Significance
Congenital adrenal hyperplasia resulting from mutations in the CYP11B1 gene, which encodes a steroidogenic enzyme 11β-hydroxylase, is a rare inherited disorder associated with hyperandrogenemia, short stature, hypertension, and virilization of female newborns. We present a comprehensive clinical, genetic, and hormonal characterization for 68 of 108 patients with a genotype from an International Consortium on Rare Steroid Disorders. We also use computational modeling to define the effect of each of the missense mutations on the structure of 11β-hydroxylase, information that can be used to predict clinical severity prenatally in high-risk mothers.
Keywords: steroid hormones, missense mutations, classic CAH, ambiguous genitalia
Abstract
Congenital adrenal hyperplasia (CAH), resulting from mutations in CYP11B1, a gene encoding 11β-hydroxylase, represents a rare autosomal recessive Mendelian disorder of aberrant sex steroid production. Unlike CAH caused by 21-hydroxylase deficiency, the disease is far more common in the Middle East and North Africa, where consanguinity is common often resulting in identical mutations. Clinically, affected female newborns are profoundly virilized (Prader score of 4/5), and both genders display significantly advanced bone ages and are oftentimes hypertensive. We find that 11-deoxycortisol, not frequently measured, is the most robust biochemical marker for diagnosing 11β-hydroxylase deficiency. Finally, computational modeling of 25 missense mutations of CYP11B1 revealed that specific modifications in the heme-binding (R374W and R448C) or substrate-binding (W116C) site of 11β-hydroxylase, or alterations in its stability (L299P and G267S), may predict severe disease. Thus, we report clinical, genetic, hormonal, and structural effects of CYP11B1 gene mutations in the largest international cohort of 108 patients with steroid 11β-hydroxylase deficiency CAH.
Congenital adrenal hyperplasia (CAH) is a Mendelian disorder transmitted as an autosomal recessive trait. The most prevalent form of CAH arises from steroid 21-hydroxylase enzyme deficiency, accounting for ∼90–95% of all cases (1, 2). In contrast, CAH caused by steroid 11β-hydroxylase deficiency is considerably rare, with a prevalence of 5–8% (3), from which we estimate an overall frequency of 1 in 100,000 live births.
Two homologous enzymes, 11β-hydroxylase and aldosterone synthase, are encoded by the CYP11B1 and CYP11B2 genes, respectively. The two genes are 40-kb apart, each comprising nine exons and mapped to chromosome 8q21-22 (3, 4) (Fig. 1A). In contrast to CYP21A2 and its CYP21A1P pseudogene, CYP11B1 and CYP11B2 are both active and do not have a pseudogene. The two encoded homologs, however, have distinct functions in cortisol and aldosterone synthesis, respectively (3). In the zona fasciculata, 11β-hydroxylase converts 11-deoxycortisol and 11-deoxycorticosterone to cortisol and corticosterone, respectively, and is regulated by adrenocorticotropic hormone secreted by the pituitary. In contrast, in the zona glomerulosa aldosterone synthase converts corticosterone to aldosterone with the intermediate production of 18-hydroxycorticosterone. These latter conversions are controlled mainly by the renin angiotensin II system and serum potassium concentration (3).
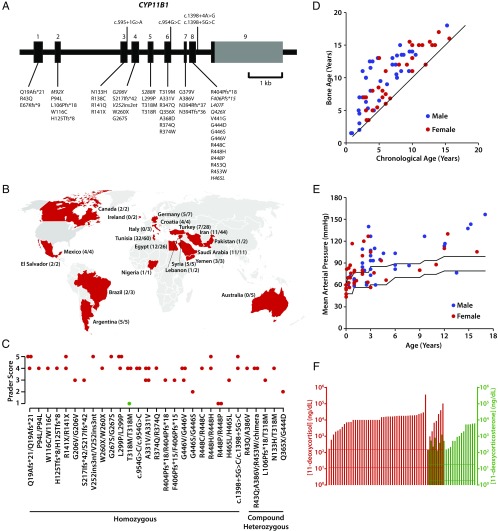
Fig. 1.
Clinical profile for patients with CAH resulting from 11β-hydroxylase deficiency from 13 nations comprising the International Consortium for Rare Steroid Disorders. (A) Structure of the human CYP11B1 gene that contains nine exons, showing mutations harbored by 108 patients in this international cohort. Previously unreported mutations are shown in italics. (B) Worldwide distribution of our cases of 11β-hydroxylase deficiency. The denominator indicates the number of patients originating from that country with 11β-hydroxylase deficiency. The numerator indicates the number of patients with 11β-hydroxylase deficiency who have been genotyped. All patients who originated from Middle East and European countries have been placed in their respective countries. It is evident from this map that the majority of patients with 11β-hydroxylase deficiency originate in the Middle East and North Africa. (C) Prader scores of patients with different genotypes (noted), both as homozygotes and compound heterozygotes. One patient (green) has been treated prenatally with dexamethasone. (D) Bone age versus chronological age in male (blue) and female (red) patients. The black line represents a slope of 1 (bone age = chronological age). Of note is that almost all patients show evidence of advanced bone age. (E) Mean arterial pressure (1/3 systolic pressure + 2/3 diastolic pressure) shown for both males and female patients of various ages. The two lines indicate the age-appropriate upper and lower limits of the normal range. (F) Measurements of 11-deoxycortisol (red) and 11-deoxycorticosterone (green) in our patient cohort. Shown also are normal reference ranges for both hormones (horizontal lines; Esoterix).
Deficiency of 11β-hydroxylase prevents the conversion of 11-deoxycortisol to cortisol and 11-deoxycorticosterone to corticosterone. This results in high levels of 11-deoxycortisol and 11-deoxycorticosterone, respectively, which are shunted into the androgen synthesis pathway, resulting in high levels of the androgenic steroid, androstenedione. Female newborns are thus profoundly virilized and exhibit significant masculinization of the external genitalia (5). Precocious puberty, rapid somatic growth, and rapid skeletal maturation because of hyperandrogenemia occur in both genders. Accumulation of the potent mineralocorticoid 11-deoxycorticosterone also leads to hypertension, which is not seen with 21-hydroxylase deficiency.
We recently published evidence for genotype–phenotype concordance in 1,507 families with 21-hydroxylase deficiency; however, 7% of the patients demonstrated nonconcordance (6). In a separate study, we used computational modeling to define in silico the structural changes that each mutation in the CYP21A2 gene induces in the 21-hydroxylase enzyme, and by correlating these changes with phenotype, defined structural derangements underpinning the clinical variants of CAH, namely salt-wasting, simple virilizing, and nonclassic CAH (7).
Studies reporting the clinical and hormonal phenotype of 11β-hydroxylase deficiency have been restricted because of small patient numbers, consistent with the rarity of this disease. As the International Consortium of Rare Steroid Disorders, we now provide extensive data on the demographics, genotype, phenotype, and hormonal profile of CAH patients with 11β-hydroxylase deficiency. Although we had 220 patients reported to us with clinical data, only 108 patients had been genotyped and are reported herein.
Our dataset confirms ethnic and geographical predominance of 11β-hydroxylase deficiency in Middle East and North African nations, in contrast to 21-hydroxylase deficiency that affects mainly Eastern Europeans of Jewish descent, with 1 in 27 patients having mild, nonclassic CAH (8). Our data also show that mostly all CAH patients with 11β-hydroxylase deficiency display rapid skeletal maturation, with their bone age exceeding chronological age; show higher Prader scores than newborns with 21-hydroxylase deficiency; and commonly suffer from hypertension. Computational modeling indicates that, as with 21-hydroxylase deficiency, mutation-induced changes in the heme- or substrate binding regions of 11β-hydroxylase and mutations affecting enzyme stability cause severe CAH.
Results
CAH resulting from 11β-hydroxylase deficiency is a rare disorder with reported prevalence of 1 in 100,000 live births (2, 3). In our international cohort, the disease was confined mainly to Middle East and North African nations, where consanguinity is common. Of note, 58% of patients were from consanguineous marriages (Table 1) (9–13). The median age of diagnosis was 1.08 y (range: 0–17 y), with 80% of females being diagnosed because of ambiguous genitalia at birth. In contrast, 84% of males presented later with precocious puberty, and the remaining cases were diagnosed upon screening triggered by hypertension, hyperpigmentation, or family history. Of the 14 46, XX patients who were assigned as males at birth, 10 were subsequently reassigned to the female sex.
Table 1.
Number of patients with congenital adrenal hyperplasia because of steroid 11β hydroxylase deficiency in Tunisia, Iran, Egypt, Turkey, and Saudi Arabia
Fig. 1A shows that, in our cohort of 108 patients, there were 31 missense, 5 nonsense, 1 insertion, and 9 frameshift mutations of the CYP11B1 gene, as well as 4 splice-site mutations. Country distribution of these patients is shown in Fig. 1B. Mutations in 56 patients have been published in smaller collaborative studies (14–21). In our current cohort, 30 patients with the G379V/G379V mutation were Arab Berbers from Tunisia, 4 with G446V/G446V were from Egypt, and 4 with Q19Afs*21/Q19Afs*21 were from Saudi Arabia. Only five patients with the genotype A331V/A331V were Sephardic Jews living in the United States (Table 1).
Fig. 1 and Table S1 provide detailed clinical and hormonal evaluations. Of the 108 patients studied, 55 were females, but Prader scores were reported for 39 patients. Of these, eight females had a Prader score of 5 (21%) (Fig. 1C). Notably, their external genitalia displayed significant masculinization, with the urethra opening at the tip of the phallus. Of the 39 females, 18 had Prader scores of 4 (46%). In a different subset of 70 patients, all had advanced bone age, with males significantly exceeding females (bone age/chronological age; males: 2 ± 0.82, n = 40; females: 1.43 ± 0.44, n = 30) (P = 0.0008) (Fig. 1D).
Table S1.
Clinical and hormonal evaluations of our cohort of patients with 11β-hydroxylase deficiency
| Patient code | Genotype | Karyotype | Sex | Ethnicity | Age at diagnosis (y) | Prader score | BA/CA (y) | SD from midparental target height | Age at which BP was measured (y) | Baseline SBP/DBP | DOC (ng/dL) | S (ng/dL) | Presentation | Ref. |
| ARG1A | R43Q; A386V; R453W/CYP11B2-CYP11B1 chimera | 46,XX | F | Caucasian (Argentine/Argentine) | 0 | 4 | 5.45/4.7 | −0.744 | 0.25 | 98/56 | ND | 15,000 | Ambiguous genitalia | |
| ARG1B | R43Q; A386V; R453W/CYP11B2-CYP11B1 chimera | 46,XX | F | Caucasian (Argentine/Argentine) | 0 | 4 | 12/9.2 | 0.031 | 0.333 | 100/75 | ND | 2,990† | Ambiguous genitalia | |
| ARG2 | R453W/R374Q | 46,XY | M | Caucasian (Argentine/Argentine) | 3 | N/A | 8/3.7 | −0.951 | 3.6 | 90/45 | ND | 5,270† | Overgrowth and virilization | |
| ARG3A | R138C/L407F | 46,XY | M | Hispanic (Argentine/Argentine) | 7.7 | N/A | 13.4/7.7 | −1.63 | 7.7 | 120/70 | ND | 790† | Gynecomastia | |
| ARG3B | R138C/L407F | 46,XY | M | Hispanic (Argentine/Argentine) | 3.3 | N/A | 5.3/3.3 | −1.51 | ND | ND | ND | 480† | Overgrowth and virilization | |
| BRA1 | Q356X/Q356X | 46,XX | M‡ | Bras/Bahia | 1 | Not given | BA not advanced | 0.62 | 36 | 160/110 | ND | 31,500† | Atypical genitalia | |
| BRA2 | G267S/G267S | 46,XX | M‡ | Bras/Bahia | Not given | 5 | 11/6 | −0.54 | 6 | 100/60 | ND | 1,900 | Atypical genitalia | |
| BRA4 | R404Pfs*18/R404Pfs*18 | 46,XX | F | Lebanese | 0 | 3 | ND | ND | 11 | 140/110 | ND | 11,560† | Ambiguous genitalia | (18) |
| CAN1 | R448H/R448H | 46,XY | M | Aboriginal, Canadian, Half Metis | 1.95 | N/A | 13.5/8.7 | Still growing | 2 | 122/66 | 528 | 12,400 | Sepsis, precocious pubertal development/growth | |
| CAN2 | R448H/R448H | 46,XY | M | Aboriginal, Canadian | 1.41 | N/A | 15/8.1 | Still growing | 1 | 96/40 | 403 | 23,300 | Increased phallus length | |
| CAN3 | c.1200+1G>A/c.1200+1G>A | 46,XX | F | Arabic Pakistani | 2.5 | 2 | 7.1/2.5 | Still growing | 2.5 | 118/57 | 1,709 | 62,200 | Virilization and hypertension | |
| DEU1 | R453Q/M92X | 46,XY | M | German | 0 | N/A | 13.5/10.5 | Still growing | 1 | 91/55 | 5,300 | 603† | Suspicion at screening | |
| DEU2 | A368D/P94L | 46,XY | M | Caucasian | 1.75 | N/A | 5/1.9 | Still growing | 13.5 | 110/60 | 820 | 6,800 | Pubarche | (17) |
| EGY1 | R347Q/R347Q | 46,XX | M‡ | Caucasian/Egypt | 0 | 4 | 2/2 | Still growing | 3 | 70/50 | ND | 18,000 | Atypical genitalia | |
| EGY2 | G446V/G446V | 46,XX | F | Caucasian/Egyptian | 0 | 4 | ND | Still growing | 1 | 94/70 | ND | 17,900 | Atypical genitalia | |
| EGY3 | G446V/G446V | 46,XX | F | Caucasian/Egyptian | 0.08 | 4 | 3.5/3.4 | Still growing | 0.5 | 90/60 | ND | 17,600 | Atypical genitalia | |
| EGY4 | G446V/G446V | 46,XX | F | Caucasian/Egyptian | 0.08 | 3 | 5/4 | Still growing | 1 | 90/50 | ND | 2,700 | Atypical genitalia | |
| EGY5 | G446V/G446V | 46,XY | M | Caucasian/Egyptian | 17 | N/A | Epiphysis fused | ND | 17 | 225/125 | ND | 19,000 | Intracranial hemorrhage | |
| EGY6 | R448H/R448H | 46,XX | M‡ | Caucasian/Egyptian | 0 | 4 | 6.5/5 | Still growing | 1 | 75/55 | ND | 16,100 | Atypical genitalia | |
| EGY7 | R448H/R448H | 46,XY | M | Caucasian/Egyptian | 5 | N/A | 12/7.5 | Still growing | ND | ND | ND | 17,000 | Pseudoprecocious puberty | |
| EGY8 | R448H/R448H | 46,XX | M‡ | Caucasian/Egyptian | 3 | 5 | 9/6.2 | Still growing | ND | ND | N/A | 19,000 | Atypical genitalia | |
| EGY9 | N133H/T319M | 46,XX | F | Caucasian/Egyptian | 0.16 | 4 | 7.5/6 | Still growing | 0.333 | 83/53 | ND | 16,200 | Atypical genitalia | |
| EGY10 | N133H/T319M | 46,XY | M | Caucasian/Egyptian | 0.08 | N/A | 5/4.2 | Still growing | 3 | 115/65 | ND | 18,200 | Salt wasting | |
| EGY11 | N133H/T319M | 46,XY | M | Caucasian/Egyptian | 3 | N/A | 9.5/5.2 | Still growing | 3 | 160/110 | ND | 5,600 | Pseudoprecocious puberty | |
| HRV1 | T318R/R141Q | 46,XY | M | Croatian | 3 | N/A | 6.3/3 | −1.258 | 2.5 | 90/45 | 6,138 | ND | Precocious puberty | (15) |
| HRV2A | E67Kfs*9/R448H | 46,XY | M | Croatian | 3 | N/A | 7/3 | −0.279 | 3 | 100/60 | 5,273.8 | ND | Precocious puberty | (15) |
| HRV2B | E67Kfs*9/R448H | 46,XY | M | Croatian | 0.25 | N/A | ND | −0.699 | 0.25 | 80/50 | 5,476 | ND | Brother of HRV2A | (15) |
| HRV3 | R448H/c.1398+4A>G | 46,XY | M | Croatian | 2.5 | N/A | 7/2.5 | Still growing | 2.5 | 90/50 | 5,936.6 | ND | Precocious puberty | (19) |
| IRN1 | R43Q/A386V | 46,XX | F | Persian, Iranian | 0 | 4 | ND | ND | 0.75 | 170/90 | ND | ND | Not given | |
| IRN2A | R374W/R374W | 46,XY | M | Kordish/Iranian | 3.58 | N/A | 12.5/3.5 | −0.734 | 9.58 | 160/110 | ND | ND | Macrophallus | |
| IRN2B | R374W/R374W | 46,XY | M | Kordish/Iranian | 1.16 | N/A | 13.5/8.4 | −1.15 | 14.89 | 170/121 | ND | ND | Macrophallus | |
| IRN3 | N394Rfs*37/R448H | 46,XY | M | Persian, Iranian | 15.25 | N/A | 18/15.2 | −1.433 | 15.25 | 190/120 | ND | ND | Hypertension, severe hyperpigmentation | |
| IRN4 | R448C/R448C | 46,XX | F | Persian, Iranian | 1.83 | 4 | ND | 0.697 | 2.16 | 160/100 | ND | ND | Not given | |
| IRN5 | R448C/R448C | 46,XY | M | Persian, Iranian | 0.91 | N/A | 10/7.7 | ND | 4.16 | 120/82 | ND | ND | High 17OHP, hypertension | |
| IRN6 | R448C/R448C | 46,XY | M | Tork Azari, Iranian | 2.58 | N/A | 7/2.5 | 0.699 | 2.75 | 180/120 | ND | ND | Macrophallus, hyperpigmentation, hypertension | |
| IRN7 | V252ins3nt/V252ins3nt | 46,XY | M | Persian, Iranian | 2.75 | N/A | 11/9.5 | −0.454 | 12.08 | 150/100 | ND | ND | Macrophallus, growth of pubic hair (Tanner 3) | |
| IRN8 | V252ins3nt/V252ins3nt | 46,XX (83%) 45,XO (17%) | M‡ | Persian, Iranian | 0 | 5 | ND | 0.155 | ND | ND | ND | ND | Ambiguous genitalia | |
| IRN31 | W116C/W116C | 46,XX | F | Persian, Iranian | 0 | 4 | 6.83/6.6 | −0.465 | 12.42 | 170/110 | ND | ND | Ambiguous genitalia | |
| IRN32 | N394Rfs*37/N394Rfs*37 | 46,XY | M | Persian, Iranian | 1.75 | N/A | 11/8.3 | −0.139 | ND | ND | ND | ND | Macrophallus, acne, virilization | |
| SAU1A | H465L/H465L | 46,XX | F | Saudi | 0 | 3 | 3.5/3.1 | Still growing | 0.5 | 105/78 | ND | 3,720 | Ambiguous genitalia | |
| SAU1B | H465L/H465L | 46,XY | M | Saudi | 3 | N/A | 11.5/3.3 | Still growing | 3 | 132/95 | ND | 19,800 | Precocious puberty | |
| SAU2A | Q19Afs*21/Q19Afs*21 | 46,XX | M | Saudi | 14 | 5 | 17/14 | −0.31 | 11 | 126/69 | ND | 18,000 | Family screen as his sister was diagnosed | (20) |
| SAU2B | Q19Afs*21/Q19Afs*21 | 46,XX | M | Saudi | 10 | 5 | 13/10 | N/A | 2.66 | 95/48 | ND | 16,500 | Family screen as his sister was diagnosed | (20) |
| SAU2C | Q19Afs*21/Q19Afs*21 | 46,XX | M‡ | Saudi | 16 | 4 | ND | N/A | 16 | 140/88 | ND | 7,590 | Breast Tanner IV, short stature, hypertension | (20) |
| SAU3 | Q19Afs*21/Q19Afs*21 | 46,XY | M | Saudi | 7 | N/A | 14/10 | N/A | 6 | 114/74 | ND | 16,500 | Family screen as his sister was diagnosed | (20) |
| SAU4A | R448P/R448P | 46,XX | F | Saudi | 7 | 1 | 12/12 | Still growing | 9 | 103/69 | ND | 1,270 | Clitoromegaly, diagnosed at birth with 21OHD | |
| SAU4B | R448P/R448P | 46,XX | F | Saudi | 0 | 1 | 5/3 | Still growing | 0.069 | 97/74 | ND | 23,200 | Clitoromegaly | |
| SAU5 | R448P/R448P | 46,XY | M | Saudi | 7 | N/A | 12.5/4 | Still growing | 2.25 | 118/61 | ND | 2,210 | Precocious puberty | |
| SAU6 | W260X/W260X | 46,XX | ND | Saudi | 1.33 | 4 | 13/5 | Still growing | 0.5833 | 98/65 | ND | 621 | Ambiguous genitalia | |
| SAU7 | G206V/G206V | 46,XX | ND | Saudi | 1.08 | 3 | ND | ND | 1.25 | 106/46 | ND | 7,430 | Ambiguous genitalia | |
| TUN1 | S217Ifs*42/S217Ifs*42 | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | 3 | ND | ND | 2 | 120/90 | ND | 13,400 | Ambiguous genitalia, melanodermia | (21) |
| TUN2A | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | Not given | ND | ND | 2 | 120/60 | ND | ND | Ambiguous genitalia, hyperpigmentation | |
| TUN2B | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | Not given | ND | ND | 6 | 140/100 | ND | ND | Ambiguous genitalia | |
| TUN3A | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Sidi Bouzid) | 0 | Not given | ND | ND | ND | Normotensive | ND | ND | Ambiguous genitalia | |
| TUN3B | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | Not given | 12/8 | Still growing | ND | ND | ND | ND | Ambiguous genitalia, hyperpigmentation, virilization | |
| TUN3C | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 1.75 | N/A | 12/12 | ND | 2 | 120/90 | ND | ND | Melanodermia, macrogenitosomia | |
| TUN3D | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 0 | N/A | 3/2 | Still growing | 6 | 135/80 | ND | ND | Hirsutism, precocious puberty | |
| TUN3E | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 1.08 | N/A | BA was advanced | ND | ND | Hypertensive | ND | ND | Melanodermia, precocious puberty | |
| TUN4A | G379V/ND | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | Not given | 6/6 | Still growing | ND | Normotensive | ND | ND | Ambiguous genitalia | |
| TUN4B | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 1.08 | 3 | 6/3 | ND | 3 | 135/90 | ND | ND | Ambiguous genitalia, precocious puberty | |
| TUN5A | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | 4 | 8/4 | Still growing | ND | ND | ND | ND | Ambiguous genitalia | |
| TUN5B | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2 | N/A | BA was advanced | ND | 12 | 160/90 | ND | ND | Hyperpigmentation, precocious puberty, melanodermia | |
| TUN5C | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2 | N/A | ND | ND | 3.5 | 130/100 | ND | ND | Macrogenitosomia, precocious puberty, hyperpigmentation | |
| TUN5D | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 0 | N/A | ND | Still growing | 0.5 | 105/55 | ND | ND | Melanodermia, hypertension | |
| TUN6 | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | Not given | BA not advanced | ND | ND | ND | ND | ND | Ambiguous genitalia, hyperpigmentation | |
| TUN7 | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | 4 | ND | Still growing | ND | ND | ND | ND | Ambiguous genitalia | |
| TUN8A | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Kairouan) | 0 | Not given | ND | Died at 4 months of age | ND | ND | ND | ND | Ambiguous genitalia | |
| TUN8B | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 1.16 | N/A | BA was advanced | Still growing | ND | ND | ND | ND | Acceleration of growth, melanodermia | |
| TUN9A | G379V/G379V | 46,XX | ND | Arab-Berber/Tunisian (Nabeul) | 0 | Not given | ND | Still growing | ND | ND | ND | ND | Ambiguous genitalia | |
| TUN9B | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Nabeul) | 0 | N/A | 10/3 | ND | ND | Hypertensive | ND | ND | Precocious puberty | |
| TUN10 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2.25 | N/A | ND | ND | 2 | 135/96 | ND | ND | Hyperpigmentation, macrogenitosomia | |
| TUN11A | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2.5 | N/A | BA was advanced | ND | 6 | 120/95 | ND | ND | Pubic hair, magrogenitosomia | |
| TUN11B | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 3.4 | N/A | BA was advanced | ND | ND | ND | ND | ND | Precocious puberty, hypertension | |
| TUN12 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2 | N/A | BA was advanced | ND | 3 | 120/90 | ND | ND | Precocious puberty, macrogenitosomia | |
| TUN13 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 0.42 | N/A | BA was advanced | ND | 4 | 120/95 | ND | ND | Precocious puberty, macrogenitosomia | |
| TUN14 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2.16 | N/A | BA was advanced | ND | 2.2 | 120/60 | ND | ND | Precocious puberty, macrogenitosomia | |
| TUN15 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 0 | N/A | ND | ND | ND | ND | ND | ND | Precocious puberty, macrogenitosomia | |
| TUN16A | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2 | N/A | 7/2 | ND | 3 | 120/80 | ND | ND | Precocious puberty, hirsutism | |
| TUN16B | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 0 | N/A | 1.5/0.75 | ND | ND | ND | ND | ND | Hirsutism | |
| TUN17 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 0.08 | N/A | N/A | ND | ND | ND | ND | ND | Hirsutism, precocious puberty | |
| TUN18 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 0.42 | N/A | 12/7 | ND | ND | ND | ND | ND | Acceleration of growth, melanodermia | |
| TUN19 | G379V/G379V | 46,XY | M | Arab-Berber/Tunisian (Kairouan) | 2 | N/A | ND | ND | ND | ND | ND | ND | Macrogenitosomia, precocious puberty | |
| TUR1 | R141X/R141X | 46,XX | F | Caucasian/Turkish | 0.41 | 4 | ND | Still growing | 0.416 | 90/40 | ND | 10,000 | Ambiguous genitalia | (16) |
| TUR2 | R141X/R141X | 46,XX | M | Caucasian/Turkish | 4.2 | 5 | 15.5/9.6 | ND | 4.3 | 120/80 | ND | 10,000 | Accelerated growth, penis enlargement, pubic hair | (16) |
| TUR4 | L299P/L299P | 46,XX | M | Caucasian/Turkish | 2.7 | 5 | 7/5.5 | ND | 2.7 | 160/110 | ND | 36,200 | Penis enlargement, pubic hair, nonpalpable gonads | (16) |
| TUR5A | L299P/L299P | 46,XX | M‡ | Caucasian/Turkish | 2.2 | 5 | 4.5/2 | ND | ND | ND | ND | 29,500 | Ambiguous genitalia | (16) |
| TUR5B | L299P/L299P | 46,XY | M | Caucasian/Turkish | 2 | N/A | 8.5/4.9 | ND | 2 | 130/70 | ND | 10,000 | Penis enlargement, pubic hair | (16) |
| TUR8A | G446S/G446S | 46,XX | F | Caucasian/Turkish | 4.9 | 2 | 4.9/8.5 | 0.279 | 4.9 | 80/60 | ND | 17,900 | Premature adrenarche after index case (her brother) | (16) |
| TUR8B | G446S/G446S | 46,XY | M | Caucasian/Turkish | 7.2 | N/A | 13/7 | −1.11 | ND | ND | ND | 14,700 | Premature adrenarche, facial acne | (16) |
| USA1A | A331V/A331V | 46,XX | M‡ | Sephardic Jewish/Egyptian | 0 | 4 | 8/5.5 | 0.465 | 0 | 76/45 | 146 | ND | Sibling/ambiguous genitalia | (14) |
| USA1B | A331V/A331V | 46,XX | F | Sephardic Jewish/Egyptian | 1.16 | 3 | 15.5/12.6 | 0.17 | ND | ND | 8,820 | ND | Ambiguous genitalia | (14) |
| USA2 | A331V/A331V | 46,XX | F | Sephardic Jewish, Turkish/Syrian | 0 | 4 | 11/10 | 0.5 | ND | ND | 61† | 170 | Ambiguous genitalia | |
| USA3 | A331V/A331V | 46,XX | F | Sephardic Jewish, Syrian | 0 | 3 | 15/13.4 | −0.155 | 3.42 | 110/60 | 1,030 | ND | Ambiguous genitalia | |
| USA4 | A331V/A331V | 46,XX | F | Sephardic Jewish/Egyptian | 0 | ND | ND | −0.279 | ND | ND | 132† | ND | Ambiguous genitalia | |
| USA5 | A331V/S288R | 46,XY | M | Sephardic Jewish/Syrian | 5 | N/A | 14/9.6 | −0.335 | 6 | 115/65 | 177 | 280† | Premature adrenarche | |
| USA6A | T318M/T318M | 46,XX | F | Yemeni | 0 | 4 | 15/13 | −1.67 | 1.02 | 110/65 | 2013 | ND | Ambiguous genitalia | (14) |
| USA6B | T318M/T318M | 46,XX | F | Yemeni | 0 | 4 | 15/11 | −0.139 | 0 | 80/50 | 525 | ND | Sibling/ambiguous genitalia | (14) |
| USA6C | T318M/T318M | 46,XX | F | Yemeni | Prenatal | 1§ | 15/10 | −2 | ND | ND | 158† | ND | Sibling | (14) |
| USA7 | L106Pfs*18/T318M | 46,XX | F | German, Dutch/Canadian Dutch | 0 | 3 | 16/15.6 | −0.372 | 2 | 101/62 | 222.7 | ND | Ambiguous genitalia | |
| USA8A | F406Pfs*15/F406Pfs*15 | 46,XX | F | Mexican | 0 | ND | ND | Still growing | 0.0055 | 67/38 | 36† | ND | Ambiguous genitalia | |
| USA8B | F406Pfs*15/F406Pfs*15 | 46,XX | F | Mexican | 0 | ND | ND | Still growing | 0.0055 | 61/35 | 1,260 | 39,900 | Ambiguous genitalia | |
| USA9 | F406Pfs*15/F406Pfs*15 | 46,XX | M‡ | Mexican/Mexican | 0 | 3 | ND | Still growing | 0.0027 | 73/44 | 232 | 2,000 | Ambiguous genitalia | |
| USA11 | Q365X/G444D | 46,XX | F | African American | 0 | 2 | 15/11.5 | −1.34 | 12 | 150/90 | 921 | ND | Ambiguous genitalia | (14) |
| USA12 | c.595+1G>A/V441G | 46,XY | M | Caucasian (non-Jewish) Welsh/Irish | 1.5 | N/A | 10.5/7.6 | −0.548 | 3.31 | 120/80 | 1,586 | 58† | Rapid growth | |
| USA13 | Q426X/Q426X | 46,XY | M | Mexican | 1 | N/A | 2.5/4.4 | Still growing | ND | ND | 84† | 25,450 | Public hair but diagnosis only at 2.5 years | |
| USA14 | L106Pfs*18/L106Pfs*18 | 46,XY | M | Jewish, German/English, Cherokee, French Indian | 4.83 | N/A | 13/4.8 | ND | ND | ND | 551 | 1,000 | Premature adrenarche | |
| USA18A | N394Tfs*36/N394Tfs*36 | 46,XY | M | Hispanic, El Salvadoran | 1.5 | N/A | 7/1.5 | ND | ND | ND | ND | 19,600 | Premature adrenarche | (38) |
| USA18B | N394Tfs*36/N394Tfs*36 | 46,XY | M | Hispanic, El Salvadoran | 0.08 | N/A | 6/5.7 | ND | ND | ND | ND | 7,080 | Sibling | (38) |
BA, bone age; BP, blood pressure; CA, chronological age; DBP, diastolic blood pressure; DOC, 11-deoxycorticosterone; N/A, not applicable; ND, not determined; S, 11-deoxycortisol (compound S); SBP, systolic blood pressure; SD, standard deviation.
On medication.
Reassigned to female after being reared as male.
Prenatally treated.
Measurement of baseline mean arterial pressures (MAP) in another subset of 70 patients showed that 38 were hypertensive, with MAPs exceeding the upper limit of normal for age (Fig. 1E). There was no significant difference in MAP between males (n = 34) and females (n = 36). There was a trend for higher MAPs in older children, but sporadic high MAPs were noted even in younger patients. Adrenal steroid hormones were measured by mass spectrometry in the local laboratory of the country. Serum 11-deoxycortisol and 11-deoxycorticosterone levels were all elevated, consistent with 11β-hydroxylase deficiency (Fig. 1F and Table S1).
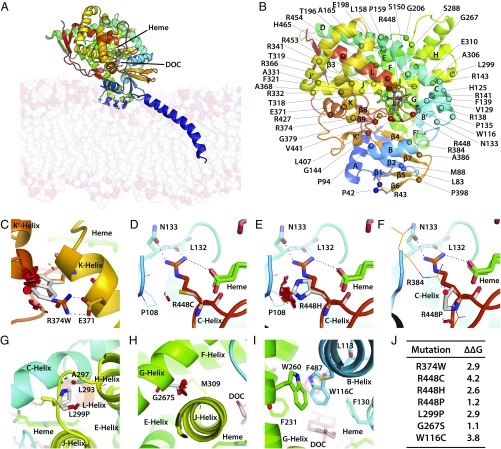
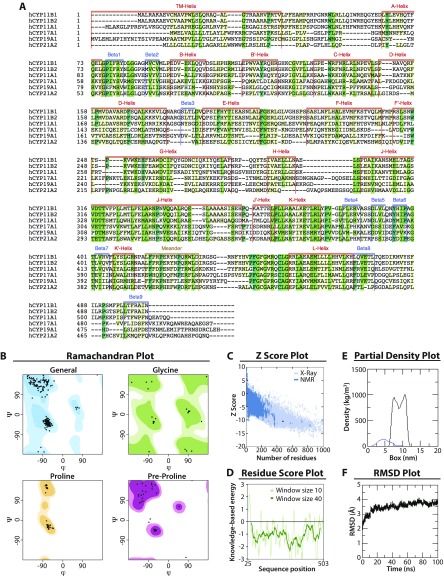
To understand whether the clinical phenotype of 11β-hydroxylase deficiency could be predicted by examining changes in enzyme structure induced by a given CYP11B1 mutation, we performed in silico analytics using the human CYP11B1 model constructed using the homologous CYP11B2 crystal structure as a template (PDB ID code 4DVQ), which exhibited 93% sequence similarity over 478 residues. We have previously shown that the clinical phenotype of patients with 21-hydroxylase deficiency correlates with the extent of functional loss induced by a given mutation (7). For example, missense mutations within 5 Å of the heme- or substrate-binding region or those affecting stability of 21-hydroxylase resulted in severe salt-wasting CAH, whereas mutations disrupting conserved hydrophobic patches or transmembrane interactions caused the less severe, simple virilizing disease (7). Our model for CYP11B1 exhibited structural features of a classic steroid-synthesizing cytochrome, with a triangular prism structure containing 16 α-helices and 9 β-sheets (Fig. 2 A and B and Fig. S1).
Fig. 2.
Modeling severe disruptive mutations affecting heme- or substrate-binding or CYP11B1 stability. Human CYP11B1 model constructed using the homologous CYP11B2 crystal structure as a template (PDB ID code 4DVQ), shown as side view showing the inner mitochondrial membrane bilayer and the bound heme (green) and deoxycorticosterone (DOC, pink) (A). Also shown are the positions of the α-helices, β-sheets, and the mutations analyzed in this study (B). R374 creates an ion-pair interaction with E371 at the end of the K-helix, which continues into a loop that forms a part of the heme-binding site (C). Mutation of this ion pair R374-E371 is disruptive. Mutations affecting the heme-binding site may not all be as disruptive, depending on the mutated residue. For example, residue R448, located on a loop between the meander region and L-helix, forms a part of the heme-binding site, and creates hydrogen bonds with the propionate tail of heme, the backbone atoms of L132, and the side chain of N133. Its mutation to Cys (D) or His (E) causes severe disruption, whereas substitution to Pro does not (F). Pro enhances rigidity of the loop, whereas the loop in the Cys/His mutants moves away from the vicinity of heme. L299 is located on the turn between the H- and J-helices, and its mutation to a more rigid Pro side chain decreases flexibility of the turn, thus impairing enzyme stability (G). G267S is located on the G-helix, and its mutation to Ser results in lost flexibility, as side chains of the bulkier Ser does not fit between G- and J-helices, causing steric clashes with residues on J-helix, significantly impairing enzyme stability (H). W116 is located on B′-helix, with its aromatic indole side chain surrounded by the aromatic side chains of F130, F231, W260, F287, and hydrophobic side chain of L113; these residues form the roof of the binding site of the aromatic substrate. A mutation to Cys disrupts the substrate-binding site (I). (J) The change (∆∆G kcal/mol) in protein stability upon mutation of each residue. A positive energy value indicates that the mutation is likely to be disruptive. The disks represent the pairwise atomic van der Waals radii overlap. Green disks represent almost in contact or slightly overlapping and red disks represent significant overlap.
Fig. S1.
Computational model of human CYP11B1. (A) Multiple sequence alignment of steroid synthesizing cytochrome P450s. The sequences of human CYP11B1 (Uniprot id P15538), CYP11B2 (Uniprot id P19099), CYP11A1 (Uniprot id 05108), CYP17A1 (Uniprot id P05093), CYP19A1 (Uniprot id P11511), and CYP21A2 (Uniprot id P08686) were aligned using Clustal Omega (www.ebi.ac.uk/Tools/msa/clustalo/). The secondary structure elements were extracted by performing a structural alignment of CYP17A1 (PDB ID code 3RUK), CYP19A1 (PDB ID code 3S79), and CYP11B2 (PDB ID code 4DVQ) crystal structures. (B) Ramachandran plot of the human CYP11B1 model showing residues in favored (dark shade), allowed (light shade), or disallowed (white) regions. (C) The z-score of our human CYP11B1 model is −10.46 and falls within the range of all experimentally determined protein chains in the PDB, indicating high overall quality of the model. (D) The residue score plot, which plots energy as a function of amino acid sequence position, highlights local model quality of the human CYP11B1 model. A positive value corresponds to erroneous part of the input structure. The plot is smoothed by calculating the average energy over each 40- (dark green) or 10- (light green) residue bin (dark green). (E) Partial density of protein (blue) and lipids (black) in the simulation box. (F) Rmsd of the protein measured over 100 ns of simulation time shows a stable structure.
We analyzed each mutation in relation to its severity in terms of causing advanced bone age, poor Prader scores, and elevated MAPs. Patients with mutations of R374 showed high Prader scores of 4, advanced bone age, and severe hypertension. Structural analysis showed that R374, a conserved residue across the cytochrome family, creates an ion-pair interaction with E371 at the end of the K-helix. The helix continues into a loop that forms a part of the heme-binding site. The ion pair R374-E371 is responsible for maintaining the heme-binding site, and its mutation is therefore predicted to be disruptive (Fig. 2C).
However, unlike the CYP21A2 gene, not all mutations within the heme-binding site of CYP11B1 yield a severe phenotype. For example, residue R448, located on a loop between the meander region and L-helix, forms a part of the heme-binding site. It creates hydrogen bonds with the propionate tail of heme, the backbone atoms of L132, and the side chain of N133 (Fig. 2 D–F). However, whereas its mutation is expected to result in a loss of these hydrogen bonds, the clinical phenotype depends on whether the mutant residue is Cys, His, or Pro. The R448C mutation was associated with profoundly advanced bone age and severe hypertension, and the Prader scores of the R448C and R448H were 4/5. In contrast, mutation to Pro, which has a rigid backbone structure, preserves the local structure around the propionate tails. Thus, R448P was very mild, with a Prader score of 1.
Certain mutations were also found to adversely affect enzyme stability, and were therefore not well tolerated. L299 is a conserved residue located on the turn between the H- and J-helices, and its mutation to a more rigid Pro side chain was found to decrease flexibility of the turn, thus impairing enzyme stability (Fig. 2G). Patients homozygous for this mutation showed Prader scores between 4 and 5, advanced bone ages, and high MAPs. Another mutation, G267S, likewise produced severe virilization (Prader score 5) and marked increases in bone age (MAPs not available). The residue G267S is located on the G-helix, and its mutation to Ser resulted in lost flexibility, as side chains of the bulkier Ser did not fit between the G- and J-helices. This caused steric clashes with residues on J-helix, significantly impairing enzyme stability (Fig. 2H).
In addition to mutations affecting the heme-binding region or enzyme stability, we also identified a mutation, W116C, which affects substrate-binding. W116 is located on B′-helix, with its aromatic indole side chain surrounded by the aromatic side chains of F130, F231, W260, F487, and hydrophobic side chain of L113. These residues together form the roof of the binding site of the aromatic substrate, and a mutation to Cys was found to disrupt the substrate-binding site (Fig. 2I). CAH patients with this mutation had Prader scores of 4 and were severely hypertensive, but one patient had an unexplained normal bone age.
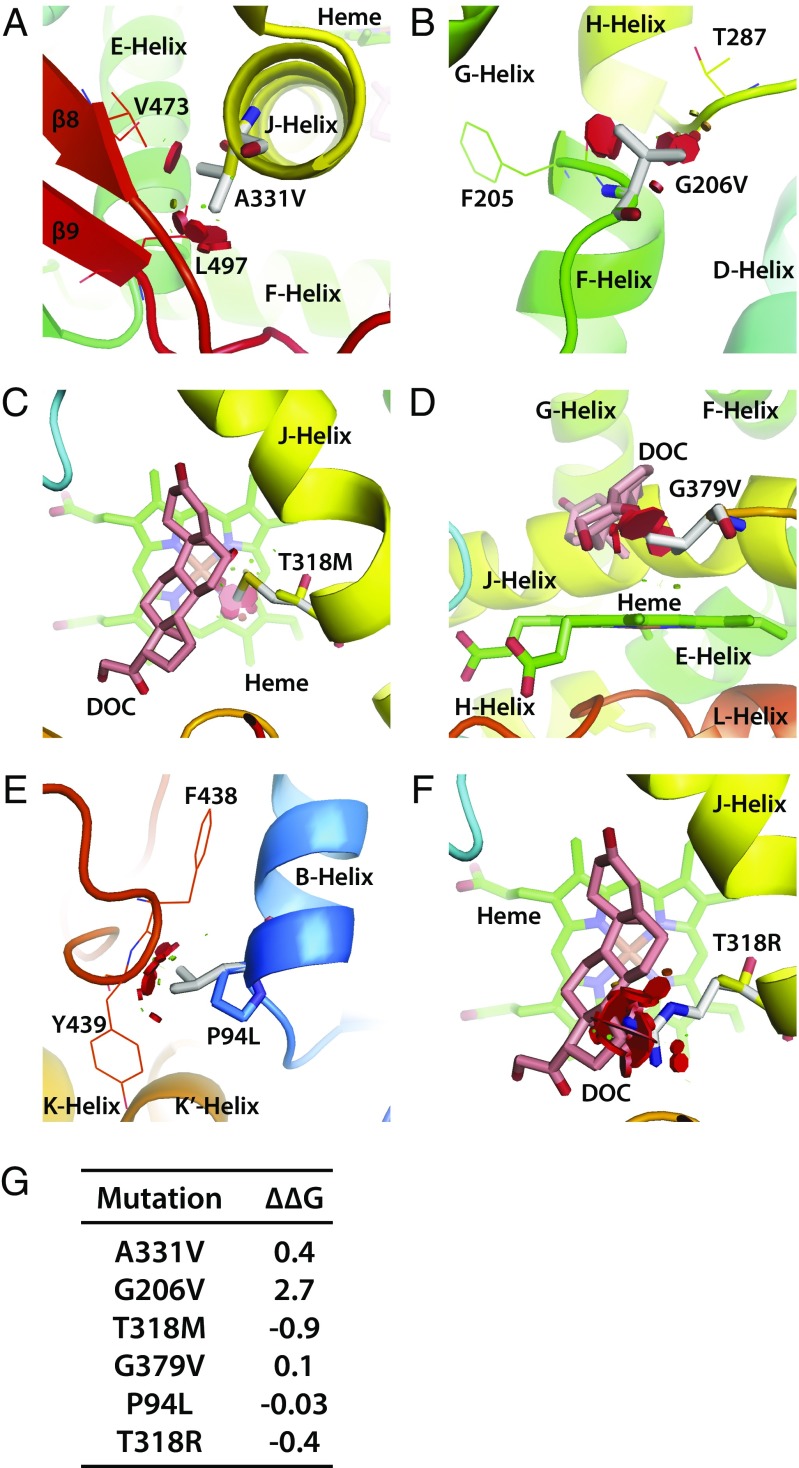
We also identified mutations that were not as severe, wherein 11β-hydroxylase structure was not as adversely affected in silico. For example, we found that the end of the J-helix contained a conserved residue, A331, which when mutated to Val, clashed sterically with residues on the β5-sheet (Fig. 3A). Patients with A331V displayed Prader scores between 2 and 4, modest increases in bone age, and normal MAPs. Another relatively “mild” mutation was G206V, in which patients had Prader scores of 3 but with normal MAPs. The highly conserved and flexible residue G206 is located at the end of the E-helix. When mutated to Val, the side chains clashed sterically with F205 and T287, but only mildly impairing enzyme stability (Fig. 3B).
Fig. 3.
Modeling of CYP11B1 mutations that do not yield a severe phenotype. A331 is a conserved residue at the end of the J-helix. When mutated to Val, it clashes sterically with residues on β5 sheet (A). G206 is located at the end of the E-helix. When mutated to Val, the side chains clash sterically with F205 and T287, but only mildly impairing enzyme stability (B). Both mutations yielded mild phenotypes. With certain mutations the three clinical parameters, Prader scores, bone age, and MAP, did not match. T318 is a highly conserved residue located on the J-helix. Hydrophobic side chains of the mutated Met residue sterically clash with heme to obstruct the ligand-binding site (C). G379, located on a loop between K-helix and β4-sheet, is positioned adjacent to the substrate-binding site. The isopropyl side chain of the mutated residue Val obstructs and thus misaligns the substrate (D). P94, located on the B-helix, when mutated to Leu, causes the hydrophobic side chain to clash sterically with the loop between the α17- and α18-helices. The rigid amino acid side chain also maintains the structural conformation between B′-helix and β2-sheet (E). With the T318R mutation, the charged guanidinium side chains of Lys clash sterically with heme to obstruct the ligand-binding site (F). (G) The change (∆∆G kcal/mol) in protein stability upon mutation of each residue. The disks represent the pairwise atomic van der Waals radii overlap. Green disks represent almost in contact or slightly overlapping, and red disks represent significant overlap.
We also investigated mutations for which severity of the three clinical parameters, namely Prader scores, bone age, and MAP, did not correlate with the structural disruption caused by mutations. Mutation of T318, a highly conserved residue located on the J-helix, to Met resulted in a Prader score of 4, but with normal bone age and mild hypertension. Computationally, the hydrophobic side chains clashed sterically with heme to obstruct the ligand-binding site, but the enzyme appeared by and large intact and stable (Fig. 3C). In contrast, G379, located on a loop between the K-helix and β4-sheet, is positioned adjacent to the substrate-binding site. The isopropyl side chain of the mutated residue Val was found to obstruct and thus misalign the substrate, again without severely affecting the enzyme (Fig. 3D). Clinically, this mutation was associated with advanced bone age but relatively mild hypertension.
Two Prader 4 mutations, namely P94L and T318R, for which we have limited clinical information in our cohort, also deserve mention. The P94 residue is located on the B-helix. When mutated to Leu, the hydrophobic side chain clashes sterically with the loop between the α17- and α18-helices. The rigid amino acid side chain also maintains the structural conformation between the B′-helix and β2-sheet (Fig. 3E). In case of the T318R mutation, the charged guanidinium side chains of Lys clashed sterically with heme to obstruct the ligand-binding site (Fig. 3F).
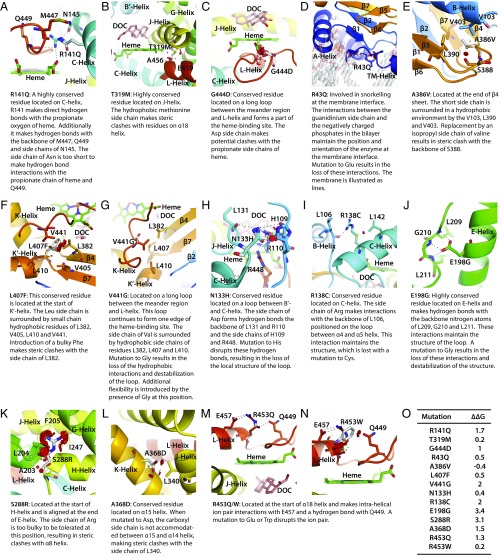
Fig. 4 is a compendium of structural changes induced by mutations that constitute compound heterozygote genotypes. The overall phenotypic severity in compound heterozygotes will depend on the relative contribution of each mutated protein. We also provide details of structural changes induced by mutations published by others (Figs. S2 and S3). Finally, two nonsense mutations, wherein the protein produced should be truncated, namely homozygous R141X and W260X mutations, were found to be associated with expectedly high Prader scores of 4/5. Similarly, patients with truncated frameshift mutations, namely F406Pfs*19 and Q19Afs*21, also had high Prader scores ranging from 3 to 5. However, the noted MAPs for all four genotypes were surprisingly normal.
Fig. 4.
Modeling of CYP11B1 mutations harbored by compound heterozygotes. The following mutations were modeled: R141Q (A); T319M (B); G444D (C); R43Q (D); A386V (E); L407F (F); V441G (G); N133H (H); R138C (I); E198G (J); S288R (K); A368D (L); R453Q (M); and R453W (N). (O) The change (∆∆G kcal/mol) in protein stability upon mutation of each residue. The disks represent the pairwise atomic van der Waals radii overlap. Green disks represent almost in contact or slightly overlapping, and red disks represent significant overlap.
Fig. S2.
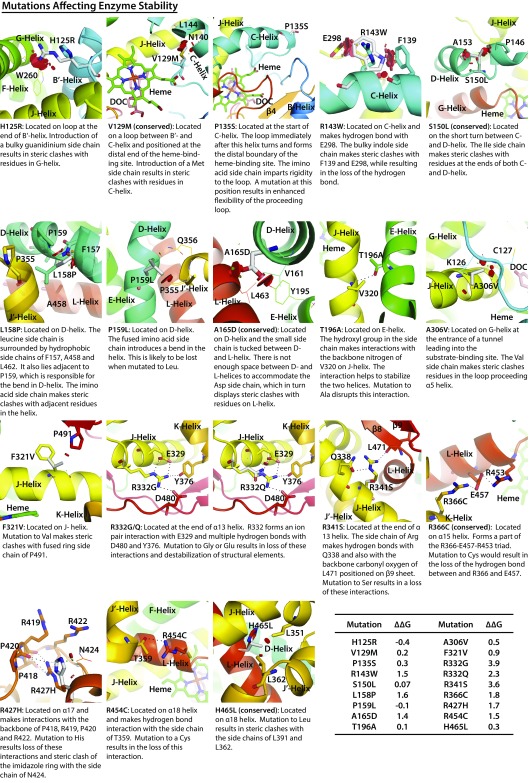
Modeling of CYP11B1 mutations affecting enzyme stability. The following mutations were modeled: H125R, V129M, P135S, R143W, S150L, L158P, P159L, A165D, T196A, A306V, F321V, R332G, R332Q, R341S, R366C, R427H, R454C, and H465L. Also shown is the change (∆∆G kcal/mol) in protein stability upon mutation of each residue. The disks represent the pairwise atomic van der Waals radii overlap. Green disks represent almost in contact or slightly overlapping, and red disks represent significant overlap.
Fig. S3.
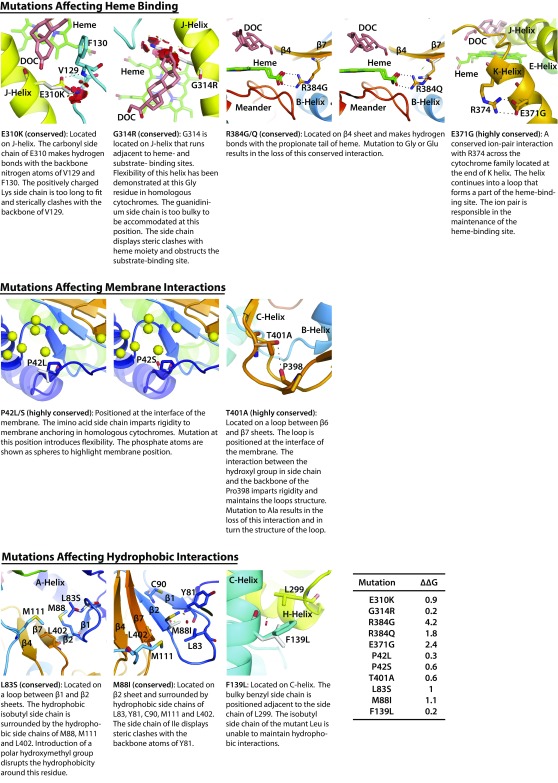
Modeling of CYP11B1 mutations affecting heme-binding, membrane, or hydrophobic interactions. CYP11B1 mutations affecting heme-binding (E310K, G314R, R384G, R384Q, and E371G), membrane (P42L, P42S, and T401A), and hydrophobic (L83S, M88I, and F139L) interactions were modeled. Also shown is the change (∆∆G kcal/mol) in protein stability upon mutation of each residue. The disks represent the pairwise atomic van der Waals radii overlap. Green disks represent almost in contact or slightly overlapping, and red disks represent significant overlap.
Discussion
This study provides a detailed description of the largest international, heterogeneous cohort of 108 CAH patients with 11β-hydroxylase deficiency from 11 countries (Fig. 1B). In contrast to CAH resulting from 21-hydroxylase deficiency, the prevalence of which is particularly high in Eastern European Jews, 11β-hydroxylase deficiency is common in the Middle East and North Africa. We report a spectrum of clinical severities across mutation types. Because performing in vitro expression studies for this large number of mutations is an enormous effort, we instead attempted, using computational modeling, to correlate the induced in silico changes in 11β-hydroxylase structure with clinical phenotype, which is predictive, not confirmatory.
We found that 41 compound heterozygotes or homozygotes for select missense or nonsense mutations—namely P49L, R141Q, W260X, G267S, L299P, T318M, T318R, A331V, Q356X, A368D, R374Q, V441G, G444D, G446V, and R448H—present with moderate to severe classic CAH because of 11β-hydroxylase, confirming prior results (3, 14–17, 22–24). These patients were mainly from Croatia, Tunisia, Africa, Turkey, and Saudi Arabia. Prader scores for females were consistently 4 or 5, but serum 11-deoxycorticosterone, 11-deoxycortisol, and androstenedione levels, albeit elevated, were highly variable. Bone age was advanced in most cases, but hypertension was diagnosed only in ∼59% of patients.
Even with the concordance noted above, we failed to establish a previously documented phenotype in the N133H/T319M mutation (3, 25). Based on in vitro data, advanced bone age, and a Tanner score of 2, a N133H/T319M female was previously assigned a diagnosis of nonclassic CAH because of 11β-hydroxylase. We found instead that three patients of Egyptian origin, two males and one female with the identical genotype N133H/T319M, present with classic disease, which we have documented both clinically and hormonally. Each patient had grossly elevated serum 11-deoxycortisol and androstenedione levels and advanced bone age. The one female had Prader score 4, and one patient had hypertension.
Comparison of our International Consortium on Rare Steroid Disorders with our mainly United States-based, 1,507-patient, 21-hydroxylase deficiency cohort (6) reveals fundamental differences in clinical phenotype. First, 11β-hydroxylase deficiency is far less common, and is localized to the Middle East and North Africa. Second, females with 11β-hydroxylase deficiency are more virilized than those with 21-hydroxylase deficiency. A Prader score of 5, indicating complete masculinization of the external genitalia, including a urethral opening at the tip of the phallus, is more frequent in 11β-hydroxylase deficiency than in 21 hydroxylase deficiency. The extent of masculinization, however, correlates poorly with the accompanying gross hyperandrogenemia. Third, and related to virilization, it is more common that a 46, XX 11β-hydroxylase–deficient newborn female is assigned a male gender. In our cohort, most such 46, XX females, misassigned to the male gender at birth, were reared as males, although some were subsequently reassigned to the female gender. Fourth, 21-hydroxylase deficiency is not associated with hypertension, whereas 59% of 11β-hydroxylase–deficient patients in whom blood pressures were measured were hypertensive (26, 27). Correlations between 11-deoxycorticosterone and hypertension were nonetheless poor. Fifth, whereas 17-hydroxyprogesterone is a gold standard for the biochemical diagnosis of 21-hydroxylase deficiency (1), we find that the most robust serum marker for 11β-hydroxylase deficiency is the accumulated substrate 11-deoxycortisol. However, as this steroid is not routinely measured, we predict that 11β-hydroxylase deficiency is underdiagnosed. Finally, one patient with full blown CAH was interestingly a heterozygote with a common mutation G379V. Possibilities for disease in this patient include a genotyping error, a cryptic intronic mutation, or a dominant-negative effect.
To study genotype–structure–phenotype concordance, we examined whether mutations in the CYP11B1 gene could induce specific changes in 11β-hydroxylase that would correlate with CAH severity. We have recently used computational modeling to establish structural aberrations in 21-hydroxylase induced by mutations that explained distinct phenotypes, namely salt-wasting, simple virilizing, and nonclassic CAH (7). Most notably, missense mutations that affected residues within 5 Å of the heme- or substrate-binding site or altered protein stability led to salt-wasting CAH, whereas mutations affecting conserved hydrophobic patches or altering the transmembrane region caused simple virilizing disease. Mild nonclassic CAH resulted from interference in oxidoreductase interactions, salt-bridge and hydrogen-bonding networks, and nonconserved hydrophobic clusters.
Here, a similar evaluation using a CYP11B1 model, derived from the crystal structure of CYP11B2, revealed three groups of mutations that caused severe disease. Mutations that altered the heme-binding site, such as R374W and R448H/C, resulted in high Prader scores (4/5), severe hypertension, and profoundly advanced bone age. Similar clinical manifestations arose from mutations that affected enzyme stability, such as L299P and G267S, or interfered with substrate-binding, notably W116C. With that said, there were mutations where the three key clinical parameters, Prader scores, bone age, and MAP, did not correlate with structural disruption caused by mutations. For example, the T318M mutation was associated with a Prader score of 4, but with normal bone age and mild hypertension. Similarly, G379V appeared to be associated with advanced bone age but relatively mild hypertension.
In summary, we report that CAH caused by 11β-hydroxylase deficiency is far less frequent than that arising from 21-hroxylase deficiency, thus requiring an international collaborative cohort to obtain sufficient number of patients for analysis. The disease was most prevalent in Tunisia (60 patients in a population of 11 million), where consanguinity is common (Table 1). However, as 11-deoxycortisol is rarely measured, the overall prevalence could in fact be an underestimate. Our cohort of 108 patients nonetheless revealed greater morbidity than 21-hydroxylase deficiency. There was profound masculinization, significant early growth arrest, and overt hypertension. Finally, although we were able to correlate structural changes of certain highly disruptive mutations of 11β-hydroxylase with the severity of CAH, unlike 21-hydroxylase deficiency, this strategy could not be applied universally.
Methods
The studies were approved by the Icahn School of Medicine’s Institutional Review Board (IRB) (PI: M.I.N), and by local hospital IRBs, as confirmed by on-site investigators comprising the International Consortium of Rare Steroid Disorders. Informed consent was obtained. To those who agreed to be part of the Consortium, we sent data entry forms, asking for information on demographics, consanguinity, clinical presentation, adrenal steroid hormone levels, and CYP11B1 mutations, at the time of diagnosis. We also requested information on bone age, Prader scores, and baseline blood pressure measurements. We received entry forms from investigators from 13 nations with data on 108 patients with CYP11B1 mutations confirming 11β-hydroxylase deficiency (Fig. 1B and Table S1). Of these, 66 patients have complete datasets. Leading endocrinologists in Australia, Canada, Chile, Dominican Republic, Finland, France, Greece, Italy, Japan, Qatar, Spain, Sweden, Switzerland, United Arab Emirates, and United Kingdom reported no CAH patients with 11β-hydroxylase deficiency. Similarly and to our surprise, seven leading endocrinologists in California, Connecticut, Michigan, New York, Pennsylvania, and Texas did not have a single patient.
Each mutation was analyzed for its ability to disrupt the CYP11B1 enzyme using molecular dynamics modeling (SI Methods). The change (∆∆G kcal/mol) in protein stability upon mutation of a single residue was calculated using the Molsoft ICM-Pro software (www.molsoft.com). The free energy of the unfolded and misfolded states is approximated by a sum of the residue-specific energies that were derived empirically using experimental data. Mutation of a given residue was followed by Monte Carlo simulations with flexible side chains for the mutated residue and its neighboring residues. The rest of the protein structure was considered rigid. A positive energy value thus indicates that the mutation is likely to be destabilizing (28).
SI Methods
The sequences of human steroidogenic cytochrome P450s were downloaded from UniProt (CYP11B1: id P15538; CYP11B2: id P19099; CYP11A1: id 05108; CYP17A1: id P05093; CYP19A1: id P11511; and CYP21A2: id P08686) and aligned using Clustal Omega (29, 30). A consensus of secondary structure elements was obtained by performing a structural alignment of CYP17A1 (PDB ID Code 3RUK), CYP19A1 (PDB ID code 3S79), and CYP11B2 (PDB ID code 4DVQ) crystal structures. The secondary structure prediction highlighted that the first 35 residues constitute the transmembrane helix, for which no structural template was available. Residues 1–35 were built separately as α-helix and subsequently attached to the model following membrane insertion. The remaining CYP11B1 model was constructed using the CYP11B2 structure as the template and exhibited 93% sequence similarity over 478 residues. The model was generated using the Modeler v9.16 (31) and stereochemical parameters checked using PROCHECK and PROSA (32, 33). The final model was chosen based on the low-energy function and low Cα rmsd overlap between the template and the model. The coordinates of heme and substrate were retained from the template and built in situ. Several rounds of minimization were performed to relieve steric clashes between side chains. The final Cα rmsd between the template and the model was 0.45 Å.
To determine how CYP11B1 orients on the membrane, a self-assembly protocol was used (34). The transmembrane α-helix (residues 1–35), was converted to a coarse-grain model and preinserted in a pre-equilibrated dipalmitoylphosphatidylcholine (DPPC) bilayer. DPPC was used as a representative of the phosphatidylcholine lipids family. The remaining model (residues 36–503) was converted into a coarse-grained representation and attached to the transmembrane helix. The system was simulated for 1 μs, following which the coarse-grained model was converted back to atomistic resolution using reverse conversion protocol (35) and subsequently used for further studies.
Molecular dynamic simulations were performed using Gromacs 4.6 as engine (36). The protein forcefield and DPPC lipid parameters were adopted from a Martini forcefield (37). An elastic bond force constant of 500 kJ⋅mol−1⋅nm−2 was used to maintain an elastic network model between a cut-off of 0.5 and 0.9 nm. The simulation was carried out in a 13.5 × 13.5 × 12.7-nm3 box that contained 567 DPPC lipids and 12,718 coarse-grained water molecules using the NPT ensemble. A Berendsen thermostat was used to regulate the temperature at 325 K and pressure at 1 bar. An isothermal compressibility of 3:5 105 bar1 was applied in all box directions. The van der Waals and Coulombic interaction cut-offs were set at 1.2 nm and LINCS algorithm used to constrain all bond lengths. The electrostatics was calculated using a shift method in connection with the periodic boundary conditions. The time step was set at 40 fs and simulation was run for 1 μs, following which the system (protein + DPPC lipids) were converted to get atomistic details of the self-assembly using a protocol of Stansfeld and Sansom (35). The coarse-grained structure was initially mapped to a fine-grained structure and then back-mapped to atomistic resolution. Within the simulated time, the protein peripherally embeds on to one leaflet of the bilayer. A short burst of energy minimization was carried out to relieve any steric clashes between the lipids and the protein side chains. The atomistic system is subjected to two rounds of 1-ns NVT (number, volume, temperature) and NPT (number, pressure, temperature) equilibrations each, with time steps of 0.002 ns, using a V-rescale algorithm for temperature coupling and Nose–Hoover temperature coupling and Parrinello–Rahman for pressure coupling in the NPT protocol. The production run was carried out for 100 ns without any restraints with a time step of 0.002 ns.
Acknowledgments
The study was supported by the Maria I. New Children Hormone Foundation; and NIH Grants DK80459 (to M.Z. and S.L.), AG40132 (to M.Z.), AR06592 (to M.Z.), and AR06066 (to M.Z.). S.H. received funding from a University College London Excellence fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621082114/-/DCSupplemental.
References
- 1.New MI, Lekarev O, Mancenido D, Parsa A, Yuen T. Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. In: New MI, et al., editors. Genetic Steroid Disorders. Academic; San Diego: 2014. pp. 29–51. [Google Scholar]
- 2.Mancenido D, New MI. The history of prenatal diagnosis of congenital adrenal hyperplasia. In: New MI, et al., editors. Genetic Steroid Disorders. Academic; San Diego: 2014. pp. 53–62. [Google Scholar]
- 3.White PC. Steroid 11β-hydroxylase deficiency and related disorders. In: New MI, et al., editors. Genetic Steroid Disorders. Academic; San Diego: 2014. pp. 71–85. [Google Scholar]
- 4.Chua SC, et al. Cloning of cDNA encoding steroid 11 beta-hydroxylase (P450c11) Proc Natl Acad Sci USA. 1987;84(20):7193–7197. doi: 10.1073/pnas.84.20.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zachmann M, Tassinari D, Prader A. Clinical and biochemical variability of congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. A study of 25 patients. J Clin Endocrinol Metab. 1983;56(2):222–229. doi: 10.1210/jcem-56-2-222. [DOI] [PubMed] [Google Scholar]
- 6.New MI, et al. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 2013;110(7):2611–2616. doi: 10.1073/pnas.1300057110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haider S, et al. Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc Natl Acad Sci USA. 2013;110(7):2605–2610. doi: 10.1073/pnas.1221133110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speiser PW, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37(4):650–667. [PMC free article] [PubMed] [Google Scholar]
- 9.Tadmouri GO, et al. Consanguinity and reproductive health among Arabs. Reprod Health. 2009;6:17. doi: 10.1186/1742-4755-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadat M, Ansari-Lari M, Farhud DD. Consanguineous marriage in Iran. Ann Hum Biol. 2004;31(2):263–269. doi: 10.1080/03014460310001652211. [DOI] [PubMed] [Google Scholar]
- 11.Hafez M, et al. Consanguineous matings in the Egyptian population. J Med Genet. 1983;20(1):58–60. doi: 10.1136/jmg.20.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alper OM, et al. Consanguineous marriages in the province of Antalya, Turkey. Ann Genet. 2004;47(2):129–138. doi: 10.1016/j.anngen.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 13.al-Abdulkareem AA, Ballal SG. Consanguineous marriage in an urban area of Saudi Arabia: Rates and adverse health effects on the offspring. J Community Health. 1998;23(1):75–83. doi: 10.1023/a:1018727005707. [DOI] [PubMed] [Google Scholar]
- 14.Motaghedi R, et al. Update on the prenatal diagnosis and treatment of congenital adrenal hyperplasia due to 11beta-hydroxylase deficiency. J Pediatr Endocrinol Metab. 2005;18(2):133–142. doi: 10.1515/jpem.2005.18.2.133. [DOI] [PubMed] [Google Scholar]
- 15.Dumic K, et al. Two novel CYP11B1 gene mutations in patients from two croatian families with 11 β-hydroxylase deficiency. Int J Endocrinol. 2014;2014:185974. doi: 10.1155/2014/185974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandemir N, et al. Novel and prevalent CYP11B1 gene mutations in Turkish patients with 11-beta hydroxylase deficiency. J Steroid Biochem Mol Biol. 2017;165(Pt A):57–63. doi: 10.1016/j.jsbmb.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Krone N, et al. Analyzing the functional and structural consequences of two point mutations (P94L and A368D) in the CYP11B1 gene causing congenital adrenal hyperplasia resulting from 11-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(7):2682–2688. doi: 10.1210/jc.2006-0209. [DOI] [PubMed] [Google Scholar]
- 18.Soardi FC, et al. Novel mutations in CYP11B1 gene leading to 11 beta-hydroxylase deficiency in Brazilian patients. J Clin Endocrinol Metab. 2009;94(9):3481–3485. doi: 10.1210/jc.2008-2521. [DOI] [PubMed] [Google Scholar]
- 19.Dumic K, et al. Steroid 11-beta hydroxylase deficiency caused by compound heterozygosity for a novel mutation in intron 7 (IVS 7 DS+4A to G) in one CYP11B1 allele and R448H in exon 8 in the other. Eur J Pediatr. 2010;169(7):891–894. doi: 10.1007/s00431-009-1110-1. [DOI] [PubMed] [Google Scholar]
- 20.Bin-Abbas B, et al. Divergent gender identity in three siblings with 46XX karyotype and severely virilizing congenital adrenal hyperplasia caused by a novel CYP11B1 mutation. Endocr Pract. 2014;20(10):e191–e197. doi: 10.4158/EP14179.CR. [DOI] [PubMed] [Google Scholar]
- 21.Ben Charfeddine I, et al. Two novel CYP11B1 mutations in congenital adrenal hyperplasia due to steroid 11β hydroxylase deficiency in a Tunisian family. Gen Comp Endocrinol. 2012;175(3):514–518. doi: 10.1016/j.ygcen.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Alqahtani MA, et al. A novel mutation in the CYP11B1 gene causes steroid 11β-hydroxylase deficient congenital adrenal hyperplasia with reversible cardiomyopathy. Int J Endocrinol. 2015;2015:595164. doi: 10.1155/2015/595164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curnow KM, et al. Mutations in the CYP11B1 gene causing congenital adrenal hyperplasia and hypertension cluster in exons 6, 7, and 8. Proc Natl Acad Sci USA. 1993;90(10):4552–4556. doi: 10.1073/pnas.90.10.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krone N, et al. Congenital adrenal hyperplasia due to 11-hydroxylase deficiency: Functional characterization of two novel point mutations and a three-base pair deletion in the CYP11B1 gene. J Clin Endocrinol Metab. 2005;90(6):3724–3730. doi: 10.1210/jc.2005-0089. [DOI] [PubMed] [Google Scholar]
- 25.Joehrer K, et al. CYP11B1 mutations causing non-classic adrenal hyperplasia due to 11 beta-hydroxylase deficiency. Hum Mol Genet. 1997;6(11):1829–1834. doi: 10.1093/hmg/6.11.1829. [DOI] [PubMed] [Google Scholar]
- 26.Rösler A, Leiberman E, Cohen T. High frequency of congenital adrenal hyperplasia (classic 11 beta-hydroxylase deficiency) among Jews from Morocco. Am J Med Genet. 1992;42(6):827–834. doi: 10.1002/ajmg.1320420617. [DOI] [PubMed] [Google Scholar]
- 27.Kandemir N, Yordam N. Congenital adrenal hyperplasia in Turkey: A review of 273 patients. Acta Paediatr. 1997;86(1):22–25. doi: 10.1111/j.1651-2227.1997.tb08824.x. [DOI] [PubMed] [Google Scholar]
- 28.Rashin AA, Rashin BH, Rashin A, Abagyan R. Evaluating the energetics of empty cavities and internal mutations in proteins. Protein Sci. 1997;6(10):2143–2158. doi: 10.1002/pro.5560061009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sievers F, Higgins DG. Clustal omega. Curr Protoc Bioinformatics. 2014;48:3.13,1–3.13.16. doi: 10.1002/0471250953.bi0313s48. [DOI] [PubMed] [Google Scholar]
- 30.Sievers F, Higgins DG. Clustal omega, accurate alignment of very large numbers of sequences. Methods Mol Biol. 2014;1079:105–116. doi: 10.1007/978-1-62703-646-7_6. [DOI] [PubMed] [Google Scholar]
- 31.Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics. 2016;54:5.6.1–5.6.37. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8(4):477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 33.Wiederstein M, Sippl MJ. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35(Web Server issue):W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cojocaru V, Balali-Mood K, Sansom MS, Wade RC. Structure and dynamics of the membrane-bound cytochrome P450 2C9. PLOS Comput Biol. 2011;7(8):e1002152. doi: 10.1371/journal.pcbi.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stansfeld PJ, Sansom MS. From coarse grained to atomistic: A serial multiscale approach to membrane protein simulations. J Chem Theory Comput. 2011;7(4):1157–1166. doi: 10.1021/ct100569y. [DOI] [PubMed] [Google Scholar]
- 36.Pronk S, et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29(7):845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH. The MARTINI force field: Coarse grained model for biomolecular simulations. J Phys Chem B. 2007;111(27):7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 38.Cerame BI, et al. Prenatal diagnosis and treatment of 11β-hydroxylase deficiency congenital adrenal hyperplasia resulting in normal female genitalia. J Clin Endocrinol Metab. 1999;84(9):3129–3134. doi: 10.1210/jcem.84.9.5976. [DOI] [PubMed] [Google Scholar]