Fungicide–flavonol interaction in honey bee health

Honey bee. Image courtesy of Wikimedia Commons/John Severns.
Just as food–drug interactions can influence human metabolism, pesticides can interact with plant-derived compounds and impair honey bee physiology. Wenfu Mao et al. (pp. 2538–2543) tested the effects of diets containing the pollen-derived flavonol quercetin, which is typically consumed at vanishingly low levels during the first 3 days of larval development, on newborn bee (Apis mellifera) larvae. Compared with larvae that were fed diets without quercetin, newborn larvae raised on quercetin exhibited increased expression of genes encoding cytochrome P450 monooxygenases, which are quercetin-detoxifying enzymes typically found in older larvae and adult bees. Computer-based molecular simulations revealed that six triazole fungicides found in American beehives snugly occupied the catalytic site of CYP9Q1, a quercetin-metabolizing P450 enzyme. Feeding quercetin together with the triazole fungicide myclobutanil to adult worker bees reduced the expression of five mitochondrion-associated genes involved in energy generation; laboratory assays of P450 in intact midguts of worker bees revealed that myclobutanil reduced quercetin metabolism from 95% to 82%. Similarly, compared with worker bees that were fed quercetin alone, bees that consumed both quercetin and myclobutanil had significantly decreased ATP levels in the thorax; thoracic muscles sustain foraging flights and generate colony-warming heat during winter. According to the authors, in vivo studies might help establish whether triazole fungicides hamper honey bees’ ability to derive energy from natural food sources through unfavorable interactions with plant compounds. — P.N.
Designer enzymes
Natural enzymes are constructed from a pool of 20 genetically encoded amino acids, and enzymes typically require one or more additional small organic molecules or metal ions to function. In addition, amino acids can be added to enzymes after protein translation, and these noncanonical amino acids (NCA) can extend the catalytic repertoire of the original enzymes. Using a chemical mutagenesis strategy, Claire Windle et al. (pp. 2610–2615) incorporated 13 different NCA side chains at 12 different positions within the active site of a Staphylococcus aureus N-acetylneuraminic acid lyase to alter its function. The authors found that a modified N-acetylneuraminicacid lyase with a 2,3-dihydroxypropyl cysteine (Dpc) residue at position 190 had increased activity for the aldol reaction of erythrose with pyruvate, compared with a wild-type enzyme. Analysis of enzyme kinetics revealed that the altered substrate specificity of the Dpc-modified enzyme could not be replicated by inserting any of the 20 genetically encoded amino acids at position 190. Structural and modeling studies revealed that the shape and functionality of the Dpc side chain remodeled the active site to enable a change in substrate specificity. According to the authors, improved methods for enzyme redesign and the availability of a variety of NCA side chains could facilitate the engineering of enzymes with catalytic functions not found in nature. — L.C.
Potential treatment for synucleinopathies
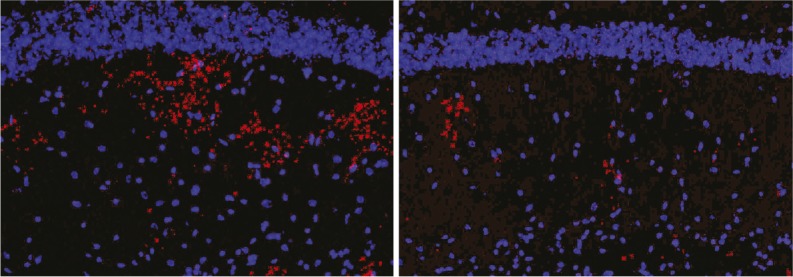
GCS inhibitor reduces α-synuclein immunoreactivity (red) in brain tissues of mice with GBA-related synucleinopathy.
Mutations in the glucocerebrosidase gene (GBA) represent the most common genetic risk factor for Parkinson’s disease (PD), and heighten the risk of PD and other synucleinopathies, a group of neurodegenerative diseases characterized by accumulation of the α-synuclein protein. GBA mutations reduce the normal breakdown of glucocerebroside lipid substrates, such as glucosylceramide and glucosylsphingosine, which can accumulate and contribute to the progression of synucleinopathies. However, how GBA mutations affect synucleinopathies such as PD remains unclear. Pablo Sardi et al. (pp. 2699–2704) used two mouse models of Gaucher-related synucleinopathy to study the potential therapeutic effects of a drug that inhibits the glucosylceramide synthase (GCS) enzyme. A presymptomatic mouse cohort was administered the GCS inhibitor drug for around 10 months, while a postsymptomatic mouse cohort received the drug for around 7 months. The results revealed that prolonged drug treatment with the GCS inhibitor decreased levels of glucosylceramide and glucosylsphingosine in the central nervous system (CNS), reduced buildup of α-synuclein in the CNS, and ameliorated memory deficits in the mice. According to the authors, limiting the synthesis of glucocerebrosides might represent a therapeutic strategy for the treatment of GBA-related synucleinopathies, including PD. — C.S.
Emotions and cognitive processing
Brain networks that contribute to conscious experiences.
Emotions have long been thought to be innately programmed by subcortical brain regions, whereas cognitive states of consciousness, such as those related to perception and memory, are believed to be rooted in various circuits involving cortical brain regions. Joseph LeDoux and Richard Brown (pp. E2016–E2025) challenge the conventional view that separate brain regions govern emotional and cognitive consciousness, and argue that emotional experiences emerge not from subcortical circuits, but from the cortical network responsible for producing cognitive states of consciousness. To illustrate their thesis, the authors build on higher-order theory, a leading theory of consciousness, with a focus on fear. The conscious experience of fear, the authors contend, involves the coalescence of various inputs in cortical cognitive circuits, including neural signals from sensory, memory, threat detection, and arousal systems in the brain as well as other body responses, to form the emotional experience. The authors’ resulting theory accounts for differences between the emotional and cognitive states of consciousness by accounting for variation in information processed by cortical brain circuits. According to the authors, the proposed theory might help illuminate how other emotions arise from cortical networks. — C.S.
Mouse model for brain effects of meditation

Experimental chamber for testing anxiety-related behavior.
Mindfulness meditation has been linked to reduced stress hormone levels, remodeling of white matter tracts around the brain’s anterior cingulate cortex (ACC), and reduced anxiety in previous studies. Using optogenetic tools, Aldis Weible et al. (pp. 2532–2537) tested whether rhythmic stimulation or suppression of ACC activity at frequencies reminiscent of meditation training influences anxiety in mice. Compared with mice that received no intervention and mice in which rhythmic ACC activity was suppressed, mice in which rhythmic ACC activity was induced in a month-long intervention over 20 sessions lasting 30 minutes each exhibited less anxiety, as indicated by their willingness to explore an illuminated environment in a well-established laboratory test of anxiety. Further, reduced anxiety was associated largely with 1 Hz and 8 Hz stimulation, but not with 40 Hz stimulation. Rhythmic stimulation of the ACC appeared not to affect the animals’ spontaneous motor activity, cognitive ability, or memory in an experimental setup, suggesting that the observed effects of rhythmic stimulation might reflect its effect on anxiety. Additional tests may help cement a link between rhythmic ACC stimulation and anxiety, but the study furnishes a potential animal model for the fine-grained analysis of the effects of some forms of meditation on the human brain, according to the authors. — P.N.
Sex bias in ancient migrations
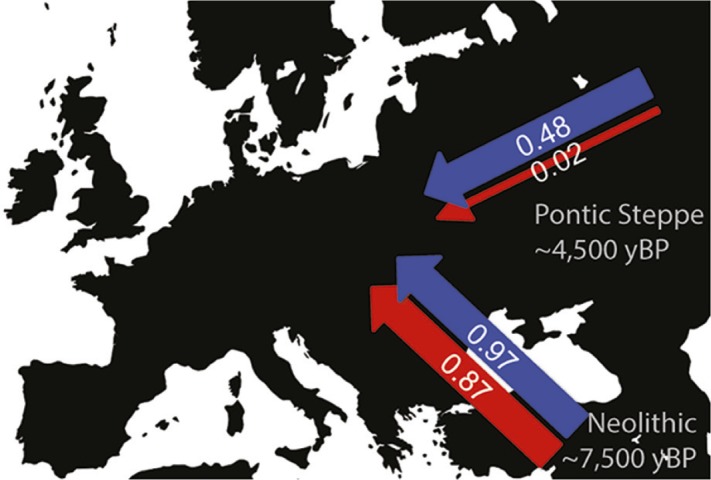
Male (blue) and female (red) contributions during the Early Neolithic and later Pontic Steppe migrations.
Different demographic histories between males and females during major events in prehistory can illuminate the social and cultural factors that give rise to such differences. Amy Goldberg et al. (pp. 2657–2662) used published genetic data comprising hundreds of thousands of genome-wide single-nucleotide polymorphisms from ancient Eurasian populations to determine the extent of sex bias in two major migrations in European prehistory. By comparing the genetic ancestry of the X chromosome with that of autosomes in the descendants of these migrations, the sex bias in each migrating population could be inferred. Anatolian farmers migrated into central Europe approximately 8,000 years ago. In the descendants of this migration, Neolithic central Europeans, the share of Anatolian ancestry in the X chromosome is similar to that in the autosomes, consistent with approximately equal numbers of males and females in the migrating Anatolian population. A second migration from the Pontic-Caspian Steppe occurred approximately 5,000 years ago. In Bronze Age central Europeans descended from this migration, the share of Pontic Steppe ancestry is much lower in the X chromosome than in autosomes, suggesting that migration from the Pontic Steppe was significantly male-biased. According to the authors, the difference in sex bias points toward different social and cultural processes driving the two migrations. — B.D.


