Abstract
The primary goal of therapy for chronic hepatitis B (CHB) is to prevent liver disease progression. Hepatitis B surface antigen (HBsAg) seroclearance or seroconversion is regarded as an optimal endpoint to discontinue treatment. However, HBsAg seroclearance occurs very rarely with nucleos(t)ide analog (NUC) treatment, and long-term, almost indefinite, NUC treatment is required for the majority of patients. In patients with drug-resistant hepatitis B virus (HBV), a combination of tenofovir disoproxil fumarate (TDF) and entecavir (ETV), which is currently regarded as the strongest combination therapy against HBV, would be potentially safe to prevent the emergence of additional HBV resistance mutations. However, long-term tolerance data are lacking, and cost may be an issue for combination therapies. Several recent, well-designed, randomized controlled trials have shown that TDF mono-therapy provides similar antiviral efficacy compared with the combination of TDF and ETV. Furthermore, no additional HBV resistance mutations emerged during TDF monotherapy for up to 96 weeks. Considering a comparable antiviral efficacy, extremely low risk of TDF-resistance, lower cost, and better safety potential, TDF monotherapy would be a reasonable choice for the treatment of drug-resistant patients with CHB.
Keywords: Adefovir, Entecavir, Lamivudine, Monotherapy, Tenofovir
INTRODUCTION
High serum hepatitis B virus (HBV) DNA levels are an independent risk factor for disease progression to cirrhosis and hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB).1,2 By contrast, reducing HBV DNA concentrations to very low or undetectable levels through long-term nucleos(t)ide analogue (NUC) therapy is associated with reduced risk of mortality and/or HCC.3–11
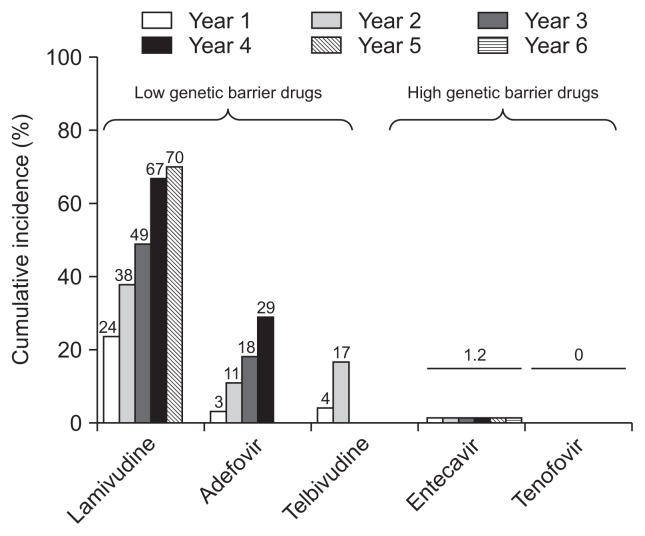
Over the past two decades, treatment of CHB has greatly improved with the availability of NUCs, including lamivudine (LAM), adefovir (ADV), entecavir (ETV), telbivudine, and tenofovir, which target particular sites of viral polymerases.12–14 Particularly, with the availability of potent NUCs, such as tenofovir disoproxil fumarate (TDF) and ETV, suppression of serum HBV DNA to levels undetectable by polymerase chain reaction assays is achievable in most NUC treatment-naïve patients in the absence of drug-resistant HBV mutants (Fig. 1).15,16 However, many patients worldwide have developed drug-resistance from the widespread use of less potent NUCs, such as LAM or ADV, which have a low genetic barrier to resistance. Patients with persistent drug-resistant HBV viremia are more likely to suffer hepatitis flares, disease progression, and to die than those without drug-resistant HBV.3,6
Fig. 1.
Cumulative incidence of hepatitis B virus resistance in pivotal trials. Adapted from EASL clinical practice guidelines. J Hepatol 2012;57:167–185.24
MECHANISM OF ANTIVIRAL DRUG RESISTANCE
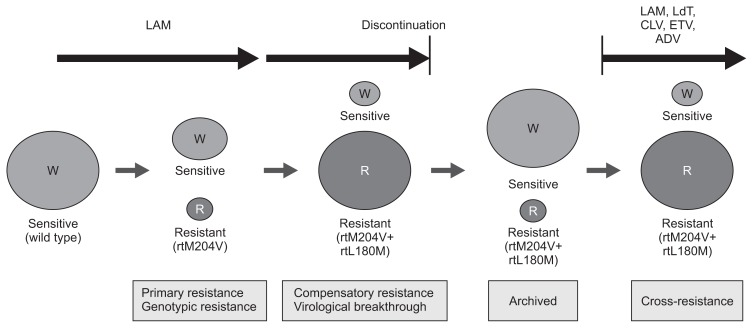
Nucleoside- and nucleotide-analogues selectively target HBV DNA polymerase, resulting in premature chain termination of viral replication. Drug-resistant strains of HBV have signature mutations in the reverse transcriptase domains of the viral polymerase gene (Table 1). Resistance mutations alter the interaction between HBV polymerase and drug, which interfere the inhibitory effect of drug on viral polymerase. After emergence of primary resistance mutations, compensatory mutations that restore replication capacity may arise (Fig. 2), as well as secondary resistance mutations that increase drug resistance when they accumulate on the same viral genome.
Table 1.
Summary of In Vitro Cross-Resistance for Hepatitis B Virus Variants
| HBV variant | Lamivudine | Entecavir | Adefovir | Tenofovir |
|---|---|---|---|---|
| Wild-type | S | S | S | S |
| M204V/I | R | I | I | S |
| L180M+M204V | R | I | I | S |
| A181T/V | R | S | R | S |
| N236T | S | S | R | I |
| A181T/V+N236T | R | S | R | I |
| L180M+M204V/I±T184 | R | R | S | S |
| L180M+M204V/I±S202 | R | R | S | S |
| L180M+M204V/I±I169T±M250 | R | R | S | S |
HBV, hepatitis B virus; S, sensitive; I, intermediate/reduced susceptibility; R, resistant.
Fig. 2.
Evolution of drug-resistant hepatitis B virus. Adapted from Bartholomeusz A, et al. Semin Liver Dis 2006;26:162–170.64; Fournier C, et al. Clin Liver Dis 2007;11:869–892.65
LAM, lamivudine; LdT, telbivudine; CLV, clevudine; ETV, entecavir; ADV, adefovir dipivoxil; W, wild type HBV strain; R, resistant HBV strain.
Once antiviral-resistant HBV mutants have been selected, they are persistently retained in the virus population even if treatment is stopped, and exerts cross-resistance to the next sequential monotherapy (Fig. 2).14,17,18 For example, ADV-resistant mutations emerge more frequently during ADV monotherapy in LAM-resistant than in treatment-naïve patients.19–21 The rate of ETV resistance increases up to 51% after 5 years of ETV treatment in patients with LAM-resistant HBV, which is in striking contrast to a 1.2% resistance rate in NUC-naïve patients.22,23 Thus, combination therapy with a nucleoside analogue (LAM, telbivudine, or ETV) and a nucleotide analogue (ADV or TDF) has generally been recommended for the treatment of patients harboring drug-resistant HBV.14,24–26 However, several recent studies including ours have suggested that TDF monotherapy is efficacious in patients with LAM-resistant, ETV-resistant, or ADV-resistant HBV.27–32
MANAGEMENT OF RESISTANCE TO LAMIVUDINE
For LAM-resistant patients, a combination of ADV and LAM showed no greater antiviral efficacy than ADV monotherapy.33 Nonetheless, ADV and LAM combination therapy had been recommended for these patients to prevent additional emergence of ADV-resistant HBV mutants.14,20,24,25,34–36
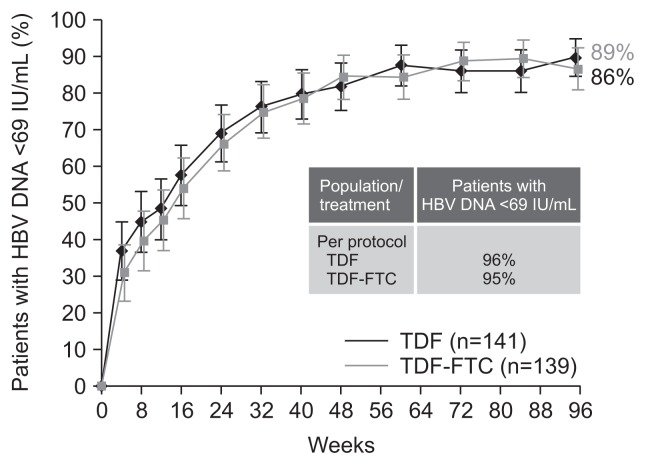
However, the situation is clearly different if TDF, that has about 30 times higher anti-HBV potency than ADV, is used instead of ADV. Treatment responses to TDF and LAM combination therapy would likely be mediated by TDF alone, since LAM may have no or minimal antiviral efficacy in the presence of LAM-resistant HBV mutants. With this combination, continued LAM treatment would likely only help prevent the development of TDF-resistance mutations, rather than increasing antiviral potency. Therefore, if there is minimal risk of TDF-resistance,16,37 the addition of LAM to TDF would theoretically provide little benefit. The hypothesis was actually proven by a recent randomized double-blind controlled trial.29 The study showed that TDF monotherapy was highly efficacious in patients with LAM-resistant HBV, which was comparable to the combination of TDF and emtricitabine, without emergence of additional resistance mutations to TDF throughout 96 weeks of treatment (Fig. 3).29,37 Entricitabine is an unapproved nucleoside analogue which has very similar anti-HBV potency and barrier to resistance development as LAM. Thus, the study results indicate that adding LAM to TDF would not provide additional antiviral benefit, and that TDF monotherapy is safe and efficacious to patients with LAM-resistant HBV.
Fig. 3.
Virologic response (hepatitis B virus [HBV] DNA <60 IU/mL) by tenofovir disoproxil fumarate (TDF) monotherapy versus TDF and emtricitabine combination therapy in lamivudine-resistant patients with chronic hepatitis B in a randomized double-blind controlled trial. Intention-to-treat population shown. Adapted from Fung S, et al. Gastroenterology 2014;146:980–988.e1, with permission from El-sevier.29
FTC, emtricitabine.
MANAGEMENT OF RESISTANCE TO LAM AND ETV
In patients with pre-existing LAM-resistance, the rate of ETV resistance increases up to 51% after 5 years of ETV treatment, which is in striking contrast to a 1.2% resistance rate in NUC-naïve patients.22,23 In addition, ETV monotherapy was suboptimal in suppressing HBV replication in patients with LAM-resistant HBV, with an unacceptably low virologic response rate (22%) seen at 12 months.38,39 This difference is because the ETV resistance barrier is lowered by the initial selection of the LAM-resistant HBV mutation, rtM204V/I.40 In vitro studies have shown that susceptibility to ETV is decreases by 10- to 250-fold when one of the ETV resistance-associated substitutions at rtT184, rtS202, or rtM250 is present in combination with rtM204V/I, and by >500-fold when two or more of these mutations are present.22,40
In vitro studies suggest that ETV-resistant HBV mutants are susceptible to TDF.17,41 These in vitro studies showed that HBV strains harboring the ETV resistance-associated substitutions, rtM204V/I, rtT184, rtS202, and/or rtM250, exhibit no cross-resistance to tenofovir.17,41 Several case reports and retrospective cohort studies also showed the clinical efficacy of TDF in ETV-resistant or ETV-refractory patients.42–44
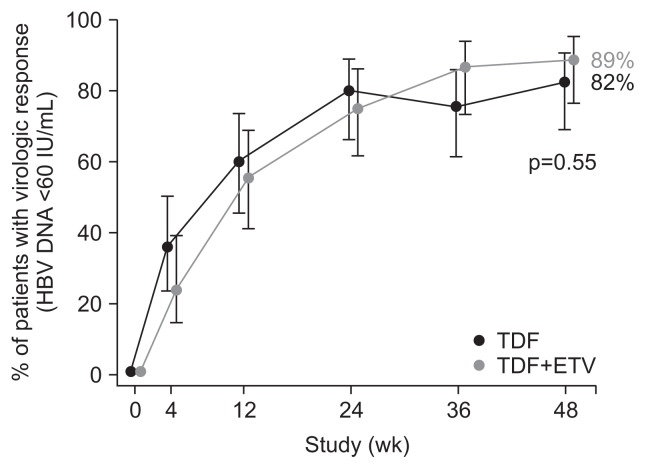
In our recent multicenter randomized trial, patients who had HBV with ETV resistance-associated mutations and serum HBV DNA concentrations >60 IU/mL were randomized to receive TDF (300 mg/day) monotherapy (n=45) or TDF and ETV (1 mg/day) combination therapy (n=45). At week 48, the proportion of patients with HBV DNA <15 IU/mL, the primary efficacy endpoint, was not significantly different between the TDF and TDF+ETV groups (71% vs 73%, p=0.81). The proportion of patients who achieved HBV DNA levels <60 IU/mL at week 48 was 82% and 89% for the TDF and TDF+ETV groups, respectively (p=0.55) (Fig. 4). The mean change in HBV DNA levels from baseline was not significantly different between groups (−3.65 log10 IU/mL vs −3.77 log10 IU/mL, p=0.69). Virologic breakthrough occurred in one patient on TDF, which was attributed to poor drug adherence. At week 48, six and three patients in the TDF and TDF+ETV groups, respectively, retained their baseline resistance mutations (p>0.99). None developed additional resistance mutations. Safety profiles were comparable in the two groups. The rate of virologic response by TDF monotherapy in our study was similar with that of a recent single arm trial with TDF+ETV in CHB patients with previous NUC treatment failure (85% had HBV DNA <50 IU/mL at week 96).45 These results support the view that TDF monotherapy may be a treatment option for patients with ETV-resistant HBV.
Fig. 4.
Virologic response (hepatitis B virus [HBV] DNA <60 IU/mL) by tenofovir disoproxil fumarate (TDF) monotherapy versus TDF and entecavir (ETV) combination therapy in ETV-resistant patients with chronic hepatitis B in a randomized controlled trial. Intention-to-treat population shown. Adapted from Lim YS, et al. Gut 2016;65:852–860.31
MANAGEMENT OF RESISTANCE TO LAM AND ADV
In vitro clonal analyses showed that multidrug-resistance mutations usually reside in the same viral genome,18,46 and antiviral sensitivities revealed that replicating clones with LAM- and ADV-associated mutations had >50-fold reduced susceptibility to combination of LAM and ADV.47,48 In fact, our previous cohort study demonstrated that, in patients with HBV resistant to LAM and ADV, combination therapy with these two drugs was not effective and was even inferior to ETV monotherapy in suppressing HBV DNA.49 However, the response to ETV monotherapy was not optimal. We demonstrated that ETV was far less effective in patients refractory to both LAM and ADV than in those with LAM mono-resistance.50
In vitro studies have shown that HBV strains expressing the ADV resistance-associated substitutions, rtA181T/V and/or rtN236T, demonstrate reduced susceptibility to tenofovir, ranging from 2.9- to 10-fold of that of the wild-type virus.46,51–53 Several cohort studies also showed reduced TDF efficacy in patients with ADV-resistant HBV.30,54 An European cohort study showed that, the probability of achieving HBV DNA levels below 400 copies/mL was significantly lower with TDF monotherapy in patients with ADV-resistant HBV and high viral load (>107 copies/mL) at baseline compared with those without ADV-resistant HBV.54 Another cohort study also showed that the efficacy of TDF was lower in patients with ADV-resistant HBV than in treatment-naïve patients, especially when they had previously failed to respond to both LAM and ADV.30 On the other hand, a combination of TDF and ETV, which is currently regarded as the strongest combination therapy against HBV, induced a virologic response in up to 90% of patients after a median 6 months of treatment regardless of preexisting ADV- or ETV-resistance.55 However, whether a combination of TDF and ETV exerts better antiviral efficacy than TDF monotherapy in patients with multidrug-resistant HBV could not be identified by these single arm studies.
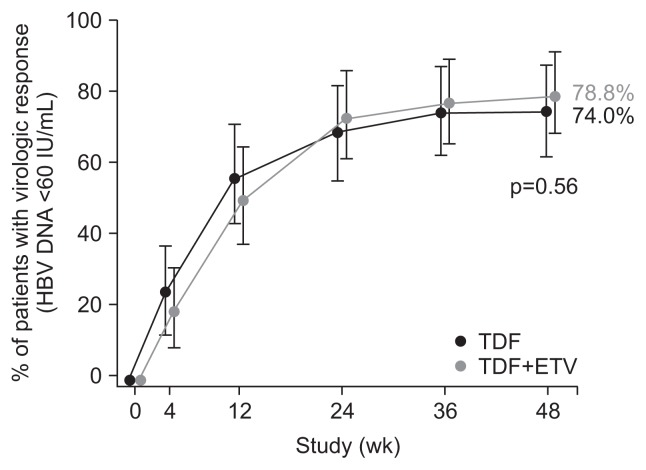
We recently performed a multicenter trial, in which, patients who had ADV-resistant HBV with serum HBV DNA levels >60 IU/mL were randomized to receive TDF (300 mg/day) monotherapy (n=50) or TDF and ETV (1 mg/day) combination therapy (TDF/ETV, n=52) for 48 weeks. All patients had ADV-resistant HBV mutations; rtA181V/T and/or rtN236T. The proportion of patients with HBV DNA <15 IU/mL was not significantly different between the TDF-TDF and TDF/ETV-TDF groups at week 48 (62% vs 63.5%, p=0.88) and at week 96 (64% vs 63.5%, p=0.96). The proportion of patients who achieved HBV DNA levels <60 IU/mL at week 48 was 74% and 78.8% for the TDF-TDF and TDF/ETV-TDF groups, respectively (p=0.56) (Fig. 5). Virologic breakthrough occurred in one patient on TDF-TDF and two patients on TDF/ETV-TDF over 96 weeks; all were attributed to poor drug adherence. At week 96, five and two patients in the TDF-TDF and TDF/ETV-TDF groups, respectively, retained some of their baseline resistance mutations (p=0.44). None developed additional resistance mutations. Safety profiles were comparable in the two groups. The results suggest that TDF monotherapy may be a treatment option for patients with ADV-resistant HBV. However, in a subgroup of patients who had double ADV-resistance mutations, i.e., both rtA181T/V and rtN236T, the decrease in serum HBV DNA levels tended to be less in the TDF group than in the TDF/ETV-TDF group. Thus, TDF plus ETV combination therapy might be more beneficial than TDF monotherapy in patients who had double ADV-resistance mutations (both rtA181T/V and rtN236T).
Fig. 5.
Virologic response (hepatitis B virus [HBV] DNA <60 IU/mL) by tenofovir disoproxil fumarate (TDF) monotherapy versus TDF and entecavir (ETV) combination therapy in adefovir-resistant patients with chronic hepatitis B in a randomized controlled trial. Intention-to-treat population shown. Adapted from Lim YS, et al. Gut 2016;65:1042–1051.32
CONCLUSIONS
The primary goal of therapy for CHB is to prevent liver disease progression, and seroclearance or seroconversion of hepatitis B surface antigen (HBsAg) is regarded as an optimal endpoint of treatment.56 However, HBsAg seroclearance occurs very rarely with NUC treatment.56 Long-term HBV DNA and HBsAg level kinetics in patients with CHB treated with potent NUCs showed that HBsAg clearance is unlikely to occur during the patient’s lifetime, even if HBV replication is well controlled.57 Thus, long-term, almost indefinite, NUC treatment is required for the majority of patients.
In patients with drug-resistant HBV, a combination of TDF and ETV would be potentially safer to prevent the emergence of resistance to TDF.58 However, long-term tolerance data are lacking and cost may be an issue for this combination. Considering a comparable antiviral efficacy, extremely low risk of TDF-resistance, lower cost, and potentially better safety profile, TDF monotherapy would be a reasonable option for the treatment of ETV-resistant patients. In fact, TDF monotherapy is now being incorporated as a primary recommendation for the treatment of patients with drug-resistant HBV in many recent practice guidelines (Table 2).24,59–62 However, the rates of virologic response in multidrug-resistant patients seem to be lower compared to those reported in previous clinical trials of TDF in treatment-naïve, LAM-resistant, or ADV-refractory patients.27,29,63 Because the combination of TDF and ETV does not seem to further increase the rate of virologic response, a more potent antiviral agent may be required for patients with HBV mutants resistant to multiple drugs including ADV.
Table 2.
Summary of Recommendations by Practice Guidelines for Patients with Drug-Resistant Hepatitis B Virus
| Resistance to | AASLD 2016 | WHO 2015 | APASL 2015 | KASL 2015 | EASL 2012 |
|---|---|---|---|---|---|
| Primary recommendations | |||||
| Lamivudine | Tenofovir | Tenofovir | Tenofovir | Tenofovir or tenofovir+nucleoside analogue | Tenofovir |
| Entecavir | Tenofovir | Tenofovir | Tenofovir | Tenofovir or tenofovir+entecavir | Tenofovir or tenofovir+entecavir |
| Adefovir (no lamivudine exposure) | Entecavir | Tenofovir | Entecavir or tenofovir | Tenofovir or tenofovir+entecavir | Entecavir or tenofovir |
| Multidrug | Tenofovir | Tenofovir | Tenofovir+entecavir | Tenofovir or tenofovir+entecavir | Tenofovir+nucleoside analogue |
| Secondary recommendations | |||||
| Lamivudine | Tenofovir+lamivudine | Adefovir+lamivudine | Adefovir+nucleoside analogue or pegylated interferon | Adefovir+lamivudine | |
| Entecavir | Tenofovir+entecavir | Adefovir+entecavir | Adefovir+entecavir | Adefovir+entecavir | |
| Adefovir (no lamivudine exposure) | Adefovir+entecavir | Tenofovir+lamivudine | Tenofovir+nucleoside analogue or adefovir+entecavir | Tenofovir | |
| Multidrug | Tenofovir+entecavir | Pegylated interferon | Tenofovir+nucleoside analogue or adefovir+entecavir | ||
AASLD, American Association for the Study of Liver; WHO, World Health Organization; APASL, Asian-Pacific Association for the Study of Liver; KASL, Korean Association for the Study of Liver; EASL, European Association for the Study of Liver.
ACKNOWLEDGEMENTS
This study was supported by grants from the Korean National Health Clinical Research (NHCR) project, Ministry of Health & Welfare, Republic of Korea (HC15C3380); the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C1061); and the Proteogenomic Research Program through the National Research Foundation (NRF-2015M3C9A1044530) of Korea funded by the Korea government.
Footnotes
CONFLICTS OF INTEREST
Y.S.L. is an advisory board member of Bayer Healthcare, Bristol-Myers Squibb, and Gilead Sciences, and receives research funding from Bayer Healthcare, Bristol-Myers Squibb, Gilead Sciences, and Novartis.
REFERENCES
- 1.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 4.Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118–125. doi: 10.1016/j.jhep.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Papatheodoridis GV, Dimou E, Dimakopoulos K, et al. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121–129. doi: 10.1002/hep.20760. [DOI] [PubMed] [Google Scholar]
- 7.Yuen MF, Seto WK, Chow DH, et al. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295–1303. [PubMed] [Google Scholar]
- 8.Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 9.Wong GL, Chan HL, Mak CW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 10.Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147:152–161. doi: 10.1053/j.gastro.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Wu CY, Lin JT, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143–151.e5. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–1341. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 15.Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 16.Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59:434–442. doi: 10.1002/hep.26686. [DOI] [PubMed] [Google Scholar]
- 17.Zoulim F, Locarnini S. Management of treatment failure in chronic hepatitis B. J Hepatol. 2012;56(Suppl 1):S112–S122. doi: 10.1016/S0168-8278(12)60012-9. [DOI] [PubMed] [Google Scholar]
- 18.Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006;44:703–712. doi: 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 19.Fung SK, Chae HB, Fontana RJ, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- 21.Yeon JE, Yoo W, Hong SP, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55:1488–1495. doi: 10.1136/gut.2005.077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenney DJ, Rose RE, Baldick CJ, et al. Two-year assessment of entecavir resistance in Lamivudine-refractory hepatitis B virus patients reveals different clinical outcomes depending on the resistance substitutions present. Antimicrob Agents Chemother. 2007;51:902–911. doi: 10.1128/AAC.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2012;18:109–162. doi: 10.3350/cmh.2012.18.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 27.Berg T, Marcellin P, Zoulim F, et al. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207–1217. doi: 10.1053/j.gastro.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 28.Berg T, Zoulim F, Moeller B, et al. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J Hepatol. 2014;60:715–722. doi: 10.1016/j.jhep.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Fung S, Kwan P, Fabri M, et al. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2014;146:980–988.e1. doi: 10.1053/j.gastro.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Patterson SJ, George J, Strasser SI, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60:247–254. doi: 10.1136/gut.2010.223206. [DOI] [PubMed] [Google Scholar]
- 31.Lim YS, Byun KS, Yoo BC, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in patients with entecavir-resistant chronic hepatitis B with multiple drug failure: results of a randomised trial. Gut. 2016;65:852–860. doi: 10.1136/gutjnl-2014-308353. [DOI] [PubMed] [Google Scholar]
- 32.Lim YS, Yoo BC, Byun KS, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in adefovir-resistant chronic hepatitis B patients with multiple drug failure: results of a randomised trial. Gut. 2016;65:1042–1051. doi: 10.1136/gutjnl-2014-308435. [DOI] [PubMed] [Google Scholar]
- 33.Peters MG, Hann HW, Martin P, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 34.Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307–313. doi: 10.1002/hep.21534. [DOI] [PubMed] [Google Scholar]
- 36.Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–1451. doi: 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 37.Corsa AC, Liu Y, Flaherty JF, et al. No resistance to tenofovir disoproxil fumarate through 96 weeks of treatment in patients with lamivudine-resistant chronic hepatitis B. Clin Gastroenterol Hepatol. 2014;12:2106–2112.e1. doi: 10.1016/j.cgh.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Chang TT, Gish RG, Hadziyannis SJ, et al. A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology. 2005;129:1198–1209. doi: 10.1053/j.gastro.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 39.Sherman M, Yurdaydin C, Sollano J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039–2049. doi: 10.1053/j.gastro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Tenney DJ, Levine SM, Rose RE, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48:3498–3507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villet S, Ollivet A, Pichoud C, et al. Stepwise process for the development of entecavir resistance in a chronic hepatitis B virus infected patient. J Hepatol. 2007;46:531–538. doi: 10.1016/j.jhep.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Pan CQ, Hu KQ, Yu AS, Chen W, Bunchorntavakul C, Reddy KR. Response to tenofovir monotherapy in chronic hepatitis B patients with prior suboptimal response to entecavir. J Viral Hepat. 2012;19:213–219. doi: 10.1111/j.1365-2893.2011.01533.x. [DOI] [PubMed] [Google Scholar]
- 43.Yip B, Chaung K, Wong CR, et al. Tenofovir monotherapy and tenofovir plus entecavir combination as rescue therapy for entecavir partial responders. Dig Dis Sci. 2012;57:3011–3016. doi: 10.1007/s10620-012-2402-2. [DOI] [PubMed] [Google Scholar]
- 44.Leemans WF, Niesters HG, van der Eijk AA, Janssen HL, Schalm SW, de Man RA. Selection of an entecavir-resistant mutant despite prolonged hepatitis B virus DNA suppression, in a chronic hepatitis B patient with preexistent lamivudine resistance: successful rescue therapy with tenofovir. Eur J Gastroenterol Hepatol. 2008;20:773–777. doi: 10.1097/MEG.0b013e3282f793d6. [DOI] [PubMed] [Google Scholar]
- 45.Zoulim F, Jablkowski MS, Diculescu M, et al. The safety and efficacy of entecavir and tenofovir combination therapy for chronic hepatitis B in patients with previous nucleos(t)ide treatment failure: week 96 results of the ENTEBE study. Hepatology. 2014;60(Suppl 4):314A–315A. doi: 10.1007/s12072-016-9737-2. [DOI] [PubMed] [Google Scholar]
- 46.Villet S, Pichoud C, Villeneuve JP, Trépo C, Zoulim F. Selection of a multiple drug-resistant hepatitis B virus strain in a liver-transplanted patient. Gastroenterology. 2006;131:1253–1261. doi: 10.1053/j.gastro.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Villeneuve JP, Durantel D, Durantel S, et al. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J Hepatol. 2003;39:1085–1089. doi: 10.1016/j.jhep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Angus P, Vaughan R, Xiong S, et al. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology. 2003;125:292–297. doi: 10.1016/S0016-5085(03)00939-9. [DOI] [PubMed] [Google Scholar]
- 49.Heo NY, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Lamivudine plus adefovir or entecavir for patients with chronic hepatitis B resistant to lamivudine and adefovir. J Hepatol. 2010;53:449–454. doi: 10.1016/j.jhep.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Shim JH, Suh DJ, Kim KM, et al. Efficacy of entecavir in patients with chronic hepatitis B resistant to both lamivudine and adefovir or to lamivudine alone. Hepatology. 2009;50:1064–1071. doi: 10.1002/hep.23145. [DOI] [PubMed] [Google Scholar]
- 51.Brunelle MN, Jacquard AC, Pichoud C, et al. Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology. 2005;41:1391–1398. doi: 10.1002/hep.20723. [DOI] [PubMed] [Google Scholar]
- 52.Villet S, Pichoud C, Billioud G, et al. Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J Hepatol. 2008;48:747–755. doi: 10.1016/j.jhep.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 53.Qi X, Xiong S, Yang H, Miller M, Delaney WE., 4th In vitro susceptibility of adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir Ther. 2007;12:355–362. [PubMed] [Google Scholar]
- 54.van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73–80. doi: 10.1002/hep.23246. [DOI] [PubMed] [Google Scholar]
- 55.Petersen J, Ratziu V, Buti M, et al. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. J Hepatol. 2012;56:520–526. doi: 10.1016/j.jhep.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63:1325–1332. doi: 10.1136/gutjnl-2013-305517. [DOI] [PubMed] [Google Scholar]
- 57.Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676–683. doi: 10.1016/j.jhep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 58.Pawlotsky JM. Is hepatitis virus resistance to antiviral drugs a threat? Gastroenterology. 2012;142:1369–1372. doi: 10.1053/j.gastro.2011.12.060. [DOI] [PubMed] [Google Scholar]
- 59.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 61.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korean Association for the Study of Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Seoul: Korean Association for the Study of Liver; 2015. [Google Scholar]
- 63.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 64.Bartholomeusz A, Locarnini SA. Antiviral drug resistance: clinical consequences and molecular aspects. Semin Liver Dis. 2006;26:162–170. doi: 10.1055/s-2006-939758. [DOI] [PubMed] [Google Scholar]
- 65.Fournier C, Zoulim F. Antiviral therapy of chronic hepatitis B: prevention of drug resistance. Clin Liver Dis. 2007;11:869–892. ix. doi: 10.1016/j.cld.2007.08.013. [DOI] [PubMed] [Google Scholar]