Significance
For many years, the development of adjuvants—compounds that boost the immunogenicity of vaccines—has been an empirical process. Adjuvants inducing a strong humoral immunity are available, but adjuvants directing the development of robust cellular immune responses are still needed. Recently, the C-type lectin receptor Mincle was found to elicit such responses on the recognition of microbial glycolipids, thereby providing a basis for the rational design of new adjuvants. Here we used a multidisciplinary approach, combining chemical synthesis, biology, and molecular modeling to decipher the molecular bases of ligand recognition by the receptor. This led us to synthesize new compounds inducing stronger immune responses than the currently available Mincle ligands that represent new powerful adjuvant molecules.
Keywords: mycobacteria, glycolipid, innate immunity
Abstract
The advances in subunit vaccines development have intensified the search for potent adjuvants, particularly adjuvants inducing cell-mediated immune responses. Identification of the C-type lectin Mincle as one of the receptors underlying the remarkable immunogenicity of the mycobacterial cell wall, via recognition of trehalose-6,6′-dimycolate (TDM), has opened avenues for the rational design of such molecules. Using a combination of chemical synthesis, biological evaluation, molecular dynamics simulations, and protein mutagenesis, we gained insight into the molecular bases of glycolipid recognition by Mincle. Unexpectedly, the fine structure of the fatty acids was found to play a key role in the binding of a glycolipid to the carbohydrate recognition domain of the lectin. Glucose and mannose esterified at O-6 by a synthetic α-ramified 32-carbon fatty acid showed agonist activity similar to that of TDM, despite their much simpler structure. Moreover, they were seen to stimulate proinflammatory cytokine production in primary human and murine cells in a Mincle-dependent fashion. Finally, they were found to induce strong Th1 and Th17 immune responses in vivo in immunization experiments in mice and conferred protection in a murine model of Mycobacterium tuberculosis infection. Here we describe the rational development of new molecules with powerful adjuvant properties.
The remarkable adjuvanticity of the mycobacterial cell wall has been known for more than a century. Complete Freund’s adjuvant (CFA), a water-in-oil emulsion of mycobacterial cell wall components, strongly elicits both humoral and cellular responses and is one of the most potent adjuvants known. Although widely used in animal models, CFA cannot be administered to humans because it is highly toxic and because its individual bioactive molecules are unknown (1). Trehalose-6,6′-dimycolate (TDM), also known as cord factor, is a highly abundant glycolipid in the mycobacterial cell wall that was identified in the 1950s as a very—if not the most—potent immunostimulatory molecule derived from mycobacteria (2, 3). Since then, the immunologic properties of TDM have been studied extensively, and structure-function analyses have resulted in the chemical synthesis of trehalose-6,6′-dibehenate (TDB) (4), an analog in which simpler fatty acids replace the complex mycolic acids (MAs). TDB induces strong cellular immune responses similar to TDM, but has lower toxicity (5, 6); however, the host receptor(s) of TDB and TDM remained unknown until very recently.
In 2009–2010, the monocyte-inducible C-type lectin (Mincle), an FcRγ-associated membrane receptor, was identified as the receptor for TDM (7, 8) and TDB (8). Ligand binding to Mincle leads to phosphorylation of the immunoreceptor tyrosine activation motif (ITAM) of the FcRγ chain and activation of NF-κB via Syk-Card9–Bcl10–Malt1 signaling (9, 10). This Mincle-dependent pathway is required for the in vivo adjuvant activity of TDM and TDB, which is characterized by robust combined Th1 and Th17 responses (8, 9, 11, 12). The crystal structures of the carbohydrate recognition domain (CRD) of bovine Mincle complexed with trehalose (13) and of the human Mincle extracellular domain (14) revealed a canonical C-type primary monosaccharide-binding site centered on a Ca2+ ion (13, 14). On one side of the primary binding site is a secondary binding site for the second glucose residue in the trehalose headgroup, and on the other side is a hydrophobic groove. This arrangement, which is not seen in other C-type CRDs, provides a docking site for a fatty acid harboring at least 10 carbons (13).
Taken together, structural, binding, and mutagenesis data support a model for TDM and TDB binding in which the two glucose units and also, surprisingly, one acyl chain interact directly with the CRD of Mincle (8, 13, 15). The precise molecular mechanisms of the glycolipid–Mincle interaction are far from being completely understood, particularly with respect to how the two complex MAs of TDM are recognized by the receptor. Nonetheless, these overall data provide a structural basis for the further development of adjuvants based on the rational design of Mincle ligands. Indeed, there is growing interest in the development of vaccine adjuvants that direct robust Th1 and Th17 responses to subunit vaccines (16).
In this study, we used a combination of strategies, including identification of the natural Mincle agonist molecules present in the mycobacterial cell wall, bioguided chemical synthesis, and molecular dynamics simulations, to better understand the molecular basis of glycolipid recognition by Mincle. Overall, our data illustrate how a C-type lectin can sense subtle structural features of fatty acids. Glucose and mannose esterified at O-6 by an α-ramified 32-carbon fatty acid have a much simpler structure than TDM, but exhibit similar agonist activity. Moreover, these compounds induce stronger Th1 and Th17 immune responses than TDB in vivo and thus represent powerful adjuvant molecules.
Results
The Mycobacterial Cell Envelope Is a Source of Mincle Ligands.
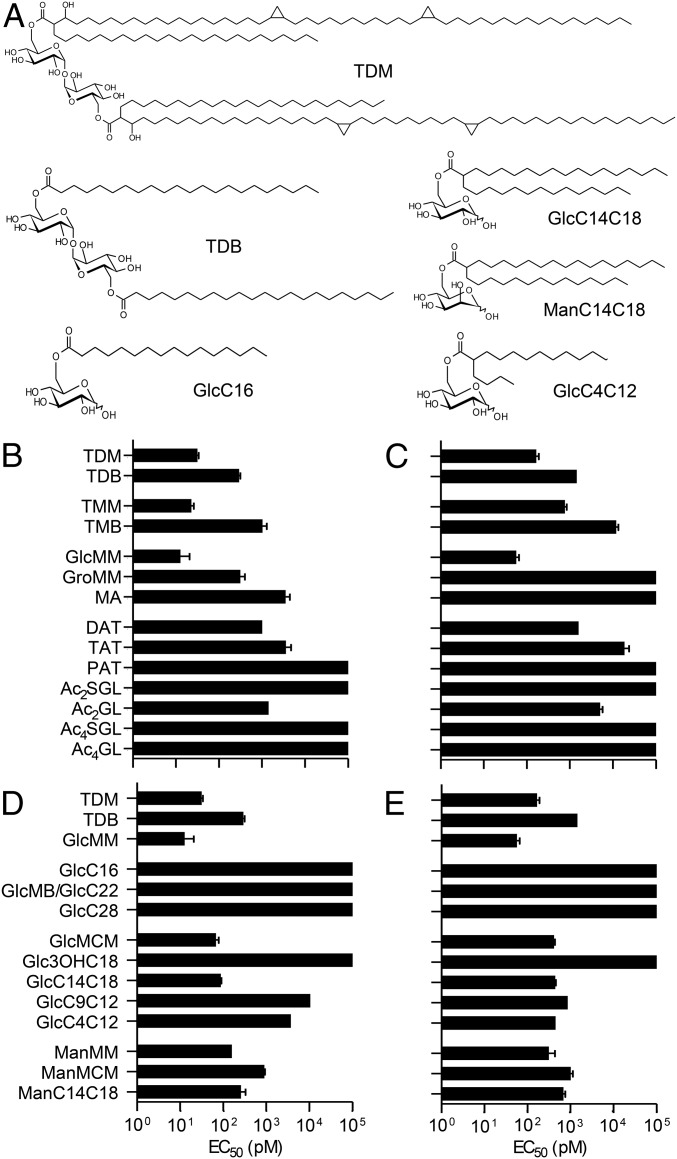
The mycobacterial ligands of Mincle identified so far are trehalose-6,6′-monomycolate (TMM) and TDM (7, 8) (Fig. 1A and SI Appendix, Fig. S1). Both the trehalose and MAs are involved in the interaction with the receptor (13, 14); however, the mycobacterial cell envelope contains many other lipids with structures based on trehalose and/or MAs (17) (SI Appendix, Fig. S1). To tentatively identify other Mincle ligands and to better understand their structure-function relationships, we investigated the capacity of these lipids to induce NF-κB activation in HEK cells expressing human Mincle (HEK-hMincle) or murine Mincle (HEK-mMincle) and an NF-κB–inducible reporter system (secreted alkaline phosphatase). As expected, TDM, its synthetic analog TDB, and TMM (Fig. 1A) induced NF-κB activation in a dose-dependent manner in HEK-hMincle and HEK-mMincle cells, but not in the parent HEK cells (SI Appendix, Fig. S2). The effective concentration (EC50) values for TDM were ∼30 pM in HEK-hMincle cells and 170 pM in HEK-mMincle cells (Fig. 1 B and C). TDB was significantly less stimulatory, with 1 log higher EC50 values.
Fig. 1.
Identification of natural and synthetic ligands of Mincle. (A) Chemical structures of TDM, TDB, GlcC14C18, ManC14C18, GlcC16, and GlcC4C12. (B–E) EC50 values determined from the activation curves of HEK-hMincle (B and D) and HEK-mMincle (C and E) after incubation with mycobacterial natural compounds (B and C) or synthetic analogs (D and E). EC50 data are presented as the mean ± SEM calculated from at least three independent experiments (SI Appendix, Fig. S2). All of the compound structures are described in SI Appendix, Fig. S1.
Among the mycobacterial MA-containing glycolipids tested, and in sharp contrast with data reported by Ishikawa et al. (7), glucose monomycolate (GlcMM) was the most stimulatory, with EC50 values even lower than those determined for TDM (∼0.5 log lower) (18, 19) (Figs. 1 B and C and SI Appendix, Fig. S2). As reported recently (20), glycerol monomycolate (GroMM) was seen to act as an agonist of human Mincle, but not of murine Mincle, and was as active as TDB in HEK-hMincle cells (Fig. 1 and SI Appendix, Fig. S1). Surprisingly, free MAs also acted as moderate agonists of the human receptor (EC50 ∼3 nM), but not the murine receptor (Fig. 1). Concerning trehalose-containing glycolipids, diacyl-trehalose (DAT) was an agonist for both the human and the murine receptor, with EC50 values similar to those of TDB (Fig. 1 and SI Appendix, Fig. S1). Further acylation of triacyl-trehalose (TAT) reduced its activity, which was fully abrogated in polyacyl-trehalose (PAT) (Fig. 1). Diacylated sulfoglycolipids (Ac2SGL) and tetracylated sulfoglycolipids (Ac4SGL) failed to activate NF-κB in any of the cell lines (Fig. 1 and SI Appendix, Fig. S1). However, removal of the sulfate group on the 2′-OH position of Ac2SGL and Ac4SGL (Ac2GL and Ac4GL, respectively) restored Mincle activation by Ac2GL at a level similar to that of DAT, although it had no effect on Mincle activation by Ac4GL (Fig. 1). Thus, the mycobacterial cell envelope was found to contain several Mincle ligands with diverse structures.
Deciphering the Structural Features Required for (Glyco)Lipid Recognition by Mincle.
Given our findings that GlcMM is at least as active as TDM in stimulating Mincle reporter cell lines, we used glucose derivatives monoacylated at position 6 to investigate the role of the fatty acid structure in ligand recognition by Mincle. Chain length was previously shown to be critical for macrophage activation by trehalose derivatives diacylated by saturated linear fatty acids, with optimal activity achieved for fatty acids containing 22 carbon atoms (behenic acid), such as TDB (21). Surprisingly, glucose monobehenate (GlcMB/GlcC22), in contrast to TDB, did not show any activity on reporter cell lines (Fig. 1 and SI Appendix, Fig. S1) and was not able to bind a soluble form of human Mincle receptor (hMincle-Fc) (SI Appendix, Fig. S3). Increasing the chain length up to 28 carbon atoms (GlcC28) did not restore any activity (Fig. 1 D and E).
MAs are complex 2-alkyl 3-hydroxy long-chain fatty acids that contain up to 90 carbon atoms and have diverse chemical functions (22, 23) (Fig. 1A). Similar fatty acids are produced by almost all members of the order Corynebacteriales, including Corynebacterium glutamicum (e.g., corynomycolic acid), albeit in a simpler form (22). Interestingly, glucose esterified by a synthetic form of corynomycolic acid with 32 carbon atoms (GlcMCM) showed potent activity and binding to hMincle-Fc (Fig. 1 and SI Appendix, Figs. S1–S3), as reported recently (24). Thus, the total number of carbon atoms in the fatty acid did not appear to be the key parameter driving Mincle activity.
To assess the impact of the 2-alkyl chain or 3-hydroxy group found in (coryno)mycolic acids, but not in simpler linear fatty acids, we synthesized glucose derivatives acylated with either 3-hydroxyoctadecanoic acid (Glc3OHC18) or 2-tetradecyloctadecanoic acid (GlcC14C18) (SI Appendix, Fig. S1). GlcC14C18 bound to hMincle-Fc and exhibited strong agonist activity, similar to that of GlcMCM (Fig. 1 and SI Appendix, Fig. S2), whereas Glc3OHC18 failed to bind to hMincle-Fc or to activate reporter cells. Taken together, these data indicate that 2-alkylation of fatty acids plays a key role in the recognition of acylated glucose by Mincle and in the induction of Mincle-mediated intracellular signaling, whereas 3-hydroxylation is not required.
Deciphering the Role of the 2-Alkyl Chain with Molecular Dynamics Simulations.
To tentatively understand the role played by 2-alkylation of fatty acids in glycolipid recognition by Mincle, we performed molecular dynamics (MD) simulations using the high-resolution crystal structures of bovine Mincle in complex with trehalose as an initial model (13). This allowed us to probe and compare the binding interactions of GlcMCM and Glc3OHC18 with Mincle, and to predict essential structural and dynamic features involved in intermolecular recognition.
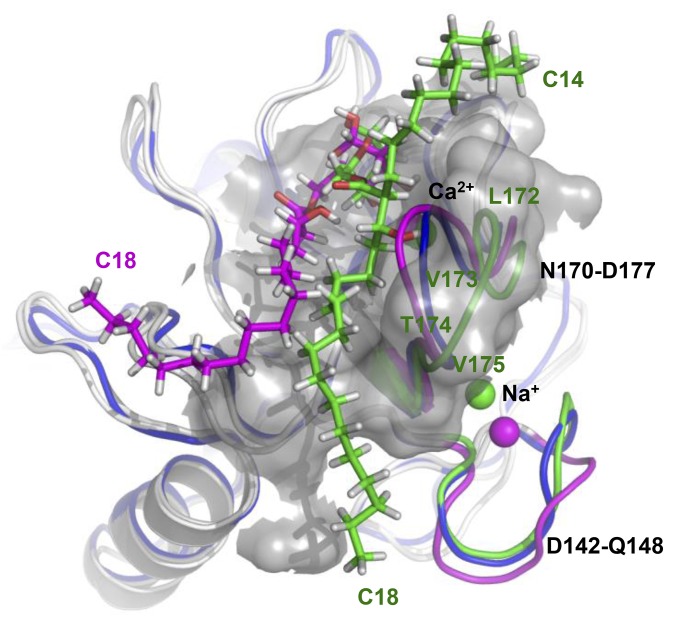
For both GlcMCM and Glc3OHC18, all hydrogen-bonding interactions with the glucose 3-OH and 4-OH groups, as well as with the Ca2+ ion, were maintained with E168, N170, E176, N192, and D193 amino acid side chains (SI Appendix, Fig. S4 A and B), in agreement with the crystallographic structure of the trehalose complex (13). In contrast, differences were observed in the flexibility of regions D142–Q148 and N170–D177 (Fig. 2), which impacted the topology of the hydrophobic groove located at the surface of the CRD. These structural regions were previously found to adopt different conformations in the crystallized forms of human Mincle (14) compared with the bovine Mincle (13). Moreover, it has been suggested that the conformation of the loop between residues N170 and D177 could play a key role in the ability of Mincle to bind to the glycolipid (15, 25).
Fig. 2.
Comparison of conformational rearrangements observed from MD simulations in regions D142–Q148 and N170–D177 of Mincle interacting with GlcMCM or Glc3OHC18. The X-ray crystallographic structure of Mincle (Protein Data Bank ID code 4KZV) is shown in blue for reference. The hydrophobic groove is represented as a molecular surface to show how the C18 alkyl chain of GlcMCM (green) binds into the cleft on conformational rearrangement of loop N170–D177, whereas Glc3OHC18 (pink) cannot be properly inserted in the groove.
During MD simulation, the N170–D177 region was found to undergo larger conformational rearrangements when two alkyl chains (GlcMCM), rather than one single chain (Glc3OHC18), were bound to Mincle (Fig. 2). The shift of loop N170–D177 observed in the MD simulation with GlcMCM widened the hydrophobic groove to better accommodate the main C18 alkyl chain inside the cleft. This conformation was maintained through van der Waals interactions with amino acid residues V194, P195, F197, F198, T174, and V175, which line the two sides of the hydrophobic groove (SI Appendix, Fig. S4A). The C14 2-alkyl chain was found to interact with a short loop stretch formed by amino acid residues L172 and V173 (Fig. 2 and SI Appendix, Fig. S4A). In the presence of one single alkyl chain, such as with Glc3OHC18, the receptor remained in a conformation similar to that observed in the crystallographic structure of the bovine form, and as a result, the alkyl chain could not be properly inserted into the groove and remained at the surface (Fig. 2). These models suggest that one 10 carbon-long chain of the fatty acid may be accommodated in the hydrophobic groove, as described earlier, whereas a second chain as short as four carbon atoms may interact with the adjacent L172–V173 loop, inducing conformational modifications of the receptor.
To obtain experimental evidence supporting these models, we synthesized glucose derivatives acylated with 2-nonyldodecanoate (GlcC9C12) or 2-butyldodecanoate (GlcC4C12) (SI Appendix, Fig. S1). Interestingly, GlcC9C12 bound to hMincle-Fc and was an agonist of Mincle, with EC50 values in the range of 1 nM for HEK-hMincle and 400 pM for HEK-mMincle (Fig. 1 D and E and SI Appendix, Fig. S3). GlcC4C12 exhibited similar agonist activity (Fig. 1A). These findings support the MD data and confirm that 2-alkylation of the fatty acid was critical for optimal binding of acylated glucose derivatives to Mincle and induction of intracellular signaling, because GlcC22 and GlcC16 were totally inactive (Fig. 1A).
To identify the role of the 3-hydroxyl group of the fatty acid in binding to Mincle, we performed MD simulations on GlcC14C18 and GlcC18 (corresponding to GlcMCM and Glc3OHC18 esterified by nonhydroxylated fatty acids, respectively). Overall, in agreement with the experimental data, the conformational dynamics of the ligands were largely unaffected by the presence of the 3-hydroxyl group (SI Appendix, Fig. S4 C and D). This finding is in agreement with the fact that no direct interaction could be observed between the receptor and the hydroxyl group of the fatty acid.
To further strengthen our conclusions, we mutated in the human Mincle protein one particular amino acid residue (A174; equivalent to V173 in the bovine protein) (SI Appendix, Fig. S5A), directly involved in the binding of the two alkyl chains of GlcMCM or GlcC14C18 and affecting the topology of the hydrophobic binding sites. Mutagenesis of A174 in the human protein into a phenylalanine residue led to a weak and similar improvement in both TDB and GlcC14C18 binding (SI Appendix, Fig. S5B), suggesting that introduction of the aromatic side chain at position 174 can slightly improve binding of the alkyl chain in the main hydrophobic groove, but does not significantly interfere with binding of the 2-alkyl chain in the short hydrophobic groove. Interestingly, in agreement with MD simulations, introduction of a proline led to a decrease of the relative binding of the protein to GlcC14C18 vs. TDB (i.e., for a compound with a 2-alkylated fatty acid vs. a compound with linear fatty acids). Indeed, the rigidity of proline residues is known to constrain the protein backbone conformation, and thus the presence of such a residue might restrain the conformational rearrangements of the N170–D177 region assumed, from MD simulations, to be required for productive binding of the 2-alkyl chain.
Mannose Derivatives Are Ligands of Mincle.
Previously reported crystal structures exhibited a canonical C-type sugar-binding site (13, 14), suggesting that Mincle can bind glucose as well as mannose derivatives. Thus, we synthesized mannose monomycolate (ManMM), mannose monocorynomycolate (ManMCM), and mannose 2-tetradecyloctadecanoate (ManC14C18), analogs of the most active glucose derivatives. These molecules bound to hMincle-Fc (SI Appendix, Fig. S3) and strongly stimulated the reporter cell lines, although they were globally weaker agonists of the receptor than their corresponding glucose derivatives (EC50 ∼1 log higher) (Fig. 1 D and E). In contrast, natural mannosylated glycolipids found in the mycobacterial cell envelope, such as phosphatidyl-myo-inositol dimannosides and β-d-mannosyl phosphomycoketide, were not recognized by the receptor (SI Appendix, Figs. S2 and S6).
Proinflammatory Properties of the Mincle Ligands.
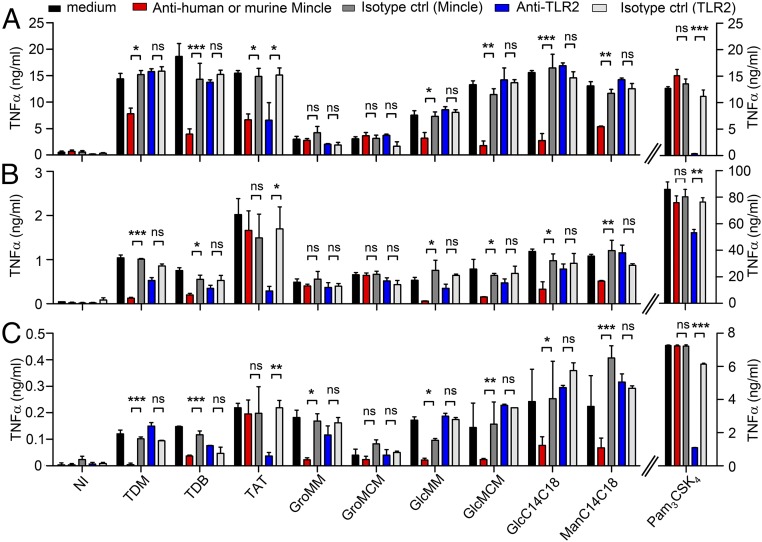
We next tested the ability of the newly identified Mincle ligands to induce the secretion of proinflammatory cytokines by primary murine or human cells. TDM and TDB strongly induced the production of tumor necrosis factor (TNF)-α (Fig. 3A) and IL-6 (SI Appendix, Fig. S7) by murine bone marrow-derived dendritic cells (BMDCs) in a Mincle-dependent fashion, as determined by antibody-blocking experiments. In contrast, stimulation of murine bone marrow-derived macrophages (BMDMs) (Fig. 3B) and human monocyte-derived macrophages (moMΦs) (Fig. 3C) was much weaker and required previous priming by IFN-γ, although these cells strongly expressed Mincle at their surfaces (SI Appendix, Fig. S8). Mincle ligands did not induce either TNF-α or IL-6 production by human monocytes or monocyte-derived dendritic cells (moDCs), even after priming by IFN-γ. The other natural ligands of Mincle—GlcMM, GroMM, and TAT—induced TNF-α production by BMDCs, BMDMs, and moMΦs (Fig. 3).
Fig. 3.
Newly identified Mincle ligands induce proinflammatory cytokines by human and mouse primary cells. BMDCs (A), BMDMs (B), and moMФs (C) were stimulated with 1 µg of plate-bound lipid for 18 h, and TNF-α release in the culture supernatant was determined by ELISA. Mincle and TLR2 dependence were investigated by preincubating cells for 30 min at 37 °C with 5 µg/mL anti-mMincle, anti-hMincle, anti-TLR2, or isotype control antibodies before stimulation with lipid. The Pam3CSK4 lipopeptide served as a control TLR2 ligand at a concentration of 100 ng/mL. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
As reported recently (20), stimulation by GroMM was dependent on Mincle in human cells, but not murine cells (Fig. 3B). Cytokine production induced by TAT was dependent on Toll-like receptor (TLR) 2 signaling, partially in BMDCs (Fig. 3A) but completely in BMDMs and moMΦs (Figs. 3 B and C). These data are in agreement with the higher relative magnitude of TLR2 vs. Mincle signaling in BMDMs and moMΦs compared with BMDCs, as determined by stimulation with Pam3CSK4 vs. Mincle ligands (Fig. 3). Accordingly, TAT induced higher levels of cytokines in BMDMs and moMΦs than in BMDCs. Interestingly, the new synthetic Mincle ligands GlcMCM, GlcC14C18, and ManC14C18 efficiently stimulated TNF-α (Fig. 3) and IL-6 (SI Appendix, Fig. S7) production.
Adjuvanticity of the Synthetic Mincle Ligands.
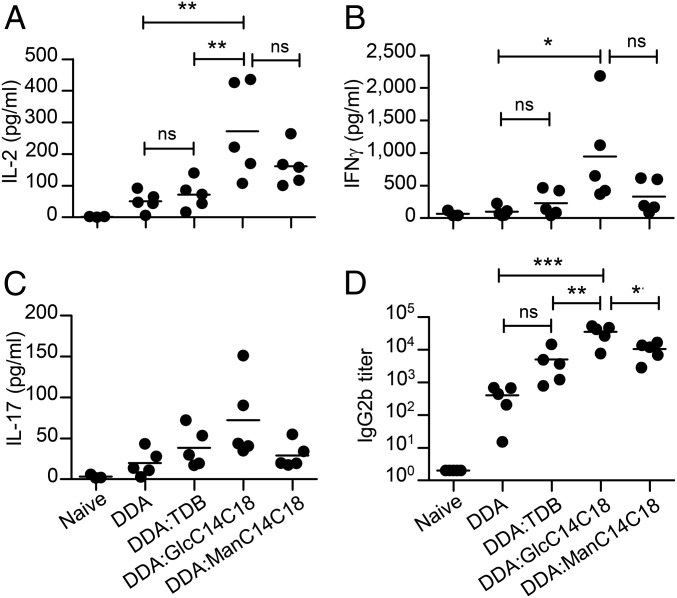
We tested the adjuvant potential of the two strongest and most structurally simple synthetic Mincle ligands, GlcC14C18 and ManC14C18. Incorporation of TDB into cationic liposomes composed of the quaternary ammonium compound dimethyldioctadecylammonium (DDA) has been previously shown to produce an adjuvant, CAF01, that induces strong specific Th1 and Th17 Mincle-dependent immune responses against antigens from Mycobacterium tuberculosis, HIV, influenza, or Chlamydia (9, 11, 26–28). Mice were immunized against the M. tuberculosis model antigen Ag85A in liposomes composed of DDA and glycolipids at a ratio of 25:1, which was previously shown to be suboptimal for CAF01 (11), and the immune response was monitored by restimulation of splenocytes with the antigen. Accordingly, the addition of TDB at this suboptimal concentration did not result in a significant increase in IL-2, IFN-γ, or IL-17 production compared with DDA alone (Fig. 4 and SI Appendix, Fig. S9). In contrast, the addition of GlcC14C18 to DDA resulted in significant increases of IL-2, IFN-γ, and IL-17. Interestingly, the addition of ManC14C18 to DDA produced a similar trend, although without reaching statistical significance. The adjuvanticity of GlcC14C18 was abrogated in Mincle-knockout mice (SI Appendix, Fig. S9).
Fig. 4.
GlcC14C18 and ManC14C18 have adjuvant activity in vivo. Mice (n = 5) were immunized intradermally three times with 10 µg of the M. tuberculosis antigen Ag85A in DDA, DDA/TDB, DDA/GlcC14C18, or DDA/ManC14C18. The immune response was monitored 3 wk after the last immunization. IL-2 (A), IFN-γ (B), and IL-17 (C) release in the culture supernatant of splenocytes after restimulation with Ag85A, along with serum anti-Ag85A IgG2b titers (D), were determined by ELISA. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
We also investigated the ability of GlcC14C18 and ManC14C18 to induce antibody responses. Mice receiving GlcC14C18 or ManC14C18 compared with DDA alone showed increases in Ag85A-specific IgG2b titers of ∼80-fold and ∼25-fold, respectively (Fig. 4D and SI Appendix, Fig. S10), whereas IgG1 titers were not affected (SI Appendix, Fig. S10), a pattern typically characteristic of a Th1 immune response (11). Finally, we tested the potency of GlcC14C18 in an M. tuberculosis challenge experiment in vivo, using mice vaccinated with the same DDA-based liposome preparations. Interestingly, liposomes containing GlcC14C18 induced the same level of protection as those containing TDB at the optimal concentration (SI Appendix, Fig. S11).
Discussion
Mincle is one of the main receptors underlying the remarkable adjuvanticity of the mycobacterial cell wall (7, 8). Its identification as a receptor for TDM has provided important insight into the formation of the characteristic granulomas in tuberculosis, and also has opened avenues for the rational design of new adjuvants (29).
Searching for Mincle ligands provides a molecular basis for identification of the natural bioactive molecules present in the mycobacterial cell wall. Using human or murine Mincle reporter cell lines, we have identified, in addition to TDM and GroMM, several other mycobacterial glycolipid ligands of the receptor, including GlcMM, DAT, and TAT. GlcMM is the strongest stimulatory compound identified. These data are in sharp contrast with a previous report indicating that GlcMM, tentatively generated by TDM hydrolysis with a trehalase enzyme, is not active (7). We were not able to address this discrepancy, however, because in the present study trehalase was not active on TDM, and so GlcMM could not be generated in this fashion. As previously observed with GroMM (20), we found that free MAs were weak agonists of the human receptor, but not of the murine receptor. Synthetic methyl 5-mycoloyl-arabinofuranoside (AraMeMM), which mimics MAs linked to arabinogalactan, modestly activated the receptor (SI Appendix, Fig. S6), indicating that MAs linked to the cell wall, in addition to those born by extractable lipids, may contribute to mycobacterial adjuvanticity via Mincle recognition. Overall, lipids, recognized by either Mincle or Toll-like receptors (30), appear to largely contribute to the exceptional immunogenic activity of the M. tuberculosis cell envelope (1), which seems to have been conserved by evolution to preserve the bacillus antigenicity required for late-stage pulmonary immunopathology and bacilli transmission (31, 32).
Crystal structures have revealed that Mincle contains a canonical C-type primary monosaccharide-binding site centered on a Ca2+ ion (13, 14), flanked on one side by a secondary monosaccharide-binding site and on the other side by a hydrophobic groove that provides a docking site for one short fatty acid (13). Regarding the polar head of the ligands, one of the two glucose residues in the bound trehalose molecule occupies the primary binding site, with the equatorial 3-OH and 4-OH groups forming coordination bonds with the Ca2+ and hydrogen bonds with four of the amino acid side chains that also ligate Ca2+, mimicking the coordination of mannose and N-acetylglucosamine residues in other C-type CRD-ligand complexes. Accordingly, synthetic acylated mannose derivatives are recognized by Mincle, albeit less efficiently than the corresponding acylated glucose derivatives. DAT and TAT, in which 3-OH is acylated, or GroMM, AraMeMM, and free MAs, which have a reduced capacity to establish coordination and hydrogen bonds within the primary binding site, demonstrated a greatly reduced capacity to bind the soluble receptor and to activate signaling. In the secondary binding site, glucose 2-OH is part of a cooperative hydrogen-bonding network in which it accepts a hydrogen bond from Arg-182 and donates to Glu-135, thereby bridging these two side chains (13). Accordingly, Ac2SGL and PAT, in which 2-OH of the second glucose unit is substituted by a sulfate group or a fatty acid, respectively, did not activate Mincle signaling. Removal of the sulfate group on Ac2SGL restored activity similar to that observed for DAT.
With regard to the alkyl moiety of the ligands, examination of the surface of the Mincle CRD in the crystal structure revealed a hydrophobic groove directly adjacent to the 6-OH group of the glucose residue in the primary binding site, and the modeling study suggested that at least six carbon atoms would be accommodated within the groove (13). Although these structural data did not address recognition of the two complex MAs of TDM by Mincle, which might require the cooperation of other, as-yet unidentified receptors, they did provide a basis for investigating the influence of fatty acid structure in monoacylated glycolipids.
Surprisingly, whereas GlcMM was as active as TDM, GlcMB/GlcC22, in contrast to TDB, did not show any activity. Actually, the 2-alkyl chain found in MAs was mandatory for recognition of monoacylated glucose derivatives, and increasing the chain length of a saturated linear fatty acid did not compensate for a lack of the 2-alkyl chain. In contrast, the characteristic 3-hydroxy group of these complex fatty acids was dispensable. The role of 2-alkylation of fatty acids in glycolipid recognition by Mincle was revealed by MD simulations. Indeed, in the case of ligands containing a single alkyl chain, the latter could not be properly inserted into the hydrophobic groove and instead remained at the surface. In contrast, binding of the 2-alkyl chain to a short hydrophobic groove formed by the amino acid residues L172 and V173 induced a shift of loop N170–D177, resulting in a widening of the main hydrophobic groove, thereby enabling better accommodation of the main alkyl chain inside the cleft. Accordingly, A174P mutation in the human protein (position 173 in the bovine form), which might restrain the conformational flexibility of the N170–D177 loop, significantly decreased binding of GlcC14C18. These data illustrate how a C-type lectin can sense subtle structural features of fatty acids.
Taken together, structure-function relationship studies, crystallography, and molecular modeling data from our group and others indicate that optimal recognition of glycolipids by Mincle involves two interactions: the interaction of two monosaccharides with the carbohydrate primary and secondary binding sites, and the interaction of two alkyl chains, borne either by the same branched fatty acid or by independent fatty acids, with two hydrophobic grooves at the surface of the CRD. At least three of these four binding sites must be occupied for effective recognition of the ligand, but interactions with the secondary glucose-binding site seem to be weaker than those induced by binding with the second hydrophobic groove; note the relative ability of GlcC14C18 vs. TMB vs. GlcMB/C22 to stimulate reporter cell lines. Key amino acid residues forming the ligand-binding site in human (14) and bovine (13) Mincle are conserved in a wide range of mammalian species, including mouse, suggesting that the receptor binding properties are conserved (33). Accordingly, only subtle differences in ligand binding between the human and mouse receptors were observed.
The newly identified natural Mincle ligands, as well as rationally designed synthetic ligands, stimulated cytokine production in human and mouse primary cells in a Mincle-dependent fashion. However, Mincle-dependent cytokine production in macrophages (e.g., BMDMs and moMΦs), was much weaker than in BMDCs and required previous priming by IFN-γ. Macrophages actually exhibited a bias toward TLR2 vs. Mincle signaling compared with BMDCs. In addition, human cells appeared to be surprisingly poorly responsive to Mincle stimulation compared with mouse cells, although this effect might be donor-dependent (34). This result is in contrast with data from Andersen et al. (26), who recently reported that the CAF01 liposomal adjuvant system, composed of DDA and TDB, promotes long-lived M. tuberculosis-specific T-cell responses in humans, in agreement with previous data obtained in mice (27). This finding may suggest that the local immune cells in humans, in contrast to in vitro monocyte-derived cells, efficiently respond to Mincle stimulation. This human trial further validates the rational design of Mincle ligands as an approach to developing new adjuvants that direct the development of robust Th17 and Th1 responses to subunit vaccines.
Interestingly, our two strongest and most structurally simple synthetic Mincle ligands, GlcC14C18 and ManC14C18, exhibited a stronger adjuvant effect than TDB. Moreover, the most potent of these ligands, GlcC14C18, which is less toxic than TDB on host cells (SI Appendix, Fig. S12), was found to induce protective immunity in a mouse model of M. tuberculosis infection. Its broader applicability merits further testing in vaccination studies in which cellular immunity is required.
Materials and Methods
Purification of Natural Mycobacterial Lipids and Chemical Synthesis.
Lipids were purified from M. tuberculosis H37Rv. Details of the chemical synthesis and analytical data are provided in SI Appendix, Materials and Methods.
Mincle Reporter Cell Line Experiments.
Glycolipids (1 mg/mL in isopropanol) were added to 96-well plates (Nunc) in serial dilutions from 1 µg to 1 ng/well, followed by evaporation of the solvents (7). Reporter cells (5 × 104/well), maintained as described in SI Appendix, Materials and Methods, were stimulated for 24 h, after which alkaline phosphatase activity was measured by mixing 20 µL of the culture supernatant and 180 µL of Quanti-Blue (InvivoGen) and reading OD at 630 nm.
Primary Cell Activation.
Glycolipids were added to plates at 1 µg/well (see above). Cells, generated as described in SI Appendix, Materials and Methods, were distributed at 105 cells/well (moMФs and BMDMs) or 2 × 105 cells/well (BMDCs and moDCs). After 18 h, cytokines were assayed in the culture supernatant by sandwich ELISA using a commercially available kit (eBioscience).
Computational Procedures.
Molecular models were derived from the high-resolution crystal structures of bovine Mincle in complex with trehalose (Protein Data Bank ID code 4KZV) (13). Molecular dynamics simulations were carried out for 10 ns in explicit water at 303K using AMBER11. Details are provided in SI Appendix, Materials and Methods.
Immunization of Mice.
Adult female C57BL/6 mice (8-10 wk old) were injected intradermally at the base of the tail three times, with a 2-wk interval between the injections (0.1 mL/dose), as described in SI Appendix, Materials and Methods. Spleens and blood were harvested at 21 d after the last immunization.
Supplementary Material
Acknowledgments
We thank Rui Appelberg (University of Porto) for providing the Ag85A protein and helping design the mouse immunization experiments, and Ruben P. van Summeren and Adrian Minaard (University of Groningen) for providing the synthetic β-d-mannosyl phosphomycoketide. This work was performed using the high-performance computing resources of the Computing Center of Région Midi-Pyrénées (CALMIP). Support for this research was provided by the Association Nationale de la Recherche et de la Technologie (ANRT), CNRS, Université Paul Sabatier, the Fondation Bettencourt-Schueller, and Fondation pour la Recherche Médicale (fellowship, to S.S.-G.). A.D. was the recipient of a CIFRE doctoral fellowship from ANRT and InvivoGen.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.S. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612421114/-/DCSupplemental.
References
- 1.Kasmar AG, Layre E, Moody DB. Lipid adjuvants and antigens embedded in the mycobacterial cell envelope. In: Norazmi MN, Acosta A, Sarmiento ME, editors. The Art & Science of Tuberculosis Vaccine Development. 2nd Ed. Oxford Univ Press; Oxford, UK: 2014. pp. 123–149. [Google Scholar]
- 2.Noll H, Bloch H, Asselineau J, Lederer E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- 3.Bloch H. Studies on the virulence of tubercle bacilli: The relationship of the physiological state of the organisms to their pathogenicity. J Exp Med. 1950;92(6):507–526. doi: 10.1084/jem.92.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaire G, Tenu JP, Petit JF, Lederer E. Natural and synthetic trehalose diesters as immunomodulators. Med Res Rev. 1986;6(3):243–274. doi: 10.1002/med.2610060302. [DOI] [PubMed] [Google Scholar]
- 5.Ryll R, Kumazawa Y, Yano I. Immunological properties of trehalose dimycolate (cord factor) and other mycolic acid-containing glycolipids: A review. Microbiol Immunol. 2001;45(12):801–811. doi: 10.1111/j.1348-0421.2001.tb01319.x. [DOI] [PubMed] [Google Scholar]
- 6.Lima VM, et al. Role of trehalose dimycolate in recruitment of cells and modulation of production of cytokines and NO in tuberculosis. Infect Immun. 2001;69(9):5305–5312. doi: 10.1128/IAI.69.9.5305-5312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa E, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206(13):2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenen H, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184(6):2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werninghaus K, et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9–dependent innate immune activation. J Exp Med. 2009;206(1):89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki S, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9(10):1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 11.Davidsen J, et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718(1-2):22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Shenderov K, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol. 2013;190(11):5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinberg H, et al. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J Biol Chem. 2013;288(40):28457–28465. doi: 10.1074/jbc.M113.497149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa A, et al. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc Natl Acad Sci USA. 2013;110(43):17438–17443. doi: 10.1073/pnas.1312649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinberg H, et al. Binding sites for acylated trehalose analogs of glycolipid ligands on an extended carbohydrate recognition domain of the macrophage receptor Mincle. J Biol Chem. 2016;291(40):21222–21233. doi: 10.1074/jbc.M116.749515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang R, Schoenen H, Desel C. Targeting Syk-Card9–activating C-type lectin receptors by vaccine adjuvants: Findings, implications and open questions. Immunobiology. 2011;216(11):1184–1191. doi: 10.1016/j.imbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 18.Prandi J. A convenient synthesis of glucose monomycolate. Carbohydr Res. 2012;347(1):151–154. doi: 10.1016/j.carres.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Sahb MM, Al Dulayymi JR, Baird MS. Glucose monomycolates based on single synthetic mycolic acids. Chem Phys Lipids. 2015;190:9–14. doi: 10.1016/j.chemphyslip.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Hattori Y, et al. Glycerol monomycolate is a novel ligand for the human, but not mouse, macrophage-inducible C-type lectin, Mincle. J Biol Chem. 2014;289(22):15405–15412. doi: 10.1074/jbc.M114.566489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan AA, et al. Long-chain lipids are required for the innate immune recognition of trehalose diesters by macrophages. ChemBioChem. 2011;12(17):2572–2576. doi: 10.1002/cbic.201100451. [DOI] [PubMed] [Google Scholar]
- 22.Marrakchi H, Lanéelle MA, Daffé M. Mycolic acids: Structures, biosynthesis, and beyond. Chem Biol. 2014;21(1):67–85. doi: 10.1016/j.chembiol.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Vander Beken S, et al. Molecular structure of the Mycobacterium tuberculosis virulence factor, mycolic acid, determines the elicited inflammatory pattern. Eur J Immunol. 2011;41(2):450–460. doi: 10.1002/eji.201040719. [DOI] [PubMed] [Google Scholar]
- 24.van der Peet PL, Gunawan C, Torigoe S, Yamasaki S, Williams SJ. Corynomycolic acid-containing glycolipids signal through the pattern recognition receptor Mincle. Chem Commun (Camb) 2015;51(24):5100–5103. doi: 10.1039/c5cc00085h. [DOI] [PubMed] [Google Scholar]
- 25.Jégouzo SA, et al. Defining the conformation of human mincle that interacts with mycobacterial trehalose dimycolate. Glycobiology. 2014;24(12):1291–1300. doi: 10.1093/glycob/cwu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dissel JT, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32(52):7098–7107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Lindenstrøm T, et al. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun. 2012;80(10):3533–3544. doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenkrands I, et al. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine. 2011;29(37):6283–6291. doi: 10.1016/j.vaccine.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 29.Matsunaga I, Moody DB. Mincle is a long-sought receptor for mycobacterial cord factor. J Exp Med. 2009;206(13):2865–2868. doi: 10.1084/jem.20092533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray A, Cot M, Puzo G, Gilleron M, Nigou J. Bacterial cell wall macroamphiphiles: Pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie. 2013;95(1):33–42. doi: 10.1016/j.biochi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Russell DG. The evolutionary pressures that have molded Mycobacterium tuberculosis into an infectious adjuvant. Curr Opin Microbiol. 2013;16(1):78–84. doi: 10.1016/j.mib.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behr MA, Divangahi M. Freund’s adjuvant, NOD2, and mycobacteria. Curr Opin Microbiol. 2015;23:126–132. doi: 10.1016/j.mib.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Rambaruth ND, Jégouzo SA, Marlor H, Taylor ME, Drickamer K. Mouse mincle: Characterization as a model for human mincle and evolutionary implications. Molecules. 2015;20(4):6670–6682. doi: 10.3390/molecules20046670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostrop J, et al. Contribution of MINCLE-SYK signaling to activation of primary human APCs by mycobacterial cord factor and the novel adjuvant TDB. J Immunol. 2015;195(5):2417–2428. doi: 10.4049/jimmunol.1500102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.