2 min, followed by 35 cycles of 95 °C for 15 s, 64 °C for 30 s, and 72 °C for 30 s. The PCR product was digested with KpnI (Takara Bio), and its fragmentation was analyzed by a capillary electrophoresis system (MultiNA; Shimadzu).
Candidate positive clones were further analyzed by genomic DNA sequencing using direct sequencing or a TA cloning method. The lack of functional β2AR was also confirmed by assessing isoproterenol- or formoterol-stimulated cAMP production. After meeting the criteria for functional characterization, a cell line devoid of functional βarr1/2 and β2AR was established.
Confocal Microscopy.
For colocalization studies, HEK293 DKO cells were transfected with N-terminal SNAP-tagged receptors (New England Biolabs) (16) and GFP-βarr1 (WT or ΔFLR) (35) 48 h before the experiments. The day before the experiments, cells were plated on poly-d-lysine–coated, 35-mm, glass-bottomed dishes (MatTek) and were starved for at least 2 h in serum-free medium before the experiments. For the colocalization studies, SNAP-tagged receptors were labeled with SNAP-Surface 649 (New England Biolabs) for 10–15 min and then washed with FluoroBrite DMEM (Life Technologies). All experiments were conducted at 37 °C in FluoroBrite DMEM (Life Technologies). Before or at different time points during stimulation of the receptors, cells were fixed with ice-cold 6% formaldehyde (Sigma–Aldrich) diluted in Dulbecco’s PBS (DPBS). Confocal images were obtained on a Zeiss LSM510 laser-scanning microscope using multitrack sequential excitation (488–633 nm) and emission [515–540 nm for GFP-βarr1 (WT or ΔFLR) or 650 nm for SNAP-Surface 649] filter sets.
BRET Assay.
To measure the recruitment of βarr1 to the plasma membrane, cells were cotransfected with Flag-tagged β2AR, Flag-β2V2R, or V2R along with RlucII-βarr1 (WT or ΔFLR) and rGFP-CAAX, which serves as a BRET acceptor tethered to the plasma membrane. To assess receptor-mediated βarr1 endosomal targeting, cells were cotransfected with Flag-β2AR, Flag-β2V2R, or Flag-V2R, along with RlucII-βarr1 (WT or ΔFLR) and rGFP-FYVE, which serves as a BRET acceptor at early endosomes. Cells were transfected and seeded on poly-d-lysine–coated, 96-well, white microplates (Greiner). Forty-eight hours later, cells were washed with DPBS, followed by addition of Tyrode’s buffer [137 mM NaCl, 0.9 mM KCl, 1 mM MgCl2, 11.9 mM NaHCO3, 3.6 mM NaH2PO4, 25 mM Hepes, 5.5 mM glucose, 1 mM CaCl2 (pH 7.4)]. Cells were subsequently stimulated with BI-167107 (1 μM for β2AR and β2V2R) or arginine vasopressin (AVP; 100 nM for V2R) for 30 min at 37 °C. The Rluc substrate coelenterazine 400a (2.5 μM; NanoLight Technology) was added 5 min before BRET measurement. All BRET measurements were performed using a Synergy Neo (BioTek) microplate reader with an acceptor filter (515 ± 30 nm) and donor filter (400 ± 80 nm).
The net BRET signal was defined as the BRET signal obtained in presence of rGFP-tagged biosensor subtracted by the signal obtained with luciferase alone (RlucII-tagged biosensor). BRET titration curves were performed by cotransfecting a constant amount of the RlucII-tagged biosensor with increasing amounts of the rGFP-tagged biosensor. Net BRET values were expressed as a function of the total expression level of the rGFP-tagged biosensor (recorded using FlexStationII) over the total expression level of the RlucII-tagged biosensor (detected by the Synergy Neo microplate reader) for each transfection condition.
Measurement of Cell Surface Expression by ELISA.
For all BRET experiments, equal surface expression in DKO HEK293 cells for the different receptors was determined by ELISA. Culture medium was removed, and cells were were fixed with DPBS containing 3.7% formaldehyde for 5 min. Cells were washed three times in washing solution (0.2% BSA in DPBS), and nonspecific binding sites were blocked by incubating cells for 45 min in blocking solution (1% BSA in DPBS). Cells were washed three times and incubated for 45 min with anti-Flag M2 monoclonal antibody (Sigma–Aldrich, 1:10,000 in blocking solution). After three washes, blocking solution was added for 15 min. Cells were then incubated for 45 min with anti-mouse HRP-conjugated antibody (1:1,000 in blocking solution; GE Healthcare). After three washes with washing buffer, followed by four washes with DPBS, HRP activity was detected by incubating cells with o-phenylenediamine dihydrochloride (Sigma–Aldrich). Adding 0.6 M HCl stopped the reaction, and absorbance was read at 492 nm using a Tecan GENios multifunction microplate reader (MTX Lab Systems, Inc.).
Coimmunoprecipitation Experiments.
To detect the effects of βarr1 (WT or ΔFLR) on BI or AVP-induced βarr1–c-Src complex formation downstream of β2AR, β2V2R, and V2R, HEK293 cells were cotransfected with Flag- tagged β2AR, β2V2R, or V2R, along with HA-βarr1 (WT or ΔFLR) and c-Src. Forty-eight hours after transfection, cells were starved for 4 h and then stimulated with BI (100 nM for β2AR and β2V2R) or AVP (1 μM for V2R) for 10 min. Subsequently, the cells were placed on ice, and dithiobis(succinimidyl propionate) cross-linking reagent (1.2 mM; Thermo Fisher Scientific) was added drop-wise. The cell lysates were subjected to coimmunoprecipitation using anti-HA beads and incubated overnight at 4 °C. Immune complexes were analyzed by Western blotting with anti-HA antibody (Santa Cruz Biotechnology) and monoclonal anti-Src antibody (36D10; Cell Signaling).
GST–c-Src Pull-Down Assay.
In vitro pull-down experiments to assess binding of c-Src to βarr1 were performed as previously described (19). In detail, 200 nM 6×His-βarr1 (WT or ΔFLR) was mixed with or without a threefold molar ratio of V2Rpp (Tufts University Analytical Core Facility) and incubated in binding buffer [20 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 2 mM EDTA, 1 mM DTT] at room temperature for 30 min. After the incubation, GST–c-Src–3D protein was added to a final concentration of 200 nM and incubated for another 1 h at room temperature. Then, the reaction mixture was further incubated with 10 μL of Glutathione Sepharose beads for 2 h at 4 °C with end-over-end rotation. The Glutathione Sepharose beads were collected and washed four times with cell lysis buffer. The samples were boiled for 10 min in 2×SDS loading buffer. The βarr1–c-Src complexes were analyzed by Western blotting with anti-His antibody (Abcam), and anti-GST antibody (Cell Signaling).
Fluorescence Resonance Energy Transfer Assay.
DKO HEK293 cells expressing β2AR endogenously were transiently transfected with ICUE2 (22), GRK2-CAAX, and HA-βarr1 (WT or ΔFLR). Triple-knockout HEK293 cell lines were transiently transfected with β2V2R or V2R, along with ICUE2 (22), GRK2-CAAX, and HA-βarr1 (WT or ΔFLR). The cells were subsequently plated in poly-d-lysine–coated, 35-mm, glass-bottomed dishes (MatTek). Forty-eight hours after transfection, the cells were washed once in DPBS, followed by addition of imaging buffer [10 mM Hepes, 125 mM NaCl, 5 mM KCl, 1.5 mM MgCl2, 1.5 mM CaCl2, 10 mM glucose, 0.2% BSA (pH 7.4)]. Cells were imaged in the dark, on a 37 °C temperature-controlled stage, for the entire experiment using a DeltaVision Deconvolution microscope (GE Healthcare) with a Coolsnap HQ2 CCD camera (Photometrics) controlled by SoftWoRx 6.1 (GE Healthcare). Dual-emission ratio imaging used a CFP/YFP dichroic mirror, and 470 ± 24-nm and 535 ±± 25-nm emission filters for CFP and YFP, respectively. Exposure times were 200 ms for CFP and 100 ms for YFP, and images were taken every 15 s. Experiments were initiated by addition of agonist, and ICUE2 fluorescence resonance energy transfer (FRET) was followed for 30 min. For the analysis of whole-cell cAMP responses, responses were averaged from a field of cells (≥44 transfected cells), and these responses were averaged across experimental replicates. Herein, analysis ignores subcellular gradients of cAMP, and instead reports the average concentration of cAMP experienced by diffusible proteins, such as ICUE2. Background corrections of fluorescent images were carried out by subtracting the intensity of the background from the emission intensities of fluorescent cells expressing the reporters. All responses represent a decrease in FRET ratio (FRETCFP/YFP/CFP) and were normalized to cAMP responses stimulated by addition of 50 μM forskolin plus 200 μM 3-isobutyl-1-methylxanthine (IBMX). Baseline experiments were conducted to define “no response” and to correct for bleaching of the fluorophores.
Surface Expression Measured by Radioligand Whole-Cell Binding.
Obtaining endogenous and matched surface expression was confirmed for FRET by whole-cell–binding assays using cell membrane-impermeable radioligands. HEK293 cells were grown until confluent in six-well plates. Cells were washed once in cold DPBS, followed by cell detachment using 0.02% EDTA. Cells were resuspended in MEM/0.1% BSA/10 mM Hepes (pH 7.4) and centrifuged at 1,700 × g for 5 min. The cell pellet was resuspended in MEM/0.1% BSA/10 mM Hepes, and samples were transferred to a 96-square-well plate (Labnet International, Inc.). For β2AR- and β2V2R-expressing cells, 30 nM radioactive [3H]-CGP 12177 (PerkinElmer) was added to each sample. For V2R-expressing cells, 4 nM radioactive [3H]-(Arg8)-vasopressin (PerkinElmer) was added to the sample. Nonspecific binding was determined by addition of 10 μM propranolol (Sigma–Aldrich) to β2AR- and β2V2R-expressing cells. Nonspecific binding was determined by addition of 30 μM AVP to V2R-expressing cells. Cells were incubated on ice for 3 h, followed by transfer to Whatman GF-B filter paper, and washed four times with cold wash buffer [50 mM Tris (pH 7.4), 12.5 mM MgCl2, 2 mM EDTA]. Filter paper containing sample was mixed with scintillation fluid (Research Products International Corp.) and counted on a β-counter for 3 min (PerkinElmer). Protein concentration was determined by BCA assay (Pierce). For these desensitization experiments, protein expression of HA-βarr1 (WT or ΔFLR) and GRK2-CAAX was matched by Western blotting for each receptor.
Data and Statistical Analysis.
All graphs were generated and analyzed using GraphPad Prism 7. Data represent the mean ± SEM of three or more independent experiments.
Acknowledgments
We thank L. Barak for generous gifts of plasmids encoding GFP-βarr1. We thank C.-R. Liang, L.-L. Gu, and J.-M. Shan for synthesizing BI-167107 compound. We thank C. Cahill, P. Achacoso, S. Johnson, C. Le Gouill, A. Laperrière, M. Walters, M. DeLong, M. Plue, T. Milledge, D. Capel, and X. Jiang for support and discussion. This work received support from NIH Grants F30HL129803 (to T.J.C.), T32HL007101 (to A.W.K.), DK090165 (to G.S.), and HL16037 (to R.J.L.); the Danish Council for Independent Research & Lundbeck Foundation (A.R.B.T.); a Canadian Institutes of Health Research (CIHR) postdoctoral fellowship (to B.P.) and CIHR Grant MOP10501 (to M.B.); JST, PRESTO (to A.I.), and AMED-CREST (to J.A.); and a Howard Hughes Medical Institute (HHMI) Medical Research Fellowship (to A.H.N.). R.J.L. is an HHMI Investigator and a cofounder and shareholder of Trevena. M.B. holds a Canada Research Chair in Signal Transduction and Molecular Pharmacology.
Footnotes
Conflict of interest statement: R.J.L. is a cofounder and shareholder of Trevena.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701529114/-/DCSupplemental.
References
- 1.Lefkowitz RJ. The superfamily of heptahelical receptors. Nat Cell Biol. 2000;2(7):E133–E136. doi: 10.1038/35017152. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture) Angew Chem Int Ed Engl. 2013;52(25):6366–6378. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- 3.Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 4.Moore CAC, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 5.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 6.Laporte SA, et al. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96(7):3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36(9):457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375(Pt 3):503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 10.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: Roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17(4):159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y, et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523(7562):561–567. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukla AK, et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512(7513):218–222. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson SSG, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271(5247):363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 15.Szczepek M, et al. Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat Commun. 2014;5:4801. doi: 10.1038/ncomms5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen AR, et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166(4):907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Namkung Y, et al. Monitoring G protein-coupled receptor and β-arrestin trafficking in live cells using enhanced bystander BRET. Nat Commun. 2016;7:12178. doi: 10.1038/ncomms12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 19.Yang F, et al. Phospho-selective mechanisms of arrestin conformations and functions revealed by unnatural amino acid incorporation and (19)F-NMR. Nat Commun. 2015;6:8202. doi: 10.1038/ncomms9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talbot J, Joly E, Prentki M, Buteau J. β-Arrestin1-mediated recruitment of c-Src underlies the proliferative action of glucagon-like peptide-1 in pancreatic β INS832/13 cells. Mol Cell Endocrinol. 2012;364(1-2):65–70. doi: 10.1016/j.mce.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: A protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 22.Violin JD, et al. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283(5):2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 23.Feinstein TN, et al. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013;288(39):27849–27860. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumari P, et al. Functional competence of a partially engaged GPCR-β-arrestin complex. Nat Commun. 2016;7:13416. doi: 10.1038/ncomms13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, et al. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem. 1999;274(16):10999–11006. doi: 10.1074/jbc.274.16.10999. [DOI] [PubMed] [Google Scholar]
- 26.Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2(4):924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 27.Pardon E, et al. A general protocol for the generation of nanobodies for structural biology. Nat Protoc. 2014;9(3):674–693. doi: 10.1038/nprot.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla AK, et al. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497(7447):137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469(7329):175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peisley A, Skiniotis G. 2D projection analysis of GPCR complexes by negative stain electron microscopy. Methods Mol Biol. 2015;1335:29–38. doi: 10.1007/978-1-4939-2914-6_3. [DOI] [PubMed] [Google Scholar]
- 31.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128(1):82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Fang J, Chittuluru J, Asturias FJ, Penczek PA. Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure. 2012;20(2):237–247. doi: 10.1016/j.str.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassoni DL, Raab WJ, Achacoso PL, Loh CY, Wehrman TS. Measurements of β-arrestin recruitment to activated seven transmembrane receptors using enzyme complementation. Methods Mol Biol. 2012;897:181–203. doi: 10.1007/978-1-61779-909-9_9. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez-Curto E, et al. Targeted elimination of G proteins and arrestins defines their specific contributions to both intensity and duration of G protein-coupled receptor signaling. J Biol Chem. 2016;291(53):27147–27159. doi: 10.1074/jbc.M116.754887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burczyk M, et al. Phenotypic regulation of the sphingosine 1-phosphate receptor miles apart by G protein-coupled receptor kinase 2. Biochemistry. 2015;54(3):765–775. doi: 10.1021/bi501061h. [DOI] [PMC free article] [PubMed] [Google Scholar]