Significance
Meditation training has been shown to reduce anxiety, lower stress hormones, improve attention and cognition, and increase rhythmic electrical activity in brain areas related to emotional control. We describe how artificially inducing rhythmic activity influenced mouse behavior. We induced rhythms in mouse anterior cingulate cortex activity for 30 min/d over 20 d, matching protocols for studying meditation in humans. Rhythmic cortical stimulation was followed by lower scores on behavioral measures of anxiety, mirroring the reductions in stress hormones and anxiety reported in human meditation studies. No effects were observed in preference for novelty. This study provides support for the use of a mouse model for studying changes in the brain following meditation and potentially other forms of human cognitive training.
Keywords: meditation, anxiety, optogenetics, theta, mouse
Abstract
Meditation training induces changes at both the behavioral and neural levels. A month of meditation training can reduce self-reported anxiety and other dimensions of negative affect. It also can change white matter as measured by diffusion tensor imaging and increase resting-state midline frontal theta activity. The current study tests the hypothesis that imposing rhythms in the mouse anterior cingulate cortex (ACC), by using optogenetics to induce oscillations in activity, can produce behavioral changes. Mice were randomly assigned to groups and were given twenty 30-min sessions of light pulses delivered at 1, 8, or 40 Hz over 4 wk or were assigned to a no-laser control condition. Before and after the month all mice were administered a battery of behavioral tests. In the light/dark box, mice receiving cortical stimulation had more light-side entries, spent more time in the light, and made more vertical rears than mice receiving rhythmic cortical suppression or no manipulation. These effects on light/dark box exploratory behaviors are associated with reduced anxiety and were most pronounced following stimulation at 1 and 8 Hz. No effects were seen related to basic motor behavior or exploration during tests of novel object and location recognition. These data support a relationship between lower-frequency oscillations in the mouse ACC and the expression of anxiety-related behaviors, potentially analogous to effects seen with human practitioners of some forms of meditation.
One month of integrated mind body meditation (1), a form of mindfulness meditation, has been shown to reduce self-reported anxiety as measured by the Profile of Mood States, reduce the stress hormone cortisol, increase ventral anterior cingulate cortex (ACC) activity, and change the white matter pathways surrounding the ACC as measured by increased fractional anisotropy in diffusion tensor imaging studies (1–4). How might a purely mental exercise such as paying attention to the present moment work to produce these changes in behavior and brain connectivity (5)?
Some of these changes, including those to white matter, were hypothesized to be related to the finding that meditation can increase frontal theta activity even when the person is at rest (6). The theta activity may increase the number of active oligodendrocytes leading to increased myelination, thereby improving connectivity between the ACC and other limbic areas. Moreover, theta activity in humans has been uniquely correlated with glucose metabolism in the ACC (7). Because the ventral ACC connects to the amygdala and is thought to regulate its activity (8), these changes in the brain could result in the reduced anxiety found following meditation training.
To test this idea, we developed a mouse model in which various frequencies of oscillatory activity were induced in the ACC using optogenetics. Because mindfulness meditation has often been associated with reductions in anxiety and negative affect (9), we examined exploratory behavior in the light/dark box. In this ethological model of anxiety, time in the light versus dark sides is thought to be a joint effect of novelty (favoring exploration of the light side) and anxiety (favoring the dark) (10). Novel object and location recognition memory also were tested as a measure of cognitive change (11) because improved attention and memory also have been reported following meditation training (9).
To drive rhythmic activity across the ACC, we used optogenetic control of parvalbumin- expressing interneurons (PV-INs). Because PV-INs provide broad and potent inhibitory control over pyramidal cells, their activation or inactivation can control global levels of activity very effectively (12, 13). We used a PV-Cre driver line together with either a Cre-dependent Archaerhodopsin-2 (Arch) line (14) or Cre-dependent Channelrhodopsin-2 (ChR2) line (15) to provide cell type-specific hyperpolarization or depolarization, respectively. Three crosses were bred: (i) PV-Arch, in which light reduces PV-IN firing, thus stimulating global ACC excitatory activity; (ii) PV-ChR2, in which light increases PV-IN firing, thus suppressing global ACC excitatory activity; and (iii) homozygous PV-PV, to control for nonspecific light effects. For each cross, behavior also was assessed in control mice receiving no light delivery (no-laser condition). We reasoned that increased rhythmic output from the ACC would improve ACC connectivity and thus reduce anxiety and improve cognitive performance. Comparisons of PV-ChR2 and PV-PV mice enabled us to address the relative importance of rhythmic stimulation versus suppression of neural activity as well as any nonspecific laser effects.
Results
Here we evaluated the behavioral effects of optogenetically inducing rhythms in the mouse ACC. Rhythmic increases in spiking activity were induced in PV-Arch mice (n = 34) by delivering pulses at 1 Hz (200 ms per pulse), 8 Hz (5 ms per pulse), or 40 Hz (5 ms per pulse). Rhythmic decreases were induced in PV-Chr2 mice (n = 27) at the same frequencies. Light pulses also were presented to PV-PV mice (n = 15) lacking either optogenetic effector. Behaviors (Fig. S1) were quantified both before (Pre) and after (Post) a 4-wk protocol of rhythmic light delivery. Behavior from each cross was compared with a control group of mice (n = 25, drawn from all three crosses) that underwent the same Pre and Post behavioral assessments without exposure to rhythmic light pulses.
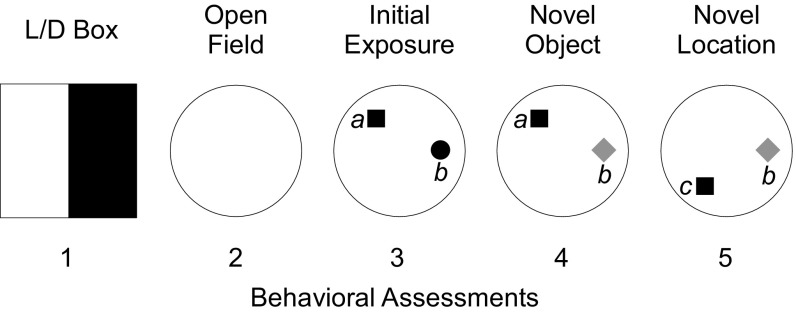
Fig. S1.
Behavioral sequence. Behaviors were quantified during five 5-min assessments. Anxiety-related behaviors were quantified in the light/dark (L/D) box during assessment 1. Mice were placed in the dark side of the apparatus. Two minutes later, a door separating the two sides was removed, and mice were able to explore both the dark and light sides of the apparatus freely for an additional 3 min. Motor behaviors were quantified in a visually cued cylindrical arena during assessment 2. Attention and short-term memory were tested during assessments 3–5. During assessment 3 (initial exposure), mice explored two objects in positions a and b. Object recognition behavior was tested during assessment 4 (novel object) by replacing the familiar object in position b with a novel object. Location recognition was tested during assessment 5 (novel location) by moving the familiar object from position a to position c.
Effects on Neural Activity.
Two groups received equivalent total illumination (1 Hz and 40 Hz, 200 ms per pulse), and two groups received equivalent pulse durations (8 Hz and 40 Hz, 5 ms per pulse) to account for those factors when assessing the effects of each manipulation. Mean responses at each frequency are illustrated in Fig. 1. Recordings were from histologically verified ACC neurons (Fig. S2A). Analyses compared (i) mean response amplitude to the light pulse, i.e., baseline versus response intervals (Fig. 1) and (ii) cumulative changes in spiking output with rhythmic light exposure (Fig. S2B).
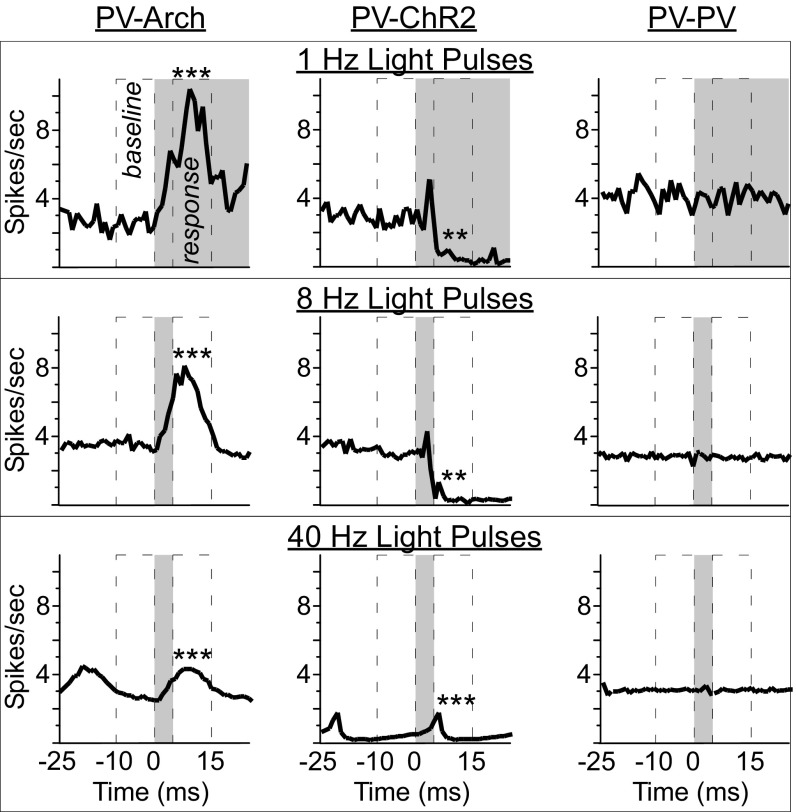
Fig. 1.
Mean responses of PV-Arch, PV-ChR2, and PV-PV mouse pyramidal neurons to light pulses delivered at 1 Hz, 8 Hz, and 40 Hz. Light pulses elicited robust phase-locked increases in spiking activity in ACC neurons of PV-Arch mice (n = 2) and decreases in neurons of PV-ChR2 mice (n = 2) at each frequency, based on comparison of the 10-ms intervals before (baseline) and after (response) light onset. No light effects were seen in cells from PV-PV mice (n = 4). In the peristimulus time histograms, gray regions indicate the period of illumination, beginning at time 0 ms. *P < 0.05; **P < 0.01; ***P < 0.001.
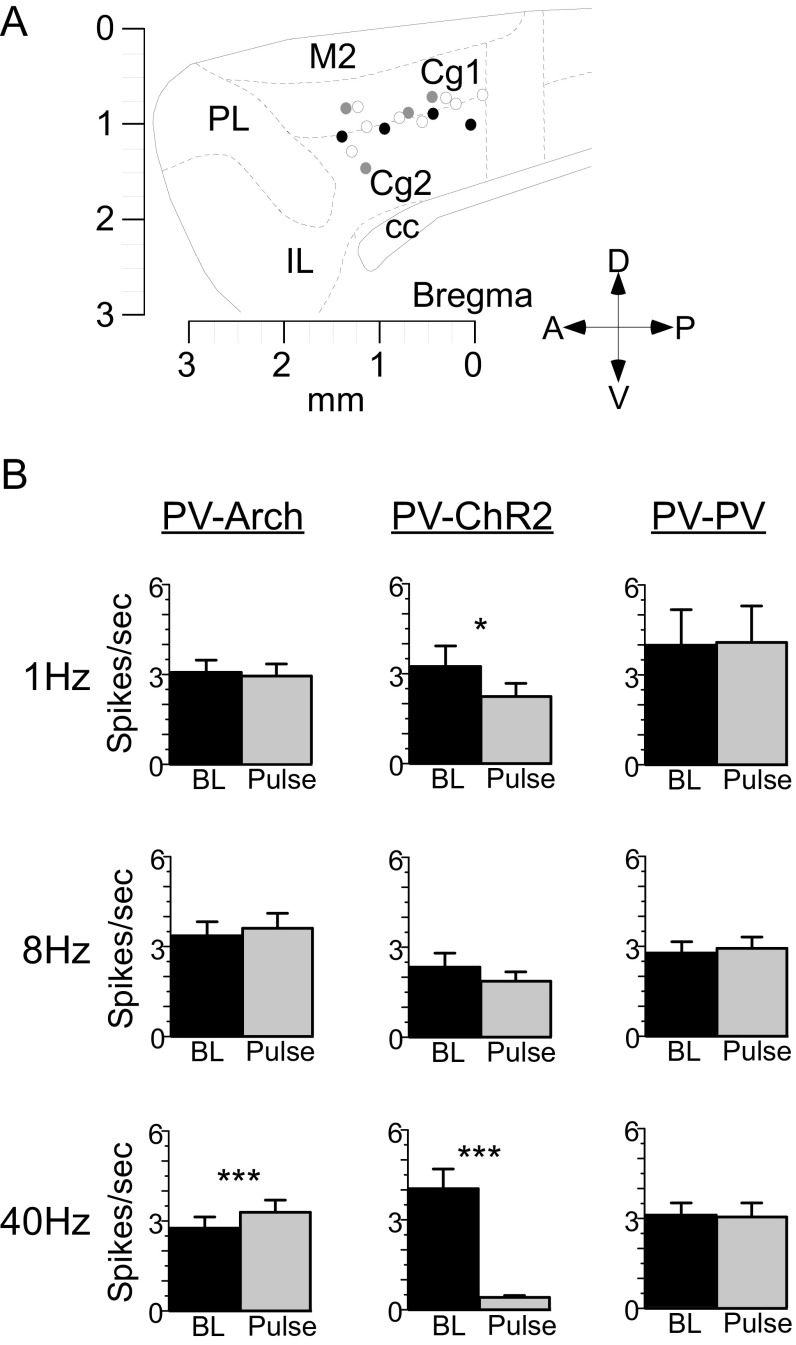
Fig. S2.
Histology for effects on neural activity and analyses of cumulative effects of rhythmic suppression and stimulation. (A) Tetrode terminations in PV-Arch (black circles), PV-ChR2 (gray circles), and PV-PV (open circles) mice. (B) Bar graphs illustrate mean overall firing during the 5-min baseline (BL; black bars) and 5-min rhythmic pulse (Pulse; gray bars) intervals. In PV-Arch mice, 5-ms pulses of light presented at 40 Hz (41 cells) produced a significant increase in total spiking activity over the 5-min pulse interval compared with baseline. In PV-ChR2 mice, 200-ms pulses presented at 1 Hz (26 cells) and 5-ms pulses presented at 40 Hz (47 cells) both produced a significant decrease in total spiking activity over the 5-min pulse interval. Light pulses produced no effect on cumulative spiking activity in PV-PV neurons. Cell counts and statistics for all comparisons are included in Table S1. Cc, corpus callosum; Cg1/Cg2, dorsal/ventral ACC; IL, infralimbic cortex; M2, secondary motor cortex; PL, prelimbic cortex. *P < 0.05; ***P < 0.001.
In PV-Arch mice (n = 2), rhythmic light pulses induced robust phase-locked increases in putative pyramidal neuron firing at each frequency (Fig. 1 and Table S1). In PV-ChR2 mice (n = 2), rhythmic light pulses induced phase-locked suppression in spiking activity at each frequency (Fig. 1 and Table S1). No effects were detected in neuronal activity of PV-PV mice (n = 4) (Fig. 1 and Table S1). Therefore, our method of inducing rhythmic activity was successful and specific to mice expressing either Arch or ChR2.
Table S1.
Statistical comparisons of mean firing rates preceding (−10 to 0 ms) and shortly following (6 to 15 ms) laser pulse onsets (paired t tests)
| Frequency | PV-Arch | PV-ChR2 | PV-PV | |||
| −10 to 0 ms | 6 to 5 ms | −10 to 0 ms | 6 to 15 ms | −10 to 0 ms | 6 to 15 ms | |
| 1 Hz | 2 mice, 43 cells | 2 mice, 26 cells | 4 mice, 70 cells | |||
| df = 42, t = −5.5, P < 0.0001 | df = 25, t = 3.1, P = 0.004 | df = 69, t = −1.0, P = 0.34 | ||||
| 2.5 ± 0.4 Hz | 7.5 ± 1.2 Hz | 2.8 ± 0.7 Hz | 0.4 ± 0.1 Hz | 4.0 ± 1.2 Hz | 4.2 ± 1.2 Hz | |
| 8 Hz | 2 mice, 39 cells | 2 mice, 21 cells | 4 mice, 64 cells | |||
| df = 38, t = −3.6, P = 0.001 | df = 20, t = 3.8, P = 0.001 | df = 63, t = 0.8, P = 0.42 | ||||
| 3.6 ± 0.5 Hz | 6.5 ± 1.1 Hz | 3.0 ± 0.6 Hz | 0.4 ± 0.1 Hz | 2.9 ± 0.4 Hz | 2.9 ± 0.4 Hz | |
| 40 Hz | 2 mice, 41 cells | 2 mice, 47 cells | 4 mice, 62 cells | |||
| df = 40, t = −6.1, P < 0.0001 | df = 46, t = 3.9, P = 0.0003 | df = 61, t = −0.4, P = 0.67 | ||||
| 2.9 ± 0.4 Hz | 4.0 ± 0.9 Hz | 0.3 ± 0.1 Hz | 0.2 ± 0.1 Hz | 3.0 ± 0.5 Hz | 3.1 ± 0.5 Hz | |
Significant effects in bold type.
To determine if any of the differences found in behavior were caused by changes in overall spiking output rather than by rhythms, we examined the cumulative change in activity over a 5-min pulse interval compared with a 5-min baseline period (Fig. S2B). In PV-Arch mice, rhythmic stimulation at 40 Hz produced a cumulative increase in spiking output during the pulse interval (SI Results, Effects on Neural Activity). In PV-ChR2 mice, rhythmic suppression at both 1 Hz and 40 Hz produced a cumulative decrease in activity.
Behavior.
Light/dark box.
The light/dark box is viewed as an ethological model of anxiety (10), placing into competition the drives to remain safe and to explore novel environments. The apparatus has two compartments (Fig. S1), one dark and enclosed, the other well-lit with transparent walls and an open top. Following 2 min of habituation in the dark, a barrier between the two sides was removed, enabling the mouse to explore the light side freely. Light-side exploration is typically reduced in anxious mice (10, 16). We held novelty constant by giving all groups the same exposure to the experimental box, so differences among groups following the laser treatment should reflect their relative anxiety. We analyzed the time to the first entry into the light side (latency), the total number of entries into the light side (entries), the time elapsed during exploration of the light side (duration), and the total number of vertical rears (verticals) for PV-Arch (n = 34), PV-ChR2 (n = 27), PV-PV (n = 15), and no-laser control mice (n = 25) before (Pre) and after (Post) the 4-wk protocol.
ANOVAs revealed differences in exploratory behavior (Table S2) between Pre and Post assessments that differed across groups. Effects for time (Pre × Post) were highly significant for all measures (all comparisons P < 0.0001). Each group exhibited significant decreases for duration, total entries, and verticals during the Post versus the Pre assessment (Table S3), representing a reduction in exploratory behavior. Significant interactions (time × group) were seen for duration [F(3,97) = 5.81, P = 0.001] and total entries [F(3,97) = 3.6, P = 0.02], and a trend was seen for latency (P = 0.10), indicating that the reduction in exploratory behavior differed in extent among groups. Interactions also were seen when analyzing by frequency (time × frequency), with significant effects for latency [F(11,89) = 2.37, P = 0.01], total entries [F(11,89) = 3.53, P = 0.0004], and duration [F(11,89) = 3.61, P = 0.0003] and a strong trend for verticals (P = 0.051). These decreases in exploratory behavior presumably resulted from the apparatus no longer being novel, reducing the impetus for exploration.
Table S2.
Means ± SE for exploratory measures in the light/dark box
| Group | Pre | Post | ||||||
| Latency, s | No. entries | Duration, s | No. verticals | Latency, s | No. entries | Duration, s | No. verticals | |
| PV-Arch | 7.6 ± 0.9 | 8.0 ± 0.4 | 80.4 ± 3.6 | 12.2 ± 1.2 | 11.5 ± 3.9 | 6.9 ± 0.5 | 54.4 ± 3.5 | 6.9 ± 0.7 |
| PV-ChR2 | 7.5 ± 1.3 | 7.8 ± 0.5 | 82.4 ± 4.4 | 12.4 ± 1.2 | 19.5 ± 8.0 | 4.8 ± 0.5 | 38.7 ± 4.5 | 4.3 ± 0.8 |
| PV-PV | 6.0 ± 1.0 | 8.5 ± 0.5 | 82.9 ± 4.3 | 10.0 ± 1.2 | 38.0 ± 15.5 | 4.3 ± 0.9 | 27.2 ± 5.8 | 2.3 ± 0.7 |
| No laser | 6.9 ± 0.9 | 8.0 ± 0.5 | 77.0 ± 4.4 | 10.3 ± 1.2 | 13.6 ± 4.3 | 4.5 ± 0.5 | 30.8 ± 3.0 | 3.9 ± 0.7 |
Table S3.
Pre versus Post comparisons for exploratory measures in the light/dark box (paired t tests)
| Group | Latency, s | No. entries | Duration, s | No. verticals |
| PV-Arch | n.s. | df = 33, t = 2.4, P = 0.02 | df = 33, t = 6.7, P < 0.0001 | df = 33, t = 4.3, P = 0.0001 |
| PV-ChR2 | n.s. | df = 26, t = 4.7, P < 0.0001 | df = 26, t = 7.9, P < 0.0001 | df = 26, t = 6.0, P < 0.0001 |
| PV-PV | n.s. | df = 14, t = 4.9, P = 0.0002 | df = 14, t = 11.6, P < 0.0001 | df = 14, t = 5.3, P < 0.0001 |
| No laser | n.s. | df = 24, t = 5.0, P < 0.0001 | df = 24, t = 8.9, P < 0.0001 | df = 24, t = 6.9, P < 0.0001 |
n.s., not significant.
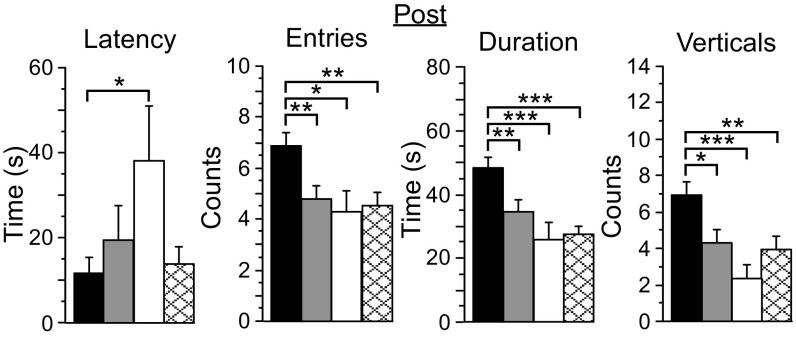
During the Pre assessment, light-side exploration was similar among groups (Fig. S3 and Table S2). During the Post assessment, however, PV-Arch mice that received rhythmic cortical stimulation made more light-side entries, scored higher for duration, and performed more verticals than mice from the other three groups (Fig. 2, SI Results, Behavior, and Table S2), including no-stimulation controls (no-laser group) and mice receiving rhythmic suppression of cortical activity (PV-ChR2 group). The latency to first light-side entry was largely similar among groups, although PV-PV mice were significantly delayed compared with PV-Arch mice (df = 47, t = 2.2, P = 0.04). Therefore, PV-Arch mice exhibited greater exploratory behavior following the 4-wk protocol.
Fig. S3.
Exploratory behavior in the light/dark box before the 4-wk protocol (Pre) was comparable among groups. During the Pre light/dark box assessment, PV-Arch (n = 34), PV-ChR2 (n = 27), PV-PV (n = 15), and no-laser (n = 25) mice scored similarly in various measures of exploratory behavior including time to first light-side entry (latency), number of light-side entries, duration of light-side exploration, and total vertical rears (verticals).
Fig. 2.
Exploration differs significantly among groups following the induction of oscillatory activity. PV-Arch mice (n = 34, black bars) exhibited significantly reduced latency and more total entries into the light side of the light/dark box, explored the light side for longer (duration), and exhibited more vertical rears than mice from the PV-ChR2 (n = 27, gray bars), PV-PV (n = 15, white bars), and no-laser (n = 25, cross-hatched bars) groups. Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
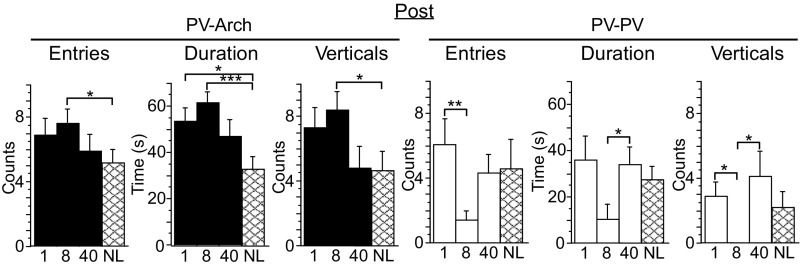
The differences seen with PV-Arch mice during the Post assessment were frequency specific (Fig. 3 and SI Results, Behavior). Compared with the no-laser control group, mice exposed to 8-Hz pulses consistently scored higher for total entries, duration, and verticals. An increase in duration was also seen for mice exposed to 1-Hz pulses. The mice exposed to 40-Hz pulses were similar to the no-laser controls. These data demonstrate that increased exploration by PV-Arch mice during the Post assessment was associated specifically with cortical stimulation at 1 Hz and 8 Hz.
Fig. 3.
Increased exploration in PV-Arch mice was associated with 1-Hz and 8-Hz cortical stimulation. Compared with PV-Arch no-laser mice (NL, n = 11, cross-hatched bars, left three panels), mice exposed to 8-Hz pulses (n = 13, black “8” bars) scored higher for total entries into the light side of the light/dark box, total duration of light-side exploration, and total vertical rears. Additionally, mice exposed to 1-Hz pulses (n = 13, black “1” bars) scored higher for duration. No differences were seen between PV-Arch exposed to 40-Hz pulses (n = 11, black “40” bars) and the no-laser control group (data not shown). For PV-ChR2 mice, no differences relative to the no-laser control group were seen at any frequency. For PV-PV mice, no differences relative to the no-laser control group were seen at any frequency (n = 5, cross-hatched bars, right three panels), although some differences were seen for mice exposed to 1-Hz pulses (n = 5, white “1” bars) and 40-Hz pulses (n = 5, white “40” bars) relative to mice exposed to 8-Hz pulses (n = 5, white “8” bars). Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
No frequency-specific effects were seen for PV-ChR2 or PV-PV mice relative to no-laser controls. Surprisingly, differences between frequencies were seen in the PV-PV group (Fig. 3, and SI Results, Behavior). PV-PV mice exposed to 1-Hz pulses exhibited more entries than mice exposed to 8-Hz pulses, in part because the mice exposed to 1-Hz pulses did not show a decline in entries compared with the Pre assessment (entries Pre: 8.1 ± 1.1; Post: 7.9 ± 1.4). PV-PV mice exposed to 1-Hz and 40-Hz pulses both scored higher for duration and verticals than mice exposed to 8-Hz pulses.
Motor output.
To assess basic motor output, we examined movement through a uniformly illuminated cylindrical arena (Fig. S1). Quantified behaviors included total distance traveled (distance), mean run speed (speed), total time immobile, number of immobile periods, and number of rotations. The results are summarized in Fig. 4 and Table S4.
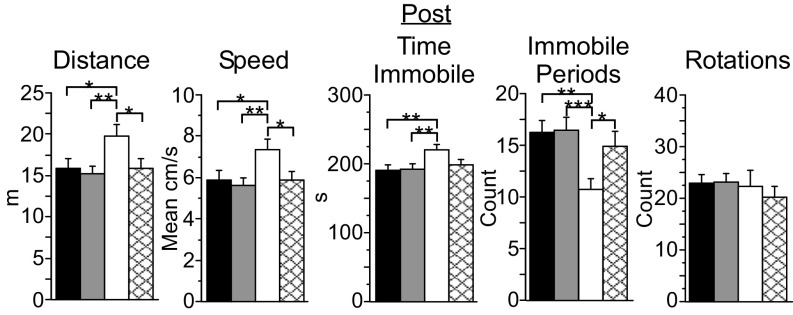
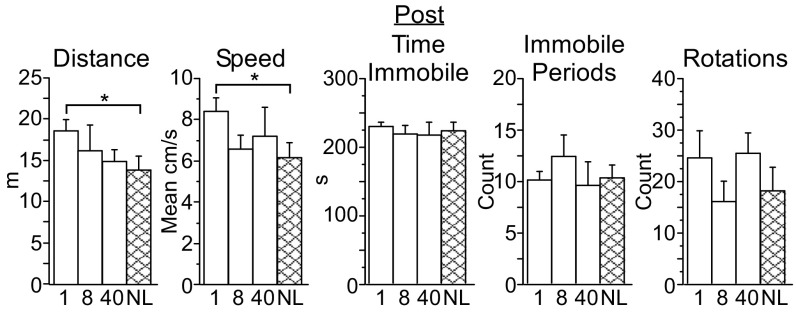
Fig. 4.
PV-PV mice exhibit distinct arena behavior following the 4-wk protocol (Post). PV-PV mice (n = 15, white bars) scored higher for total distance traveled, mean speed, and total time immobile and had fewer immobile periods than mice from the PV-Arch (n = 34, black bars), PV-ChR2 (n = 27, gray bars), and no-laser (n = 25, cross-hatched bars) groups. Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Table S4.
Means ± SE for motor output behaviors in the cylindrical arena
| Group | Pre | Post | ||||||||
| Distance, m | Speed, cm/s | No. rotations | Immobile, s | No. times immobile | Distance, m | Speed, cm/s | No rotations | Immobile, s | No times immobile | |
| PV-Arch | 21.8 ± 1.0 | 8.1 ± 0.4 | 26.1 ± 2.0 | 233 ± 6 | 8.8 ± 0.9 | 15.9 ± 1.2 | 5.9 ± 0.4 | 22.9 ± 1.7 | 191 ± 7 | 16.2 ± 1.2 |
| PV-ChR2 | 21.0 ± 1.1 | 7.8 ± 0.4 | 27.8 ± 2.0 | 231 ± 4 | 9.4 ± 0.9 | 15.2 ± 0.9 | 5.6 ± 0.3 | 23.1 ± 1.7 | 193 ± 7 | 16.5 ± 1.2 |
| PV-PV | 20.6 ± 1.3 | 7.6 ± 0.5 | 21.5 ± 2.4 | 223 ± 5 | 11.4 ± 1.1 | 20.2 ± 1.4 | 7.5 ± 0.5 | 22.4 ± 3.1 | 221 ± 7 | 10.8 ± 1.0 |
| No laser | 21.2 ± 1.1 | 7.8 ± 0.4 | 25.8 ± 1.9 | 236 ± 5 | 8.7 ± 1.0 | 15.9 ± 1.2 | 5.9 ± 0.4 | 20.3 ± 2.1 | 198 ± 8 | 14.9 ± 1.4 |
ANOVAs revealed highly significant effects for time (Pre × Post) for all measures (all comparisons P < 0.0001) except rotations. Significant interactions (time × group) were observed for distance [F(3,97) = 2.91, P = 0.04], mean speed [F(3,97) = 3.1, P = 0.03], time immobile [F(3,97) = 4.5, P = 0.005], and total immobile periods [F(3,97) = 6.0, P = 0.0009]. When separated by frequency (time × frequency), significant interactions were seen for time immobile [f(11,93) = 1.95, p = 0.04] and total immobile periods [F(11,93) = 2.4, P = 0.01], with trends for distance (P = 0.10) and mean speed (P = 0.08).
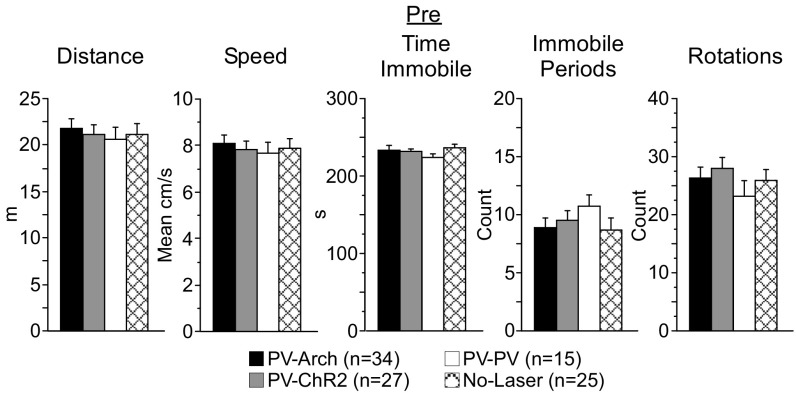
During the Pre assessment, behavior in the arena was comparable among PV-Arch, PV-ChR2, PV-PV, and no-laser mice (Fig. S4). Following the 4-wk protocol, PV-Arch, PV-ChR2, and no-laser mice all demonstrated significant decreases in distance traveled and mean speed and increases in immobile periods and time immobile (all t test comparisons yielding P ≤ 0.0003; Table S4 for mean data). No-laser mice made fewer rotations (df = 24, t = 2.3, P = 0.03), with trends toward a similar reduction for PV-Arch and PV-ChR2 mice (P = 0.18 and P = 0.06, respectively). The changes also were similar in magnitude, with no significant between-group differences observed for any of the arena behaviors. These changes indicate (i) that optogenetic manipulation of ACC activity had no subsequent effect on spontaneous motor output and (ii) reduced exploration of the arena that, as with the Post light/dark box assessment, likely reflects the loss of novelty.
Fig. S4.
Motor output in the arena before the 4-wk protocol (Pre) was comparable among groups. During the Pre arena assessment, PV-Arch (n = 34), PV-ChR2 (n = 27), PV-PV (n = 15), and no-laser (n = 25) mice scored similarly in various measures of motor output including total distance traveled, mean run speed, total time immobile, number of immobile periods, and the total number of rotations (clockwise and counterclockwise).
In contrast to the other three groups, the arena behavior of PV-PV mice during the Post assessment was almost identical to that seen 4 wk earlier during the Pre assessment (Table S4), resulting in the group differences illustrated in Fig. 4. During the Post arena assessment, PV-PV mice exhibited greater total distance traveled and greater mean speed than PV-Arch, PV-ChR2, and no-laser mice (Fig. 4 and SI Results, Behavior). PV-PV mice also exhibited fewer immobile periods but more time immobile (Fig. 4 and SI Results, Behavior).
What might account for the differences in PV-PV motor output? As noted above, PV-PV mice behaved similarly during Pre and Post assessments, in contrast to PV-Arch, PV-ChR2, and no-laser mice. When the data were broken down by frequency, no significant differences were observed between Pre and Post assessments for any behavior for PV-PV mice exposed to1 Hz, 8 Hz, or 40 Hz pulses and no-laser mice. This result cannot be attributed simply to the lower number of mice in each PV-PV group, because the SEs observed at each frequency for each behavior Pre and Post are comparable to those for the PV-Arch, PV-ChR2, and no-laser groups (each of which dis have more mice per group). Therefore, the difference in PV-PV motor output during the Post assessment appears to be partly attributable to a difference specific to that cross, rather than to an effect of light. However, during the Post assessment, PV-PV mice exposed to 1-Hz pulses did score higher than no-laser mice in distance traveled and mean speed (Fig. 5 and SI Results, Behavior), indicating that an effect of laser cannot be ruled out for some differences in PV-PV motor output.
Fig. 5.
PV-PV mice exposed to 1-Hz pulses show greater distance traveled and mean speed in the arena following the 4-wk protocol (Post). Although PV-PV mice exposed to 8-Hz (n = 5, “8” bars) or 40-Hz (n = 5, “40” bars) pulses did not differ from the no-laser control group (n = 5, cross-hatched bars), the differences in distance and speed between the mice exposed to 1-Hz pulses (n = 5, “1” bars) and no-laser mice (NL, cross-hatched bars) indicate that a light-specific effect cannot be ruled out in PV-PV mice. Error bars indicate SEM. *P < 0.05.
Novel object and novel location recognition.
To assess attention, basic cognition, and short-term memory, we quantified novel object and novel location recognition behavior (Fig. S1). During initial exposure, mice explored a pair of objects (familiar) in the same arena in which motor output data were collected. Next, mice explored one familiar and one novel object to assess object recognition memory. Finally, mice explored the same objects but with the familiar object moved to a novel location. This sequence is illustrated in Fig. S1. The time spent exploring each object was compared as a measure of preference for one object/position over another.
ANOVAs revealed a significant effect for time (Pre × Post) for each object/location in assessments 3–5 (most comparisons P < 0.0001; novel location F = 5.8, P = 0.02), reflecting the general decrease in the time spent exploring the objects during the Post session (Table S5). A significant interaction (time × group) was detected for exploration of both objects during the test of object recognition memory [familiar object: F(3,97) = 6.4, P = 0.0005; novel object: F(3,97) = 3.6, P = 0.02]. An interaction also was seen when sorting by frequency (time × frequency) during this test [familiar object: F(11,89) = 3.3, P = 0.0008; novel object: F(11,89) = 2.2, P = 0.02]. No interactions (by group or by frequency) were detected for either object during the test of location recognition memory.
Table S5.
Quantification of object and location recognition behavior (time in seconds ± SE)
| Group | Pre | Post | ||||||||||
| Initial exposure | Object recognition | Location recognition | Initial exposure | Object recognition | Location recognition | |||||||
| a | b | a | NO | b | NL | a | b | a | NO | b | NL | |
| PV-Arch | 46.7 ± 4 | 38.4 ± 4 | 13.7 ± 2 | 21.6 ± 2 | 14.2 ± 2 | 21.5 ± 3 | 29.2 ± 3 | 24.2 ± 3 | 7.2 ± 1 | 11.1 ± 1 | 8.6 ± 2 | 15.6 ± 2 |
| PV-ChR2 | 34.3 ± 4 | 31.2 ± 3 | 10.9 ± 4 | 14.2 ± 4 | 7.8 ± 2 | 15.5 ± 2 | 23.9 ± 4 | 21.6 ± 3 | 8.8 ± 2 | 13.6 ± 2 | 8.0 ± 2 | 12.9 ± 2 |
| PV-PV | 47.9 ± 5 | 43.4 ± 4 | 26.3 ± 4 | 28.1 ± 4 | 13.1 ± 3 | 25.5 ± 5 | 22.8 ± 4 | 15.4 ± 3 | 6.9 ± 2 | 12.2 ± 3 | 8.6 ± 3 | 20.5 ± 4 |
| No laser | 49.2 ± 5 | 41.8 ± 4 | 14.7 ± 2 | 24.8 ± 4 | 15.2 ± 2 | 19.6 ± 3 | 25.0 ± 2 | 23.2 ± 3 | 8.1 ± 1 | 11.3 ± 2 | 6.8 ± 1 | 17.3 ± 2 |
a, b: positions corresponding to familiar and novel objects, respectively, during the object recognition test; NL, novel location; NO, novel object.
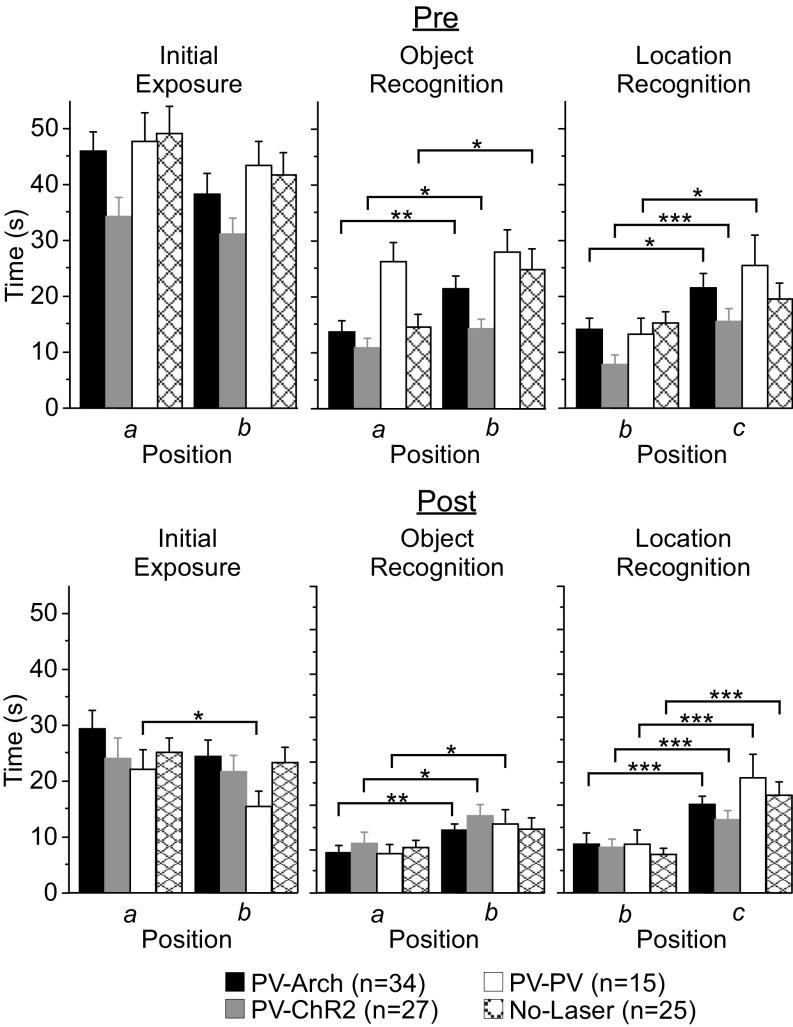
During the Pre assessment, PV-Arch, PV-ChR2, and no-laser mice all recognized the novel object (Fig. S5 and Table S6), whereas PV-Arch, PV-ChR2, and PV-PV mice all recognized the novel location. PV-PV mice and no-laser mice failed to exhibit object recognition and location recognition, respectively, although in both cases a modest preference for the novel stimulus was observed.
Fig. S5.
Object exploration times before (Pre) and after (Post) the 4-wk protocol. During the Pre assessment, PV-Arch (n = 34), PV-ChR2 (n = 27), and no-laser (n = 25) mice exhibited significant object recognition behavior, and PV-Arch, PV-ChR2, and PV-PV (n = 15) mice exhibited significant location recognition behavior. During the Post assessment, PV-Arch, PV-ChR2, and PV-PV mice exhibited significant object recognition behavior, and all four groups exhibited significant location recognition behavior. a, b: positions corresponding to familiar and novel objects, respectively, during the object recognition test; b, c: positions corresponding to the familiar and novel locations, respectively, during the location recognition test. *P < 0.05; **P < 0.01; ***P < 0.001.
Table S6.
Statistical group comparisons for object and location recognition memory (paired t tests)
| Group | Pre | Post | ||
| Object recognition | Location recognition | Object recognition | Location recognition | |
| PV-Arch | df = 33, t = 3.0, P = 0.005 | df = 33, t = 2.7, P = 0.01 | df = 32, t = 3, P = 0.005 | df = 33, t = 4.8, P < 0.0001 |
| PV-ChR2 | df = 26, t = 2.2, P = 0.04 | df = 26, t = 4.7, P < 0.0001 | df = 24, t = 2.3, P = 0.03 | df = 26, t = 2.9, P = 0.007 |
| PV-PV | n.s. | df = 14, t = 2.2, P = 0.04 | df = 13, t = 2.5, P = 0.02 | df = 14, t = 3.0, P = 0.008 |
| No laser | df = 24, t = 2.4, P = 0.02 | n.s. | n.s. | df = 24, t = 5.3, P < 0.0001 |
n.s., not significant.
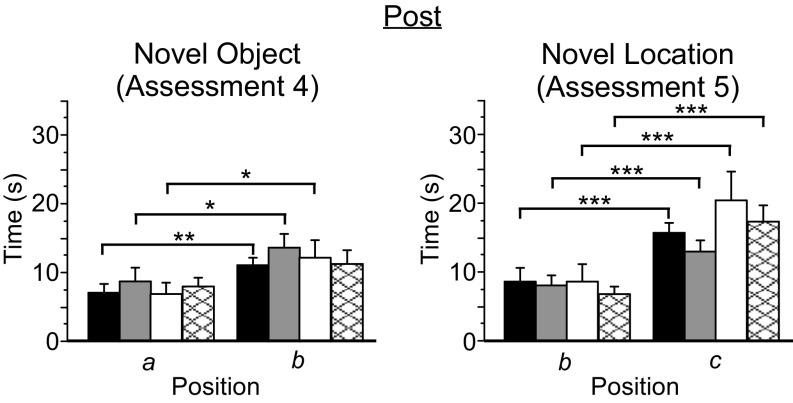
During the Post assessment, PV-Arch, PV-ChR2, and no-laser mice explored the two initial exposure objects comparably (Table S5), whereas PV-PV mice were biased toward the object serving as the familiar stimulus in the subsequent object recognition test. However, as with PV-Arch and PV-ChR2 mice, PV-PV mice exhibited a significant preference for the novel object, indicating that the bias seen during initial exposure did not interfere with, or spuriously account for, novel object recognition (Table S6). No-laser mice failed to exhibit significant object recognition behavior following the 4-wk protocol, although a strong trend was observed (P = 0.051) (Fig. 6). All four groups exhibited robust location recognition behavior. Comparisons of the “strength” of novelty preference, either as a ratio of novel/familiar or simple difference between novel and familiar exploration times, yielded little evidence of group effects. The ratio for novel object preference was greater for PV-PV mice than for no-laser mice (df = 38, t = 2.2; P = 0.04). The difference in novel location preference was greater for no-laser mice than for PV-ChR2 mice (df = 50, t = 2.2, P = 0.03). No other differences in the strength of preference for either object or location novelty were observed.
Fig. 6.
Rhythmic manipulation of cortical activity did not alter object or location recognition memory. Mice were tested for novel object and novel location recognition memory before and after the 4-wk protocol. Greater exploration times are interpreted as novelty recognition. Following the 4-wk protocol (Post), PV-Arch (n = 34, black bars), PV-ChR2 (n = 27, gray bars), and PV-PV (n = 15, white bars) mice all recognized the novel object; a strong trend toward object recognition (P = 0.051) was also exhibited by no-laser mice (n = 25, cross-hatched bars). All four groups exhibited robust location recognition. a, b: positions corresponding to familiar and novel objects, respectively, during the object recognition test; b, c: positions corresponding to the familiar and novel locations, respectively, during the location recognition test. Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In previous work it was found that integrated mind body meditation training (20 sessions of 30 min each) reduced measures of negative affect such as self-reported anxiety (1). Such training also has been found to increase rhythmic activity in the ventral ACC and improve connectivity in white matter tracts related to the ACC (1, 3, 4). In the current study we examined how inducing rhythmic activity at different frequencies in the mouse ACC influenced a range of behaviors, including light/dark box exploration (an assay of anxiety), motor output in the open arena, and novel object and location recognition (tests of attention and short-term memory). Each behavior was measured before and after 4 wk of rhythmic manipulation of ACC activity. All mice, including the nonstimulated controls, exhibited changes in behavior following the 4-wk protocol consistent with an overall reduction of exploration on the second occasion of being tested compared with the first occasion. However, we found that light/dark box exploration by PV-Arch mice that underwent rhythmic stimulation remained elevated compared with mice receiving rhythmic suppression or no manipulation. In contrast, behavior of PV-Arch mice in the open arena was comparable to each of the other groups, as was object exploration and performance during the tests of recognition memory. Given the absence of (i) a motor phenotype (e.g., hyperactivity), (ii) any generalized changes in exploratory behavior, or (iii) evidence of cognitive impairment or inability to recall past experience, the data suggest that rhythmic cortical stimulation may facilitate a reduction in anxiety. This finding supports our hypothesis that increased output from the ACC would be critical to behavior change. Nonetheless, we recognize that the light/dark box behavior is complex and that other tests of anxiety, such as extinction of conditioned fear, should be examined in future studies to help further constrain the nature of the effects observed here.
The greater light/dark box exploration seen in PV-Arch mice depended on the frequency of stimulation (Fig. 3). Stimulation at 8 Hz was associated with the most exploration. The effects of 1-Hz stimulation were in the same direction as with 8-Hz stimulation but were less robust. Stimulation at 40 Hz produced little or no difference from no-light stimulation, indicating that the effects on behavior were related to frequency rather than to the cumulative increase in spiking output. The absence of a behavioral effect with rhythmic suppression in PV-ChR2 mice confirms the importance of phase-locked increases in spiking activity rather than simply the induction of oscillations, per se. Overall, these data support our prediction that 8-Hz (theta) stimulation would be most effective, but the effect was not completely specific because 1-Hz stimulation produced similar results.
What factor(s) might underlie the possible decreased anxiety exhibited by PV-Arch mice following 1-Hz and 8-Hz stimulation? According to some views, the ACC serves to regulate limbic areas to which it is connected (8, 17). It is reasonable to propose that altering the strength and/or efficiency of those connections could influence behavior. Learning is associated with changes in synaptic transmission that may be induced artificially with 1-Hz and 8-Hz stimulation (18, 19). Learning also induces structural changes in white matter (20–23). Recently, it was shown that these white matter changes likely result from the formation of new myelin-producing oligodendrocytes (24). Our stimulation of cortex at 1 Hz and 8 Hz may drive the generation of new oligodendrocytes, resulting in increased myelination of fibers linking the ACC with other limbic areas and consequently reducing anxiety-related behaviors. The effects of rhythmic cortical stimulation on light/dark box exploration are consistent with ventral ACC involvement in regulating anxiety, fear, and stress (25–29), and chronic exposure to anxiety/stress induces changes in neurotransmission and long-term potentiation in the rodent ACC (26, 30, 31). Validation of the hypothesis linking 1- to 8-Hz cortical stimulation with increased myelination and decreased anxiety will require quantifying the generation of new oligodendrocytes and the density of myelin in mice that undergo stimulation.
Demonstrating that rhythmic cortical stimulation had no effect on motor output in the arena was essential for the interpretation of the light/dark box results. The ACC is reciprocally connected with primary and secondary motor areas and is often associated with motor processes (32–35). In the mouse, secondary motor cortex is situated immediately lateral to the ACC and likely was exposed to light, albeit to a lesser degree, during the 4-wk protocol. Any hyper-locomotor activity or other motor phenotype during the Post assessment would have complicated interpretation of the light/dark box exploration data. The absence of any such phenotype confirms the specificity of the effect to a reduction of anxiety-associated behavior.
The absence of an effect on recognition memory did not match our expectation based on previous observations of improved cognition with meditation (1, 2). However, it fits with the finding that meditation training, in comparison with relaxation, increased activity in the ventral rather than the dorsal ACC. This part of the ACC is connected with limbic areas and is thought to control mainly emotional activity (8). Moreover, it is not inconsistent with the broader literature. In rodents and humans, the ACC is active during object exploration and exposure to novelty (36–43). However, it is unclear whether the ACC is necessary for recognition memory (44–46). Whether the stimulation protocol used here would improve performance on more complex cognitive tasks or behaviors known to be dependent on the ACC remains to be determined.
We consider some hypotheses for the results obtained with the PV-PV animals. First, the Pvalb-IRES-Cre animals were originally generated by insertion of an internal ribosome entry site (IRES)-Cre cassette into the 3′ UTR of the parvalbumin (Pvalb) locus (47). Although this strategy effectively mimics the exact expression of a gene, endogenous expression of the target locus could be disrupted. That said, defects in endogenous parvalbumin expression that could account for our results have not been reported (48). Second, the PV, Arch, and ChR2 mouse lines, although all maintained on a C57BL6/J background, have been bred separately and thus could have undetected spontaneous mutations that have become fixed (genetic drift) that could result in physiological and behavioral differences. In light of these possibilities, and given the absence of an effect on spiking activity in PV-PV mice with light delivery, we feel the line-specific no-laser controls are more relevant to the effect of pulse frequency.
In previous work it was found that meditation training reduced measures of negative affect such as self-reported anxiety (1). Meditation training also has been found to increase activity in the ventral ACC and to improve connectivity in white matter tracts related to the ACC (1, 3, 4). Our current results show that rhythmic stimulation of frontal cortical activity in an animal model can produce some of the affective changes found in humans. It is important to note that our previous studies of human meditation used only one form of mindfulness training, and that other forms of meditation have been associated with rhythmic changes in other brain regions. For example, a study of experienced Buddhist meditators found increases in gamma frequency rhythms in parietal–occipital cortical regions (49). Nonetheless, although there is no reason to believe that theta stimulation accounts for all the effects of meditation, it may prove useful to have a plausible account of how meditation and lower frequency oscillations could lead to such physical changes in the brain as altered white matter (3, 4, 6). Future studies may examine the brain mechanisms that support these behavioral changes in our mouse model. It also will be possible to explore experimental manipulation of frontal theta activity by less invasive external stimulation in humans as a further test of the idea that frontal theta activity may be the effective mechanism in changing white matter following meditation.
Methods
All studies were conducted with protocols approved by the University of Oregon Institutional Animal Care and Use Committees in compliance with National Institutes of Health guidelines for the care and use of experimental animals.
Mice.
We assessed the effects of rhythmic suppression or stimulation of cortical activity in mice expressing the light-gated ion channel Channelrhodopsin-2 or the proton pump Archaerhodopsin in parvalbumin-positive inhibitory interneurons. Parents were homozygous for Pvalb-IRES-Cre (PV; 008069; The Jackson Laboratory), Rosa-CAG-LSL-ChR2(H134R)-EYFP-WPRE (ChR2; 012569; The Jackson Laboratory), or Rosa-CAG-LSL-Arch-GFP-WPRE (Arch; 012735; The Jackson Laboratory). Optogenetic suppression of the ACC was performed using offspring heterozygous for both PV and ChR2 alleles (PV-ChR2). Optogenetic stimulation was performed using offspring heterozygous for both PV and Arch alleles (PV-Arch). Mice homozygous for Pvalb-IRES-Cre (PV-PV) were used to control for nonspecific effects of pulsed light delivery. Data were collected from both male and female mice aged 8–12 wk at the time of surgery. A total of 47 PV-Arch, 38 PV-ChR2, and 25 PV-PV mice were included in the behavioral and single-neuron recording experiments.
Surgery.
To study the behavioral effects of rhythmic cortical activity manipulations, mice were implanted with a two-by-two array of optic fibers (200-µm diameter) overlying each hemisphere of the ACC. For recording single-neuron activity, a four-tetrode array with a single 200-µm optic fiber was implanted in the ACC.
Behavioral Data Acquisition and Stimulus Delivery.
Mice were run through a sequence of five 5-min assessments both before and after a 4-wk protocol (for a detailed description, SI Methods and Fig. S1. The only changes before (Pre) and after (Post) the 4-wk protocol were in the set of objects used and their positions on the arena floor relative to the visual cues during assessments 3–5 (i.e., one set of objects was used before the 4-wk protocol, and another set was used after the protocol). For optogenetic manipulations, 520-nm wavelength modules were set to 9.5 mW for PV-Arch mice, and 445-nm wavelength modules were set to 6.3-mW for PV-ChR2 mice. The effective spread for each wavelength at these intensities is 1.5 mm in diameter (14, 50, 51).
Assessments 1 and 3–5 were hand-scored separately by at least two viewers blind to experimental condition. Assessment 2 was quantified by the commercial tracking software package Any-maze (Stoelting Co.). All analyses of behavioral data were performed using the statistical analysis program StatView. ANOVAs were used to assess effects of time (Pre versus Post) and interactions by group or frequency. Paired t tests were used to assess within-group differences following the 4-wk protocol. Unpaired t tests were used for between-group comparisons and for within-group comparisons for frequency effects versus no-laser controls (for details, SI Methods).
Single-Neuron Recording and Analysis.
Single-neuron data were analyzed during a 5-min baseline interval followed by a 5-min pulsed-light interval for each frequency. Data were collected from each mouse at 1-Hz, 8-Hz, and 40-Hz pulse frequencies. For PV-PV mice, data were collected using either 445-nm or 520-nm wavelength light to control for nonspecific wavelength effects. Analyses were limited to well-isolated putative pyramidal neurons [SI Methods for interneuron exclusion criteria and cluster isolation metrics (52–54)]. Mean firing rates were calculated for each 5-min interval and were analyzed using paired t tests. To identify significant phase-locked changes in firing rate associated with the laser, paired t tests compared spiking activity during a 10-ms response interval 6–15 ms following the onset of each laser pulse with a 10-ms baseline interval immediately preceding each pulse (the 5-ms interval following the pulse was excluded to control for potentially confounding photoelectric artifacts). Unpaired t tests were used for between-frequency and between-group comparisons. Between-group comparisons for the size of the change were calculated using a simple difference between response and baseline firing rates.
SI Methods
Mice.
In the present study we assessed the effects of rhythmic suppression or stimulation of cortical activity in mice expressing the light-gated ion channel Channelrhodopsin-2 or the proton pump Archaerhodopsin in parvalbumin-positive inhibitory interneurons. Three transgenic lines were used in this study. The Pvalb-IRES-Cre line (008069; The Jackson Laboratory; PV) has a targeted Cre knockin to the 3′ UTR of exon 5 of the endogenous parvalbumin locus. The Rosa-CAG-LSL-ChR2(H134R)-EYFP-WPRE line (012569; The Jackson Laboratory; ChR2) has Cre-dependent expression of a channelrhodopsin-2/EYFP fusion protein. The Rosa-CAG-LSL-Arch-GFP-WPRE line (012735; The Jackson Laboratory; Arch) has Cre-dependent expression of an Archaerhodopsin-3/GFP fusion protein. Optogenetic suppression of ACC was performed using offspring heterozygous for both PV and ChR2 alleles (PV-ChR2). Optogenetic stimulation was performed using offspring heterozygous for both PV and Arch alleles (PV-Arch). A third group of mice was homozygous for the PV allele (PV-PV). For the behavioral experiments, mice were divided into four groups: 34 PV-Arch (16 female, 18 male), 27 PV-ChR2 (20 female, seven male), 15 PV-PV (six female, nine male), and 25 no-laser controls (13 female, 12 male). The no-laser controls included mice from each of the three transgenic groups. Each frequency of light delivery included a mix of males and females.
Surgery.
Surgical anesthesia was induced and maintained with isoflurane (1.25–2.0%). Depth of anesthesia was verified with a deep tail-pinch. In all animals, the scalp and fascia from anteroposterior (AP) +5.0 mm to lambda were removed, and the skull was covered with a thin layer of cyanoacrylate (Vetbond; World Precision Instruments) before hardware was implanted. To study the behavioral effects of rhythmic cortical activity manipulations, mice were implanted with a two-by-two array of optic fibers (200 µm in diameter). A rectangular opening (approximately 2.5 × 0.8 mm) was made in the skull on both sides of the midline, extending forward from bregma. The fiber array was implanted flush with the dura, immediately dorsal to the ACC, with a pair of fibers overlying each hemisphere. The center-to-center distance was 0.8 mm across the midline and 1.1 mm along the rostral–caudal axis. The caudal pair of fibers was located approximately 0.6 mm anterior to bregma. For recording single-neuron activity, a four-tetrode optrode array was implanted in the ACC centered at AP 0.8 mm and mediolateral 0.4 mm to a depth of approximately 0.8 mm. A 200-µm optic fiber was fixed adjacent to the bundle 1.0 mm dorsal to the tetrode tips. All hardware was fixed in place with Grip Cement (Dentsply). Mice were administered carprofen (5.0 mg/kg) postoperatively to minimize discomfort and were allowed 7 d of postoperative recovery.
Behavioral Data Acquisition and Stimulus Delivery.
The present study involved examining whether and how chronic rhythmic manipulation of ACC activity would influence basic measures of anxiety, motor output, attention, and short-term memory. Mice were run through a sequence of five 5-min behavioral assessments both before and after a 4-wk protocol involving rhythmic manipulations of ACC activity. All assessments were recorded on video. Mice were placed in a holding cage between assessments. The five-assessment sequence is illustrated in Fig. S1.
Behavioral measures of anxiety were quantified during assessment 1 as mice explored the light/dark box (Maze Engineers). The apparatus was 40 cm wide × 40 cm long × 34.5 cm high and was divided into two halves of equal dimensions, one dark and enclosed and the other well lit with transparent walls and an open top. Mice were placed singly in the dark side of the apparatus for 2 min. Then the door separating the two sides was removed, and mice were allowed to explore both sides for the next 3 min. The time to the first entry into the light side (latency), total entries into the light side (entries), time elapsed exploring the light side (duration), and the total number of vertical rears (verticals) were quantified. Basic motor behavior was quantified during assessment 2 as mice explored a visually cued, cylindrical arena 60 cm in diameter. Moderate illumination was diffuse and consistent throughout the arena. The quantified behaviors included total distance traveled (in meters), mean run speed (in centimeters per second), total rotations, time spent immobile (in seconds), and total number of immobile episodes. Assessments 3–5 were carried out in the same arena and assessed novel object and novel location recognition memory. During assessment 3, mice explored two different objects for 5 min. In assessment 4, one of the two objects was replaced with a novel object. In assessment 5, the second of the two original objects was shifted to a novel position. The total time spent exploring each object was quantified to provide a measure of preference for one object over another. This sequence of assessments was repeated following the 4-wk pulsed-light protocol. The only changes were the set of objects and their positions on the arena floor relative to the visual cues during assessments 3–5 (i.e., one set of objects was used before the 4-wk protocol, and a different set was used afterwards).
Light/dark box behavior (assessment 1) and the three object exploration sessions (assessments 3–5) were hand-scored separately by at least two viewers blind to the experimental condition. Behavior in the open arena (assessment 2) was quantified by the commercial tracking software package Any-maze (Stoelting Co.). All analyses of behavioral data were performed using the statistical analysis program StatView. ANOVAs were used to assess effects of time (Pre versus Post) and interactions by group or frequency. Unpaired t tests were used for between-group comparisons. If a difference between groups was detected, a within-group analysis was performed, comparing each frequency with the no-laser control group to determine whether the difference was frequency specific. Paired t tests were used to assess within-group effects of time (Pre versus Post). For object exploration sessions (assessments 3–5) (Fig. S1), paired t tests were performed to measure preference for one object/location versus another based on total time explored. Unpaired t tests were used to compare the relative “strength” of the recognition behavior between groups. Here, strength was assessed both as a ratio of novel/familiar and as the simple difference between exploration times (novel − familiar). For light/dark box analyses, mice that did not enter the light chamber (3 of 101 mice, two from the PV-PV group and one from the PV-ChR2 group) were scored a time to first light entry of 180 s and were scored 0 s for the remaining timed measures and 0 vertical rears.
The initial five assessments were always performed on a Friday. The following Monday, mice began once-daily 30-min pulsed-light sessions that were conducted 5 d/wk for 4 wk. Mice from all three genetic backgrounds were assigned to a 1-Hz (200 ms/pulse), 8 Hz (5 ms/pulse), 40 Hz (5 ms/pulse), or no-laser condition. Four optic fiber tethers were fit to the fiber array, and the mice were placed in a dark, sound-attenuating chamber. The other ends of the tethers were fastened to individually adjustable laser modules that were calibrated before each session. For PV-Arch mice, 520-nm wavelength modules were set to 9.5 mW. For PV-ChR2 mice, 445-nm wavelength modules were set to 6.3 mW. These intensities were selected to produce an effective spread through a volume of tissue approximately 1.5 mm in diameter, thus restricting the direct effect predominantly to the ACC (50, 51). Half of the PV-PV mice received pulses at the 520-nm wavelength, and half received pulses at the 445-nm wavelength.
Single Neuron Recording and Analysis.
We implanted optrode arrays in the ACC of a set of mice to assess how pulsed-light delivery altered single-neuron spiking activity in PV-Arch, PV-ChR2, and PV-PV mice. Arrays consisted of four tetrodes passed through a pair (oriented along the rostro–caudal axis) of 28-gauge stainless steel hypodermic tubes (two tetrodes per tube). Tetrodes were made using 18-µm (25-µm coated) tungsten wire (California Fine Wire). A 200-µm optic fiber was mounted immediately lateral to the tubes, terminating 1 mm dorsal to the tetrode tips. The entire array was mounted on a custom microdrive.
Single-neuron recordings were 10 min in duration and consisted of 5-min baseline and pulsed-light intervals. Data were collected from each mouse at 1-Hz, 8-Hz, and 40-Hz pulse frequencies. For PV-PV mice, data were collected using either blue (445-nm wavelength) or green (520-nm wavelength) light to control for any potential nonspecific effects related to wavelength. To identify significant changes in firing rate associated with the laser, paired t tests compared the spiking activity during a 10-ms baseline interval immediately preceding each pulse with the spiking activity in a 10-ms response interval 6–15 ms following the onset of each laser pulse (the 5-ms interval beginning with laser onset was excluded to control for potentially confounding photoelectric artifacts). Unpaired t tests were used for between-frequency and between-group comparisons. Between-group comparisons for the size of the change were calculated using a simple difference between response and baseline firing rates. Cumulative changes in spiking activity were calculated using paired t tests comparing the 5-min baseline and pulsed-light intervals.
Analyses were performed on spiking activity of putative pyramidal neurons. Only cells with mean waveform widths ≥300 µs (peak–trough), corresponding to those typically seen for pyramidal neurons (52), were included in the present analyses. When recording from PV-ChR2 mice, cells responding robustly during the laser pulse were identified as parvalbumin-expressing inhibitory interneurons and also were excluded from further analysis (53). A threshold was also set for cluster quality, so that only those cells with an L-ratio <1.5 were included (54).
Histology.
Following physiology experiments, mice were killed with a lethal dose of sodium pentobarbital and then were transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were extracted, placed in fixative for a minimum of two additional days, and then sectioned to verify the placement of tetrodes. Only recordings histologically verified as originating from the ACC were included in the present study (Fig. S2A).
SI Results
Effects on Neural Activity.
In PV-Arch mice (n = 2), rhythmic light pulses induced phase-locked increases in putative pyramidal neuron firing across frequencies [1 Hz (43 cells): df = 42, t = 5.5, P < 0.0001; 8 Hz (39 cells): df = 38, t = 3.6, P = 0.001; 40 Hz (41 cells): df = 40, t = 6.1, P < 0.0001] (Fig. 1 and Table S1). The phase-locked bursts of activity with 1-Hz and 8-Hz pulses did not differ significantly, although both exceeded that seen with 40-Hz trains (df = 110, t = 4.1, P < 0.0001 and df = 98, t = 3.2, P = 0.002, respectively). In PV-ChR2 mice, light pulses produced significant suppression of pyramidal neuron activity across frequencies [1 Hz (26 cells): df = 25, t = 3.1, P = 0.004; 8 Hz (21 cells): df = 20, t = 3.8, P = 0.001; 40 Hz (47 cells): df = 46, t = 3.9, P = 0.0003] (Fig. 1 and Table S1).
To determine if differences found in behavior were caused by changes in overall spiking output rather than by rhythms, we examined the cumulative change in activity over a 5-min pulse interval compared with a 5-min baseline (Fig. S2B). In PV-Arch mice, 40-Hz rhythmic stimulation produced a cumulative increase in spiking output during the pulse interval (df = 40, t = 3.7, P = 0.0007). In PV-ChR2 mice, rhythmic suppression at both 1 Hz and 40 Hz produced a general decrease in activity throughout the entire pulse interval (df = 25, t = 2.7, P = 0.01 and df = 46, t = 5.5, P < 0.0001, respectively).
No effects were seen in neurons of PV-PV mice in response to any of the three pulse frequencies (1 Hz: 70 cells, 8 Hz: 64 cells, 40 Hz: 62 cells) (Fig. 1, Fig. S2B, and Table S1). No differences among the three frequencies were seen during the baseline or response intervals. No differences were seen between the two populations of PV-PV neurons exposed to blue or green wavelengths.
Behavior.
Light/dark box.
Unpaired t tests were performed to compare group behavior in the light/dark box following the 4-wk protocol (Post). PV-Arch mice performed more total entries than PV-ChR2 mice (df = 59, t = 2.7, P = 0.01), PV-PV mice (df = 47, t = 2.2, P = 0.03), and no-laser controls (df = 57, t = 2.9, P = 0.005); had greater duration exploring the light side than PV-ChR2 mice (df = 59, t = 2.8, P = 0.007), PV-PV mice (df = 47, t = 3.9, P = 0.0003), and no-laser controls (df = 57, t = 4.9, P < 0.0001); and performed more total vertical rears than PV-ChR2 mice (df = 59, t = 2.5, P = 0.02), PV-PV mice (df = 47, t = 3.2, P = 0.003), and no-laser controls (df = 57, t = 2.8, P = 0.007). Fig. S3 and Tables S2 and S3.
Unpaired t tests were performed to compare the behavior in the light/dark box of PV-Arch mice at each pulse frequency to the behavior of PV-Arch no-laser control mice following the 4-wk protocol (Post). Compared with PV-Arch no-laser control mice, PV-Arch mice exposed to 8-Hz pulses exhibited more total entries (df = 22, t = 2.2, P = 0.04), had greater duration exploring the light side (df = 22, t = 4.1, P = 0.0004), and performed more total vertical rears (df = 22, t = 2.3, P = 0.03). Compared with no-laser control mice, PV-Arch mice exposed to 1-Hz pulses had greater duration exploring the light side (df = 19, t = 2.6, P = 0.02). Fig. S3 and Tables S2 and S3.
Unpaired t tests were performed to compare behavior in the light/dark box of PV-PV mice exposed to pulses at different frequencies following the 4-wk protocol. Compared with PV-PV mice exposed to 8-Hz pulses, PV-PV mice exposed to 1-Hz pulses exhibited more total entries (df = 8, t = 3.1, P = 0.02) and more total vertical rears (df = 8, t = 2.7, P = 0.03). Compared with PV-PV mice exposed to 8-Hz pulses, PV-PV mice exposed to 40-Hz pulses had greater duration exploring the light side (df = 8, t = 2.32, P < 0.05) and more total vertical rears (df = 8, t = 2.6, P = 0.03).
Motor output.
Unpaired t tests were performed to compare group behavior in the open arena following the 4-wk protocol (Post). PV-PV mice exhibited a greater total distance traveled than PV-ChR2 mice (df = 40, t = −3.5, P = 0.001), PV-Arch mice (df = 47, t = −2.4, P = 0.02), and no-laser mice (df = 38; t = −2.7, P = 0.01); exhibited a greater mean speed than PV-ChR2 mice (df = 40, t = −3.4, P = 0.001), PV-Arch mice (df = 47, t = −2.4, P = 0.02), and no-laser mice (df = 38, t = −2.7, P = 0.01); exhibited more time immobile than PV-ChR2 mice (df = 40, t = −3.0, P = 0.004), PV-Arch mice (df = 47, t = −2.8, P = 0.008), and no-laser mice (df = 38, t = −2.1, P = 0.04); and exhibited more immobile periods than PV-ChR2 mice (df = 40, t = −3.7, P = 0.0006), PV-Arch mice (df = 47, t = −3.4, P = 0.001), and no-laser mice (df = 38, t = −2.4, P = 0.02). Fig. S4 and Table S4.
Novel object and novel location recognition.
SI Summary
Rhythmic light delivery induced robust phase-locked increases and decreases in pyramidal neuron spiking activity in PV-Arch and PV-ChR2 mice, respectively. Light pulses had no discernible effect on the spiking activity of neurons in PV-PV mice. This manipulation, performed 30 min/d, 5 d/wk, for 4 wk, was associated with changes in the behavior of PV-Arch mice that were not observed in mice from the other two groups or in no-laser controls.
All mice exhibited changes in behavior following the 4-wk protocol, including less exploration in the light/dark box, less object exploration during initial exposure and tests of recognition memory, and generally less activity in the open arena. We attribute these changes to the loss of novelty of each of the environments during the second round of testing compared with the first round.
In the light/dark box, the likely consequence of reduced novelty following the 4-wk protocol is that the innate drive to remain safe in the dark half became stronger than the drive to explore (i.e., anxiety outweighs curiosity). However, PV-Arch mice that underwent rhythmic cortical stimulation exhibited more exploratory behaviors following the 4-wk protocol than did PV-ChR2, PV-PV, and no-laser control mice. This effect was driven primarily by behavior from mice exposed to 1-Hz and 8-Hz pulses and may be interpreted as evidence for decreased anxiety following 4 wk of cortical stimulation at these frequencies. Importantly, behavior of PV-Arch mice exposed to 40-Hz pulses was comparable to that of no-laser controls, indicating that 40-Hz cortical stimulation had no significant effect (positive or negative) on anxiety/exploration. Other observations support the specificity of the stimulation effect. First, the inferred reduction of anxiety is not likely attributable to impaired/altered exploration, per se, because PV-Arch mice explored objects comparably to other groups during the initial exposure and the subsequent tests of object and location recognition memory. Second, these recognition memory tests also indicate that PV-Arch mice had normal basic cognitive ability and short-term memory (neither enhanced nor impaired compared with other groups). Third, PV-Arch mice exhibited behaviors in the open arena that were similar to those of PV-ChR2 and no laser mice, indicating that cortical stimulation did not result in an abnormal motor phenotype such as hyperactivity that could skew rates of exploration. Thus, the greater exploratory activity by PV-Arch mice exposed to 1-Hz and 8-Hz pulses may indeed reflect a reduction of anxiety.
No effects were seen with rhythmic cortical suppression in the PV-ChR2 mice for the light/dark box assessments or any other behaviors assessed, indicating that the effects seen in PV-Arch mice were attributable specifically to rhythmic increases of cortical activity rather than simply the induction of rhythmic oscillations. Some unexpected behavioral results were observed with PV-PV mice, including a frequency-specific decrease in exploratory activity in the light/dark box and a generalized motor phenotype in the open arena.
Acknowledgments
We thank Miya Ott and Andrew Mosman for their assistance with scoring of behavior and Prof. Yiyuan Tang, Texas Tech University, and Prof. Gary Lynch, University of California, Irvine, for their consultations on this work. This research was funded by Office of Naval Research Grants N00014-15-1-2022 and N00014-15-2148 to the University of Oregon.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700756114/-/DCSupplemental.
References
- 1.Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci USA. 2007;104(43):17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang YY, Posner MI. Attention training and attention state training. Trends Cogn Sci. 2009;13(5):222–227. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Tang YY, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA. 2010;107(35):15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang YY, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci USA. 2012;109(26):10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posner MI, Tang YY, Lynch G. Mechanisms of white matter change induced by meditation training. Front Psychol. 2014;5:1220. doi: 10.3389/fpsyg.2014.01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: An EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40(6):939–949. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- 8.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 9.Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16(4):213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 10.Hart PC, et al. In: Experimental Models of Anxiety for Drug Discovery and Brain Research. Mouse Models for Drug Discovery, Methods in Molecular Biology. Proetzel G, Wiles MV, editors. Humana; New York: 2010. [DOI] [PubMed] [Google Scholar]
- 11.Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16(12):1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- 12.Lien AD, Scanziani M. In vivo labeling of constellations of functionally identified neurons for targeted in vitro recordings. Front Neural Circuits. 2011;5:16. doi: 10.3389/fncir.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73(1):159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 17.Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29(4):1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez J, Morales IS, Villarreal DM, Derrick BE. Low-frequency stimulation induces long-term depression and slow onset long-term potentiation at perforant path-dentate gyrus synapses in vivo. J Neurophysiol. 2014;111(6):1259–1273. doi: 10.1152/jn.00941.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grover LM, Kim E, Cooke JD, Holmes WR. LTP in hippocampal area CA1 is induced by burst stimulation over a broad frequency range centered around delta. Learn Mem. 2009;16(1):69–81. doi: 10.1101/lm.1179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bengtsson SL, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, et al. Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp. 2011;32(1):10–21. doi: 10.1002/hbm.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampaio-Baptista C, et al. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci. 2013;33(50):19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenzie IA, et al. Motor skill learning requires active central myelination. Science. 2014;346(6207):318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filiou MD, et al. Proteomics and metabolomics analysis of a trait anxiety mouse model reveals divergent mitochondrial pathways. Biol Psychiatry. 2011;70(11):1074–1082. doi: 10.1016/j.biopsych.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Ito H, Nagano M, Suzuki H, Murakoshi T. Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology. 2010;58(4-5):746–757. doi: 10.1016/j.neuropharm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Kim SS, et al. Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain. 2011;4:6. doi: 10.1186/1756-6606-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanzenberger RR, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61(9):1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen NK, et al. Effect of neuropeptide Y Y2 receptor deletion on emotional stress-induced neuronal activation in mice. Synapse. 2009;63(3):236–246. doi: 10.1002/syn.20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga K, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron. 2015;85(2):377–389. doi: 10.1016/j.neuron.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzawa-Yanagida K, et al. Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology. 2008;33(8):1952–1965. doi: 10.1038/sj.npp.1301590. [DOI] [PubMed] [Google Scholar]
- 32.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77(4-5):677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- 33.Kollias SS, Alkadhi H, Jaermann T, Crelier G, Hepp-Reymond MC. Identification of multiple nonprimary motor cortical areas with simple movements. Brain Res Brain Res Rev. 2001;36(2-3):185–195. doi: 10.1016/s0165-0173(01)00094-7. [DOI] [PubMed] [Google Scholar]
- 34.Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 35.Picard N, Strick PL. Motor areas of the medial wall: A review of their location and functional activation. Cereb Cortex. 1996;6(3):342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 36.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 37.Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- 38.Kabbaj M, Akil H. Individual differences in novelty-seeking behavior in rats: A c-fos study. Neuroscience. 2001;106(3):535–545. doi: 10.1016/s0306-4522(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 39.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: Differential expression of stress-related molecules. J Neurosci. 2000;20(18):6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol. 1999;38(2):234–246. [PubMed] [Google Scholar]
- 41.Papa M, Pellicano MP, Welzl H, Sadile AG. Distributed changes in c-Fos and c-Jun immunoreactivity in the rat brain associated with arousal and habituation to novelty. Brain Res Bull. 1993;32(5):509–515. doi: 10.1016/0361-9230(93)90299-q. [DOI] [PubMed] [Google Scholar]
- 42.Weible AP, Rowland DC, Monaghan CK, Wolfgang NT, Kentros CG. Neural correlates of long-term object memory in the mouse anterior cingulate cortex. J Neurosci. 2012;32(16):5598–5608. doi: 10.1523/JNEUROSCI.5265-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weible AP, Rowland DC, Pang R, Kentros C. Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. J Neurophysiol. 2009;102(4):2055–2068. doi: 10.1152/jn.00214.2009. [DOI] [PubMed] [Google Scholar]
- 44.Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20(3):249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- 45.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113(3):509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 46.Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35(7):999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 47.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3(5):e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris JA, et al. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits. 2014;8:76. doi: 10.3389/fncir.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrarelli F, et al. Experienced mindfulness meditators exhibit higher parietal-occipital EEG gamma activity during NREM sleep. PLoS One. 2013;8(8):e73417. doi: 10.1371/journal.pone.0073417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weible AP, Liu C, Niell CM, Wehr M. Auditory cortex is required for fear potentiation of gap detection. J Neurosci. 2014;34(46):15437–15445. doi: 10.1523/JNEUROSCI.3408-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weible AP, et al. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr Biol. 2014;24(13):1447–1455. doi: 10.1016/j.cub.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox SE, Ranck JB., Jr Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res. 1981;41(3-4):399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- 53.Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci. 2013;33(34):13713–13723. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience. 2005;131(1):1–11. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]