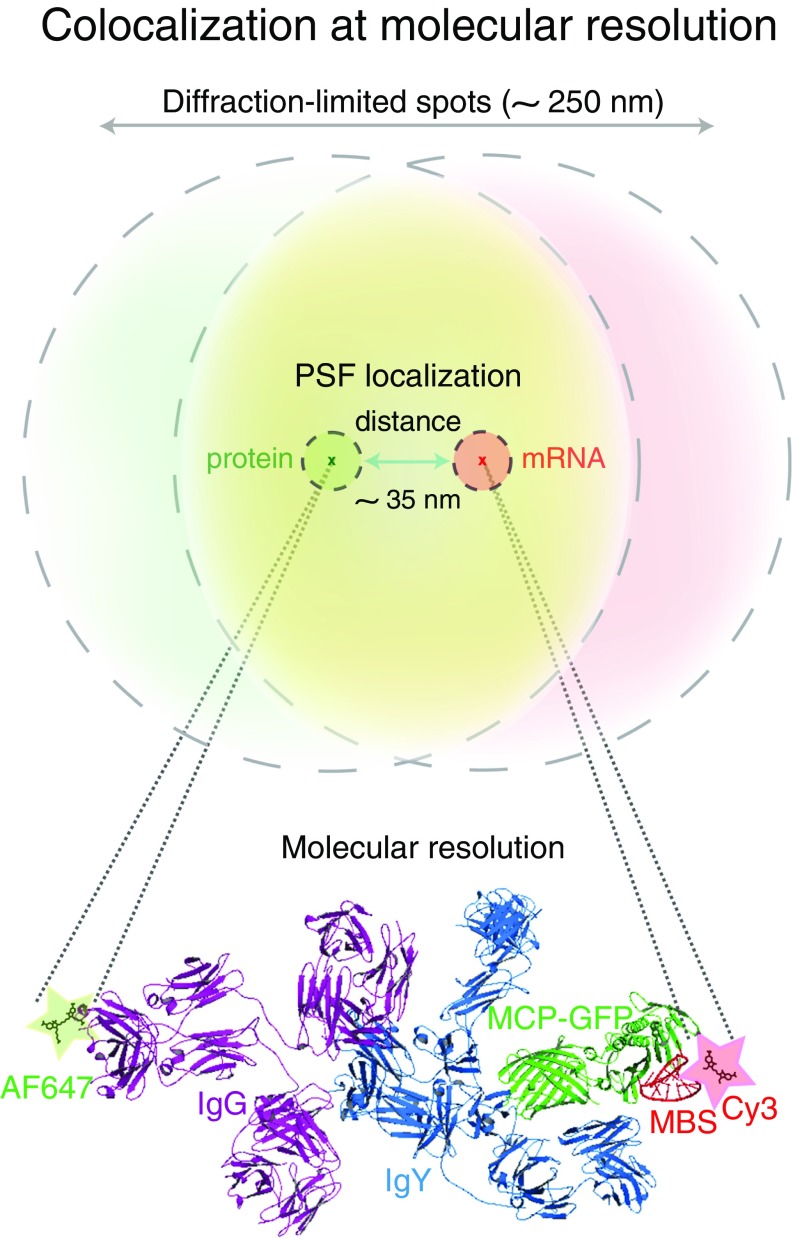
Significance
Gene expression is regulated by interactions between mRNAs and RNA-binding proteins. This functionality depends on their ability to specifically recognize and bind RNAs within the cell. Thus, elucidation of RNA–protein interactions is an area of active research. Technological developments allowed the study of these associations in living cells by ensemble biochemistry approaches. However, these traditional methods to investigate RNA–protein association may report adventitious associations and importantly, lack information about cellular spatial information. In this work, we provide an approach that can integrate information from biochemical interactions with visualization of these physical contacts within neurons.
Keywords: super registration, chromatic aberration correction, smFISH-IF
Abstract
RNA–protein interactions are essential for proper gene expression regulation, particularly in neurons with unique spatial constraints. Currently, these interactions are defined biochemically, but a method is needed to evaluate them quantitatively within morphological context. Colocalization of two-color labels using wide-field microscopy is a method to infer these interactions. However, because of chromatic aberrations in the objective lens, this approach lacks the resolution to determine whether two molecules are physically in contact or simply nearby by chance. Here, we developed a robust super registration methodology that corrected the chromatic aberration across the entire image field to within 10 nm, which is capable of determining whether two molecules are physically interacting or simply in proximity by random chance. We applied this approach to image single-molecule FISH in combination with immunofluorescence (smFISH-IF) and determined whether the association between an mRNA and binding protein(s) within a neuron was significant or accidental. We evaluated several mRNA-binding proteins identified from RNA pulldown assays to determine which of these exhibit bona fide interactions. Surprisingly, many known mRNA-binding proteins did not bind the mRNA in situ, indicating that adventitious interactions are significant using existing technology. This method provides an ability to evaluate two-color registration compatible with the scale of molecular interactions.
RNA-binding proteins (RBPs) specifically recognize and bind with RNA, regulating its lifecycle (1, 2). Dysfunctional RNA–protein interaction represents one of the causes of genetic disorders that vary from neurodevelopmental and neurodegenerative diseases to cancer (3–9). Traditionally, RNA–protein interactions have been investigated by ensemble biochemistry approaches, including affinity purification and cross-linking and immunoprecipitation-based techniques (reviewed in refs. 10 and 11). However, these methods may report adventitious RNA–protein associations that would occur after lysis of cells (12, 13), or functionally important complexes may not survive the procedure. Importantly, ensemble biochemistry studies lack morphological information, particularly essential for neurons.
Currently, there is no method to verify whether these biochemical techniques determine real interactions that take place in the cell. Standard wide-field microscopy has been used to reveal interactions by “colocalizing” two fluorescent tags. Technically, “colocalization” refers to two or more fluorescent molecules emitting different wavelengths of light that superimpose within an indeterminate microscopic resolution. Biologically, colocalization implies the association between these molecules. However, their physical association occurs at a dimension not usually achievable by light microscopy because it occurs below the diffraction limit (∼250 nm). Thus, as currently practiced, colocalization is a suggestion of spatial correlation but does not rule out random association. Here, we derive a method to define colocalization precisely as a nonrandom physical association of two labels at a resolution consistent with their molecular dimensions. We used fluorescent beads with sizes below the diffraction limit of light to determine the characteristics of the objective and derived a correction algorithm to coregister their centers of each point spread function (PSF) at different wavelengths across the field of view (FOV) with nanometer precision, a process that we refer to as “super registration.”
We tested the method using proteins known to bind mRNA in hippocampal neurons. Specifically, we used β-actin and spinophilin mRNAs and two proteins that have been previously shown to bind to them: an endogenous protein [zipcode binding protein 1 (ZBP1)] (14–17) and an engineered protein that binds the MS2 binding sites (MBSs) inserted into the 3′-UTR of β-actin mRNA [MS2 Capsid Protein (MCP)] (18, 19). As a negative control, we used an mRNA that binds neither of these two proteins. We used these controls to develop a method to assess the significance of binding. We then tested whether RBPs isolated biochemically with a standard RNA pulldown met the binding test developed using this quantitative microscopic approach. The results show that, by using standard light microscopy, we can identify with high probability whether these putative binding proteins actually interact with the mRNA and how much. The approach is applicable to any two-labeled molecular species. Significantly, any standard fluorescence microscope can achieve this super registration methodology by simple calibration of the objective lens coupled with subsequent image analysis. This approach provides the quality control for the information obtained from biochemistry techniques.
Results
Super Registration.
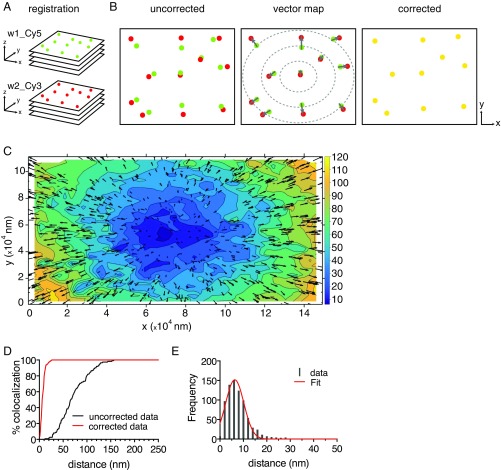
We developed a dual-color methodology that reduced systematic errors limiting previous colocalization measurements by rigorously characterizing the microscope optics (Materials and Methods and SI Results). We first imaged subdiffraction-limited fluorescent beads with a broad emission spectrum in z stacks and detected sequentially in Cy5 and Cy3 channels (Fig. 1A). The centroids of these beads were determined with subpixel precision (20). We calculated the displacement vectors between the centroid positions of each bead in the two channels as a function of its position in the field (Fig. 1 B and C). This process revealed that the chromatic aberration varied substantially from the center of the field to the edge (by as much as 120 nm) (Fig. 1 C and D) because of the inability of the planapochromatic objective lens to correct across the entire field. To compensate for this aberration, we developed a transform that reduced the error to less than 10 nm across the entire FOV (Fig. 1E, SI Results, and Fig. S1).
Fig. 1.
Super registration procedure for dual-color localization microscopy. (A) Registration. A poly–l-lysine–coated surface was sparsely loaded with 100-nm-diameter fluorescent beads, and z stacks were acquired in Cy5 (green) and Cy3 (red) channels with a wide-field microscope. (B) Chromatic aberration correction. Localization of the center of each spectrally separated PSF was determined by a Gaussian curve fitting using FISH_QUANT software (20); then all centroids were allocated in pairs, and distances were measured by using MATLAB custom algorithms (Materials and Methods and SI Results). A vector transformation map (affine transformation matrix) was used to then correct the images for chromatic aberration. Arrows illustrate displacement vectors. Yellow circles illustrate corrected images. (C) Objective contour distortion map of chromatic aberration. The actual distortion determined by the vector map in B for the specific objective used in this study. The entire FOV is represented (in nanometers). Vectors in black indicate chromatic shift direction and magnitude (Cy5 to Cy3). Cooler colors require minimal correction; warmer colors indicate major correction (in nanometers). (D) Percentages of colocalization between spectrally separated centroids before (black line) and after (red line) correction was applied to the entire FOV. (E) Distribution of observed distances of centroid pairs in two-color images after correction. Data are shown as gray bars, and the Gaussian fit is the red line. Mean of distribution = 7.86 ± 0.21 nm. Error, SEM.
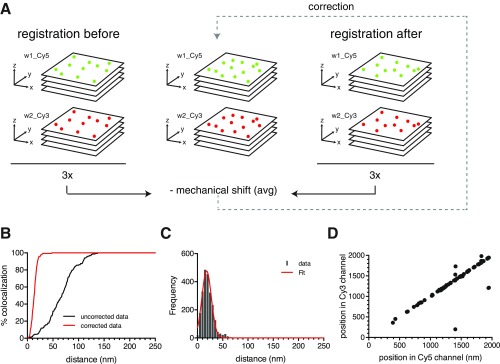
Fig. S1.
Mechanical shift correction for dual-color localization microscopy. (A) Schematic representation of the super registration procedure for dual-color wide-field microscopy used to correct for microscope instability. In addition to the chromatic aberration correction, images were also corrected for mechanical shifts using an average displacement measurement calculated before and after image acquisition. Subdiffraction fluorescent beads were imaged through z stacks in Cy5 (green) and Cy3 (red) channels in between the registration of beads that were imaged in the same wavelengths (before and after registration). Localization of the center of each spectrally separated PSF was determined by a Gaussian fit using FISH_QUANT software (20); all centroids were segregated by pairs, and their distances were measured using MATLAB custom algorithms. (B) Percentage of colocalization between centroids before (black line) and after (red line) correction was applied to the entire FOV. (C) Distribution of observed distances of centroid pairs in two-color images after correction. Data are shown as gray bars, and Gaussian fit is the red line. Mean of distribution = 20.45 ± 0.22 nm. Error, SEM. (D) Scatterplot shows equidistant positions between localized centroids in Cy5 and Cy3 channels.
Imaging Physical Contact Between MBS-Containing β-Actin mRNA and MCP.
To provide a standard model system for calibration of protein binding, we used mRNA tagged with MBS (19) to visualize single mRNA molecules and their associated RBPs within fixed cells. Neurons derived from a mouse in which 24 MBSs were integrated into the 3′-UTR of the β-actin gene were cultured in vitro for 14–21 d (18). The fluorescent capsid protein MCP-GFP was introduced by lentivirus infection and specifically binds to MBS with high affinity (14, 21, 22). To confirm the intracellular association between MCP-GFP and single–β-actin mRNA molecules within the cell, we performed single-molecule FISH in combination with immunofluorescence (smFISH-IF) in neurons (Fig. 2 A and B). We found that MBS (β-actin mRNA) molecules overlapped with MCP signal, both of which appeared as diffraction-limited spots. Neurons derived from WT mice were used as a negative control for MCP association because they have no MBS. We observed the MCP-GFP in the nucleus (MCP has a nuclear localization signal) of these lentivirus-infected WT neurons but did not observe any MCP-GFP spots in dendrites, confirming that its association with the mRNA was MBS-dependent (Fig. S2 A–C). These results indicate that both MCP-GFP (protein) and MBS (β-actin mRNA) are detected in close proximity within dendrites, consistent with their expected intermolecular interaction.
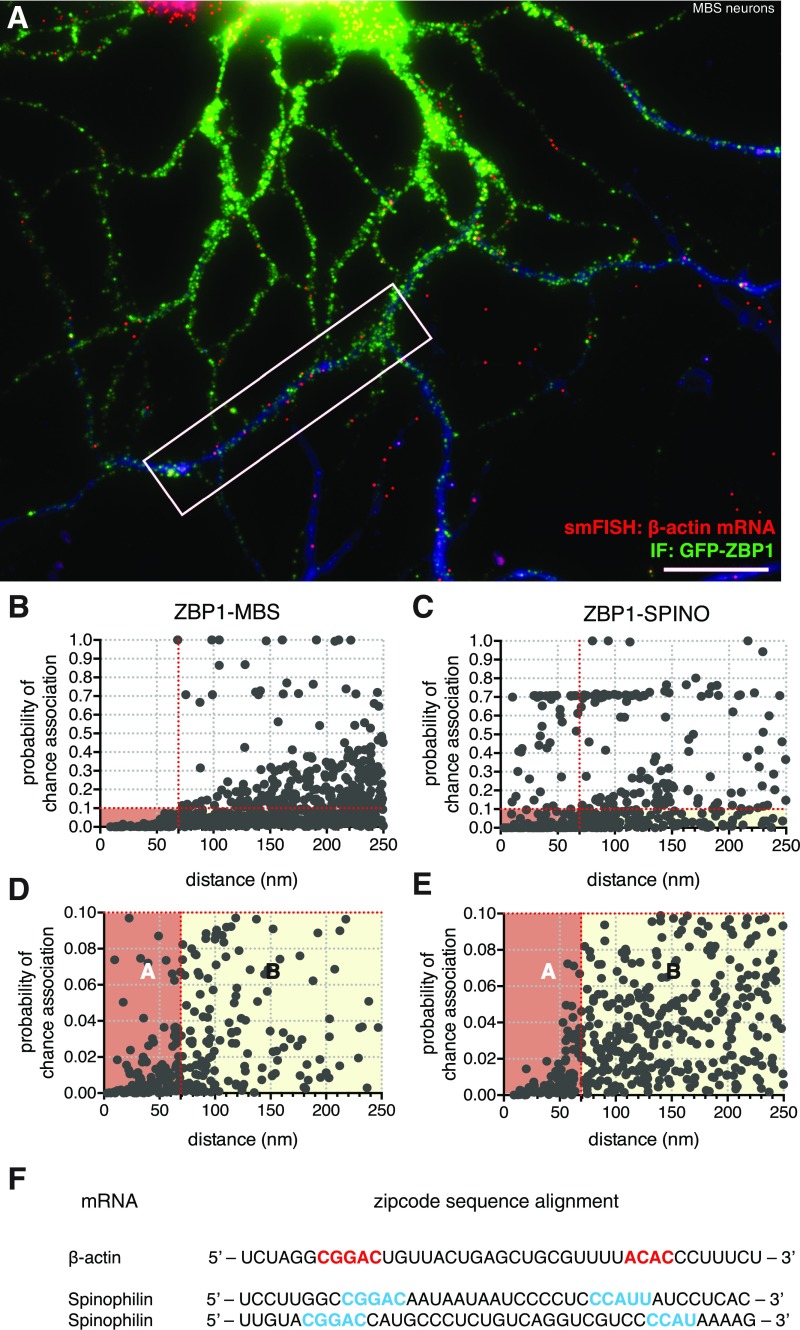
Fig. 2.
Determining significance of association between MCP and endogenous MBS-containing β-actin mRNA. (A) Schematic representation of smFISH-IF on β-actin mRNP: 24 MBSs are present in β-actin 3′-UTR. Two MBSs separated by linker regions (gray) are illustrated for simplicity. Cy3-labeled RNA FISH probes (MBS probes are shown as red stars) hybridized to linker regions as described (18) are depicted. The MCP fused to GFP (gray circles and green barrels, respectively) is bound to the MBS as a dimer and can be detected by IF using antibodies against GFP and Alexa Fluor 647 (AF647)-conjugated secondary antibodies (illustrated with green stars). (B and C) Representative smFISH-IF images from dissociated hippocampal neurons from MBS mice expressing MCP-GFP by lentivirus infection were probed for (B) β-actin mRNA (MBS FISH probes, Cy3; red) or (C) CaMKII mRNA (CaMKII FISH probes, Cy3; red) and IF for MCP-GFP (GFP antibody, AF647; green). (B) A nonexpressing MCP-GFP neuron only showed FISH signal (red). MAP2 is shown in blue as a dendrite marker. (C) The image shows discrete fluorescent particles detected by both smFISH and IF throughout the dendrite that rarely overlap because the MCP does not bind CaMKII mRNA but binds β-actin mRNA with MBS in its 3′-UTR. Images are representative of four independent experiments, with over 15–20 dendrites observed in each experiment. (Scale bars: 5 μm.) (D) Schematic representation of a neuron and the super registration method that measures the significance of each mRNA–protein pair (red and green circles, respectively; magnified). The circle represents the nearest red circle (mRNA). The simulation measures the frequency that the number of green circles (protein) within this area would fall within distances less than d by chance. (Inset) The shaded area represents probability of chance association < 0.1 (the frequency for the illustrated pair based on 10,000 simulations). Every pair with this probability within 250 nm (the diffraction limit) is a single point in F and G. Complete data are in Fig. S2 E and F. (E) Curve of association between an mRNA and a binding protein was calculated as the cumulative ratio of association for intermolecular distances (in the range between 0 and 250 nm) that were less than a given observed distance. The ratio of association was calculated between the number of molecular pairs that can be found in proximity at each given nanometer of distance (and probability of chance association < 0.1) and the total number of molecular pairs within 250 nm (F and G). Red arrows show the distance wherein the mRNA–protein association for MCP-MBS and MCP-CaMKII is maximally separated [optimal distance (OD) = 69 nm] (Materials and Methods, Measurement of Association). Black line, MCP-MBS; dotted gray line, MCP-CaMKII. (F and G) Scatterplots show the probability of chance association between molecules for (F) MCP-GFP and β-actin mRNA (MBS) in MCP-MBS and (G) MCP-GFP and CaMKII mRNA (CaMKII) in MCP-CaMKII. Box A (pink) includes the associated molecules that have a probability of chance association < 0.1 and a distance less than the optimal distance of 69 nm (red vertical line; E). Box A includes the molecules that are physically likely to be in contact. Box B (light yellow) includes molecules with a probability of chance association < 0.1 but at distances greater than the optimal distance and within the diffraction limit of 250 nm. Box B includes the molecules that would be detected as positives by standard colocalization. The total numbers of intermolecular pairs in box A are 614 for MCP-MBS and 21 for MCP-CaMKII. The total numbers of pairs in box B are 120 for MCP-MBS and 111 for MCP-CaMKII (Fig. S2 E and F). (H) Distribution of observed distances for MCP-MBS (gray bars; Gaussian fit in red line) and MCP-CaMKII (MCP is bound to MBS on β-actin mRNA; black bars) after correction. Mean of observed distance was 34.58 ± 0.65 nm for MCP-MBS. Mean observed distance was 541.96 ± 8.14 nm for MCP-CaMKII (chance association) (Fig. S2D). Error, SEM.
Fig. S2.
MCP is associated with endogenous β-actin mRNA in MBS cells. (A–C) Representative smFISH-IF images in WT neurons (control): dissociated hippocampal neurons derived from WT mice (A and B) expressing or (C) not expressing MCP-GFP were probed for IF for MCP-GFP (GFP antibody; green) and smFISH using the following FISH probes: (A) MBS probes (Cy3; red) and (B and C) β-actin ORF probes (Cy3; red). In WT neurons, β-actin mRNA did not have MBS in its 3′-UTR; thus, MCP-GFP did not bind the mRNA, and it is retained in the nucleus because of a nuclear localization signal signal. (A) No discrete fluorescent signal was detected in either channel. (B and C) Only fluorescent spots in smFISH channel were detected using β-actin ORF probes. MAP2 is shown in blue as a dendrite marker. Images are representative of two independent experiments, with over 20 dendrites observed in each experiment. (Scale bars: 10 μm.) (D) Distribution of observed distances for MCP-MBS (<50 nm; gray bars) and MCP-CaMKII (>150 nm; black bars). The higher observed distances between MCP and CaMKII mRNA suggest a random association. (E and F) Scatterplots showed the probability of chance association between molecules for (E) MCP-GFP and β-actin mRNA (MBS) in MCP-MBS and (F) MCP-GFP and CaMKII mRNA (CaMKII) in MCP-CaMKII. Boxes A and B are expanded in Fig. 2 F and G, respectively, for better visualization. (G and H) Histograms of signal intensity for (G) MCP and (H) MBS. Gray bars indicate total population, and red bars indicate physically associated mRNA and protein molecules defined by box A.
Redefining Colocalization: Significance of RNA–Protein Association.
To ensure that the overlapping spots of single-molecule FISH (smFISH) to the MBS and immunofluorescence (IF) to the MCP-GFP did not occur by chance, we measured the likelihood of finding these two molecules in close proximity. To address this quantification, we included the negative control for RNA–protein association (in this case, MCP-GFP) and a dendritically localized transcript without MBS (CaMKII mRNA) (Fig. 2C). After performing smFISH-IF for CaMKII and MCP-GFP, we observed few events of close proximity between the two molecules at distances less than 150 nm compared with MBS and MCP-GFP (Fig. 2 B and C and Fig. S2D). At increasingly larger distances (>150 nm), the spots are more likely to overlap by chance. In addition, any colocalization above 150 nm is not only a random event but occurs at a distance that is not relevant for physical contact.
The higher the local molecular density, the more likely that any colocalization could occur by chance and hence, influence the level of specificity and significance for observed colocalization events. Therefore, we designed an analysis that accounted for the local density around each of the associated pairs of labeled molecules: in this case, mRNA (Fig. 2D, red in expanded circle) and protein (Materials and Methods, Fig. 2D, green in expanded circle, and SI Results). We compared the observed intermolecular distances for each pair with a simulated Monte Carlo random distribution of the two colors at similar concentrations. This procedure provided a measurement to evaluate the significance compared with a randomized distribution. We expressed this probability of chance association when the simulation yielded a distance that was less than the observed distance (Fig. 2D, Inset). The lower the probability of chance association, the higher the probability that the observed colocalization reveals an intermolecular association that is statistically significant. Consistent with this result, we found that most MCP-GFP and MBS signals showed a high significance (probability of chance association is <0.1). In contrast, most MCP-GFP and CaMKII signals did not show significant association (Fig. 2 F and G and Fig. S2 E and F).
To obtain this probability measurement, we calculated association between the two molecules as a function of their distances apart for positive and negative controls (Materials and Methods, Fig. 2E, and SI Results). For the positive control, 85% of the observed distances from the labeled probes to the MBS and from the antibodies to the MCP-GFP were within 69 nm. In contrast, 15% of the observed associations in the negative control (MCP-GFP and the CaMKII probes) occurred at this distance (Fig. 2E, black and dotted gray lines, respectively). The 69-nm cutoff was determined to be the optimal distance between molecules, where the difference between the detection of association for the positive control and the detection of association for the negative control was the greatest (Fig. 2E, red arrows). Within this distance, we defined a probability of chance association less than 10% (<0.1) that represented mRNA–protein molecules that were likely to interact (Materials and Methods, box A in Fig. 2 F and G, SI Results, and Fig. S2 E–H). In this analysis, we found that there were mRNA–protein molecules with a probability of chance association less than 10% (because they were increased relative to the negative control) but that they were not in relevant proximity for a molecular interaction (i.e., distances ranging from optimal distance to 250 nm; box B). For MCP-GFP and MBS, the mean observed distance was 34.58 ± 0.66 nm (Fig. 2H). This measurement includes the distance from the labeled antibodies detecting MCP-GFP to the labeled oligonucleotide probes used to detect β-actin mRNA (using MBS FISH probes). A molecular model for the physical association of MCP-GFP and MBS using available crystal structures in PyMOL indicated that the antibodies positioned the fluorescent label ∼25 nm away from the MCP-GFP. This model supports the conclusion that standard wide-field microscopy is capable of resolving a bona fide mRNA–protein complex (Fig. 3).
Fig. 3.
Association between β-actin mRNA (MBS) and MCP as a molecular model mRNP. Schematic representation of overlapping red (RNA) and green (protein) diffraction-limited spots in a wide-field image and the molecular scale with nanometer precision of MCP-GFP and β-actin (MBS) interaction. By measuring and fitting a Gaussian curve to the PSF, the position in x, y, and z of its center can be determined accurately with high spatial resolution (compare outer dotted line with inner dotted line). One Cy3-labeled MBS (red), MCP-GFP (green), primary antibody (IgY; light blue), and Alexa Fluor 647-labeled secondary antibody (IgG; purple) are depicted. The mean observed distance between labeled antibody and labeled RNA FISH probes is 34.58 nm (Fig. 2H). The distance for MCP-GFP to β-actin mRNA is estimated at 7 nm. The drawing of the molecules was generated in PyMol software with the help of published structure data (22, 44).
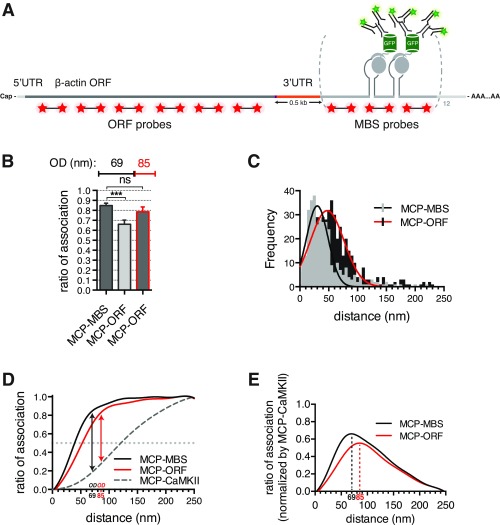
The precision of the registration showed that physical distances between the location where the protein is positioned relative to the FISH probes could be mapped within 10–20 nm depending on their separation, showing that this approach can serve as a “molecular ruler” (SI Results and Fig. S3).
Fig. S3.
Super registration as a molecular ruler. (A) Schematic representation of MBS-containing β-actin mRNA. Labeled RNA FISH MBS and ORF probes (red stars), MCP-GFP (gray circles and green barrels), and antibodies (green) are depicted. Two MBSs separated by linker regions (gray) are illustrated for simplicity. Distance between the stop codon and MBS is ∼500 nt (3′-UTR; shown in orange). (B) Ratio of association for MCP-GFP and β-actin mRNA in neurons comparing MBS or ORF probe sets (MCP-MBS and MCP-ORF, respectively) shows that optimal distance with the ORF probes is 85 nm. Optimal distance is in nanometers. Error bar, SD. Unpaired t test; ns indicates P > 0.05. OD, optimal distance. ***P < 0.001. (C) Distribution of observed distances for MCP-MBS (light-gray bars and black line) and MCP-ORF (black bars and red line) shows the shift consistent with the increased distance from the MCP to the ORF. (D) Curves of association for MCP-MBS (black line), MCP-ORF (red line), and MCP-CaMKII (dotted gray line) show that the curves converge at 85 nm. (E) Normalization of the curves of association for MCP-GFP and β-actin mRNA [using MBS FISH probes; black line (MCP-MBS)] and MCP-GFP and β-actin mRNA [D; using ORF FISH probes; red line (MCP-ORF)] reveals where the mRNA–protein association is maximally separated, defining the optimal distance. Optimal distance is 69 nm using MBS FISH probes (dotted black line; MCP-MBS) and 85 nm using ORF FISH probes (dotted red line; MCP-ORF).
Application to the Interaction Between ZBP1 and Its mRNA Targets.
We then tested this analytical technique on a bona fide endogenous complex: the well-characterized interaction between β-actin mRNA and ZBP1, the protein that binds to its bipartite zipcode sequence element present in the 3′-UTR (14, 16). MBS neuronal cultures infected with lentivirus encoding GFP fused to ZBP1 showed discrete particles along mature dendrites, reminiscent of dendritically transported mRNA granules with different sizes and signal intensities (Fig. 4 A and C and Fig. S4 A and F). Analysis of the images revealed that the overlap between β-actin mRNA (FISH signal) and ZBP1-GFP (IF signal) was 27% (Fig. 4 E and F and Fig. S4 B and D). This association of ZBP1-GFP with the mRNA is less than that of MCP-GFP, which has essentially a longer off rate. Other than β-actin mRNA, other targets for ZBP1 have been described (16). For instance, spinophilin, a zipcode-containing mRNA, was enriched in pulldown experiments for ZBP1 from brain extracts and localized to mature dendrites dependent on ZBP1 (16). In support of this finding, we observed ZBP1-GFP in close proximity with spinophilin mRNA within mature dendrites (Fig. 4 B, D, E, and G and Fig. S4 C, E, and F). Our findings showed one population of interacting molecules from 0 to 69 nm and another from 69 to 100 nm, consistent with this mRNA having two putative zipcodes (Fig. 4 B and G and Fig. S4F). The ZBP1-GFP molecules bound to spinophilin mRNA molecules at optimal distance < 69 nm were greater than those bound to β-actin mRNA (using MBS FISH probes) (Fig. 4E). These results show that this imaging method has the resolution to determine where in the dendrite a direct interaction occurs between an RBP, such as ZBP1, and its mRNA targets and its relative degree of association compared with MBS-MCP.
Fig. 4.
Association between ZBP1 and endogenous mRNA targets at molecular resolution. (A) Schematic representation of β-actin mRNA showing MBS and the zipcode (blue) bound by ZBP1 (light-blue oval) in the 3′-UTR. Two MBSs separated by linker regions (gray) are illustrated for simplicity. Cy3-labeled RNA FISH probes (MBS probes; red stars) and antibodies are also depicted. (B) Schematic representation of spinophilin mRNA showing two putative zipcodes (blue) bound by ZBP1 (light-blue ovals) in the 3′-UTR. Cy3-labeled RNA FISH probes (red stars) and antibodies are also depicted. (C and D) Representative smFISH-IF images in dissociated hippocampal neurons from MBS mice expressing GFP-ZBP1 detected by GFP antibody (green) combined with smFISH for (C) β-actin mRNA (MBS FISH probes; red) and (D) spinophilin mRNA (red). Distal dendrites were analyzed where both smFISH and IF detected discrete fluorescent spots. Yellow arrowheads show sites of molecular interaction as defined by box A in Fig. 2 (probability of chance association < 0.1 and optimal distance = 69 nm); white arrowheads show nonassociated molecules as defined by box B in Fig. 2 (distances between optimal distance and 250 nm). MAP2 is shown in blue as a dendrite marker. Images are representative of (C) five and (D) two independent experiments, with over 20 dendrites observed in each experiment. (Scale bars: 5 μm.) (E) Ratios of association for ZBP1-MBS and ZBP1-SPINO in neurons compared with the standard model MCP-MBS and MCP-CaMKII (negative control). The dotted red line indicates background association as defined by MCP-CaMKII. Error bar, SD. Unpaired t test. *P < 0.05; ****P < 0.0001. (F and G) Distribution of observed distances for GFP-ZBP1 and β-actin mRNA (ZBP1-MBS) in F and GFP-ZBP1 with spinophilin mRNA (ZBP1-SPINO) in G after correction. Gray bars and red lines show associated molecules as defined by box A (optimal distance < 69 nm); black bars show nonassociated molecules as defined by box B (distances between optimal distance and 250 nm). Mean of observed distance was 45.44 ± 1.80 nm for ZBP1-MBS in F and 41.00 ± 1.53 nm for ZBP1-SPINO in G. Error, SEM.
Fig. S4.
smFISH-IF shows association between ZBP1 and endogenous mRNA targets within neurons. (A) FOV of the representative smFISH-IF image shown in Fig. 4C: dissociated hippocampal neurons from MBS mice expressing GFP-ZBP1 detected by GFP antibody (green) combined with smFISH for β-actin mRNA (MBS probes; red). ZBP1 is highly expressed in soma and proximal dendrites and less expressed in distal dendrites, showing a puncta-like pattern. Only distal dendrites were analyzed where both smFISH and IF detected discrete fluorescent spots. smFISH-IF spot signals were dilated by one pixel for visualization. MAP2 is shown in blue as a dendrite marker. (Scale bar: 20 μm.) Inset is shown in Fig. 4C. (B and C) Scatterplots show the probability of chance association for GFP-ZBP1 and β-actin mRNA (ZBP1-MBS) in B and GFP-ZBP1 with spinophilin mRNA (ZBP1-SPINO) in C. Boxes A and B are expanded in D and E, respectively, for better visualization. Box A (pink) shows the associated molecules that have a probability of chance association < 0.1 and a distance less than the optimal distance of 69 nm (red vertical lines). Box A includes the molecules that are physically likely to be in contact. Box B (light yellow) shows molecules with a probability of chance association < 0.1 but at distances greater than the optimal distance and within the diffraction limit of 250 nm. (F) Zipcode sequence alignment for β-actin and spinophilin 3′-UTRs as was described in ref. 16. Spinophilin 3′-UTR showed two putative ZBP1 KH34 binding elements (zipcodes; depicted in light blue) that have the same spatial arrangement as the unique bipartite zipcode in β-actin 3′-UTR (shown in red).
Validation of β-Actin mRNA-Associated Factors.
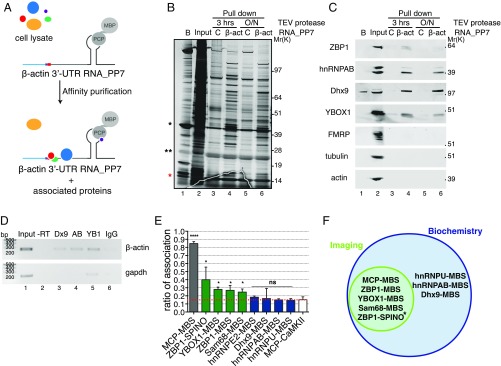
To evaluate the efficacy of this approach to validate putative RNA–protein interactions, we isolated additional binding proteins for β-actin mRNA by a typical pulldown assay. By using in vitro transcribed Pseudomonas aeruginosa PP7 bacteriophage (PP7)-tagged zipcode-containing β-actin 3′-UTR RNA as bait, we captured stably associated proteins from mammalian cell extracts (Fig. 5 A and B). Proteins specifically bound to β-actin 3′-UTR RNA were eluted, separated by SDS/PAGE, and analyzed by liquid chromatography–MS/MS (LC-MS/MS). Gene ontology analysis revealed that proteins found associated with β-actin 3′-UTR were principally involved in RNA posttranscriptional modification, protein synthesis, gene expression, and RNA trafficking functions (Dataset S1 and Fig. S5 A and B). In addition to ZBP1, we found heterogeneous nuclear ribonucleoproteins (hnRNPs) AB, A0, A3, A1, L, D, DL, UL1, U, Q1 (Syncrip), and R; Y-Box binding protein 1 (YBOX1); Cold shock domain-containing protein A; ATP-dependent RNA helicase A (Dhx9); IMP2; IIF2; Staufen 1 & 2; PABP1; Src-associated in mitosis 68 kDa (Sam68); Myelin expression factor 2-like; UPF1; eIF3; and several SR proteins. We also found the motor-related protein Myosin Regulatory Light Chain 2.
Fig. 5.
Validation of β-actin 3′-UTR affinity purification of associated proteins. (A) Schematic representation of β-actin 3′-UTR pulldown strategy. In vitro-transcribed PP7-tagged zipcode-containing β-actin 3′-UTR RNA was incubated with mouse embryonic fibroblast cell lysates, affinity-purified on amylose magnetic resin, and incubated with TEV protease either for 3 h or overnight (O/N) to identify protein components that interact with β-actin mRNA and ZBP1 protein. β-Actin 3′-UTR containing one PP7 binding site (gray) bound by PP7 coat protein (PCP) fused to MBP (gray circles), the zipcode element (red), and the coding region (light blue) are depicted. (B) Silver-stained SDS/PAGE gel of proteins specifically bound to β-actin 3′-UTR RNA isolated from mouse embryonic fibroblast extracts using either a control (C; lanes 3 and 5) or β-actin 3′-UTR (lanes 4 and 6) as a bait. A list of proteins identified by LC-MS/MS is summarized in Fig. S5B and Dataset S1. Molecular weight (Mr) is shown. Beads (B; lane 1) indicate proteins remained bound to beads after TEV elution. Input (lane 2) indicates 3 μg total protein. Lanes 3–6 show 60% of pulldown eluates. Red asterisk indicates PCP. *MBP-PCP; **TEV protease. (C) Western blot analyses of indicated proteins in input and pulldown eluates on TEV protease digestion for 3 h or overnight (O/N) as indicated. Molecular weight (Mr) is shown. Beads (B; lane 1) indicate proteins that remained bound to beads after TEV elution. Input (lane 2) indicates 30 μg total protein. Lanes 3–6 show 40% of pulldown eluates. The results shown are representative of three independent experiments. (D) RIP. Enrichment of (Upper) endogenous β-actin and (Lower) GAPDH mRNAs in Dhx9 (Dx9), hnRNPAB (AB), and YBOX1 (YB1) immunoprecipitations (lanes 3–5) compared with IgG control (lane 6). A PCR carried out without reverse transcriptase (−RT) is shown in lane 2. (E) Summary of association of the indicated mRNA and proteins by smFISH-IF in dendrites. Dotted red line indicates background association defined by MCP-CaMKII. Error bar, SD. Unpaired t test; ns indicates P > 0.05. *P < 0.05; ****P < 0.0001. (F) Venn diagram showing mRNA and protein association validated by both imaging and biochemistry approaches in this work. *mRNA–protein association validated by biochemistry in ref. 16.
Fig. S5.
Protein(s) associated with β-actin 3′-UTR by affinity purification. (A) GO analysis and (B) subcellular location and type of RNA-binding domain present in the identified proteins associated with β-actin 3′-UTR by affinity purification coupled to LC-MS/MS analysis shown in Fig. 5. C, cytoplasm; CSD, cold shock domain; DZF, domain associated with zinc fingers; KH, K homology domain; N, nucleus; RGG box, glycine–arginine-rich domain; RRM, RNA recognition motif. (C) Western blot analysis of indicated proteins in input and pulldown eluates. β-Act, β-actin 3′-UTR RNA; C, control RNA. Molecular weight (Mr) is shown. (D–J) Observed distances for the indicated proteins and mRNAs shown in this study: (D) YBOX1-MBS, (E) Sam68-MBS, (F) hnRNPE2-MBS, (G) Dhx9-MBS, (H) hnRNPAB-MBS, (I) hnRNPU-MBS, and (J) MCP-CaMKII. Gray bars and red lines indicate associated molecules that have a probability of chance association < 0.1 and a distance less than optimal distance (=69 nm) as defined by box A; black bars indicate molecules with a probability of chance association < 0.1 but at distances greater than the optimal distance and within the diffraction limit of 250 nm as defined by box B (histograms for MCP-MBS in Fig. 2H, ZBP1-MBS in Fig. 4F, and ZBP1-SPINO in Fig. 4G). Ratio of association was calculated as the ratio of the physically associated molecules as defined by box A to the total population of boxes A and B.
We confirmed the association between β-actin 3′-UTR RNA and proteins identified by standard biochemical techniques, such as Western blot (Fig. 5C and Fig. S5C) and RNA immunoprecipitation (RIP) (Fig. 5D). ZBP1, hnRNPAB (23), Dhx9, YBOX1, and Sam68 (24) showed a significant interaction with β-actin 3′-UTR RNA compared with the control RNA. Non-RBPs, such as tubulin or actin, were not detected in pulldown eluates, indicating enrichment in specific binders. FMRP, a prominent neuronal mRNA-binding protein (25), was not detected by either LC-MS/MS or Western blot analysis. While Western blots in Fig. 5C highlighted the specificity of protein–RNA interactions found by LC-MS/MS, endogenous β-actin mRNA was found in eluates of immunoprecipitations carried out by specific antibodies against Dhx9, hnRNPAB, and YBOX1 (Fig. 5D). Binding of ZBP1, hnRNPAB, YBOX1, and Sam68 was precluded when a β-actin 3′-UTR RNA containing a deletion of the zipcode sequence region was used, suggesting that they bound to the zipcode or were part of a zipcode binding complex (Fig. S6).
Fig. S6.
Protein(s) associated with β-actin 3′-UTR RNA bind to the zipcode region. (A) Schematic representation of the β-actin 3′-UTR and β-actin 3′-UTR containing a deletion of the zipcode sequence region (Δzip) RNAs used for pulldown. In vitro-transcribed PP7-tagged zipcode-containing β-actin 3′-UTR and Δzip RNAs were incubated with MEF cell lysates and affinity-purified on amylose magnetic resin to identify protein components that interact with β-actin mRNA and ZBP1 protein. (B) Sequence alignment for β-actin 3′-UTR and Δzip RNAs. (C) Silver-stained SDS/PAGE gel of proteins isolated from MEF cell extracts using control (C), β-actin 3′-UTR (β-act), or Δzip 3′-UTR RNAs as a bait. (D) Western blot analysis of indicated proteins in input and pulldown eluates. WT indicates MEF cell extracts derived from WT mice, and KO indicates MEF cell extracts derived from ZBP1 KO mice. Molecular weight is shown.*MBP-PCP; **TEV protease.
Finally, we tested the identified RNA–protein associations by super registration microscopy. YBOX1, Sam68, hnRNPE2, hnRNPU, hnRNPAB, and Dhx9 IF combined with smFISH for β-actin mRNA (using MBS FISH probes) was performed in fixed neurons, and intermolecular distances were calculated (Fig. 5 E and F and Figs. S5 D–J and S7 and Table S1). RNA–protein associations ranged from 10 to 40% for all of the identified factors analyzed with β-actin mRNA in hippocampal dendrites (Fig. 5E and Table S1). ZBP1, YBOX1, and Sam68 were associated with β-actin mRNA; however, Dhx9, hnRNPE2, hnRNPU, and hnRNPAB were nonspecific in their interactions, similar to the association of CaMKII (15%). We assumed similar molecular conformations and dye orientations for each pair and used the optimal distance less than 69 nm previously determined. Therefore, two-color imaging can critically evaluate whether single molecules of mRNA make bona fide physical contacts with putative binding proteins.
Fig. S7.
Proteins associated with β-actin 3′-UTR by smFISH-IF. Representative smFISH-IF images in dissociated hippocampal neurons from MBS mice detected by smFISH for β-actin mRNA (MBS FISH probes; red) combined with IF for the indicated proteins (green): (A) YBOX1, (B) Sam68, (C) Dhx9, (D) hnRNPU, (E) hnRNPAB, and (F) hnRNPE2. Yellow arrowheads show sites of molecular interaction as defined by box A in Fig. 2 (probability of chance association < 0.1 and optimal distance = 69 nm); white arrowheads show nonassociated molecules as defined by box B in Fig. 2 (distances between optimal distance and 250 nm). MAP2 is shown in blue as a dendrite marker. Images are representative of (A, D, and E) two and (B, C, and F) three independent experiments, with over 15–20 dendrites observed in each experiment. (Scale bars: 5 μm.)
Table S1.
Validation of β-actin 3′-UTR–associated proteins
| Protein–mRNA | Imaging | Biochemistry: MS/WB/RIP | ||
| Association | Ratio of association | Mean observed distance (nm) | ||
| MCP-MBS | Yes | 0.85 | 34.58 ± 0.65 | Yes |
| ZBP1-SPINO | Yes | 0.40 | 41.00 ± 1.53 | Yes* |
| YBOX1-MBS | Yes | 0.28 | 48.01 ± 3.29 | Yes |
| ZBP1-MBS | Yes | 0.27 | 45.44 ± 1.80 | Yes |
| Sam68-MBS | Yes | 0.25 | 47.52 ± 2.52 | Yes |
| hnRNPE2-MBS | No | 0.18 | 41.38 ± 2.38 | No |
| Dhx9-MBS | No | 0.17 | 42.27 ± 3.85 | Yes |
| hnRNPAB-MBS | No | 0.15 | 51.03 ± 2.78 | Yes |
| hnRNPU-MBS | No | 0.15 | 48.03 ± 3.38 | Yes |
Summary of the ratios of association between mRNA and protein molecules and the mean observed distance calculated by this imaging technique. Intermolecular associations suggested by biochemistry techniques [LC-MS/MS (MS), Western blot (WB), and RIP] are also shown for comparison. Mean observed distance was calculated using the molecular interactions as defined by box A (probability of chance association < 0.1 and optimal distance = 69 nm).
mRNA–protein association validated by biochemistry in ref. 16.
SI Results
Super Registration.
The premise of super registration is that we need to compensate for the intrinsic inability of optics to correct completely for chromatic aberration and other factors that influence the optical path in a way that interferes with the fidelity of detecting centroid positions using single-molecule localization techniques. We found that minimizing the influence of and compensating for these aspects were essential for achieving exquisite alignment of multiple fluorescence channels to 10-nm precision.
Chromatic aberration is a common optical problem that occurs when wavelengths of different color are focused at different positions in the focal plane. Using high-quality superplanapochromatic objectives does minimize this optical distortion. However, these lenses still do not provide a perfectly corrected image from edge to edge of the FOV of a typical detector (144.48 × 110.08 μm). The objective’s correction works best just at the center of the FOV. Therefore, to compensate for the objective’s chromatic aberration across the entire FOV, we mapped the optical distortion as a function of position by observing subdiffraction limit-sized fluorescent beads that have a broad emission spectrum (TetraSpeck Fluorescent Microspheres, 100-nm diameter; Life Technologies). Multiple fields of beads (n = 760 beads) were imaged in three dimensions sequentially in the Cy5 and Cy3 channels (Fig. 1A). Then, centroids of the PSF of the beads were localized with subpixel precision in each channel (Materials and Methods, Single-Molecule Localization). The Cy5 channel centroid positions in x and y were compared with the Cy3 channel centroid positions in x and y, and the displacement vectors between the centroid positions of each bead in the two channels were calculated (Fig. 1B). For simplicity of analysis, only the x–y plane was taking into account because the imaging was done with z sectioning. Ideally, the displacement vectors would be zero across the entire FOV. However, we observed an offset caused by chromatic aberration. We determined the displacement vectors in each orthogonal axis independently as a function of the position in the FOV. For the objectives that we used, the function that best fitted the displacement data for the x and y axes was a plane. This finding was consistent with the observation of a radial optical aberration, in which the magnitude of the distortion increased as a function of the position from the center of the FOV (where centroid positions in x and y were practically identical in the two channels) toward the edges (Fig. 1 B and C). Eqs. 1 and 2 described the distortion that was fitted to a plane in x and y independently, where k is the plane’s slopes. R2 = 0.9404 for the x-axis fit, and R2 = 0.9546 for the y-axis fit. The fitted polynomial functions were used to determine chromatic aberration in any position in the FOV:
| [S1] |
and
| [S2] |
As a result of knowing centroid positions in x and y with high precision at multiple locations in the two channels, we were able to generate a unique vector map that characterized the chromatic aberration of the specific objective that we used relative to the image detector. Because the coordinates of the vector map were relative to the position of the camera, it was important to secure the camera’s position to prevent rotational movement of the detector. Interestingly, we also found that this function could be different for every type of objective, even of the same model, and therefore, this calibration needs to be applied to every objective lens to obtain super registration (Materials and Methods, Objective Testing). We compensated for the objective’s chromatic aberration between Cy5 and Cy3 using an affine transformation, resulting in a mean registration error of 7.86 ± 0.21 nm (for the entire FOV) (Fig. 1). The main contribution to having a mean registration error that is more than zero is uncertainty in the centroid localization (because of signal to noise ratio) detected by FISH_QUANT (Materials and Methods, Single-Molecule Localization). The mean registration error was 65.46 ± 1.07 nm (for the entire FOV) without chromatic aberration correction.
In addition to chromatic aberration, there are other influences that cause two colors to diverge from each other, such as room environmental conditions, vibration of the apparatus by motorized components that change the position of filter sets and the z position of the objective or specimen during z-stack acquisition, having the plane of the coverslip not parallel to plane of the microscope slide, mismatches in the refractive index, cross-talk, postacquisition image analysis that performs single-molecule localization, etc. It is not possible to completely correct for all of these factors. However, we compensated for mechanical instability during imaging acquisition. Each day, we imaged at least three fields of subdiffraction limit-sized fluorescent beads both before and after smFISH-IF images were acquired (Fig. S1). After chromatic aberration, correction was applied; we calculated the mean displacement vector between the centroid positions in x and y in the two channels and estimated v, the average linear offset in x and y axes caused by mechanical shift (Eqs. 3 and 4):
| [S3] |
and
| [S4] |
Thus, we used the displacements dx and dy (Eqs. 3 and 4) to compensate for both chromatic aberration (described in Eqs. 1 and 2) and mechanical shift between Cy5 and Cy3 channels using an affine transformation. To evaluate the mechanical stability of the system, we performed an experiment, in which we applied the procedure that corrects for chromatic aberration and mechanical shifts, but rather than imaging smFISH-IF, we imaged diffraction limited-sized beads (25 fields, n = 2,300 beads). Whereas without any correction, the mean registration error was 65.21 ± 0.61 nm (for the entire FOV), the mean error was 43.54 ± 0.39 nm (for the entire FOV) when only chromatic aberration was corrected and 20.45 ± 0.22 nm (for the entire FOV) when both chromatic aberration and mechanical shift corrections were applied (Fig. S1). These results show that both chromatic aberration and mechanical shift need to be corrected and also suggest that the optical path alignment setup and room environment were stable during imaging acquisition. Importantly, this approach does not require fiduciary markers (beads) within the biological sample field and thus, avoids introducing noise that interferes with the accurate detection of single molecules. It is worth mentioning that this method corrects for chromatic aberration on the optical system and not inside the cell, and it only applies to fixed samples using homogenous refractive index. Therefore, this super registration method improves the confidence with which we can determine that two labeled objects are “colocalized” at molecular resolution.
Measurement of Intermolecular Distances and Determining the Significance of Association.
After the centroid positions in x and y were corrected for chromatic aberration and mechanical shifts in the Cy5 and Cy3 channels (as described above in SI Results, Super Registration), we first used a nearest neighbor algorithm to pair molecules between the two channels (pairing). This pairing procedure had the assumptions that no molecule could be a member of more than one pair at a time and that some molecules may remain unpaired. Then, we measured the Euclidean distance between the centroid positions in each pair. Finally, to ensure that the molecules in a pair did not occur by chance, we measured the likelihood of finding the two molecules in close proximity. We started with the assumption that the smaller the distance between the molecules in the pair and the farther away the pair was from its molecular neighbors, the more significance one can assign to the likelihood that that pair of molecules was associated. Conventionally, one would perform a Monte Carlo simulation, in which all of the molecules were randomly positioned repeatedly within the ROI, resulting in a distribution of simulated intermolecular distances. This approach uses the global molecular population within an ROI, which overestimates the significance of the interactions by homogenizing the local context of molecular densities. For this reason, we developed a method to determine the probability of molecular association for each molecular pair based on the intermolecular distances observed and the proximal context within the cell (i.e., where the RNA–protein interactions are taking place).
We measured the significance of an association from the perspective of the channel in which the molecules were less abundant [i.e., mRNA (red circles) in Fig. 2D]. However, it is also possible to make the observation from either channel or even combine the results as a sum of squares. After an intermolecular pair is identified, the geometric boundary for the analysis was the distance from the red molecule of the pair to the next closest red molecule (Fig. 2D, outer dashed line). We then performed a 10,000-iteration Monte Carlo simulation using the number of molecules counted from both channels (red and green) within that area. We obtained a distribution of 10,000 distances (one for every iteration), each of which was the smallest intermolecular distance measured among the randomly positioned molecules. We then calculated a percentile rank as the ratio of the number of times that the simulation yielded a distance that was less than or equal to the observed distance to the total number of simulations. This percentile rank expressed the probability of chance association with values that ranged from zero to one. The probability of chance association described the likelihood that two molecules in a pair would have been the same distance apart (or closer) than observed distance if randomly positioned given the local molecular density in each channel. The lower the probability of chance association, the more significant the association. We measured the significance of association using the molecular density immediately adjacent to the association observed by limiting the area where the simulation was performed. We also measured the significance of association in the cell body of neurons, where the molecular density is higher than in dendrites. We found that the ratios of association for MCP-MBS in cell body and dendrites were comparable (87% in cell body vs. 85% in dendrites). This result suggests that this statistical analysis is capable of determining molecular interactions in crowding areas of the cell with similar significant association. Thus, the results were independent of the ROI and global spatial molecular distribution by either number or density in either channel, yielding a more robust evaluation of the molecular associations’ significance. It is important to note that the statistical analysis breaks down when there is so much crowding that the optimal distance cannot distinguish between associations by chance and meaningful associations. Because the signal to noise of the optimal distance is about 6:1 (Fig. 2E), the concentration of one component would need to be six times higher, and molecular crowding would require that the space be almost entirely filled with a single molecule, not a biologically relevant situation. If this scenario was the case, we could compensate by reducing the optimal distance to less than 69 nm, thereby reducing the effective amount of the higher component.
Registration as a Molecular Ruler.
The precision of the registration measured for the antibodies to the MCP-GFP and the probes to the MBS suggests that they should be able to detect when fluorescent signals from smFISH were positioned at different physical distances along the mRNA. To test whether these intermolecular distances could be measured accurately, we imaged β-actin mRNA by using specific RNA FISH probes to the ORF (at 500-nt average distance from the MBS) and anti-GFP antibodies to the MCP-GFP (Fig. S3A). We found that the ratio of association at the optimal distance (=69 nm) between β-actin mRNA and MCP-GFP decreased to 65% compared with 85% for MBS probes (Fig. S3B). The interaction distances were shifted to higher values, suggesting a longer optimal distance (85 nm compared with 69 nm using the MBS FISH probes) (Fig. S3 C–E). After the new optimal distance of 85 nm was taken into account, the association recovered to 80% (Fig. S3B). These results show that physical distances between the locations where two fluorophores are positioned could be mapped with 10- to 20-nm precision. This finding has implications for evaluating the distance between any two pairs of fluors depending on their molecular separation, and it could be used in this work to standardize the evaluation of real RNA–protein interactions, regardless of where they may bind on the mRNA.
Discussion
In this study, we provide an approach to ascertain the physical interaction between single mRNAs and binding proteins in situ in single cells using standard wide-field microscopy. The flowchart is illustrated in Fig. 6. This imaging method extends biochemical-based studies on RNA–protein interactions by providing spatial information about where in the cells these interactions are likely to occur. This morphological information is especially important in neurons, in which RNA regulatory mechanisms play an essential role in the regulation of localized gene expression.
Fig. 6.
Flowchart illustrating the steps to determine whether mRNA and protein molecules physically interact within cells. OD, optimal distance.
The analysis of colocalization has, as its basis, the likelihood of finding two molecules in close proximity. For instance, colocalization is deduced by the merging of two colors (e.g., a yellow spot when comparing red with green pseudocolors). However, a yellow spot may not indicate real association between molecules. First, the resolution may not be sufficient to determine the true distance between the colors. Second, the overlap may have occurred by chance dependent on the concentrations of each of the molecules. By this same reasoning, two molecules may be colocalized even if a merged signal is not apparent because of chromatic aberration or disparities in the brightness of each component. In this work, we have developed a quantitative image acquisition and analysis method that measures the distance between labeled molecules and the likelihood of their physical association independent of their intensities.
Various statistical methods have been proposed to address colocalization using single-molecule imaging. A dominant method is the Ripley’s K function method (reviewed in ref. 26), which tests spatial randomness through the computation of its quantiles. This method and its derivatives have been developed to create a fast and robust statistical test. However, this approach is limited because the region of interest (ROI) requires straight lines at its edges to account for edge-effect biases and may not be as accurate as the more computationally expensive Monte Carlo simulation. Because neuronal structure is highly irregular and small sets of pairing events require quantitative characterization, we centered our study on the interaction between individual mRNAs and proteins without analyzing the global spatial molecule distribution through an ROI. Therefore, the imaging analysis described here allows an objective quantification of the probability of molecular association, and it is independent of the molecular density within the cell.
Chemical and UV cross-linking followed by RNA sequencing after immunoprecipitation has been used to identify putative mRNA–protein associations (27–32). However, although these techniques show that these molecules can interact, it does not provide evidence of a stable in vivo complex; the molecules may come in contact transiently on cell disruption or be artificially stabilized by cross-linking (33, 34). In contrast, imaging at the single-molecule and cellular level provides evidence of a biologically relevant interaction. In addition, the percentage binding can be represented spatially in unmodified cells: where in the cell this binding is likely to occur.
This imaging method can characterize and validate protein components of a specific messenger ribonucleoprotein (mRNP). In addition to the well-known ZBP1, we found other proteins that bound to the zipcode-containing β-actin 3′-UTR using a PP7 stem loop to pull down the RNA. From the list of protein candidates that bound the β-actin 3′-UTR, the presence of YBOX1, hnRNPAB, and Dhx9 was consistent with its presence in ZBP1/IMP1 ribonucleoprotein particle (RNP) granules (35, 36). Sam68 has also previously been found to bind to β-actin mRNA in neurons and regulate its translation (24, 37, 38). More importantly, the approach will be instrumental in ruling out false positive associations. For instance, hnRNPAB has been shown to bind AU-rich response elements commonly present in 3′-UTRs (39–42), and we find it associated with β-actin 3′-UTR by affinity purification. However, this approach reveals that hnRNPAB and β-actin mRNA do not interact except by chance in dendrites. Similarly, hnRNPU and Dhx9, an RNA helicase mostly enriched in the nucleus, also do not associate with β-actin mRNA except by accident in dendrites in contrast to results that suggested specific binding using biochemical techniques (Fig. 5 and Table S1). It should be noted, however, that the observations do not negate the possibility of a physiologically significant effect of these proteins because a transient interaction may be sufficient for a protein to modify an RNA or promote formation of a complex, even if the interaction occurs statistically by chance. Nonetheless, this method clearly identifies proteins (ZBP1, YBOX1, and Sam68) that are stably associated with β-actin mRNA at intermolecular distances below 69 nm, the threshold for distinguishing physically meaningful interactions. However, it is also possible that proteins in a large complex (>69 nm) may be associated but may not be in physical contact with the mRNA. In addition, the association of ZBP1-GFP with β-actin mRNA may be underestimated because there was competition with the endogenous ZBP1 for β-actin mRNA binding. ZBP1 also dissociates from the mRNA depending on its phosphorylation (15, 43). Finally, the detection of the ZBP1-GFP by antibodies would be less efficient than direct labeling of mCherry-ZBP1 in cells derived from a KO mouse, where all ZBP1 is labeled (43).
Identifying bona fide RNA–protein associations in situ is important for investigating their roles in a variety of molecular and subcellular events, such as local translation in synaptic plasticity. The RNA–protein interactome can be explored with the methodology described here. smFISH-IF can be generally applied to any combination of mRNA and binding protein(s), allowing single mRNP complex observation at cellular sites of mRNP assembly. Notably, endogenous mRNAs and proteins can be directly investigated by using RNA FISH probes and antibodies commercially available without genetic manipulation of the cells. Importantly, this approach can be achieved by simple fluorescence microscopes and does not require laser illumination, EM-CCD cameras, long imaging acquisition times, deconvolution, or image reconstruction. Thus, this imaging method will be an essential technique to complement biochemical studies because the spatial relationship within the cell is preserved.
Materials and Methods
Mouse Hippocampal Neuron Culture.
Animal work was performed in accordance with Institutional Animal Care and Use Committee protocols at the Albert Einstein College of Medicine. Postnatal mouse hippocampal tissue was isolated from homozygous MBS knock-in (18) newborn pups (P0–P1). Hippocampi were placed in 0.25% trypsin for 15 min at 37 °C. Tissue was triturated, plated onto poly–d-lysine (Sigma-Aldrich)–coated glass-bottom dishes (MatTek) at 45,000 cells per dish, and cultured in Neurobasal A Media (Life Technologies) supplemented with B-27 (Life Technologies), GlutaMax (Life Technologies), and Primocin (InvivoGen). Hippocampal neurons from WT mouse embryos [embryonic day (E) 18; BrainBits, LLC] were prepared as above. Dissociated mouse hippocampal neurons were infected with lentivirus expressing MCP-GFP or ZBP1-GFP at 5 d in vitro.
smFISH-IF.
Combining smFISH with IF required multiple conditions to accommodate both reagents. For fixation, permeabilization, and staining, mouse postnatal hippocampal neuronal cells infected on DIV5 with lentivirus encoding for tandem dimer MCP-GFP were fixed at DIV14–DIV21 with ice-cold 4% (vol/vol) paraformaldehyde and 4% (wt/vol) sucrose in 1× PBS supplemented with 1 mM MgCl2 and 0.1 mM CaCl2 (PBS-MC) for 20 min, quenched in 50 mM glycine, and permeabilized with ice-cold 0.1% Triton X-100 (28314; Thermo Scientific) and 0.5% UltraPure BSA (AM2616; Life Technologies) in 1× PBS-MC for 15 min. After incubation with 10% (vol/vol) formamide, 2× SSC, and 0.5% UltraPure BSA in RNase-free water for 30 min at room temperature, cells were incubated for 3 h at 37 °C with either 10 ng (Invitrogen) or 50 nM (Stellaris RNA FISH Probes; Biosearch Technologies) labeled mix probe sets (Dataset S2) and primary antibody against GFP from Aves Labs, Inc. (GFP-1010) at 1/5,000 dilution in Hybridization Buffer [10% formamide, 1 mg/mL Escherichia coli tRNA, 10% dextrane sulfate, 20 mg/mL BSA, 2× SSC, 2 mM Vanadyl Ribonucleoside Complex, 10 U/mL Superase.In (Ambion) in RNase-free water]. Then, cells were quickly washed and incubated twice with Alexa Fluor 647-conjugated secondary antibody (Life Technologies) at 1/1,000 dilution in 10% formamide and 2× SSC in RNase-free water for 20 min at 37 °C. After four 2× SSC washes, DNA was counterstained with DAPI (0.5 μg/mL in 2× SSC; Sigma-Aldrich), and after a final wash, cells were mounted using ProLong Gold Antifade Reagent (Life Technologies). smFISH-IF spot signals were dilated by one pixel for visualization.
Microscope Setup.
Images were taken using an upright, wide-field Olympus BX-63 Microscope equipped with a SuperApochromatic 60×/1.35 N.A. Olympus Objective (UPLSAPO60XO), an X-Cite 120 PC Lamp (EXFO), an ORCA-R2 Digital Interline CCD Camera (C10600-10B; Hamamatsu) mounted using U-CMT and 1X-TVAD Olympus c-Mount Adapters, and zero-pixel shift filter sets: DAPI-5060C-Zero, FITC-5050A-Zero, Cy3-4040C-Zero, and Cy5-4040C-Zero from Semrock. The resulting image pixel size was 107.5 nm, and the z-step size (along the optical axis) used for all optical sectioning acquisition was 200 nm. To position the specimen more accurately along the optical axis (in z) and minimize mechanical vibration, a PZMU-2000 Piezo-Z Top Plate from Applied Scientific Instrumentation was used. A webcam was used to monitor the automated acquisition remotely to avoid turbulence and temperature fluctuations in the microscope environment. To improve optical stability, we used a vibration isolation table (TMC) and ensured that airflow did not affect the microscope stand. The environmental control system maintained constant temperature (20 °C ± 1 °C) and low humidity (35 ± 5% relative humidity) during a given experimental day. Metamorph software (Molecular Devices) was used for controlling microscope automation and image acquisition.
Super Registration.
We compensated for the objective’s chromatic aberration across the entire FOV using a map that described the optical distortion as a function of position by observing subdiffraction limit-sized fluorescent beads that have broad emission spectra (TetraSpeck Fluorescent Microspheres, 100-nm diameter; Life Technologies). Multiple fields of beads (n = 760 beads) were imaged in three dimensions sequentially in Cy5 and Cy3 channels. Then, centroids of the PSF of the beads were localized with subpixel precision in each channel (Materials and Methods, Single-Molecule Localization). The Cy5 channel centroid positions in x and y were compared with the Cy3 channel centroid positions in x and y, and the displacement vectors between the centroid positions of each bead in the two channels were calculated. The displacement vectors were determined in each orthogonal axis independently as a function of the position in the FOV. The objective’s chromatic aberration between Cy5 and Cy3 was compensated for using an affine transformation. A detailed description of the super registration can be found in SI Results.
Bead Preparation.
Beads were diluted with distilled water and uniformly suspended by sonication before they were loaded to a poly–l-lysine–coated coverslip. After the beads settled and dried, Prolong Gold Mounting Media Reagent (Life Technologies) was added and left overnight on a level surface in the dark, and then the coverslip was sealed with nail polish.
Objective Testing.
The optical calibration on six matched objectives acquired from Olympus was tested. All of these 60× objective lenses showed unique variations in their chromatic aberrations. Each objective lens was unique, in that its performance characteristics had its own “fingerprint” for optical distortion across the FOV. The objective that required the least total chromatic correction in our optical path was used for this study (UPLSAPO60XO, 4K020 serial number).
Single-Molecule Localization.
To determine the centroid position of single molecules, we used FISH_QUANT software (20) (free and available online). Briefly, after background subtraction, the software fitted a 3D Gaussian function to the PSF of the single molecule, which yielded centroid coordinates in each channel with subpixel accuracy (<20 nm). Autofluorescent and nonspecific signal were excluded by thresholding the intensity and by the width of the 3D Gaussian curve.
Measurement of Intermolecular Distances and Determining the Significance of Association.
Software was written in MATLAB (MathWorks) to identify centroid pairs using nearest neighbor algorithm (pairing), measure intermolecular distances (in nanometers), and provide significance of association for each pair of molecules between the two channels. The method determined the probability of chance association for each intermolecular pair based on the intermolecular distances observed and the local molecular density within the cell. Details are in SI Results.
Measurement of Association.
The following procedure determined the largest distance that two molecules could be separated and still be considered physically associated. First, the intermolecular distances and significance of association from a positive and negative control were calculated [in this case, MCP-GFP and MBS (MCP-MBS) and MCP-GFP and CaMKII (MCP-CaMKII), respectively (as described above in Materials and Methods, Measurement of Intermolecular Distances and Determining the Significance of Association)]. Second, the molecular pairs that exhibited the most significant probability of chance association (<0.1) and that had a intermolecular distance < 250 nm (diffraction limit) were selected. The cumulative ratio of association for intermolecular distances (in the range between 0 and 250 nm) that were less than or equal to a given observed distance was plotted (for both positive and negative controls separately) (Fig. 2E). The distance wherein the difference was the highest between the detection of association for MCP-MBS (“signal”) and the detection of association for MCP-CaMKII (“noise”) defined the optimal distance (in this case, 69 nm) (Fig. 2E, red arrows). At the optimal distance, the signal to noise ratio is maximized. Thus, we used the distance of 69 nm as the optimal distance in the analysis of RNA–protein interaction unless otherwise noted. Only the molecular pairs with probability of chance association < 0.1 and intermolecular distances less than optimal distance were considered associated and defined the population of pairs included in box A (Fig. 2 F and G). Box B was defined as the population of molecular pairs with probability of chance association < 0.1 but at intermolecular distances in the range from the optimal distance to 250 nm. Finally, the ratio of association between molecules of mRNA and protein was expressed as the ratio of the population of box A to the population of boxes A and B combined. Optimal distance is dependent on both the positive and negative controls analyzed.
The interacting labeled molecules included in box A showed intensities that were representative of the total molecular population analyzed (Fig. S2 G and H). This result indicates that this imaging is able to identify bona fide mRNA–protein associations based on the spatial position of their fluorophores, independent of their intensities.
Imaging Analysis Software.
All image analysis was performed with existing software packages and custom algorithm programs written in MATLAB (MathWorks). The code provides (i) chromatic aberration and mechanical shift corrections (super registration), (ii) identification of centroid pairs (pairing) and measurement of intermolecular distances (in nanometers), (iii) evaluation of the probability of chance association, and (iv) ratio of association as described in this work. The software is able to read FISH_QUANT (20)-detected spot files (version 3D_v1) and import all of the centroid positions in x and y along with the corresponding ROI chosen. It can import as many ROIs as the image has at once. The code (version 1.0) is available online through our website (open access for anyone to use without restriction).
PP7-Based RNA Affinity Purification (Pulldown).
Amylose magnetic resin (NEB) was washed twice and incubated with recombinant purified protein Maltose Binding Protein (MBP)-PP7 and preheated PP7–β-actin 3′-UTR RNA (ratio 1:1) in binding buffer [20 mM Tris, pH 7.2, 200 mM NaCl, 1 mM EDTA, pH 8.0, 1 mM DTT, 0.01 mg/mL tRNA, 0.01% IGEPAL CA-630 (Sigma-Aldrich)] for 1 h at 4 °C with constant rotation. The pulldown was then performed by adding cell extract aliquots (5–30 mg total protein) supplemented with 100 mM NaCl and 0.01 mg/mL tRNA to the RNA immobilized to the beads through the MBP-PP7 protein followed by incubation at 4 °C for 2 h with constant rotation. Total protein aliquots used in pulldown procedures varied and are listed in figures. We use 1.5-mL nonstick microcentrifuge tubes when working with small volumes or 15-mL sterile polypropylene centrifuge tubes with larger volumes. After pulldown, the magnetic beads were washed five times (1-mL volume washes) with ice-cold wash buffer (20 mM Tris, pH 7.2, 200 mM NaCl, 1 mM EDTA, pH 8.0, 1 mM DTT, 0.01% IGEPAL CA-630) and transferred to a new tube in the last wash step. For RNP complex elution from the beads, Tobacco Etch virus (TEV) protease was added to the beads followed by 3 h of incubation at 4 °C with rotation. Alternatively, 500 μL 0.5 M NH4OH supplemented with 0.5 mM EDTA, pH 8.0, was added to the beads followed by a 20-min incubation at room temperature with rotation. After beads were removed, eluate fractions were lyophilized in the Eppendorf Vacufuge speed vac for at least 4 h at room temperature. For protein analysis using SDS/PAGE, the eluates were incubated with appropriate volume of 4× protein sample buffer (Invitrogen) supplemented with 50 mM DTT and heated at 70 °C for 10 min. Construction of the PP7-tagged β-actin 3′-UTR RNA, PP7-MBP recombinant protein purification, and cell extract preparation can be found in SI Materials and Methods.
Additional information includes SI Materials and Methods, SI Results, Datasets S1 and S2, Figs. S1–S7, and Table S1.
SI Materials and Methods
Mouse Embryonic Fibroblast Cell Culture and Cell Lysis Procedure.
Mouse embryonic fibroblasts (MEFs) were isolated from E14 embryos, immortalized with SV40 large T antigen as previously described in ref. 18, and maintained in 10-cm culture dishes with DMEM (Invitrogen) containing 10% heat-inactivated FBS (Sigma-Aldrich) and 1% penicillin and streptomycin (Invitrogen) at 37 °C and 5% CO2.
For pulldown experiments, 300 million cells were grown in 15-cm dish (∼50 dishes per condition). Healthy and not density-arrested cell cultures (70–80% confluence) were rinsed twice with ice-cold 1× PBS, collected in 2 mL ice-cold 1× PBS containing 1 mM PMSF per dish using a cell scraper, transferred into ice-cold 15-mL sterile polypropylene centrifuge tubes, and centrifuged at 300 × g for 10 min at 4 °C. Then, cell pellet was washed once with 10 mL ice-cold 1× PBS containing 1 mM PMSF, flash-frozen in liquid nitrogen, and stored at −80 °C until cell lysis.
For cell lysis, cell pellets were thawed on the addition of 3 vol packed cell volume ice-cold complete lysis buffer [50 mM Tris⋅HCl, pH 7.4, 100 mM NaCl, 1 mM MgCl2, 0.1 mM CaCl2, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS supplemented with 1mM PMSF, 1mM DTT, Protease Inhibitor mixture (Roche), 100 U/mL RNaseOUT (Invitrogen)], incubated for 10 min on ice (swelling), and frozen/thawed twice in liquid nitrogen. Cell debris was pelleted by centrifugation at maximum speed for 10 min at 4 °C, and the supernatant was removed and transferred to a new ice-cold tube. Total protein concentration was determined by using Coomassie Plus (Bradford) Assay Reagent (Thermo Scientific).
Construction of the PP7-Tagged β-Actin 3′-UTR RNA.
A fragment containing the last 60 nt of the ORF and the first 90 nt of the 3′-UTR of β-actin mRNA was amplified by PCR from the pcDNA3-b-actin-3′-UTR plasmid by using the following primers: T7_actbFwd: 5′-CTAATACGACTCACTATAGGGGCAAGCAGGAGTACGATGAGTCC-3′; actb_3UTR_pp7_1Rev(actbpp7R): 5′-taGGAGCGACGCCATATCGTCTGCTCCtataGCCATGCCAATGTTGTCTC-3′; and T7_actb_middleFwd: 5′-CTAATACGACTCACTATAGGGCGGTGAAGGCGACAGCAGTTGG-3′. Control RNA was prepared from pLacZ plasmid by using the following primers: T7_LacZFwd: 5′-CTAATACGACTCACTATAGGGCAGCCCTTCCCGGCTGTGCCG-3′ and LacZpp7Rev: 5′-taGGAGCGACGCCATATCGTCTGCTCCtataATCAGCGACTGATCCACCCAGTCC-3′.
T7 promoter (bold) and PP7 stem loop (underlined) sequence were added into the forward and reverse primers, respectively. The PCR product obtained was then in vitro-transcribed by using the MEGAshortscript T7 Transcription Kit (Ambion) following the manufacturer’s instructions.
PP7-MBP Recombinant Protein Purification.
PP7 coat protein was cloned by PCR into a derivative of pMalc vector (New England BioLabs) that contains a TEV protease site after the MBP. A C-terminal 6xHis tag was added by PCR to ensure purification of the intact fusion protein as was described previously in ref. 14. The vector was transformed into Escherichia coli strain Rosetta2 (EMD Biosciences), and recombinant protein was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside for 4 h at 37 °C. Cell pellets were resuspended in lysis buffer (50 mM Tris, pH 7.5, 1.5 M NaCl, 1 mM EDTA, 1 mM DTT) supplemented with one Complete EDTA-Free Protease Inhibitor tablet (Roche) and lysed by sonication. Cell debris was removed by centrifugation, and the soluble fusion protein was purified by amylose affinity chromatography (New England BioLabs) followed by either TALON affinity (Clontech) or anion exchange (GE Healthcare) chromatography.
Staining of Gels, MS, and Western Blot Analysis.
After SDS/PAGE, protein gels were stained by either silver staining (SilverQuest TM Staining Kit; Invitrogen) or a fast and sensitive Coomassie dye (GelCode Blue Safe Protein Stain; Thermo Scientific). Gel lanes were excised in slices and analyzed by LC-MS/MS at the Proteomic Resource Center at The Rockefeller University. In parallel, candidate proteins were identified by Western blot analysis; 10 μL eluates were separated in SDS/PAGE and transferred to nitrocellulose membranes (Life Technologies). After blocking in 1% milk in 1× PBS-Tween, membranes were incubated with primary antibody in blocking solution before they were washed and incubated with IR-labeled secondary antibodies for 40 min at room temperature. Signal was detected by using Odyssey Infrared Imaging System (LI-COR Biosciences).
Gene Ontology Analysis.
IPA Knowledge Base 9 (Ingenuity Systems; www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/) was used to investigate the functional relationship among the proteins identified by the RNA affinity purification procedure. The enrichment of gene ontology (GO) terms of selected genes to molecular and cellular function categories was determined. The P value, based on a right-tailed Fisher’s exact test, considered the number of identified genes and the total number of molecules known to be associated with these categories in the IPA Knowledge Base. Only statistically significantly enriched GO terms with P value less than 0.05 were considered.
RIP.
Cells were scraped, rinsed with ice-cold 1× PBS, and lysed in ice-cold 10 mM Hepes-KOH, pH 7.0, 100 mM KCl, 5 mM MgCl2, 0.5% Nonidet P-40 supplemented by 1 mM PMSF, 1 mM DTT, Protease Inhibitor Mixture (Roche), and 100 U/mL RNAseOUT (Invitrogen). Cell lysates were mixed with 50 μL Dynabeads-Protein A (Invitrogen) and precleared for 1 h at 4 °C (to reduce background). In parallel, prewashed Dynabeads-Protein A (50 μL per reaction tube) resuspended in 50 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, and 0.05% Nonidet P-40 (NT2 buffer) supplemented with 1 mM PMSF and 100 U/mL RNAseOUT (Invitrogen) were incubated with either anti-Ig (nonspecific control) or specific antibodies with gentle rotation for 1.5 h at 4 °C. Subsequently, magnetic beads were washed five times with NT2 buffer (1 mL) and incubated with precleared cell lysate supplemented with 200 U RNAseOUT, 1 mM DTT, and 20 mM EDTA, pH 8.0, in NT2 buffer for 3 h at 4 °C tumbling end over end. Magnetic beads were then washed five times with ice-cold NT2 buffer and resuspended in 100 μL NT2 buffer supplemented with 0.1% SDS and 30 μg Proteinase K (Invitrogen) for 30 min at 55 °C, flicking the tube occasionally. RNA was then extracted by adding phenol:chloroform:isoamyalcohol (25:24:1; Sigma-Aldrich) and precipitated overnight at −20 °C with 2-propanol supplemented with 300 mM sodium acetate, pH 5.2 and 1 μL glycogen (Roche) as a carrier. After centrifugation at 20,000 × g for 20 min at 4 °C, RNA pellet was air-dried, resuspended in RNase-free water, and subsequently treated with DNase-TURBO following the manufacturer’s specifications (Ambion). The amount of RNA was then quantified using NanoDrop (Thermo Fisher Scientific), and cDNAs were synthesized using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Equal amounts of cDNA were subjected to semiquantitative PCR using Platinum Taq Polymerase (Invitrogen) using the following specific pair of primers to detect β-actin and GAPDH mRNA as was described in ref. 18: Actb_MBS(2009-29)Fwd: 5′-GATCTGCGCGCGATCGATATCAGCGC-3′; Actb_MBS(2009-30)Rev: 5′-GCCAGCCCTGGCTGCCTCAACACCTC-3′; GAPDH(2009-15)Fwd: 5′-GAGCGAGACCCCACTAACATCAAATG-3′; and GAPDH(2009-16)Rev: 5′-CAGGATGCATTGCTGACAATCTTGAG-3′.
Plasmids and Lentivirus Generation.
Coding sequences for tandem dimer MCP-GFP (tdMCP-mEos2-GFP) and GFP-ZBP1 were cloned into the lentivirus expression vector. Lentivirus particles were produced as follows: plasmids for ENV (pMD2.VSVG), packaging (pMDLg/pRRE), REV (pRSV-Rev), and the expression vector (gift from Antonia Follenzi, Albert Einstein College of Medicine) were mixed and transfected into HEK 293T cells using Lipofectamine 2000 Reagent (Invitrogen) as per the manufacturer’s instructions. Expression of the insert was under the control of the UbC promoter. The virus-containing supernatant was harvested and concentrated using Lenti-X Concentrator (Clontech) as per the manufacturer’s instructions. The viral particles were resupended in Neurobasal A and stored at −80 °C for subsequent infection of neurons in culture. DNA constructs used in this work are available at Addgene.
RNA FISH Probes.
MBS probes (Invitrogen) were used to detect MBS cassette present in β-actin mRNA 3′-UTR in MBS cells as was described in ref. 18. Each probe was labeled at both ends with Cy3 fluorescent dye (GE Healthcare). β-Actin ORF probes (Invitrogen) were used to detect β-actin mRNA as was described in ref. 18. Each probe was labeled at both ends with Cy3 Fluorescent Dye (GE Healthcare). CaMKII probes (Stellaris RNA FISH Probes; Biosearch Technologies) were used to detect CaMKII mRNA. Each probe was labeled at the 5′ end with Quasar570 Fluorescent Dye. Spino probes (Stellaris RNA FISH Probes; Biosearch Technologies) were used to detect spinophilin mRNA. Each probe was labeled at the 5′ end with Quasar570 Fluorescent Dye. FISH probes sequences are available in Dataset S2.
Antibodies.
For Western blot, polyclonal rabbit anti-zbp1 (gift from Stefan Hüttelmaier, Martin Luther University, Medical Faculty, Institute of Molecular Medicine, Centre for Applied Medical and Human Biology Research, Halle, Germany), rabbit polyclonal anti-hnRNPA/B [M-36; Santa Cruz (sc-98810)], rabbit polyclonal anti-YB1 [Abcam (ab12148)], rabbit monoclonal anti-KHDRBS1/SAM68 [Lifespan Biosciences (EPR3232)], rabbit polyclonal anti-Sam68 [C-20; Santa Cruz (sc-333); gift from Mat Klein, Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine], rabbit polyclonal anti-RNA Helicase A [Dhx9; Abcam (ab26271)], rabbit polyclonal anti-FMRP [Abcam (ab17722)], mouse monoclonal anti–tubulin-alpha [DMA1; Sigma-Aldrich (T6199)], mouse monoclonal anti-MBP [New England BioLabs (E8032S)], mouse monoclonal anti–β-actin clone AC15 [Sigma-Aldrich (A1978)], rabbit polyclonal anti-IGFBP2/IMP2 [MBL (RN008P)], rabbit polyclonal anti-MRCL3/Myosin Regulatory Light Chain 2/MYL9 [FL-172; Santa Cruz (sc-15370)], and anti-rabbit and anti-mouse IgG (H&L; goat) antibodies IRDye680- and IRDye800-conjugated (Rockland) were used. For IF, antibodies used were chicken anti-GFP [1:5,000; Aves Labs, Inc. (GFP-1010)], rabbit polyclonal anti-MAP2 [Millipore (AB5622); dilution 1/2,500], mouse monoclonal anti-MAP2 [Sigma-Aldrich (M4403); dilution 1/1,500], rabbit anti-hnRNPA/B [M-36; Santa Cruz (sc-98810)], anti-YBOX1 [Abcam (ab12148)], anti-sam68 [Lifespan Biosciences (EPR3232)], rabbit polyclonal anti-Sam68 [C-20; Santa Cruz (sc-333)], anti-Dhx9 [Abcam (ab26271)], anti-FMRP [Abcam (ab17722)], mouse monoclonal anti-hnRNPU [Sigma-Aldrich (R6278); gift from Stefan Hüttelmaier], mouse monoclonal anti-hnRNPE2 [PCBP2; Abnova (H00005094); gift from Stefan Hüttelmaier], mouse monoclonal IGF2BP1/IMP1 [MBL (RN001M)], and polyclonal rabbit anti-zbp1 (gift from Stefan Hüttelmaier). Alexa Fluor-labeled anti-chicken, -mouse, and -rabbit secondary antibodies were used (Life Technologies; dilution 1/1,000).
Supplementary Material
Acknowledgments
We thank Jeff A. Chao for the MBP-PP7 recombinant protein and 3′-UTR β-actin_PP7 construct; Xiuhua Meng for GFP-ZBP1 cloning; Jeetayu Biswas for the molecular model in PyMOL; Young Yoon for hippocampal neuron cultures and discussion; Bin Wu for tdMCP-GFP lentivirus and discussion; Chiso Nwokafor and Melissa Lopez-Jones for mice; Aviv Bergman for mathematical modeling; Stefan Hüttelmaier for anti-zbp1, anti-hnRNPU, and anti-hnRNPE2 antibodies; Maria Vera and members of the R.H.S. laboratory for critical reading of the manuscript; Joe Fernandez and Milica Tesic Mark for proteomics (Rockefeller University); and Andrew Gerson (Olympus) and Thomas Geer (BioVision) for help with instrumentation. This work was supported by a Human Frontier Science Program Fellowship (to C.E.), NIH Grant NS083085 (to R.H.S.), and the Integrated Imaging Program of the Gruss Lipper Biophotonics Center.
Footnotes
Conflict of interest statement: The material in this manuscript is the subject of a provisional application to the US Patent and Trademark Office. It has not been licensed to any corporation, and the authors are the sole inventors.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621440114/-/DCSupplemental.
References
- 1.Eliscovich C, Buxbaum AR, Katz ZB, Singer RH. mRNA on the move: The road to its biological destiny. J Biol Chem. 2013;288(28):20361–20368. doi: 10.1074/jbc.R113.452094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582(14):1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao DI, Aldridge GM, Weiler IJ, Greenough WT. Altered mRNA transport, docking, and protein translation in neurons lacking fragile X mental retardation protein. Proc Natl Acad Sci USA. 2010;107(35):15601–15606. doi: 10.1073/pnas.1010564107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: Emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19(R1):R46–R64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu-Yesucevitz L, et al. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31(45):16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukong KE, Chang KW, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends Genet. 2008;24(8):416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Nussbacher JK, Batra R, Lagier-Tourenne C, Yeo GW. RNA-binding proteins in neurodegeneration: Seq and you shall receive. Trends Neurosci. 2015;38(4):226–236. doi: 10.1016/j.tins.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paronetto MP, Achsel T, Massiello A, Chalfant CE, Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176(7):929–939. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook KB, Hughes TR, Morris QD. High-throughput characterization of protein-RNA interactions. Brief Funct Genomics. 2015;14(1):74–89. doi: 10.1093/bfgp/elu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oeffinger M. Two steps forward--one step back: Advances in affinity purification mass spectrometry of macromolecular complexes. Proteomics. 2012;12(10):1591–1608. doi: 10.1002/pmic.201100509. [DOI] [PubMed] [Google Scholar]
- 12.Mili S, Steitz JA. Evidence for reassociation of RNA-binding proteins after cell lysis: Implications for the interpretation of immunoprecipitation analyses. RNA. 2004;10(11):1692–1694. doi: 10.1261/rna.7151404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley KJ, Steitz JA. The “Observer Effect” in genome-wide surveys of protein-RNA interactions. Mol Cell. 2013;49(4):601–604. doi: 10.1016/j.molcel.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao JA, et al. ZBP1 recognition of beta-actin zipcode induces RNA looping. Genes Dev. 2010;24(2):148–158. doi: 10.1101/gad.1862910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hüttelmaier S, et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438(7067):512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 16.Patel VL, et al. Spatial arrangement of an RNA zipcode identifies mRNAs under post-transcriptional control. Genes Dev. 2012;26(1):43–53. doi: 10.1101/gad.177428.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH. Characterization of a beta-actin mRNA zipcode-binding protein. Mol Cell Biol. 1997;17(4):2158–2165. doi: 10.1128/mcb.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lionnet T, et al. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat Methods. 2011;8(2):165–170. doi: 10.1038/nmeth.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand E, et al. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2(4):437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- 20.Mueller F, et al. FISH-quant: Automatic counting of transcripts in 3D FISH images. Nat Methods. 2013;10(4):277–278. doi: 10.1038/nmeth.2406. [DOI] [PubMed] [Google Scholar]
- 21.Wu B, Chao JA, Singer RH. Fluorescence fluctuation spectroscopy enables quantitative imaging of single mRNAs in living cells. Biophys J. 2012;102(12):2936–2944. doi: 10.1016/j.bpj.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao JA, Patskovsky Y, Almo SC, Singer RH. Structural basis for the coevolution of a viral RNA-protein complex. Nat Struct Mol Biol. 2008;15(1):103–105. doi: 10.1038/nsmb1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinnamon JR, Waddell CB, Nik S, Chen EI, Czaplinski K. Hnrpab regulates neural development and neuron cell survival after glutamate stimulation. RNA. 2012;18(4):704–719. doi: 10.1261/rna.030742.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein ME, Younts TJ, Castillo PE, Jordan BA. RNA-binding protein Sam68 controls synapse number and local β-actin mRNA metabolism in dendrites. Proc Natl Acad Sci USA. 2013;110(8):3125–3130. doi: 10.1073/pnas.1209811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassell GJ, Warren ST. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagache T, Sauvonnet N, Danglot L, Olivo-Marin JC. Statistical analysis of molecule colocalization in bioimaging. Cytometry A. 2015;87(6):568–579. doi: 10.1002/cyto.a.22629. [DOI] [PubMed] [Google Scholar]
- 27.Ule J, et al. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302(5648):1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 28.Hafner M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141(1):129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ule J, Jensen K, Mele A, Darnell RB. CLIP: A method for identifying protein-RNA interaction sites in living cells. Methods. 2005;37(4):376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Licatalosi DD, et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456(7221):464–469. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Darnell RB. Mapping in vivo protein-RNA interactions at singlenucleotide resolution from HITS-CLIP data. Nat Biotechnol. 2011;29(7):607–614. doi: 10.1038/nbt.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.König J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol. 2010;17(7):909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedersdorf MB, Keene JD. Advancing the functional utility of PAR-CLIP by quantifying background binding to mRNAs and lncRNAs. Genome Biol. 2014;15(1):R2. doi: 10.1186/gb-2014-15-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castello A, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149(6):1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Jønson L, et al. Molecular composition of IMP1 ribonucleoprotein granules. Mol Cell Proteomics. 2007;6(5):798–811. doi: 10.1074/mcp.M600346-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Weidensdorfer D, et al. Control of c-myc mRNA stability by IGF2BP1-associated cytoplasmic RNPs. RNA. 2009;15(1):104–115. doi: 10.1261/rna.1175909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Q, Taylor SJ, Shalloway D. Specificity and determinants of Sam68 RNA binding. Implications for the biological function of K homology domains. J Biol Chem. 1997;272(43):27274–27280. doi: 10.1074/jbc.272.43.27274. [DOI] [PubMed] [Google Scholar]
- 38.Itoh M, Haga I, Li QH, Fujisawa J. Identification of cellular mRNA targets for RNA-binding protein Sam68. Nucleic Acids Res. 2002;30(24):5452–5464. doi: 10.1093/nar/gkf673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snee M, Kidd GJ, Munro TP, Smith R. RNA trafficking and stabilization elements associate with multiple brain proteins. J Cell Sci. 2002;115(Pt 23):4661–4669. doi: 10.1242/jcs.00137. [DOI] [PubMed] [Google Scholar]
- 40.Raju CS, et al. In neurons, activity-dependent association of dendritically transported mRNA transcripts with the transacting factor CBF-A is mediated by A2RE/RTS elements. Mol Biol Cell. 2011;22(11):1864–1877. doi: 10.1091/mbc.E10-11-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raju CS, et al. In cultured oligodendrocytes the A/B-type hnRNP CBF-A accompanies MBP mRNA bound to mRNA trafficking sequences. Mol Biol Cell. 2008;19(7):3008–3019. doi: 10.1091/mbc.E07-10-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda N, et al. The transacting factor CBF-A/Hnrnpab binds to the A2RE/RTS element of protamine 2 mRNA and contributes to its translational regulation during mouse spermatogenesis. PLoS Genet. 2013;9(10):e1003858. doi: 10.1371/journal.pgen.1003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu B, Buxbaum AR, Katz ZB, Yoon YJ, Singer RH. Quantifying protein-mRNA interactions in single live cells. Cell. 2015;162(1):211–220. doi: 10.1016/j.cell.2015.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu D, Jaroszewski L, Li Z, Godzik A. AIDA: Ab initio domain assembly server. Nucleic Acids Res. 2014;42(Web Server issue):W308–W313. doi: 10.1093/nar/gku369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.