Significance
Multiple sclerosis (MS), an autoimmune disease that affects the central nervous system, is driven by activated T lymphocytes that invade the brain and spinal cord, leading to damage to the myelin sheaths that surround nerve axons. Current treatments are only partly successful, especially for the later stages of chronic forms of MS. Experimental autoimmune encephalomyelitis (EAE) is a useful animal model of MS, suitable for testing new approaches to treatment. By genetically altering mice to eliminate a T lymphocyte surface protein known as CD6, we show that the CD6 molecule is essential for the development of EAE. Furthermore, an anti-CD6 monoclonal antibody is effective in treating EAE in mice that express human CD6 but not mouse CD6 on their T lymphocytes.
Keywords: CD6, multiple sclerosis, EAE
Abstract
CD6 was established as a marker of T cells more than three decades ago, and recent studies have identified CD6 as a risk gene for multiple sclerosis (MS), a disease in which autoreactive T cells are integrally involved. Nevertheless, the precise role of CD6 in regulating T-cell responses is controversial and its significance in the pathogenesis of various diseases remains elusive, partly due to the lack of animals engineered to alter expression of the CD6 gene. In this report, we found that CD6 KO mice showed decreased pathogenic T-cell responses, reduced spinal cord T-cell infiltration, and attenuated disease severity in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. CD6-deficient T cells exhibited augmented activation, but also significantly reduced survival and proliferation after activation, leading to overall decreased Th1 and Th17 polarization. Activated CD6-deficient T cells also showed impaired infiltration through brain microvascular endothelial cell monolayers. Furthermore, by developing CD6 humanized mice, we identified a mouse anti-human CD6 monoclonal antibody that is highly effective in treating established EAE without depleting T cells. These results suggest that (i) CD6 is a negative regulator of T-cell activation, (ii) at the same time, CD6 is a positive regulator of activated T-cell survival/proliferation and infiltration; and (iii) CD6 is a potential new target for treating MS and potentially other T-cell–driven autoimmune conditions.
CD6 is a type I membrane protein and a member of the scavenger receptor cysteine-rich (SRCR) protein family (1). Recent genetic studies in multiple sclerosis (MS) patients from several groups have consistently linked certain CD6 gene polymorphisms to MS (2–5), identifying CD6 as a risk gene for this disease. CD6 was first cloned more than three decades ago (6) and was found to be primarily expressed on T cells (1). Human CD6 gene and amino acid sequences are highly homologous to mouse CD6, and CD6 from either human or mouse both bind the same ligand without species restrictions (7). The specific expression of CD6 on T cells and its highly conserved sequence among species strongly suggest that CD6 could be an important regulatory molecule for T cells.
Although CD6 was discovered more than 30 y ago, its function remains unclear. Previous in vitro studies using various CD6-specific monoclonal antibodies (mAbs) yielded contradictory results, suggesting that CD6 can either stimulate or suppress T-cell activation (8, 9). CD6 was also speculated to be a potential target for treating autoimmune diseases (10). However, the impetus for using CD6-targeted reagents in treating human diseases had faltered due in part to the lack of in vivo data to validate in vitro and ex vivo studies, and, to the absence of CD6 gene engineered animals to facilitate testing of CD6-targeted reagents in vivo, further hindering meaningful assessment of the conflicting implications of the in vitro studies (10). Despite the lack of clear understanding of CD6 function and the absence of CD6-related clinical trials in the United States and Europe, Itolizumab, an anti-CD6 mAb developed in Cuba, has recently been approved to treat psoriasis in India (11). So far, there is only one report of in vivo studies of CD6 using genetically engineered animals (12), and the precise role of CD6 in regulating T-cell responses needs to be clarified.
In this report, we developed CD6 KO mice, studied their pathogenic T-cell responses and disease severity in EAE (experimental autoimmune encephalomyelitis), an animal model of MS, and investigated the potential mechanisms by which CD6 regulates the development of EAE. We also developed CD6 humanized mice to evaluate human CD6-targeted reagents for future therapeutic development and identified a mAb against human CD6 that is highly effective in treating EAE.
Results
Development and Characterization of CD6 KO Mice.
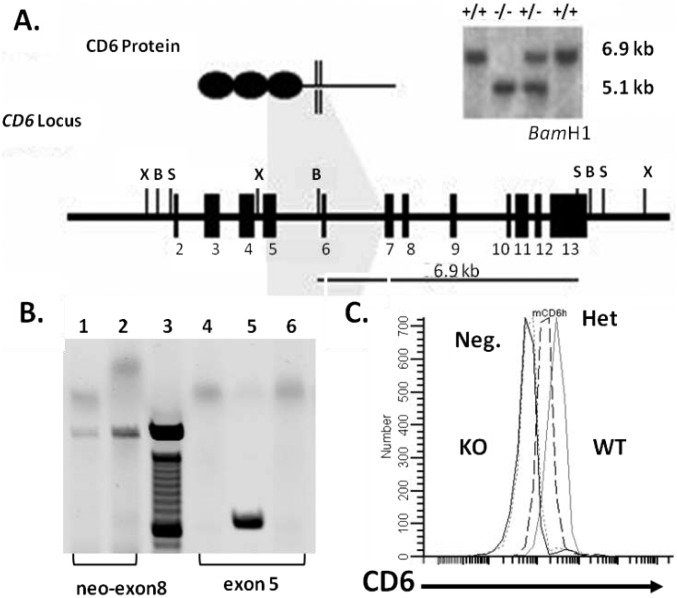
CD6 KO mice were developed by manipulating mouse embryonic stem (ES) cells using a homologous recombination strategy and blastocyst injection of the identified target ES cells. The CD6 KO mice were bred onto the DBA/1 background by 12 generations of backcrossing. PCR and Southern blot assays verified the deletion of the targeted loci (Fig. S1 A and B), and flow cytometry analysis of CD6 protein on lymphocytes confirmed the deficiency of CD6 in the CD6 KO mice (Fig. S1C).
Fig. S1.
Development of the CD6 KO mice. (A) Exons 5–7 of the CD6-coding region were replaced by a neomycin gene cassette after homologous recombination. Southern blot confirmed the recombination event. (B) CD6 KO mice genotyping. All mCD6 KO mice were genotyped with a dual PCR. The neo insert can be identified by a PCR with a neo primer (forward-5′-CTT GGG TGG AGA GGC TAT TC-3′) and an exon 8 primer (reverse-5′-AGC CAA CCT TTC TTC TGA GAG CCA-3′). Mice heterozygous for the neo insert also contains mouse CD6 exon 5 (forward-5′-TGG GCC CAA AGC ATT TAG CTT GAC-3′) and (reverse-5′-TAC AGA GAG CTT GGC AGT GCT TGA-3′). A mouse containing neo between exons 5–7 (lane 1) but also having exon 5 (lane 5) is a heterozygous KO. In contrast, a homozygous KO has neo (lane 2) but does not contain exon 5 (lane 6). Lane 3 is the 100-bp ladder, and lane 4 is a PCR negative control. (C) The absence of CD6 protein on CD6 KO mouse lymphocytes. Lymphocytes from WT, heterozygous (Het) KO, and homozygous (homo) KO mice were analyzed for CD6 expression by flow cytometry, showing reduced levels of CD6 in het KO mice (middle dotted line), and absence of CD6 in homo KO mice (left solid line). Neg, negative control.
CD6 KO Mice Are Resistant to EAE Induction.
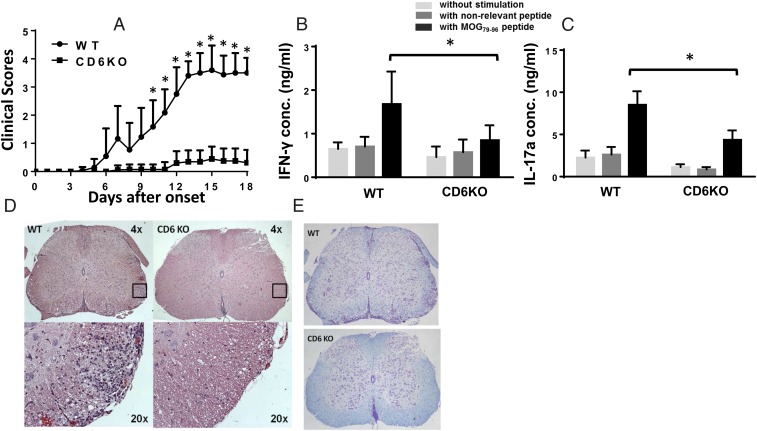
Although CD6 gene polymorphisms have been associated with MS (2–5), the precise role of CD6 in the pathogenesis of MS is unclear. To address this issue, we immunized both WT and CD6 KO mice with MOG79–98 (myelin oligodendrocyte glycoprotein) peptide in Complete Freund's Adjuvant (CFA) to induce EAE, and found that CD6 KO mice developed significantly less severe clinical signs of EAE compared with WT mice (Fig. 1A). In parallel, recall assays showed markedly decreased antigen-specific Th1 (Fig. 1B) and Th17 (Fig. 1C) responses, and spinal cord histopathology showed reduced T-cell infiltration (Fig. 1D) and diminished demyelination (Fig. 1E) in the CD6 KO mice with EAE. These results demonstrate that CD6 is required for the development of EAE, potentially by regulating pathogenic T-cell responses and/or T-cell infiltration into the central nervous system (CNS).
Fig. 1.
CD6 KO mice are protected from EAE. (A) Clinical scores of the WT and CD6 KO mice. Combined results from four experiments. n = 15 in each group. *P < 0.01. (B) MOG-specific Th1 and Th17 recall assays. Splenocytes from WT and CD6 KO mice 21 d after immunization were incubated without peptide (white bars), with 10 µg/mL of a nonrelevant peptide (IRBP1–20, gray bars) or with 10 µg/mL MOG79–96 peptide (black bars) for 72 h. IFN-γ and IL-17a levels in the culture supernatants were measured by ELISA (n = 14 in each group, *P < 0.05). (C) Spinal cord in CD6 KO mice had markedly reduced leukocyte infiltration as assessed by H&E staining. (D) Representative images of spinal cord sections from the EAE mice after H&E staining, showing that CD6 KO mice have significantly reduced cell infiltration. (Magnification: Upper, 4x; Insets shown at Lower, 20×.) (E) Representative images of spinal cord sections from the EAE mice after Luxol Blue staining, showing that CD6 KO mice had intact myelin sheath (blue staining) compared with severe demyelination in the WT mice. (Magnification: 4×.)
Lack of CD6 on T Cells Reduces Both Th1 and Th17 Differentiation in Vitro.
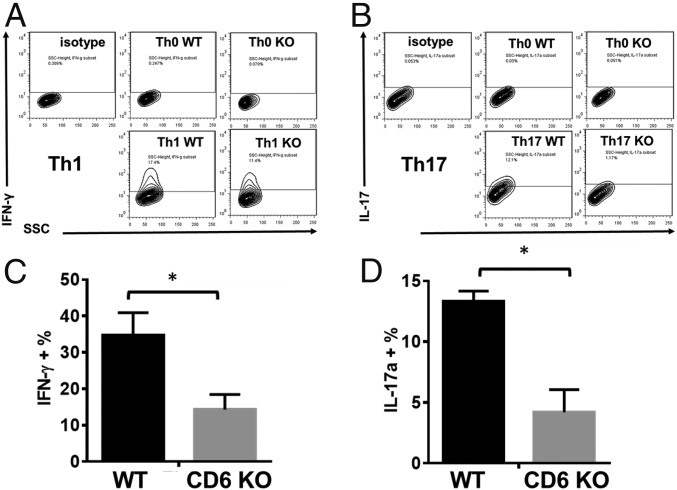
To elucidate the mechanism by which lack of CD6 reduces pathogenic Th1/Th17 responses and ameliorates disease severity in EAE, we isolated CD4+ T cells from naïve WT and CD6 KO mice, then cultured them under Th1 or Th17 polarization conditions, followed by flow cytometric analysis of intracellular IFN-γ (Th1) or IL-17 (Th17). In these experiments, CD6 KO CD4+ T cells had impaired Th1 and Th17 development compared with WT CD4+ T cells (Fig. 2).
Fig. 2.
Absence of CD6 leads to impaired Th1 and Th17 development. Purified CD4+ T cells from naïve WT and CD6 KO mice were activated and cultured under Th1 or Th17 polarization conditions for 5 d. Then the development of Th1 and Th17 cells was assessed by intracellular staining of IFN-γ or IL-17a. (A and B) Representative results of the intracellular IFN-γ or IL-17a staining within the differentiated T cells; (C and D) combined results from three different experiments. n = 3, data are mean ± SEM, *P < 0.05.
CD6 Deficiency on T Cells Enhances T-Cell Activation.
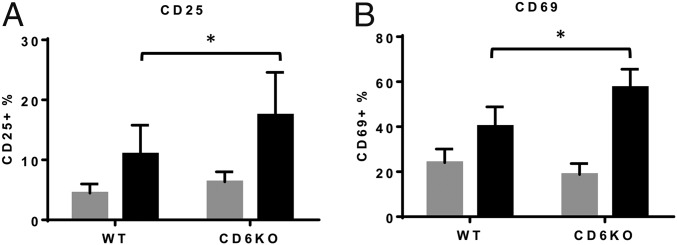
Previous studies using anti-CD6 mAbs to study the role of CD6 in T-cell activation generated conflicting results. Some of the data suggest that CD6 provides costimulatory signals to enhance T-cell activation, and some suggest that CD6 inhibits T-cell activation (8, 9). To clarify the role of CD6 in T-cell activation, we activated WT and CD6 KO T cells by using anti-CD3 and anti-CD28 mAbs for 5 h, then measured up-regulation of T-cell activation markers CD25 and CD69. We found that, compared with WT T cells, CD6 KO T cells showed augmented up-regulation of both CD25 (Fig. 3A) and CD69 (Fig. 3B), suggesting that CD6 is a negative regulator of T-cell activation.
Fig. 3.
Absence of CD6 leads to augmented T-cell activation. Purified CD4+ T cells from naïve WT and CD6 KO mice were activated by incubation with 1 μg/mL anti-CD3 and anti-CD28 mAbs, then the activation of the T cells was assessed 5 h later by measuring the up-regulation of activation markers CD25 (A) and CD69 (B) using flow cytometric analysis. Gray bars, before activation; black bars, 5 h after activation, n = 5, data are mean ± SEM, *P < 0.05.
Lack of CD6 on T Cells Reduces Activated T-Cell Survival and Proliferation.
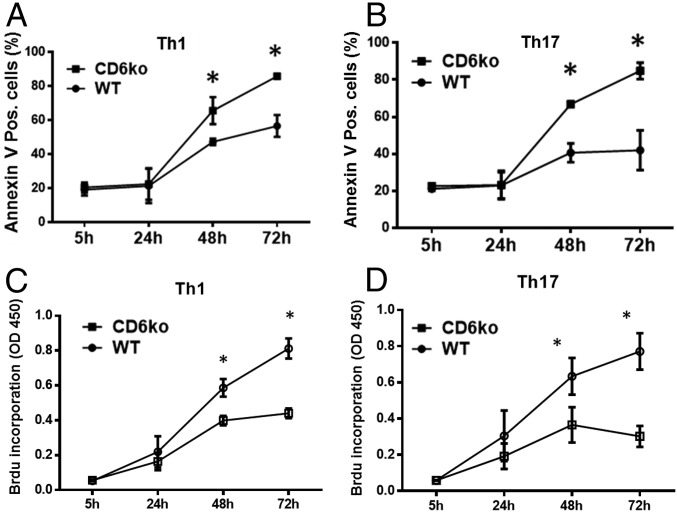
The discovery that CD6 is a negative regulator of T-cell activation appears to conflict with results from the above EAE studies that showed decreased Th1/Th17 responses in CD6 KO mice. To address this paradox, we again activated WT and CD6 KO CD4+ T cells under Th1 or Th17 polarization conditions and compared T-cell apoptosis at 5, 24, 48, and 72 h by annexin V staining. After activation under both Th1 and Th17 polarization conditions, CD6 KO T cells underwent significantly more apoptosis (annexin V+) than WT T cells (Fig. 4 A and B).
Fig. 4.
Absence of CD6 leads to reduced proliferation and enhanced apoptosis of activated T cells. Purified CD4+ T cells from naïve WT and CD6 KO mice were activated by incubation with plate-bound anti-CD3 and anti-CD28 mAbs, then cultured under the Th1 or Th17 polarization conditions. At 5, 24, 48, and 72 h time points, proliferation of the activated cells was assessed by measuring BrdU incorporation using a BrdU ELISA kit (A and B), and apoptosis of the activated T cells was assessed by staining the cells with annexin V followed by flow cytometric analysis (C and D). n = 3 in each group, data are mean ± SEM, *P < 0.05.
In addition to activation and survival, proliferation of activated T cells also governs the outcome of a T-cell response. We therefore measured proliferation of activated WT and CD6 KO T cells under Th1 or Th17 polarization conditions at 5, 24, 48, and 72 h after activation, by a BrdU incorporation assay. In the absence of CD6, activated T cells under both Th1 and Th17 polarization conditions had significantly reduced proliferation (Fig. 4 C and D).
CD6 Deficiency on T Cells Impairs T-Cell Migration Through Brain Microvascular Endothelial Cells.
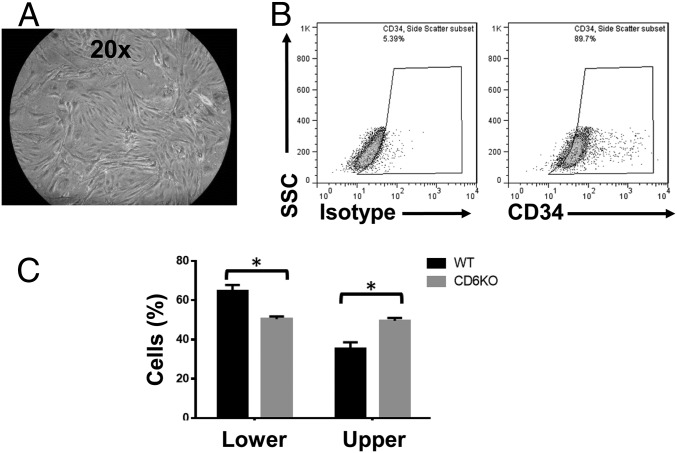
In EAE, activated pathogenic Th1 and/or Th17 cells need to migrate through the blood–brain barrier (BBB) into the CNS to initiate local inflammation, and brain microvascular endothelial cells (BMECs) are an important component of the BBB. To determine whether CD6 has an effect on activated T-cell migration through the BBB, we first isolated BMECs from naïve WT mice following an established protocol (13) (Fig. 5 A and B), then grew these cells into monolayers on culture inserts in transwell culture plates. We added carboxyfluorescein succinimidyl ester (CFSE)-labeled WT or CD6 KO T cells activated with anti-CD3/anti-CD28 mAbs onto the top of the monolayer of BMEC; CCL2 [Chemokine (C-C motif) ligand 2] was added to the bottom of the transwells to induce T-cell migration. After 18 h of incubation, WT T cells migrated better than the CD6 KO T cells through the BMEC monolayer (Fig. 5C), suggesting that CD6 on T cells is required for activated T cells to efficiently migrate through the BBB to initiate inflammation in EAE.
Fig. 5.
Absence of CD6 reduces T-cell migration through BMEC monolayers. WT DBA-1 mouse BMECs were first isolated by following an established protocol (A) and were ∼90% pure based on CD34 staining (B). The isolated BMECs were grown on culture inserts until monolayers were formed, then 0.6 × 106 of CFSE-labeled and anti-CD3/CD28 mAb-activated T cells from naïve WT and CD6 KO mice were added into the culture inserts with 20 ng/mL CCL2 in the bottom chambers to facilitate cell migration. After overnight culture, cells that remained in the culture inserts and those that migrated into the lower chambers were quantitated (C). n = 4 in each group, data are mean ± SEM, *P < 0.05.
Development of CD6 Humanized Mice.
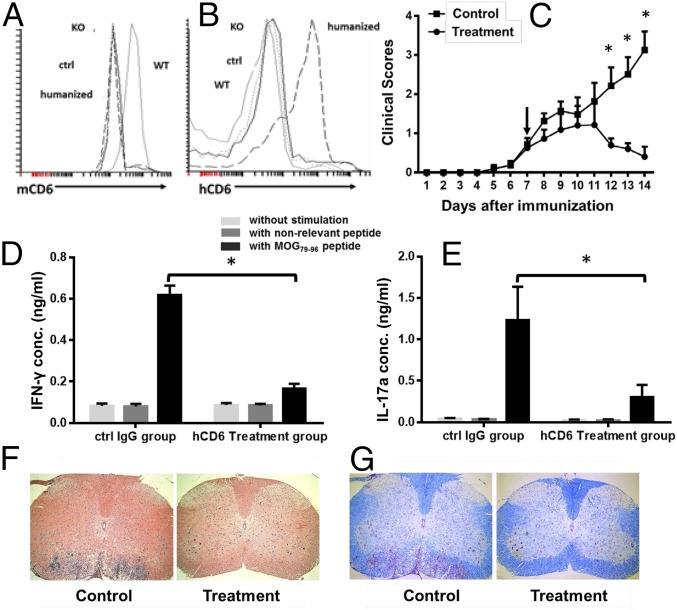
The above data suggest that CD6 could be a valid target for treating EAE. Because (i) almost all existing anti-mouse CD6 mAbs were developed in rats, and rat IgGs are highly immunogenic in mice, (ii) the identification of a disease-modifying anti-human CD6 mAb would have potential translational value, and (iii) almost all of the anti-human CD6 mAbs were developed in mice, it would be valuable to develop a CD6 humanized mouse in which mouse CD6 is replaced by human CD6. In addition, human and murine CD6 proteins are highly homologous, and previous studies demonstrate that CD6 interacts with its ligand without species restrictions (7). We therefore generated CD6 humanized mice in which mouse CD6 on T cells was replaced by human CD6. In brief, we first developed human CD6 transgenic mice (hCD6 Tg) in which the hCD6 expression is driven by a human CD2 promoter/LCR to direct expression of CD6 restricted to T cells. After verifying the expression of human CD6 protein on lymphocytes in the resultant Tg mice, we backcrossed the human CD6 Tg mice onto the DBA-1 background, then bred the human CD6 Tg mice with the mouse CD6 KO mice, and generated CD6 humanized mice that do not express mouse CD6 (Fig. 6A) but instead express human CD6 in vivo (Fig. 6B).
Fig. 6.
Mouse anti-human CD6 mAb (UMCD6) treats EAE in CD6 humanized mice. (A and B) CD6 humanized mice do not express mouse CD6 (A) but express human CD6 (B) on their lymphocytes. (C) EAE clinical scores of CD6 humanized mice treated with UMCD6 and control mouse IgGs. CD6 humanized mice were immunized to induce EAE. After mice showed mild clinical symptoms, they were randomly divided into two groups with one group receiving ∼0.4 mg per mouse anti-human CD6 IgG (UMCD6) (circles) and the other receiving 0.4 mg purified mouse IgGs (squares). Clinical scores were recorded daily. n = 14 in each group, *P < 0.05. (D and E) Th1 (D) and Th17 (E) recall assays in CD6 humanized mice treated with UMCD6 or control IgG. At the end of treatment experiments (day 14), splenocytes from the mice were collected and incubated without peptide (light gray bars), with 10 µg/mL of a nonrelevant peptide (IRBP1–20, dark gray bars) or with 10 µg/mL MOG79–96 peptide (black bars) for 72 h. IFN-γ and IL-17a levels in the culture supernatants were measured by ELISA. n = 7 in each group, *P < 0.05. (F and G) Representative images of spinal cord sections showing significantly reduced cell infiltration (F) and demyelination (G) in the UMCD6-treated mice compared with the controls. (Magnification: 4×.)
A Mouse Anti-Human CD6 mAb Reverses EAE Progression in the CD6 Humanized Mice.
To demonstrate that human CD6 can replace mouse CD6 function in vivo and that these newly developed humanized mice can be used to test CD6-targeted reagents that have potential in treating human diseases, and to evaluate our mouse anti-human CD6 mAb for its potential in treating MS, we induced EAE in the humanized CD6 mice (DBA-1 background). Humanized mice were immunized with MOG79–96 peptide in CFA together with pertussis toxin, per protocol described above, then the development of EAE was assessed by monitoring clinical scores daily. Once mice showed mild signs of EAE clinically, we randomly treated half of the mice with a mouse anti-human CD6 IgG (UMCD6) (∼0.4 mg per mouse) and the other half with the same amount of mouse IgG by i.p. injection, then continued to monitor the mice daily. We also carried out immunological and histopathological assays as described. These experiments showed that, like WT DBA-1 mice, humanized CD6 mice developed severe EAE (Fig. 6C), indicating that transgenic expression of human CD6 can replace the function of mouse CD6 in mice, and supporting our hypothesis that using CD6 humanized mice we can predict the effects of CD6-related reagents in human MS. Compared with severe EAE that developed in humanized CD6 mice treated with control mouse IgG, EAE progression in the treated mice was halted, and these mice showed little clinical evidence of EAE 7 d after treatment (Fig. 6C). Recall assays showed significantly reduced MOG-specific Th1 (Fig. 6D) and Th17 responses (Fig. 6E) in the active treatment group compared with control. Further, histopathological assays showed markedly decreased spinal cord inflammation (Fig. 6F) and reduced demyelination (Fig. 6G) in the treated mice.
UMCD6 Does Not Deplete T Cells in the Treated EAE Mice.
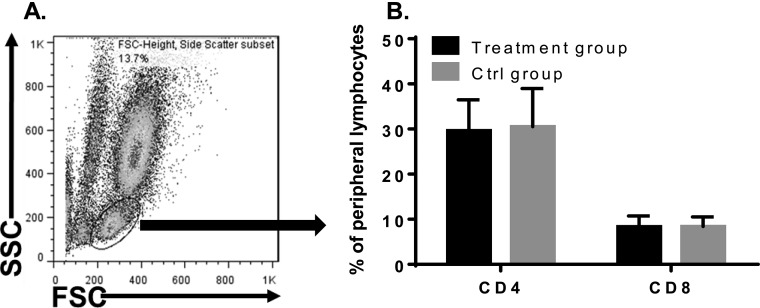
Because CD6 is present on all T cells, one possible mechanism by which the anti-CD6 mAb ameliorates EAE severity could be depletion of T cells. To test this hypothesis, we assessed CD4+ and CD8+ T-cell percentages in the UMCD6-treated and control IgG-treated mice by staining the peripheral leukocytes with anti-CD4, anti-CD8 (Biolegend), and a polyclonal anti-hCD6 IgG (R&D). These experiments found that CD4+, CD8+, and CD6+ T-cell populations did not significantly change between the treated and control groups (Fig. S2), suggesting that UMCD6 mAb attenuated EAE disease severity in the CD6 humanized mice neither by T-cell depletion nor by modulating CD6 on T cells.
Fig. S2.
UMCD6 treatment does not deplete T cells. CD4+ and CD8+ T-cell percentages in the peripheral blood were analyzed in both UMCD6 and control IgG-treated CD6 humanized mice by flow cytometry at the end of the treatment studies, showing no difference between the two groups of mice. n = 14 in each group. (A) FSC/SSC shows the lymphocyte population analyzed. (B) CD4+ and CD8+ T-cell percentages of mice treated with UMCD6 (black bars) or control IgG (gray bars).
Discussion
CD6 was one of the first identified markers of T cells, but its role in T cells remains unclear, partly because of the lack of CD6 KO mice. In this study, we developed CD6 KO mice and demonstrated that lack of CD6 protects mice from CNS injury in EAE in association with reduced pathogenic Th1/Th17 responses and decreased T-cell infiltration into the CNS. In addition, we developed CD6 humanized mice and demonstrated that human CD6 functions in these mice. Using such mice, we identified a mouse anti-human CD6 mAb (UMCD6) that is highly effective in treating EAE without depleting T cells.
Results from previous studies on the role of CD6 in regulating T cells were contradictory. In these studies, because of the lack of CD6 gene engineered animals, different anti-CD6 mAbs were used to block and/or cross-link CD6 in an effort to gain insights into the CD6 function. It has been reported that CD6 is a negative regulator of T cells (14), and we have reported that certain CD6-reactive mAbs inhibit the proliferation of activated memory T cells (9), suggesting that CD6 can also be a positive regulator of T cells. Because distinct mAbs that bind CD6 may differ in function, these mAbs might block, modulate, or cross-link CD6, and, even different concentrations of the same mAb could have opposite effects on the target protein. Therefore, it was not surprising that functional studies using different CD6-reactive mAbs gave conflicting results. In most of these studies, 3H-thymidine incorporation assays were used to assess the activation state of T cells, but levels of incorporated 3H-thymidine in the T cells are combined outcomes of T-cell activation, proliferation, and survival. In the current experiments, using CD6 KO or WT T cells, we first measured the initial up-regulation of T-cell activation markers such as CD69 and CD25 at an early time point after T-cell stimulation, and then evaluated the proliferation of the activated T cells by BrdU incorporation at later time points. We also assessed the survival of the activated WT and CD6 KO T cells by checking percentages of apoptotic cells and by quantitating the final IFN-γ– or IL-17–producing T cells. We found that CD6 KO T cells expressed higher levels of early T-cell activation markers following stimulation compared with WT T cells. Taken together, this result suggests that CD6 is a negative regulator of T-cell activation. Surprisingly, we also found that after activation, CD6 KO T cells proliferated to a lesser extent than WT T cells despite higher surface expression of activation markers. Moreover, CD6 KO T cells were more prone to apoptosis. This data are consistent with our prior observation that high CD6 expression on thymocytes was associated with reduced rates of apoptosis (15). Consequently, the cumulative effects of CD6 on T-cell activation, proliferation, and apoptosis together result in CD6 KO T cells differentiating into far fewer IFN-γ–producing Th1 or IL-17–producing Th17 cells compared with WT T cells. The observation that recall responses to MOG peptide ex vivo showed reduced IFN-γ and IL-17 secretion in CD6 KO splenocytes compared with WT splenocytes support that absence of CD6 reduces production of proinflammatory cytokines during memory responses. These data begin to clarify the complexity of the roles of CD6 in regulation of T-cell activation and differentiation.
The finding that CD6 is a negative regulator of T-cell activation and, at the same time, a positive regulator of T-cell proliferation and survival indicates that CD6 has a similar effect in regulating T cells as does CD5 (16), another member of the SRCR protein family (17). The CD6 and CD5 genes are immediately adjacent to each other, and CD6 and CD5 proteins are distributed in a similar pattern, e.g., both are primarily expressed on T cells and on some B cells such as B1a cells. Interestingly, CD5 has also been identified as a negative regulator of T-cell activation (18). CD5 KO mice likewise showed reduced disease severity in EAE after MOG peptide immunization, potentially due to the augmented apoptosis of the activated T cells lacking CD5 (16). The extent to which CD6 shares similar signaling pathways with CD5 in regulating T-cell activation, proliferation, and survival warrants further investigation.
Although certain CD6 polymorphisms have been associated with susceptibility to MS (2–5), the pathogenic role of CD6 in MS is still unclear. Surprisingly, in in vitro assays, activated T cells from patients carrying a CD6 risk allele have impaired proliferation comparing to T cells from donors carrying the nonrisk allele (19). By studying WT and CD6 KO mice in EAE, we found that the absence of CD6 protected mice from CNS injury in EAE, indicating that CD6 is required for the development of EAE, and potentially, MS. Our in vitro T-cell activation, proliferation, and survival studies provided insights into the mechanisms underlying the observed reduced MOG-specific Th1 and Th17 responses in the CD6 KO mice in EAE. It appears that after EAE induction in the CD6 KO mice, although MOG-specific T cells were initially activated more robustly, their differentiation into IFN-γ–secreting Th1 and IL-17–secreting Th17 cells was less efficient and they died faster than did T cells in WT mice, leading to reduced MOG-specific Th1/Th17 responses, and, eventually, attenuated EAE.
Our H&E studies also showed that there was significantly reduced cell infiltration in the CNS of CD6 KO mice after EAE induction. To distinguish whether CD6 expression also affected the ability of T cells to infiltrate to the CNS, we performed in vitro T-cell migration assays comparing the capacity of CD6 KO T cells and WT T cells to migrate through a monolayer of BMEC. The data showed that CD6 is also required for T cells to infiltrate with optimal efficiency through the BMEC monolayer. This result implies that CD6 is important for migration of T cells through the BBB into the CNS, which is known to be a critical step in development and/or progression of both EAE and MS. The modest impact of CD6 on the in vitro assay that we used, compared with the more robust changes seen in vivo may indicate limitations of the in vitro assay. More likely, the decrease in the leukocyte infiltrates in the CNS of CD6 KO mice is due to a combination of impaired differentiation/survival of pathogenic autoreactive T cells and impaired T-cell migration into the target organ.
CD6 is known to interact with CD166 (20), also known as activated leukocyte cell adhesion molecule, which is abundant on endothelial cells (21). Consistent with these results, it has been reported that blocking CD166 by using a mAb attenuates EAE in WT mice by reducing T-cell infiltration into the CNS (22). Taken together, these data suggest that in addition to tuning T-cell activation, proliferation, and survival, CD6 is also important for T-cell infiltration through the BBB into the CNS, which is likely another mechanism by which deficiency of CD6 leads to attenuated EAE in the CD6 KO mice. In contrast to the multiple roles for CD6 on T cells in the pathogenesis of EAE and as a target for treatment, our data indicate that the expression of CD6 on a small B-cell population in mice is likely not critical to either the pathogenesis or treatment of EAE.
Speculation that CD6 is a good target for treating autoimmune diseases including MS has existed for decades (10), but, the first and only clinical study conducted more than 30 y ago using a T-cell–depleting mouse anti-human CD6 IgM for treating MS patients was inconclusive (23). Data that CD6 deficiency leads to reduced Th1 and Th17 polarization in vitro and that CD6 KO mice are protected from CNS injury in EAE in vivo strongly argue that CD6-targeted reagents, useful for treating EAE, merit reevaluation as a potential approach to MS. Because all of the available anti-human CD6 mAbs were developed in mice, and previous studies suggest that CD6 binds to its ligand without species restrictions (7), we developed a CD6 humanized mouse in which human CD6 replaces mouse CD6 on T cells. These animals can be used to screen mouse anti-human CD6 mAbs for future development without confounding immunogenicity issues in mice. Our results that CD6 KO mice are resistant to EAE induction and that the restoration of human CD6 in the CD6 KO mice (CD6 humanized mice) is associated with severe EAE after immunization provide clear evidence that human and mouse CD6 function interchangeably in mice as predicted (7). Thus, these CD6 humanized mice are invaluable to identify effective human CD6-targeted reagents, including human CD6-targeted mAbs in the EAE model of human MS and, potentially, in other models of human autoimmune diseases.
Although there is no current CD6-related clinical trial in the United States and Europe, Itolizumab, an anti-human CD6 mAb developed in Cuba, has been effective in reducing pathogenic T-cell responses in psoriasis patients and was recently approved for treating psoriasis in India (24, 25). Itolizumab combined with methotrexate has also been reported to reduce T-cell numbers and proinflammatory cytokine levels in patients with rheumatoid arthritis, although the clinical outcomes still need to be defined (26). Surprisingly, Itolizumab binds to domain 1 of CD6 (27), and it does not block the interaction between CD6 and its currently known ligand, CD166, which binds to domain 3 of CD6 (28). Interestingly, the anti-human CD6 mAb (UMCD6) that we used to treat EAE in CD6 humanized mice also binds to domain 1 of CD6 and does not block CD6–CD166 interaction (8, 29). Thus, both in vitro and in vivo studies using anti-CD6 mAbs suggest that the CD6–CD166 interaction might not be critical for CD6 function in disease, at least not in psoriasis or MS. Instead, a new ligand that interacts with domain 1 of CD6 could be a more important CD6 partner than CD166. We reported that an antigen recognized by mAb 3A11 is a novel ligand of CD6 (30), and studies using blocking mAbs suggest that this new CD6 ligand binds to domain 1 of CD6 (29, 31), the site where both the Itolizumab and UMCD6 bind. Additional experiments to clarify the identity of this second CD6 ligand should be an important focus for future experiments.
In our treatment studies, UMCD6 did not appear to work by depleting T cells, as the previous mAb (T12) did (23), because UMCD6-treated mice had comparable CD4+ and CD8+ T cells compared with the control mice. Similar to our observations using UMCD6 in treatment of EAE in CD6 humanized mice, clinical studies in patients also showed that Itolizumab does not deplete T cells in vivo (32).
Our latest studies of B cells in CD6 KO mice showed that, unlike in humans, CD6 is not expressed on any of the B2 cells (specifically marginal and follicular B cells) in mice, neither constitutively nor after activation (33). Instead, mouse CD6 is selectively expressed on a small subset of B1a cells found in the spleen, liver, and peripheral blood, but not in the bone marrow or peritoneal cavity. In addition, we found that human CD6 is only present on B1a cells (besides T cells) in the CD6 humanized mice (Fig. S3), and that there was no difference between the control and treated CD6 humanized mice in terms of the serum levels of total IgM, total IgG, or MOG79–96-specific IgG (Fig. S4), suggesting that B cells, including the CD6+ B1a cells, might not be affected during treatment, and that the anti-CD6 mAb treatment does not ameliorate EAE by altering either Ig levels or autoantibody titers.
Fig. S3.
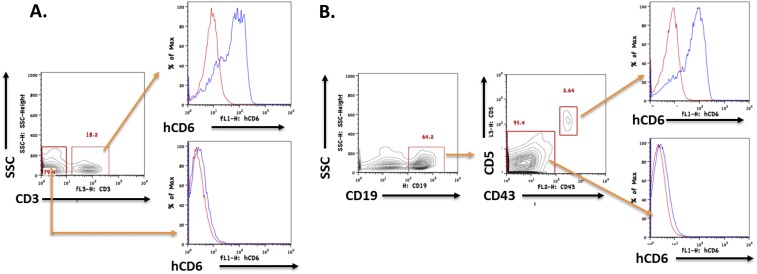
Human CD6 is expressed on B1a cells in addition to T cells in the CD6 humanized mice. Splenocytes from CD6 KO mice and CD6 humanized mice were stained for CD3, CD19, CD43, and CD5, the analyzed by flow cytometry. (A) Human CD6 is detectable on T cells (CD3+) but not any other cells (CD3−) in the spleen of CD6 humanized mice. (B) Within the B-cell (CD19+) population, human CD6 is detectable on B1a cells (CD19+CD43+CD5+) but not on B2 cells (CD19+CD43−CD5−). Red lines, cells from CD6 KO mice (controls); blue lines, cells from CD6 humanized mice.
Fig. S4.
Levels of serum total IgM, total IgG, or MOG79–96–specific IgGs are not statistically different between the mock-treated (control) and anti-CD6 mAb (UMCD6)-treated (treatment) CD6 humanized mice. Respective ELISA were used to measure levels of total IgM, total IgG, or MOG79–96–specific IgGs in sera collected 7 d after treatment (14 d after EAE induction). P > 0.05 between the groups in all measurements.
During the preparation of this manuscript, a report has used CD6−/− mice to assess the role of CD6 in T-cell development and activation (12). This study found subtle aberrations in single-positive thymocyte and mature T-cell subsets in CD6−/− TCR transgenic mice. The severity of collagen-induced arthritis (CIA) was enhanced in CD6−/− mice, in apparent contrast to our current results in the EAE model. It is worth noting that the CIA studies were conducted in C57BL/6 mice, a strain in which the incidence and severity of CIA is substantially less compared with the DBA-1 strain (the strain that we used for our EAE studies). Additional studies will be required to unravel the reasons that underlie the apparent differences between distinct autoimmune models and genetically distinct mouse strains in the role of CD6 in the development of autoimmune disease, and whether such differences are paralleled by heterogeneity in the roles of CD6 in various human autoimmune conditions. Nevertheless, the results in CIA and our current data both highlight an emerging appreciation of the potentially pivotal role of CD6 in control of T-cell driven autoimmunity. In view of evidence that human natural T-regulatory cells express little or no CD6 (34), the roles of Tregs in altered courses and outcomes of autoimmune syndromes in CD6-manipulated animals also warrant further analysis.
In summary, using WT and CD6 KO mice, we demonstrated that CD6 is required for the development of EAE. CD6 is a negative regulator of T-cell activation, but a positive regulator of T-cell proliferation and survival. Therefore, lack of CD6 leads to reduced T-cell responses in EAE. In addition, CD6 on T cells is also required for T-cell infiltration through the BBB into the CNS. By developing a CD6 humanized mice, we showed that human CD6 functions in mice, and identified UMCD6, a mouse anti-human CD6 mAb, as a potent inhibitor of EAE. These results encourage exploration of the potential of a humanized variant of an anti-CD6 antibody such as UMCD6 to become a new therapeutic for treating MS and possibly other diseases.
Materials and Methods
All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Cleveland Clinic, and all were done in accordance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and institutional guidelines. The development of CD6 KO mice, CD6 humanized mice, details of the induction and assessment of disease severity of EAE, as well as ex vivo MOG-specific Th1/Th17 recall assay, in vitro Th1/Th17 polarization, activation, proliferation and survival assays, and statistical analyses are described in SI Materials and Methods.
SI Materials and Methods
Animals.
Wild-type (WT) mice, CD6 KO mice, and CD6 humanized mice (all on DBA-1 background) were maintained under pathogen-free conditions in the animal facility of Lerner Research Institute, Cleveland Clinic. All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Cleveland Clinic, and all were done in accordance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and institutional guidelines.
Development of the CD6 KO Mice.
CD6 KO mice were developed at Bristol-Myers Squibb by following a conventional KO mice generation protocol (35), and the resultant CD6 KO mice were backcrossed with DBA-1 mice for 12 generations. In this CD6 KO mouse, exons 5–7 of the CD6 gene were replaced by a neomycin cassette after homologous recombination. To ensure the absence of CD6 protein in the CD6 KO mice, peripheral lymphocytes from CD6 KO mice and WT mice were analyzed for the presence of CD6 protein by flow cytometry. At the same time, percentages of both CD4+ and CD8+ T cells in the peripheral blood from age- and sex-matched WT and CD6 KO mice were also analyzed by flow cytometry using a BD FACSCalibur (BD Biosciences).
Induction and Assessment of Disease Severity of EAE.
EAE was induced by active immunization, and disease severity was assessed by assigning clinical scores by following published protocols (36, 37). In brief, 8- to 10-wk-old female mice were immunized at the base of the tail and in both thighs with 200 μg of mouse MOG79–96 peptide (custom synthesized by GenScript USA) emulsified in CFA (Difco Laboratories) that had been supplemented with Mycobacterium tuberculosis strain H37Ra to 4 mg/mL. Pertussis toxin (0.2 µg; List Biologic Laboratories) was injected i.p. immediately after immunization and the following day. Clinical severity was assessed daily with a 0–5 scoring system (0, no signs; 1, flaccid tail; 2, impaired righting reflex and/or gait; 3, partial hind limb paralysis; 4, total hind limb paralysis; 5, moribund or dead). Each mouse was assessed twice in a blinded fashion within the same day, and the average score from the two assessments was recorded as the composite score for that day.
Histology and Histochemical Staining of Spinal Cords.
Following killing of the mice, spinal cords were removed and fixed in 10% (vol/vol) formalin in PBS buffer for 24 h, then embedded in paraffin. Sections were cut at 5 μm on a microtome and stained with hematoxylin and eosin (H&E) to assess CNS inflammatory infiltrates, or Luxol Fast Blue to assess demyelinated areas, by following established protocols.
Th1 and Th17 Recall Assays.
Splenocytes were collected immediately following killing of the mice. After lysing the red blood cells, 0.4 × 106 splenocytes were incubated without peptide, with 10 µg/mL of a nonrelevant peptide (IRBP161–180), or with 10 µg/mL MOG79–96 peptide in 100 µL of RPMI 1640 medium with 10% (vol/vol) FBS in each well of a 96-well plate. After 72 h, IFN-γ and IL-17a levels in the culture supernatants were measured by respective ELISA (BioLegend) following manufacturer-provided protocols.
In Vitro Th1 and Th17 Polarization Assays.
CD4+ T cells were isolated from gender- and age-matched WT or CD6 KO mice by negative selection using magnetic beads (EasySep Mouse CD4+ T Cell Isolation Kit, STEMCELL Technologies), then cultured under Th1 or Th17 polarization conditions by following published protocols (38). In brief, CD4+ T cells (2 × 104 cells per well) were activated with plate-bound 5 µg/mL anti-mouse CD3 and 1 µg/mL anti-CD28 (BioLegend), then cultured at 37 °C for 5 d. For Th1 polarization, activated cells were cultured in the presence of 20 ng/mL recombinant mouse IL-2, 25 ng/mL recombinant mouse IL-12 (PeproTech), and 10 μg/mL neutralizing anti–IL-4 antibody (BioLegend). For Th17 differentiation, activated T cells were cultured in the presence of 20 ng/mL recombinant mouse IL-6, 5 ng/mL recombinant mouse TGF-β (PeproTech), and 10 μg/mL neutralizing anti–IL-4 and anti–IFN-γ antibodies (BioLegend). On days 1, 2, and 3, cells were stained with 5 μL of annexin V per sample followed by flow cytometric analysis according to a manufacturer-provided protocol (Annexin V Apoptosis Detection Kit, BD Biosciences). For the BrdU incorporation assay, cells were cultured in the presence of BrdU (10 µM), and at different time points, the proliferation of the cells was assessed by measuring BrdU incorporation using a BrdU ELISA kit (Roche). Finally, differentiated Th1 and Th17 cells (day 5) were quantitated by intracellular staining of IFN-γ and IL-17 (38).
T-Cell Activation Marker Regulation Analysis.
Purified WT or CD6 KO CD4+ T cells were activated by 1 µg/mL anti–mouse-CD3 and anti–mouse-CD28 (BioLegend); 5 h later, expression levels of activation markers CD25 and CD69 on the activated CD4+ T cells were measured by flow cytometry after staining with 2 μg/mL of both PE–anti-mouse CD69 and APC–anti-mouse CD25 mAbs (BioLegend).
Mouse Primary BMEC Isolation.
Mouse BMEC were isolated by following a published protocol (13). In brief, mouse brains were isolated, and the brainstems, cerebella, thalami, and meninges were removed under a dissecting microscope. The remaining tissue was minced and digested by 5 mg/mL collagenase CLS2 (Worthington Biochemical) in DMEM for 1 h at 37 °C, then washed with 20% (vol/vol) BSA-DMEM and centrifuged at 1,000 × g for 20 min at 4 °C. The pellet was resuspended in 1 mg/mL collagenase/dispase (Worthington Biochemical) and incubated for another 1 h at 37 °C. After the final washing, the resultant cells were cultured in endothelial cell medium (PeproTech). The isolated BMEC purity was determined by flow cytometric analysis after staining the cells with an anti-CD34 mAb (BioLegend).
Transwell T-Cell Migration Assay.
Isolated WT BMEC were cultured onto the upper chambers of 3-µm pore size culture inserts (Falcon) in a 24-well transwell plate. After the cells formed a monolayer, 0.6 × 106 nylon wool-enriched splenic T cells [activated by 1 μg/mL of both anti-mouse CD3 and anti-mouse CD28 mAbs (BioLegend)] from matched WT and CD6 KO mice were added into the inserts with 20 ng/mL of mouse CCL2 (PeproTech) in the lower chambers (to facilitate T-cell migration), and cultured at 37 °C. After 18 h, the number of T cells remaining on top of the BMEC monolayer in the culture inserts and the number of migrated cells in the bottom chamber were counted both by using a hemacytometer after Trypan blue staining and by flow cytometry. The percentages of cells remaining in the upper culture inserts and those that migrated into the lower chamber were calculated by using the following formula: % = cells in the upper inserts (or cells in the lower chambers) / (cells in the upper inserts + cells in the lower chambers) × 100%.
Development of the CD6 Humanized Mice.
CD6 humanized mice were developed by first generating a human CD6 (hCD6) transgenic (Tg) mouse in which hCD6 cDNA (kind gift of J. Parnes, Stanford University, Stanford, CA) expression is driven by a human CD2 promoter and its locus control region (39) (kind gift of D. Kioussis, National Institute for Medical Research, London), following conventional protocols at the transgenic core facility of Case Western Reserve University. A hCD6 high-expressing Tg mouse was identified by flow cytometry and bred with mCD6 KO mice to generate CD6 humanized mice (DBA-1 background). The CD6 humanized mice were typed by flow cytometry using respective anti-mCD6 and anti-hCD6 mAbs to ensure the presence of hCD6, but the absence of mCD6 on T cells.
EAE Induction and Treatment Studies.
EAE was induced in the CD6 humanized mice by active immunization with MOG79–96 peptide as described above. Seven days after immunization, when mice developed mild clinical signs of EAE, they were randomly separated into two groups. One group was injected i.p. with 0.4 mg of a mouse anti-human CD6 IgG (UMCD6) (9) (0.4 mg per mouse) in the form of diluted ascites, and the other group received the same amount of purified mouse IgG (Jackson ImmunoResearch) as controls. Mice were then monitored and clinical scores recorded daily for another week. At the end of experiments, mice were euthanized, and peripheral blood lymphocytes were analyzed for percentages of CD4+, CD8+, or CD6+ T cells, splenocytes were used to carry out antigen-specific Th1 and Th17 recall assays, and spinal cords were analyzed by the same histological and histochemical assays described above.
Human CD6 Expression on B Cells in the CD6 Humanized Mice.
Human CD6 expressions on peripheral white blood cells and spenocytes from CD6 humanized mice and CD6 KO mice were analyzed by flow cytometry after staining the cells with anti-human CD6 mAb, anti-CD3 (for T cells), anti-CD19 (for B cells), anti-CD43, and anti-CD5 (for B1a cells).
Levels of Total IgG, IgM, and MOG-specific IgG Measurements in Anti-CD6 mAb-Treated CD6 Humanized Mice with EAE.
Sera collected from the mice after treatment were diluted and used in respective ELISA to measure levels of total IgG and IgM by using commercial kits (Sigma) following manufacturer-provided protocols. To measure MOG79–96–specific IgG levels by using ELISA, plates were first coated with 20 µg/mL MOG79–96 peptide in PBS overnight at 4 °C.
Statistical Analysis.
Experiments were repeated at least twice. To determine whether statistically significant differences existed between groups, clinical scores were analyzed by using ANOVA test, whereas other results were compared by using the Student t test. A P value <0.05 was considered significant.
Acknowledgments
We thank Sherree Friend and Christopher Clegg for the generation of the CD6 KO mice; the transgenic animal core, the Clinical and Translational Science Center (UL1TR000439), and JingFang Li for the development of hCD6 Tg mice; and Gary Starling for anti-mouse CD6 Abs. This work was supported in part by NIH Grants EY025373, NS081443 (to F.L.) and AI47392 (to N.G.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615253114/-/DCSupplemental.
References
- 1.Aruffo A, Melnick MB, Linsley PS, Seed B. The lymphocyte glycoprotein CD6 contains a repeated domain structure characteristic of a new family of cell surface and secreted proteins. J Exp Med. 1991;174(4):949–952. doi: 10.1084/jem.174.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Multiple Sclerosis Genetics Consortium The genetic association of variants in CD6, TNFRSF1A and IRF8 to multiple sclerosis: A multicenter case-control study. PLoS One. 2011;6(4):e18813. doi: 10.1371/journal.pone.0018813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaminathan B, et al. Validation of the CD6 and TNFRSF1A loci as risk factors for multiple sclerosis in Spain. J Neuroimmunol. 2010;223(1-2):100–103. doi: 10.1016/j.jneuroim.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 4.De Jager PL, et al. International MS Genetics Consortium Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet. 2009;41(7):776–782. doi: 10.1038/ng.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heap GA, et al. Genome-wide analysis of allelic expression imbalance in human primary cells by high-throughput transcriptome resequencing. Hum Mol Genet. 2010;19(1):122–134. doi: 10.1093/hmg/ddp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamoun M, Kadin ME, Martin PJ, Nettleton J, Hansen JA. A novel human T cell antigen preferentially expressed on mature T cells and shared by both well and poorly differentiated B cell leukemias and lymphomas. J Immunol. 1981;127(3):987–991. [PubMed] [Google Scholar]
- 7.Bowen MA, et al. Characterization of mouse ALCAM (CD166): The CD6-binding domain is conserved in different homologs and mediates cross-species binding. Eur J Immunol. 1997;27(6):1469–1478. doi: 10.1002/eji.1830270625. [DOI] [PubMed] [Google Scholar]
- 8.Bott CM, Doshi JB, Morimoto C, Romain PL, Fox DA. Activation of human T cells through CD6: Functional effects of a novel anti-CD6 monoclonal antibody and definition of four epitopes of the CD6 glycoprotein. Int Immunol. 1993;5(7):783–792. doi: 10.1093/intimm/5.7.783. [DOI] [PubMed] [Google Scholar]
- 9.Singer NG, et al. Role of the CD6 glycoprotein in antigen-specific and autoreactive responses of cloned human T lymphocytes. Immunology. 1996;88(4):537–543. [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto M, Carmo AM. CD6 as a therapeutic target in autoimmune diseases: Successes and challenges. BioDrugs. 2013;27(3):191–202. doi: 10.1007/s40259-013-0027-4. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman K. Biocon’s first-in-class anti-CD6 mAb reaches the market. Nat Biotechnol. 2013;31(12):1062–1063. doi: 10.1038/nbt1213-1062b. [DOI] [PubMed] [Google Scholar]
- 12.Orta-Mascaró M, et al. CD6 modulates thymocyte selection and peripheral T cell homeostasis. J Exp Med. 2016;213(8):1387–1397. doi: 10.1084/jem.20151785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruck T, Bittner S, Epping L, Herrmann AM, Meuth SG. Isolation of primary murine brain microvascular endothelial cells. J Vis Exp. 2014;(93):e52204. doi: 10.3791/52204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira MI, et al. CD6 attenuates early and late signaling events, setting thresholds for T-cell activation. Eur J Immunol. 2012;42(1):195–205. doi: 10.1002/eji.201040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer NG, et al. CD6: Expression during development, apoptosis and selection of human and mouse thymocytes. Int Immunol. 2002;14(6):585–597. doi: 10.1093/intimm/dxf025. [DOI] [PubMed] [Google Scholar]
- 16.Axtell RC, Webb MS, Barnum SR, Raman C. Cutting edge: Critical role for CD5 in experimental autoimmune encephalomyelitis: Inhibition of engagement reverses disease in mice. J Immunol. 2004;173(5):2928–2932. doi: 10.4049/jimmunol.173.5.2928. [DOI] [PubMed] [Google Scholar]
- 17.Resnick D, Pearson A, Krieger M. The SRCR superfamily: A family reminiscent of the Ig superfamily. Trends Biochem Sci. 1994;19(1):5–8. doi: 10.1016/0968-0004(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 18.Tarakhovsky A, et al. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269(5223):535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 19.Kofler DM, Severson CA, Mousissian N, De Jager PL, Hafler DA. The CD6 multiple sclerosis susceptibility allele is associated with alterations in CD4+ T cell proliferation. J Immunol. 2011;187(6):3286–3291. doi: 10.4049/jimmunol.1100626. [DOI] [PubMed] [Google Scholar]
- 20.Bowen MA, et al. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med. 1995;181(6):2213–2220. doi: 10.1084/jem.181.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohneda O, et al. ALCAM (CD166): Its role in hematopoietic and endothelial development. Blood. 2001;98(7):2134–2142. doi: 10.1182/blood.v98.7.2134. [DOI] [PubMed] [Google Scholar]
- 22.Cayrol R, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9(2):137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 23.Hafler DA, et al. Immunologic responses of progressive multiple sclerosis patients treated with an anti-T-cell monoclonal antibody, anti-T12. Neurology. 1986;36(6):777–784. doi: 10.1212/wnl.36.6.777. [DOI] [PubMed] [Google Scholar]
- 24.Krupashankar DS, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: Results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol. 2014;71(3):484–492. doi: 10.1016/j.jaad.2014.01.897. [DOI] [PubMed] [Google Scholar]
- 25.Menon R, David BG. Itolizumab - a humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2015;8:215–222. doi: 10.2147/CCID.S47784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aira LE, et al. Immunological evaluation of rheumatoid arthritis patients treated with itolizumab. MAbs. 2016;8(1):187–195. doi: 10.1080/19420862.2015.1105416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso R, et al. Towards the definition of a chimpanzee and human conserved CD6 domain 1 epitope recognized by T1 monoclonal antibody. Hybridoma (Larchmt) 2008;27(4):291–301. doi: 10.1089/hyb.2008.0007. [DOI] [PubMed] [Google Scholar]
- 28.Chappell PE, et al. Structures of CD6 and its ligand CD166 give insight into their interaction. Structure. 2015;23(8):1426–1436. doi: 10.1016/j.str.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer NG, et al. CD6 dependent interactions of T cells and keratinocytes: Functional evidence for a second CD6 ligand on gamma-interferon activated keratinocytes. Immunol Lett. 1997;58(1):9–14. doi: 10.1016/s0165-2478(97)02707-7. [DOI] [PubMed] [Google Scholar]
- 30.Saifullah MK, et al. Expression and characterization of a novel CD6 ligand in cells derived from joint and epithelial tissues. J Immunol. 2004;173(10):6125–6133. doi: 10.4049/jimmunol.173.10.6125. [DOI] [PubMed] [Google Scholar]
- 31.Alonso-Ramirez R, et al. Rationale for targeting CD6 as a treatment for autoimmune diseases. Arthritis (Egypt) 2010;2010:130646. doi: 10.1155/2010/130646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez PC, et al. A clinical exploratory study with itolizumab, an anti-CD6 monoclonal antibody, in patients with rheumatoid arthritis. Results Immunol. 2012;2:204–211. doi: 10.1016/j.rinim.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enyindah-Asonye G, et al. CD6 receptor regulates intestinal ischemia/reperfusion-induced injury by modulating natural IgM-producing B1a cell self-renewal. J Biol Chem. 2017;292(2):661–671. doi: 10.1074/jbc.M116.749804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia Santana CA, Tung JW, Gulnik S. Human treg cells are characterized by low/negative CD6 expression. Cytometry A. 2014;85(10):901–908. doi: 10.1002/cyto.a.22513. [DOI] [PubMed] [Google Scholar]
- 35.Limaye A, Hall B, Kulkarni AB. Manipulation of mouse embryonic stem cells for knockout mouse production. Curr Protoc Cell Biol. 2009;44(19.13):1–24. doi: 10.1002/0471143030.cb1913s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul-Majid KB, et al. Screening of several H-2 congenic mouse strains identified H-2(q) mice as highly susceptible to MOG-induced EAE with minimal adjuvant requirement. J Neuroimmunol. 2000;111(1-2):23–33. doi: 10.1016/s0165-5728(00)00360-x. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Huang D, Nacion K, Bu H, Lin F. Augmenting DAF levels in vivo ameliorates experimental autoimmune encephalomyelitis. Mol Immunol. 2009;46(15):2885–2891. doi: 10.1016/j.molimm.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 39.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185(1):133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]