Abstract
The pathogenesis of irritable bowel syndrome (IBS), once thought to be largely psychogenic in origin, is now understood to be multifactorial. One of the reasons for this paradigm shift is the realization that gut dysbiosis, including small intestinal bacterial overgrowth (SIBO), causes IBS symptoms. Between 4% and 78% of patients with IBS and 1% and 40% of controls have SIBO; such wide variations in prevalence might result from population differences, IBS diagnostic criteria, and, most importantly, methods to diagnose SIBO. Although quantitative jejunal aspirate culture is considered the gold standard for the diagnosis of SIBO, noninvasive hydrogen breath tests have been popular. Although the glucose hydrogen breath test is highly specific, its sensitivity is low; in contrast, the early-peak criteria in the lactulose hydrogen breath test are highly nonspecific. Female gender, older age, diarrhea-predominant IBS, bloating and flatulence, proton pump inhibitor and narcotic intake, and low hemoglobin are associated with SIBO among IBS patients. Several therapeutic trials targeting gut microbes using antibiotics and probiotics have further demonstrated that not all symptoms in patients with IBS originate in the brain but rather in the gut, providing support for the micro-organic basis of IBS. A recent proof-of-concept study showing the high frequency of symptom improvement in patients with IBS with SIBO further supports this hypothesis.
Keywords: Bacterial overgrowth, Dysbiosis, Breath tests, Gastrointestinal microbiota, Probiotics, Rifaximin
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the commonest disorders encountered in Gastroenterology practice.1 IBS is manifested by abdominal pain and/or discomfort, irregular stool form and passage.2 Bloating is another common symptom of IBS.2,3 Patients with small intestinal bacterial overgrowth (SIBO), in which there is increase in bacteria equal to or greater than 105 colony forming unit per mL of upper gut aspirate,2 also experience abdominal pain or discomfort, bloating, flatulence and loose motion.4,5 In contrast to the earlier belief, SIBO is known to occur in absence of anatomical factors predisposing to it.6 A proportion of patients with IBS are known to have SIBO.7 Recent realization that SIBO may be associated with symptoms of IBS, led to a paradigm shift in understanding the pathogenesis of this condition, hitherto thought to be related largely to psychological factors,8 to more organic nature.7 Such realization is well known in other conditions as well; for example, peptic ulcer, once thought to be related to psychological stress,9 is now known to be related to infection with Helicobacter pylori.10,11 Hence, it is worthwhile reviewing the existing literature on SIBO and IBS.
GUT FLORA AND SIBO
Human gut harbor 1014 bacterial cells, which are 10 times higher than the number of cells in the human body.12 Gastrointestinal (GI) tract is considered as the most heavily colonized organ and more than 70% of microbes reside in colon.13 The human GI tract is inhabited by a vast number of microbial population including bacteria, fungi, and viruses.14 Bacteria contribute to the largest population of gut microbiota, consisting of 500 (using culture approaches) to 1,000 (by 16S rRNA gene sequencing) different bacterial species.15 The number of bacteria increases from stomach (101 to 103 bacteria/g) to the colon (1011 to 1012 bacteria/g).13 The small intestine comprises mainly of Gram positive and aerobic bacteria and the large intestine contains predominantly Gram negative and anaerobic bacteria.16 Majority of bacteria residing in the colon are strictly anaerobes (95% of total) followed by facultative anaerobes and aerobes.15 More than 50 bacterial phyla have been identified in human gut.17 Major phyla residing in the gut are Bacteroidetes and Firmicutes, whilst Proteobacteria, Verrucomicrobia, Actino-bacteria, Fusobacteria, and Cyanobacteria are present in minor proportion.13,18
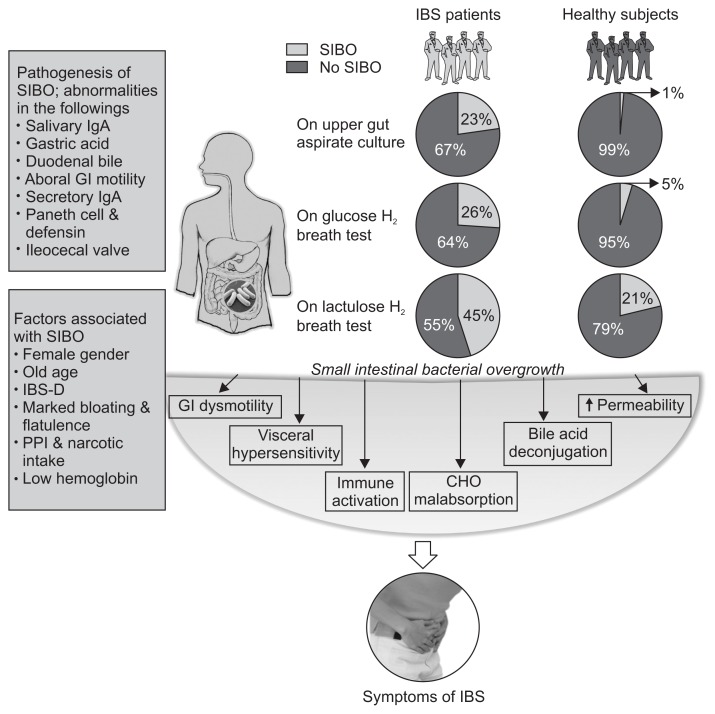
Normal gut flora may provide several beneficial effects to the host. These include fermentation of un-digested dietary residue and endogenous mucus producing short chain fatty acids, which are nutrients to the colonic epithelial cells and conservation of energy, absorption of NaCl and water, particularly from the right colon, synthesis of vitamin K, control of epithelial cell proliferation, protection against pathogens by a barrier effect and training of the immune system.19–21 One study showed that small intestine of germ free animal has thin and irregular villi, reduced crypt size, increased number of Peyer’s patches, and infiltration of leukocytes in lamina propria.22,23 Alteration in the normal flora leads to disturbance in the intestinal homeostasis.2 There are several intrinsic and extrinsic factors that prevent overgrowth of bacteria in the small intestine. Intrinsic factors include: (1) secretion of gastric juice and bile, which have antibacterial effect; (2) peristaltic movement preventing adherence of bacteria into the intestinal mucosa; (3) normal gut defense including humoral and cellular mechanisms; (4) mucin production by intestinal mucosal epithelial cell inhibiting pathogenic bacteria; (5) gut antibacterial peptides such as defensins; and (6) ileocecal valve preventing retrograde translocation of bacteria from colon to the small intestine.24–26 Extrinsic factors include diet and drugs modulating gut flora, such as pre and probiotics, gastric acid suppressants such as proton pump inhibitors (PPIs), H2 blockers, and antibiotics and drugs altering motility (prokinetics, anticholinergics, and opioids).4,22,27–30 If, there is failure of any of the above-mentioned protective mechanisms, it may lead to development of SIBO (Fig. 1).
Fig. 1.
Schematic diagram showing the frequency of small intestinal bacterial overgrowth (SIBO) using quantitative jejunal aspirate culture, glucose and lactulose hydrogen breath tests (GHBT and LHBT, respectively) among patients with irritable bowel syndrome (IBS), gut defense mechanisms that prevent the development of SIBO, factors associated with SIBO among patients with IBS, and mechanisms of IBS symptom development. As shown in the figure, the frequency of SIBO in IBS patients using LHBT (early-peak criteria) is higher than that by using upper gut aspirate culture and GHBT (LHBT [45%]; upper gut aspirate culture [23%] and GHBT [26%]). Moreover, SIBO is more frequent in healthy controls using LHBT due to false positive test results (LHBT [21%], upper gut aspirate culture [1%] and GHBT [5%]).
GI, gastrointestinal; IBS-D, irritable bowel syndrome, diarrhea-predominan; PPI, proton pump inhibitor; CHO, carbohydrate.
Though quantitative culture of the upper gut aspirate has traditionally been used as the gold standard for the diagnosis SIBO, its limitations include difficulty and invasiveness, cost, contamination by oropharyngeal flora, and inability to culture as high as 70% bacteria colonizing the gut.2,13,30,31 Moreover, distribution of bacterial overgrowth may be patchy and upper gut aspirate may not be able to detect bacterial overgrowth in distal gut.30,32 The anaerobic bacteria may not grow if air is used during endoscopy; hence, either nitrogen or carbon dioxide is better for this purpose. In one of our earlier studies in which we used air during endoscopy, of 34 of 50 patients with malabsorption syndrome in whom bacteria were cultured in jejunal aspirate, only one grew anaerobic bacteria.33 Hence, search for other less invasive and patient-friendly methods for diagnosis of SIBO continues.
In an attempt to overcome some of the limitations of the traditional culture-based method for diagnosis of SIBO, a novel technology, called culturomics, has been developed recently.15 Culturomics confer a new platform for identification of large number of bacterial colonies as well as noncultivable species in a short time duration using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF).15,34 In a recent study, using 212 different culture conditions, 340 different bacterial, 5 fungal species and one virus were identified, including 31 new species using culturomics (MALDI-TOF) technique.34,35 Thus, culturomic approaches are feasible, rapid, cost-effective and reproducible for the study of gut microbiota.15,34 However, studies on SIBO using culturomics method are lacking. Moreover, use of effective culture conditions and sequencing methods may make it rarely usable for routine clinical application.
Breath tests are popular, noninvasive and patient-friendly methods used increasingly for diagnosis of SIBO.36 Diagnostic role of hydrogen breath tests depends on the type of the substrates used; for example, lactose and fructose hydrogen breath tests are useful for carbohydrate malabsorption; on the other hand, glucose and lactulose hydrogen breath tests (GHBT and LHBT) are useful for diagnosis of SIBO, the former being more specific. Therefore, choice of the substrate while performing hydrogen breath test is important as only specific substrate diagnoses SIBO and others test for carbohydrate malabsorption.36 Hydrogen and methane gases are produced by the gut flora from the ingested substrates, particularly the colonic flora in patients with carbohydrate malabsorption and from small bowel bacteria in patients with SIBO.7,24,37 Eighty percent of the gases like hydrogen and methane are eliminated with the flatus and the remaining 20% are absorbed and exhaled by lung, which can be measured in breath.22,38 In GHBT, rise in hydrogen by 12 parts per million (ppm) above basal following administration of 50 to 100 g glucose due to bacterial fermentation of the substrate in small intestine is diagnostic of SIBO.36 A recent study showed that measuring methane does not increase the yield of hydrogen breath test to diagnose SIBO.39 In presence of SIBO, two peaks may be seen during LHBT: the first one due to bacterial fermentation of lactulose in small bowel and the second one after lactulose reaches colon.36 Since number of bacteria in colon is higher than that in the small bowel even in patients with SIBO, a rise in breath hydrogen more than 20 ppm above basal is expected from colonic fermentation of the lactulose.40 Though GHBT is highly specific (78% to 97%),41,42 it is quite insensitive (15.7% to 62%).42,43 In contrast, conventional double-peak criteria on LHBT lack sensitivity (31% to 68%) and the recently proposed early-peak criterion (rise in breath hydrogen within 90 minutes by 20 ppm above basal following lactulose ingestion) often gives false positive result with specificity of 65% to 97.9%.39,44 This is the reason for overestimation of frequency SIBO (as high as 78%) in the initial studies from United States.40 In fact, the early-peak criterion on LHBT, which was used in the initial studies on SIBO in patients with IBS, presumed that normal mouth to cecum transit time is more than 90 minutes in spite of the observation that it may be shorter.36,40 A study that combined radio-nuclide gut transit and LHBT revealed that in most patients in whom a peak in hydrogen was seen on LHBT, radio-nuclide already arrived in cecum.45 Other methods for diagnosis of SIBO include CO2 breath tests (14C or 13C D-xylose, 13C glucose and 13C cholylglycine hydrolase).7,22,46,47
Though hydrogen breath tests are quite popular for the diagnosis of SIBO, these are not free from limitations. In patients with distal SIBO, GHBT may be falsely negative as glucose gets completely absorbed in the proximal small bowel and hence, may not reach the site of SIBO.5,31,36 In patients with fast gut transit, early peak criteria proposed by Pimentel et al.40 often give false positive results.36 Fast gut transit is not uncommon, particularly in Asia. In a study from our center, median orocecal transit time in healthy subjects was 65 minutes (range, 40 to 110 minutes).48 A Taiwanese study revealed that average orocecal transit time was 85±37 minutes.49 Hence, it is important to realize that there is need to search for a noninvasive yet sensitive and specific method for diagnosis of SIBO.
FREQUENCY OF SIBO IN PATIENTS WITH IBS
There are several studies evaluating frequency of SIBO among patients with IBS as compared with controls using different diagnostic methods such as GHBT, LHBT and quantitative upper gut aspirate culture.7 Table 1 summarizes the results of these studies. As shown in the Table 1, frequency SIBO among patients with IBS ranged between 4% and 78% and that among controls, between 1% and 40%.37,39,40,45,50–68 Most case-control studies revealed that SIBO was commoner among IBS than controls; this suggests that there is significant association between SIBO and IBS (Fig. 1).
Table 1.
Prevalence of Small Intestinal Bacterial Overgrowth among Patients with Irritable Bowel Syndrome
| Study no. | Prevalence of SIBO in cases | Prevalence of SIBO in controls | Methane producers in cases | Methane producers in controls | Country | Year | Reference |
|---|---|---|---|---|---|---|---|
| Culture of jejunal aspirate (≥105 CFU/mL colonic-type bacteria) | |||||||
| 1 | 7/162 (4) | 1/26 (4) | ND | ND | Sweden | 2007 | Posserud et al.52 |
| 2 | 4/12 (33) | 0/9 | ND | ND | The Netherlands | 2008 | Kerckhoffs et al.53 |
| 3 | 15/80 (18) | 0/10 | 2/15 (13) | ND | India | 2014 | Ghoshal et al.39 |
| 4 | 42/112 (37) | ND | ND | ND | Greece | 2012 | Pyleris et al.54 |
| Culture of jejunal aspirate (≥103 CFU/mL colonic-type bacteria) | |||||||
| 5 | 62/139 (44.6) | ND | ND | ND | United States | 2015 | Erdogan et al.27 |
| Lactulose hydrogen breath test | |||||||
| 6 | 157/202 (78) | ND | ND | ND | United States | 2000 | Pimentel et al.40 |
| 7 | 64/98 (65) | ND | ND | ND | Italy | 2005 | Nucera et al.37 |
| 8 | 39/390 (10) | ND | ND | ND | Canada | 2005 | Walters and Vanner55 |
| 9 | 35/89 (39) | 1/13 (8) | ND | ND | China | 2014 | Zhao et al.56 |
| 10 | 25/40 (63) | ND | ND | ND | Canada | 2011 | Yu et al.45 |
| 11 | 34/76 (45) | 16/40 (40) | 19/76 (25) | 10/40 (25) | Korea | 2010 | Park et al.57 |
| 12 | 28/43 (65) | 4/56 (7) | 4/43 (9) | 0 | Italy | 2009 | Scarpellini et al.58 |
| 13 | 55/127 (43) | ND | ND | ND | Italy | 2008 | Carrara et al.59 |
| 14 | 89/258 (34.5) | ND | ND | ND | United States | 2009 | Mann and Limoges-Gonzales60 |
| 15 | 60/175 (34.3) | 45/150 (30) | ND | ND | India | 2008 | Rana et al.61 |
| 16 | 22/119 (18.4) | ND | ND | ND | Pakistan | 2011 | Yakoob et al.62 |
| Glucose hydrogen breath test | |||||||
| 17 | 25/225 (11.1) | 1/100 (1) | ND | ND | India | 2012 | Rana et al.67 |
| 18 | 93/204 (46) | ND | 27/204 (13) | ND | United States | 2007 | Majewski and McCallum63 |
| 19 | 105/331 (32) | 7/105 (7) | ND | ND | Rome | 2014 | Moraru et al.70 |
| 20 | 14/59 (24) | 1/37 (2.7) | 5/59 (9) | 9/37 (24) | India | 2011 | Sachdeva et al.64 |
| 21 | 11/129 (8.5) | 1/51 (2) | ND | ND | India | 2010 | Ghoshal et al.51 |
| 22 | 44/96 (45.8) | ND | ND | ND | Italy | 2006 | Cuoco and Salvangnini65 |
| 23 | 20/65 (31) | 4/102 (4) | ND | ND | Italy | 2005 | Lupascu et al.66 |
| 24 | 8/72 (11.1) | ND | ND | ND | Rome | 2013 | Moraru et al.50 |
| 25 | 49/200 (24.5) | 3/50 (6) | ND | ND | Italy | 2010 | Lombardo et al.143 |
| 26 | 38/139 (27.3) | ND | ND | ND | United States | 2015 | Erdogan et al.27 |
| 27 | 11/175 (6.2) | 1/150 (0.66) | ND | ND | India | 2012 | Rana et al.97 |
| 28 | 40/107 (37.3) | 14/107 (13) | ND | ND | Iran | 2015 | Abbasi et al.68 |
Data are presented as number (%).
SIBO, small intestinal bacterial overgrowth; CFU, colony forming unit; ND, not done.
Two meta-analyses also suggested association between IBS and SIBO. In a meta-analysis by Ford et al.,69 of the 12 studies including 1,921 patients with IBS, pooled prevalence of a positive LHBT and GHBT was 54% (95% confidence interval [CI], 32% to 76%) and 31% (95% CI, 14% to 50%), respectively. The odds ratio (OR) for any test showing positive SIBO result among patients with IBS as compared to controls was 3.45 to 4.7.69 In another meta-analysis on 11 studies, breath testing was found to be abnormal among patients with IBS than controls (OR, 4.46; 95% CI, 1.69 to 11.80). Breath testing had an overall sensitivity and specificity in separating IBS patients from healthy subjects of 44% and 84%, respectively.42 However, in this meta-analysis, authors suggested that abnormal breath test might not always mean SIBO as rise in breath hydrogen greater than 20 ppm above basal has poor specificity to diagnose SIBO.42
In last half a decade, after these meta-analyses were published, at the time of writing this review, about eight case-control studies on frequency of SIBO among patients with IBS have been published (Table 1). Two of these studies were published from United States, three from India, one each from Pakistan, Romania, and Iran.27,39,54,62,64,67,68,70 Of the five studies, which compared the frequency of SIBO among patients with IBS as compared to healthy controls, four showed significant difference. In these studies, frequency of SIBO among patients with IBS ranged between 19% and 37% and that among healthy controls between 0% and 12%.27,39,54,62,64,67,68,70 It is worthwhile evaluating the reasons for such wide variation in frequency of SIBO in these studies.
EXPLANATION FOR DIFFERENCE IN PREVALENCE OF SIBO IN IBS
Variations in prevalence of SIBO in patients with IBS and controls in several studies might be attributed to difference in geographical origin of studied population, different criteria for diagnosis of IBS (such as Manning, Rome I, II, and III), and methods for diagnosis of SIBO using different breath tests (such as nature of substrates, gases analyzed, instrument).51 Early peak criteria of LHBT give higher frequency of SIBO among patients with IBS and controls than other diagnostic methods, which might be attributed to false positive results.7,40,57,59,60 Whilst, double peak criteria on LHBT and GHBT give low frequency of SIBO in IBS patients and controls, which might be due to low sensitivity.7 Advanced noninvasive diagnostic techniques must be standardized for therapeutic management of SIBO in patients with IBS.
FACTORS ASSOCIATED WITH SIBO AMONG PATIENTS WITH IBS
There are several factors that are associated with SIBO among patients with IBS. These include female gender, older age, predominant symptom of bloating and flatulence, and diarrheal subtype of IBS.4,71 In fact, in a recent study, we found that number of bacterial colonies in the small bowel influenced Bristol stool type with higher number being associated with looser stools.39 Similar observation has been reproduced in another recent study.72 Since many patients with IBS might be taking PPIs due to overlapping dyspepsia, and PPI intake may influence development of SIBO, this may be one factor predicting occurrence of SIBO among patients with IBS.4,73 Narcotic intake might be another factor causing SIBO among patients with IBS due to slowing of gut motility.74 In one study, we found that lower value hemoglobin was associated with SIBO on GHBT.51 Subjects with older age are more susceptible to SIBO, most likely as a result of reduced GI motility, intestinal surgery, small bowel diverticulosis and use of medications.75 One previous study reported that patients older than 55 years with symptoms of IBS, particularly abdominal bloating and flatulence were more likely to be positive by GHBT.4 Abdominal bloating among patients with IBS might be due to excess gas production by bacterial fermentation of undigested carbohydrates, in addition to SIBO.76
MICROBIOLOGICAL ASPECTS OF SIBO IN IBS
SIBO can be classified into two categories based on difference in bacterial flora: (1) Gram positive flora might be due to failure of gastric acid barrier, and (2) coliform bacteria might be due to failure of intestinal clearance and small bowel anatomical alterations.52,77 Recently, one study based on culture of jejunal aspirates showed that Pseudomonas aeruginosa, Escherichia coli, Acenetobacter lwoffii, Staphylococcus species, Klebsiella pneumoniae, Streptococcus species, Acinetobacter baumannii, Enterococcus faecalis, and Enterococcus faecium were dominant bacteria among patients with SIBO.39 Pyleris et al.54 reported that of 42/112 patients with IBS having SIBO, E. coli, Enterococcus species and K. pneumoniae were the predominant species. Gram negative bacilli and Enterobacter were most common on culture of jejunal aspirates among patients with IBS.54
PATHOGENESIS OF IBS SYMPTOMS AMONG PATIENTS WITH SIBO
Though the pathophysiology of IBS remains largely enigmatic, evidence from recent studies does show that dysbiosis may contribute to development of symptoms, at least in a subset of patients.2,78 Though SIBO is a form of quantitative alteration of small bowel microbes, altered microbiota (dysbiosis) does not necessarily mean SIBO only. Dysbiosis includes qualitative alteration of gut flora (most authors reported on fecal microbiota) but also its quantitative change (SIBO).79,80 In the recent Rome IV review, importance of dysbiosis including that of SIBO has been recognized by several experts.81–83 In SIBO, bacterial fermentation of diet in the lumen produces hydrogen, methane and carbon dioxide gases, which may contribute to symptoms like distension, flatulence, abdominal pain, and bloating.2,22 Methane is known to slow gut transit resulting in constipation.84 These gases, however, may also be produced in the colon among patients without SIBO in presence of carbohydrate malabsorption.
SIBO is more often associated with diarrhea than constipation-predominant IBS.7 Mechanism of diarrhea in patients with SIBO include de-conjugation of bile salts, enterotoxic effect of bacterial metabolites, increased small intestinal permeability, deficiency of vitamin B12 and low grade inflammation resulting from immune activation in the small intestinal mucosa.22,37,85
Secondary deficiency of disaccharidases (e.g., lactase) is well known in patients with SIBO.86,87 This results in maldigestion of carbohydrates such as lactulose, sucrose and sorbitol.86,87 Moreover, fermentation of carbohydrates leads to formation of short chain fatty acids like acetic acid, propionic acid and butyric acid.88 Though short chain fatty acids are useful for colon by providing nutrients to the colonocytes, conservation of energy and absorption of water and electrolytes, in the small bowel, it inhibits nutrient absorption and inhibits jejunal motility (ileal brake) through liberation of peptide YY, neurotensin and glucagon like peptide-1, which promotes SIBO.89 Lipopolysaccharides derived from Gram negative bacteria may also affect the GI motility.90 Furthermore, bacterial derived metabolites may affect colonic motility. Likewise, formyl-methionyl-leucyl-phenylalanine may affect the enteric nervous system.91–94
Bacteria utilize intraluminal proteins leading to production of ammonia.95 Bacterial overgrowth produces a number of toxic compounds (peptidoglycans, D-lactate and serum amyloid A), which promote inflammation, may damage the brush border of the enterocytes and increases small intestinal permeability.22,95 Host response to SIBO also depends on its genetic make-up as evidenced by a recent case-control study on 209 patients with IBS and 273 healthy subjects.96 Under-producer genotypes of interleukin 1 (IL-1) receptor antagonist gene (anti-inflammatory) were associated with IBS.96 Moreover, IBS patients had higher levels of IL-1 α and β than those without SIBO.96 Another study showed higher level of proinflammatory cytokines such as IL-6 and tumor necrosis factor α among patients with IBS-D than controls.97 In addition, SIBO is associated with increased level of serum endotoxins, inflammatory cytokines and chemokines, and endogenous production of ethanol.23,95,98
Increased number of enterochromaffin cells was found in the mucosa of colon and rectum among patients with IBS than healthy controls.99 Immune activation in response to SIBO recruits increased number of intraepithelial lymphocytes, mast cells and enterochromaffin cells.100 Moreover, mediators of host immune response trigger the enteric nervous system altering GI motility and visceral hypersensitivity, which are the major pathophysiological mechanisms of IBS.101
Overgrowth of sulfate reducing bacteria may play an important role in patients with IBS.102 An association was found between bacterial derived hydrogen sulfide (H2S) and visceral hypersensitivity.103 H2S is known to act as gaseous neurotransmitters inducing the contraction of detrusor muscle in the urinary bladder.103 Recently, a study has shown that H2S produced by sulphate reducing bacteria may play role in pathogenesis of SIBO. The author suggested that breath H2S could be considered as potential noninvasive biomarker for diagnosis of SIBO among patients with IBS-D.104
Fibromyalgia, a condition associated with IBS, is also associated with SIBO.99 A study by Pimental et al.105 showed that all 42 patients with fibromyalgia had positive LHBT. This percentage was significantly higher than the control population (3/15, 20%). These data might suggest that somatic hypersensitivity is also influenced by altered gut flora.
TREATMENT OF SIBO IN PATIENTS WITH IBS
There are several approaches to treat SIBO among patients with IBS that include antibiotics, probiotics and prokinetics.106 Dietary manipulation has potential influence on the gut microbiota that may relieve some of the symptoms of SIBO.2,22,107 Recently, utility of therapeutic manipulation of gut flora using antibiotic and probiotic2 to treat IBS is being increasingly recognized and hence, worth reviewing.
1. Antibiotics
While choosing antibiotics, one should consider whether its antibacterial spectrum is broad including aerobes and anaerobes and absorption is poor reducing systemic side effects.108 Though in the past, tetracycline, doxycycline, co-trimoxazole, fluoroquinolones have all been used in the treatment of SIBO,109,110 in most of the recent studies among patients with IBS, rifaximin has been the preferred antibiotic (Table 2).52,108,111–114
Table 2.
Clinical Trials of Antibiotics among Patients with Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome
| Study no. | Antibiotics (dosage) | Duration, day | Study subject | Clinical outcome | Reference |
|---|---|---|---|---|---|
| 1 | Rifaximin (1,600 mg/day) vs rifaximin (1,200 mg/day) | 7 | 80 Patients with SIBO | Rate of normalization of GHBT was greater with higher dose of rifaximin than lower dose (80% vs 58%, p<0.05). | Scarpellini et al.108 |
| 2 | Rifaximin (group 1, 600; group 2, 800; group 3, 1,200 mg/day) | 7 | 90 Patients with SIBO and 30 patients in each group | Rate of normalization of GHBT was higher in group 3 than group 1 and 2 (60% vs 17%, p<0.001; 60% vs 27%, p<0.01). | Lauritano et al.112 |
| 3 | Neomycin (n=55) or placebo (n=56) | 7 | 111 Patients with IBS | Neomycin reduced the symptoms of IBS more often than placebo (35% vs 11%, p<0.05) and normalized lactulose hydrogen breath test result. | Pimentel et al.113 |
| 4 | Ciprofloxacin (500 mg, twice daily) | 10 | 7 Patients with SIBO | Ciprofloxacin decreased viable bacterial counts in five patients (71%), while four (57%) still fulfilled criteria for SIBO. Three patients (43%) reported at least 25% improvement in IBS symptoms. | Posserud et al.52 |
| 5 | Norfloxacin (800 mg/day) or placebo | 10 | 80 IBS patients | Norfloxacin significantly reduced the symptom scores among patients with SIBO than without but not with placebo at 1 month. Symptoms resolved to turn Rome III negative more often in SIBO patients receiving norfloxacin than placebo at 1 month (7/8, 87.5 vs 0/7, p=0.004). | Ghoshal et al.114 |
SIBO, small intestinal bacterial overgrowth; GHBT, glucose hydrogen breath test; IBS, irritable bowel syndrome.
Rifaximin is a semi-synthetic, nonabsorbable antimicrobial agent that acts against Gram positive and Gram negative aerobic and anaerobic bacteria.108 Pimentel et al.115 reported two identically designed, large, multicenter, double blind, placebo-controlled trials (TARGET 1 and TARGET 2) among patients with nonconstipating IBS (n=1,260) diagnosed by Rome II criteria. IBS subjects receiving rifaximin at a dose of 550 mg three times daily for 14 days reported adequate relief in global IBS symptoms as compared to identical placebo (TARGET 1: 40.8% vs 31.2%, p=0.01 and TARGET 2: 40.6% vs 32.2%, p=0.03).115 Moreover, rifaximin was more effective in relieving abdominal bloating than placebo (TARGET 1: 39.5% vs 28.7%, p=0.005 and TARGET 2: 41.0% vs 31.9%, p=0.02).115 The improvement in symptoms of IBS (like abdominal pain, loose or watery stool) persisted for a duration of 10 weeks after the end of 2-week treatment.115
Recently, TARGET 3 study has been completed to evaluate the efficacy and safety of retreatment with rifaximin among 636 patients with IBS-D, who had responded to rifaximin previously but developed recurrent IBS symptoms over a duration of 18 weeks follow-up.116,117 The end-point of TARGET 3 study was different from the TARGET 1 and TARGET 2 studies according to the guidelines proposed by Food and Drug Administration (FDA). TARGET 3 study included those IBS subjects who reported improvement in symptoms during at least 2 of the first 4 weeks in abdominal pain (≥30% decrease from baseline in mean weekly pain score) and stool consistency (≥50% decrease from baseline in the number of days per week with bowel movements complying with type 6 or 7 on the Bristol stool form scale).117,118 Retreatment with rifaximin showed 33% response rate as compared to 25% in placebo group (p=0.02), consistent with FDA guidelines for clinical assessment of IBS drugs.118
In a study of open level antibiotic treatment, bacterial overgrowth was eradicated in 25 out of 47 patients and symptoms of IBS like diarrhea and abdominal pain were improved.40 Moreover, 48% of the subjects were found negative for Rome I criteria.40 In another study, of the 10 patients with SIBO treated with norfloxacin, amoxicillin-clavulanic acid and Saccharomyces boulardii over a period of 7 days,119 norfloxacin and amoxicillin-clavulanic acid significantly improved the mean daily stool frequency, but not S. boulardii.119 Recently, a proof of the concept study suggested that the lower response rate of 40% among patients treated with antibiotic in TARGET I and II studies might be related to the fact that patients were not selected based on the presence or absence of SIBO. In this study, 7 of 8 (87.5%) of 15 patients with SIBO treated with norfloxacin became Rome III negative at 1 month as compared to none of those treated with placebo. Interestingly, in this study, of 40 patients treated with norfloxacin, 15 (37.5%) responded showing that when not selected according to presence of SIBO, response rate was somewhat similar to the frequency of improvement as reported in TARGET I and II study.114
In a recent meta-analysis, efficiency of rifaximin (two studies) in eradicating SIBO was 64.1% as compared to 41% with other systemic antibiotics (metronidazole or tetracycline, p=0.003).120 Another meta-analysis of eight studies showed that overall normalization rate of breath test with rifaximin was 49.5% (95% CI, 44.0 to 55.1).121 Antibiotics like metronidazole, neomycin and ciprofloxacin (four studies) showed higher response rate than placebo in normalizing breath tests with an odds ratio of 2.55 (95% CI, 1.29 to 5.04).121 Thus, evidence from above studies suggests that antibiotics can be given in IBS patients with suspected SIBO.
2. Probiotics
Probiotics are live microorganisms, which, when administered in sufficient quantities may alleviate symptoms of IBS than placebo as shown by several clinical trials.2 Probiotics may work by suppressing proinflammatory cytokines, modulating gut microbiota, sustaining the integrity of intestinal epithelium and altering the visceral hypersensitivity and brain function.28,122–125 Randomized controlled trials of probiotics among patients with SIBO are scanty. An old randomized controlled cross-over study only on 10 patients with SIBO showed that though norfloxacin and amoxicillin-clavulanic acid were effective in improving mean daily stool frequency and breath hydrogen, S. boulardii administered for one week was ineffective.119 Another study, however, showed that administration of high doses of S. boulardii for one month reduced abdominal pain, bloating, flatulence among pediatric patients with short bowel syndrome (SBS) and led to some change in bacterial flora in the stool samples suggesting that S. boulardii may impact the gut microbiota in patients with SBS.126 Furthermore, probiotics may enhance the efficiency of antibiotics. One study showed that treatment with rifaximin along with probiotic (Lactobacillus casei) improved the symptoms of SIBO more effectively than antibiotic followed by prebiotic (short chain fructo-oligosaccharide).127 Some studies recommended that treatment with rifaximin along with probiotics as a standard therapy for management of SIBO.127 Use of multispecies probiotics had shown several benefits in reliving symptoms of IBS.128 A randomized controlled trial of VSL#3 (twice daily for 8 weeks) in patients with IBS-D showed that abdominal bloating was significantly reduced as compared to placebo but not other parameters such as bowel dysfunction, colonic transit time, abdominal pain, flatulence or urgency.128,129 More studies, however, are needed to evaluate efficacy of probiotics among patients with IBS in relation to presence of SIBO.
3. Prokinetics
Since IBS is associated with alteration and gut motility, and SIBO is associated with motility disorders, prokinetics are expected to be beneficial in patients with SIBO. In an earlier study, Pimentel et al.130 showed that IBS patients with SIBO had lower frequency of migratory motor complex. Hence, it is expected that prokinetic drugs that improve small bowel motility might be useful in preventing SIBO following its successful treatment. The same group of authors showed that tegaserod, a serotonin receptor agonist, prevents the recurrence of IBS symptoms after antibiotic treatment compared to another prokinetic, erythromycin (a motilin agonist).131
4. Dietary manipulation of gut microbiota
Dietary manipulation may help patients with IBS in general and those with SIBO in particular.132,133 In patients with SIBO, bacteria in the small bowel may ferment carbohydrates such as lactose, fructose and also the dietary fermentable oligo-, di-, monosaccharides and polyols (FODMAPs), which forms gas resulting in flatulence, abdominal bloating and pain.134,135 Hence, restriction of these dietary components may improve these symptoms.136 Moreover, some preliminary data suggest that manipulation of the diet may alter gut microbiota.136 In a study, human fecal microbiota was transplanted into germ free mice that were fed low fat diet and plant polysaccharides.137 Subsequently, feeding Western diet resulted in change in composition of gut microbiota leading to increased number of Firmicutes, Clostridium species, Eubacterium, Enterococcus and decreased number of Bacteroides.137 Moreover, diet rich in complex carbohydrates favors growth of less pathogenic bacteria (Mycobacterium avium subspecies paratuberculosis and Enterobacteriaceae) than diet rich in fat or protein.138 Vegetarian diets, rich in fiber, lead to higher production of short chain fatty acids, which inhibit potentially invasive bacteria like E. coli and other members of Enterobacteriaceae.138,139 In a recent study, we found that vegetarianism was a risk factor for IBS on univariate and multivariate analysis.140 More studies are needed to evaluate effect of dietary manipulation on gut microbiota including SIBO.
CONCLUSIONS
Recent realization that SIBO play an important role in pathogenesis of symptoms in a subset of patients with IBS led to a paradigm shift in understanding this disorder, hitherto thought to be predominantly psychogenic in nature. This is further substantiated by the initiative of Rome Foundation that introduced the concept of multidimensional clinical profile in diagnosis and management of functional GI disorders including IBS.141 Though frequency of SIBO among patients with IBS varied between 4% and 78% patients, most studies reported the frequency to be higher among IBS than controls. Variation in the methods to diagnose SIBO is the most important reason for the wide variation in frequency of SIBO among patients with IBS in different studies. Quantitative jejunal aspirate culture, considered as the gold standard for the diagnosis of SIBO, is invasive and hence, hydrogen breath tests have been popularly used to diagnose SIBO.31,142 However, whereas GHBT is highly specific, it is quite insensitive. On the other hand, the early-peak criteria in LHBT is highly nonspecific.36 Hence, clinical phenotype of IBS may be used to consider treating patients empirically for possible SIBO. Diarrhea-predominant IBS (looser stool on Bristol scale), marked bloating and flatulence, older age, symptom development while on PPI therapy have been shown to be associated with SIBO among patients with IBS143; unless better noninvasive methods for diagnosis of SIBO become available, patients with these clinical predictors may be treated for possible SIBO. Currently, rifaximin is the best treatment for SIBO among patients with IBS.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Wall GC, Bryant GA, Bottenberg MM, Maki ED, Miesner AR. Irritable bowel syndrome: a concise review of current treatment concepts. World J Gastroenterol. 2014;20:8796–8806. doi: 10.3748/wjg.v20.i27.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghoshal UC, Shukla R, Ghoshal U, Gwee KA, Ng SC, Quigley EM. The gut microbiota and irritable bowel syndrome: friend or foe? Int J Inflam. 2012;2012:151085. doi: 10.1155/2012/151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malinen E, Krogius-Kurikka L, Lyra A, et al. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. 2010;16:4532–4540. doi: 10.3748/wjg.v16.i36.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddymasu SC, Sostarich S, McCallum RW. Small intestinal bacterial overgrowth in irritable bowel syndrome: are there any predictors? BMC Gastroenterol. 2010;10:23. doi: 10.1186/1471-230X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 6.Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y) 2007;3:112–122. [PMC free article] [PubMed] [Google Scholar]
- 7.Ghoshal UC, Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hype. World J Gastroenterol. 2014;20:2482–2491. doi: 10.3748/wjg.v20.i10.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grover M, Kanazawa M, Palsson OS, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol Motil. 2008;20:998–1008. doi: 10.1111/j.1365-2982.2008.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levenstein S, Rosenstock S, Jacobsen RK, Jorgensen T. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol. 2015;13:498–506.e1. doi: 10.1016/j.cgh.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 10.Safavi M, Sabourian R, Foroumadi A. Treatment of Helicobacter pylori infection: current and future insights. World J Clin Cases. 2016;4:5–19. doi: 10.12998/wjcc.v4.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Tan RZ, Chen Y, et al. CagA promotes proliferation and secretion of extracellular matrix by inhibiting signaling pathway of apoptosis in rat glomerular mesangial cells. Ren Fail. 2016;38:458–464. doi: 10.3109/0886022X.2016.1138831. [DOI] [PubMed] [Google Scholar]
- 12.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal UC, Park H, Gwee KA. Bugs and irritable bowel syndrome: the good, the bad and the ugly. J Gastroenterol Hepatol. 2010;25:244–251. doi: 10.1111/j.1440-1746.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- 15.Lagier JC, Million M, Hugon P, Armougom F, Raoult D. Human gut microbiota: repertoire and variations. Front Cell Infect Microbiol. 2012;2:136. doi: 10.3389/fcimb.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigam D. Microbial interactions with humans and animals. Int J Microbiol Allied Sci. 2015;2:1–17. [Google Scholar]
- 17.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schloss PD, Handelsman J. Status of the microbial census. Microbiol Mol Biol Rev. 2004;68:686–691. doi: 10.1128/MMBR.68.4.686-691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyas U, Ranganathan N. Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol Res Pract. 2012;2012:872716. doi: 10.1155/2012/872716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulet O, Joly F. Intestinal microbiota in short bowel syndrome. Gastroenterol Clin Biol. 2010;34(Suppl 1):S37–S43. doi: 10.1016/S0399-8320(10)70019-1. [DOI] [PubMed] [Google Scholar]
- 22.Bures J, Cyrany J, Kohoutova D, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978–2990. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderhoof JA, Young RJ. Etiology and pathogenesis of bacterial overgrowth. Clinical manifestations and diagnosis of bacterial overgrowth: treatment of bacterial overgrowth. UpToDate Online. 2010;18 [Google Scholar]
- 24.Gabrielli M, D’Angelo G, Di Rienzo T, Scarpellini E, Ojetti V. Diagnosis of small intestinal bacterial overgrowth in the clinical practice. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 2):30–35. [PubMed] [Google Scholar]
- 25.Hao WL, Lee YK. Microflora of the gastrointestinal tract: a review. Methods Mol Biol. 2004;268:491–502. doi: 10.1385/1-59259-766-1:491. [DOI] [PubMed] [Google Scholar]
- 26.Riordan SM, McIver CJ, Wakefield D, Duncombe VM, Thomas MC, Bolin TD. Small intestinal mucosal immunity and morphometry in luminal overgrowth of indigenous gut flora. Am J Gastroenterol. 2001;96:494–500. doi: 10.1111/j.1572-0241.2001.03533.x. [DOI] [PubMed] [Google Scholar]
- 27.Erdogan A, Rao SS, Gulley D, Jacobs C, Lee YY, Badger C. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil. 2015;27:481–489. doi: 10.1111/nmo.12516. [DOI] [PubMed] [Google Scholar]
- 28.Chey WD, Maneerattaporn M, Saad R. Pharmacologic and complementary and alternative medicine therapies for irritable bowel syndrome. Gut Liver. 2011;5:253–266. doi: 10.5009/gnl.2011.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with rifaximin. World J Gastroenterol. 2009;15:2628–2631. doi: 10.3748/wjg.15.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler TR, Cole CR. Small bowel bacterial overgrowth in adults: a potential contributor to intestinal failure. Curr Gastroenterol Rep. 2007;9:463–467. doi: 10.1007/s11894-007-0060-x. [DOI] [PubMed] [Google Scholar]
- 31.Ghoshal UC, Ghoshal U, Das K, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with orocecal transit time. Indian J Gastroenterol. 2006;25:6–10. [PubMed] [Google Scholar]
- 32.Britton E, McLaughlin JT. Ageing and the gut. Proc Nutr Soc. 2013;72:173–177. doi: 10.1017/S0029665112002807. [DOI] [PubMed] [Google Scholar]
- 33.Ghoshal U, Ghoshal UC, Ranjan P, Naik SR, Ayyagari A. Spectrum and antibiotic sensitivity of bacteria contaminating the upper gut in patients with malabsorption syndrome from the tropics. BMC Gastroenterol. 2003;3:9. doi: 10.1186/1471-230X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagier JC, Armougom F, Million M, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 35.Kokcha S, Mishra AK, Lagier JC, et al. Non contiguous-finished genome sequence and description of Bacillus timonensis sp. nov. Stand Genomic Sci. 2012;6:346–355. doi: 10.4056/sigs.2776064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312–317. doi: 10.5056/jnm.2011.17.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nucera G, Gabrielli M, Lupascu A, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;21:1391–1395. doi: 10.1111/j.1365-2036.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- 38.Abraczinskas D, Goldfinger SE. Intestinal gas and bloating. Up-ToDate Online. 2010:18. [Google Scholar]
- 39.Ghoshal UC, Srivastava D, Ghoshal U, Misra A. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur J Gastroenterol Hepatol. 2014;26:753–760. doi: 10.1097/MEG.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 40.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 41.Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–988. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- 42.Shah ED, Basseri RJ, Chong K, Pimentel M. Abnormal breath testing in IBS: a meta-analysis. Dig Dis Sci. 2010;55:2441–2449. doi: 10.1007/s10620-010-1276-4. [DOI] [PubMed] [Google Scholar]
- 43.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojetti V, Di Rienzo TA, D’Angelo G, et al. Early peak of hydrogen during lactose breath test predicts intestinal motility. Open J Gastroenterol. 2014;4:40–44. doi: 10.4236/ojgas.2014.41007. [DOI] [Google Scholar]
- 45.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]
- 46.Santavirta J. Lactulose hydrogen and [14C]xylose breath tests in patients with ileoanal anastomosis. Int J Colorectal Dis. 1991;6:208–211. doi: 10.1007/BF00341392. [DOI] [PubMed] [Google Scholar]
- 47.Banik GD, Maity A, Som S, et al. Diagnosis of small intestinal bacterial overgrowth in irritable bowel syndrome patients using high-precision stable 13 CO 2/12 CO 2 isotope ratios in exhaled breath. J Anal At Spectrom. 2014;29:1918–1924. doi: 10.1039/C4JA00186A. [DOI] [Google Scholar]
- 48.Ghoshal UC, Ghoshal U, Ayyagari A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol. 2003;18:540–547. doi: 10.1046/j.1440-1746.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 49.Lu CL, Chen CY, Chang FY, Lee SD. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: an Oriental study. Clin Sci (Lond) 1998;95:165–169. doi: 10.1042/cs0950165. [DOI] [PubMed] [Google Scholar]
- 50.Moraru IG, Portincasa P, Moraru AG, Diculescu M, Dumitraşcu DL. Small intestinal bacterial overgrowth produces symptoms in irritable bowel syndrome which are improved by rifaximin: a pilot study. Rom J Intern Med. 2013;51:143–147. [PubMed] [Google Scholar]
- 51.Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16:40–46. doi: 10.5056/jnm.2010.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerckhoffs AP, Visser MR, Samsom M, et al. Critical evaluation of diagnosing bacterial overgrowth in the proximal small intestine. J Clin Gastroenterol. 2008;42:1095–1102. doi: 10.1097/MCG.0b013e31818474d7. [DOI] [PubMed] [Google Scholar]
- 54.Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, Koussoulas V, Barbatzas C, Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: relationship with irritable bowel syndrome. Dig Dis Sci. 2012;57:1321–1329. doi: 10.1007/s10620-012-2033-7. [DOI] [PubMed] [Google Scholar]
- 55.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–1570. doi: 10.1111/j.1572-0241.2005.40795.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhao J, Zheng X, Chu H, et al. A study of the methodological and clinical validity of the combined lactulose hydrogen breath test with scintigraphic orocecal transit test for diagnosing small intestinal bacterial overgrowth in IBS patients. Neurogastroenterol Motil. 2014;26:794–802. doi: 10.1111/nmo.12331. [DOI] [PubMed] [Google Scholar]
- 57.Park JS, Yu JH, Lim HC, et al. Usefulness of lactulose breath test for the prediction of small intestinal bacterial overgrowth in irritable bowel syndrome. Korean J Gastroenterol. 2010;56:242–248. doi: 10.4166/kjg.2010.56.4.242. [DOI] [PubMed] [Google Scholar]
- 58.Scarpellini E, Giorgio V, Gabrielli M, et al. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: a case-control study. J Pediatr. 2009;155:416–420. doi: 10.1016/j.jpeds.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 59.Carrara M, Desideri S, Azzurro M, et al. Small intestine bacterial overgrowth in patients with irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2008;12:197–202. [PubMed] [Google Scholar]
- 60.Mann NS, Limoges-Gonzales M. The prevalence of small intestinal bacterial overgrowth in irritable bowel syndrome. Hepatogastroenterology. 2009;56:718–721. [PubMed] [Google Scholar]
- 61.Rana SV, Sinha SK, Sikander A, Bhasin DK, Singh K. Study of small intestinal bacterial overgrowth in North Indian patients with irritable bowel syndrome: a case control study. Trop Gastroenterol. 2008;29:23–25. [PubMed] [Google Scholar]
- 62.Yakoob J, Abbas Z, Khan R, Hamid S, Awan S, Jafri W. Small intestinal bacterial overgrowth and lactose intolerance contribute to irritable bowel syndrome symptomatology in Pakistan. Saudi J Gastroenterol. 2011;17:371–375. doi: 10.4103/1319-3767.87176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139–142. [PubMed] [Google Scholar]
- 64.Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol. 2011;26(Suppl 3):135–138. doi: 10.1111/j.1440-1746.2011.06654.x. [DOI] [PubMed] [Google Scholar]
- 65.Cuoco L, Salvagnini M. Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with rifaximin. Minerva Gastroenterol Dietol. 2006;52:89–95. [PubMed] [Google Scholar]
- 66.Lupascu A, Gabrielli M, Lauritano EC, et al. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:1157–1160. doi: 10.1111/j.1365-2036.2005.02690.x. [DOI] [PubMed] [Google Scholar]
- 67.Rana SV, Sharma S, Kaur J, Sinha SK, Singh K. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion. 2012;85:243–247. doi: 10.1159/000336174. [DOI] [PubMed] [Google Scholar]
- 68.Abbasi MH, Zahedi M, Darvish Moghadam S, Shafieipour S, HayatBakhsh Abbasi M. Small bowel bacterial overgrowth in patients with irritable bowel syndrome: the first study in iran. Middle East J Dig Dis. 2015;7:36–40. [PMC free article] [PubMed] [Google Scholar]
- 69.Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. doi: 10.1016/j.cgh.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 70.Moraru IG, Moraru AG, Andrei M, et al. Small intestinal bacterial overgrowth is associated to symptoms in irritable bowel syndrome: evidence from a multicentre study in Romania. Rom J Intern Med. 2014;52:143–150. [PubMed] [Google Scholar]
- 71.Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Treat Options Gastroenterol. 2004;7:19–28. doi: 10.1007/s11938-004-0022-4. [DOI] [PubMed] [Google Scholar]
- 72.Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spiegel BM, Chey WD, Chang L. Bacterial overgrowth and irritable bowel syndrome: unifying hypothesis or a spurious consequence of proton pump inhibitors? Am J Gastroenterol. 2008;103:2972–2976. doi: 10.1111/j.1572-0241.2008.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choung RS, Ruff KC, Malhotra A, et al. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33:1059–1067. doi: 10.1111/j.1365-2036.2011.04625.x. [DOI] [PubMed] [Google Scholar]
- 75.Gasbarrini A, Scarpellini E, Gabrielli M, Tortora A, Purchiaroni F, Ojetti V. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33:1378–1379. doi: 10.1111/j.1365-2036.2011.04657.x. [DOI] [PubMed] [Google Scholar]
- 76.Haderstorfer B, Psycholgin D, Whitehead WE, Schuster MM. Intestinal gas production from bacterial fermentation of undigested carbohydrate in irritable bowel syndrome. Am J Gastroenterol. 1989;84:375–378. [PubMed] [Google Scholar]
- 77.Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013;4:223–231. doi: 10.1177/2040622313496126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shukla R, Ghoshal U, Dhole TN, Ghoshal UC. Fecal microbiota in patients with irritable bowel syndrome compared with healthy controls using real-time polymerase chain reaction: an evidence of dysbiosis. Dig Dis Sci. 2015;60:2953–2962. doi: 10.1007/s10620-015-3607-y. [DOI] [PubMed] [Google Scholar]
- 79.Park H. The role of small intestinal bacterial overgrowth in the pathophysiology of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:3–4. doi: 10.5056/jnm.2010.16.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi CH, Chang SK. Role of small intestinal bacterial overgrowth in functional gastrointestinal disorders. J Neurogastroenterol Motil. 2016;22:3–5. doi: 10.5056/jnm15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barbara G, Feinle-Bisset C, Ghoshal UC, et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150:1305–1318.e8. doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 82.Francisconi CF, Sperber AD, Fang X, et al. Multicultural aspects in functional gastrointestinal disorders (FGIDs) Gastroenterology. 2016;150:1344–1354. doi: 10.1053/j.gastro.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 84.Pimentel M, Gunsalus RP, Rao SS, Zhang H. Methanogens in human health and disease. Am J Gastroenterol Suppl. 2012;1:28–33. doi: 10.1038/ajgsup.2012.6. [DOI] [Google Scholar]
- 85.Fan X, Sellin JH. Review article: small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhoea. Aliment Pharmacol Ther. 2009;29:1069–1077. doi: 10.1111/j.1365-2036.2009.03970.x. [DOI] [PubMed] [Google Scholar]
- 86.Riepe SP, Goldstein J, Alpers DH. Effect of secreted Bacteroides proteases on human intestinal brush border hydrolases. J Clin Invest. 1980;66:314–322. doi: 10.1172/JCI109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zaidel O, Lin HC. Uninvited guests: the impact of small intestinal bacterial overgrowth on nutritional status. Pract Gastroenterol. 2003;27:27–34. [Google Scholar]
- 88.Bala L, Ghoshal UC, Ghoshal U, et al. Malabsorption syndrome with and without small intestinal bacterial overgrowth: a study on upper-gut aspirate using 1H NMR spectroscopy. Magn Reson Med. 2006;56:738–744. doi: 10.1002/mrm.21041. [DOI] [PubMed] [Google Scholar]
- 89.Ghoshal UC, Kumar S, Misra A, Choudhuri G. Pathogenesis of tropical sprue: a pilot study of antroduodenal manometry, duodenocaecal transit time & fat-induced ileal brake. Indian J Med Res. 2013;137:63–72. [PMC free article] [PubMed] [Google Scholar]
- 90.Donowitz JR, Petri WA., Jr Pediatric small intestine bacterial overgrowth in low-income countries. Trends Mol Med. 2015;21:6–15. doi: 10.1016/j.molmed.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malbert CH. The ileocolonic sphincter. Neurogastroenterol Motil. 2005;17(Suppl 1):41–49. doi: 10.1111/j.1365-2982.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 93.Dass NB, John AK, Bassil AK, et al. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19:66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 94.Barbara G, Stanghellini V, Brandi G, et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 95.Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol. 2006;4:11–20. doi: 10.1016/j.cgh.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 96.Srivastava D, Ghoshal U, Mittal RD, Ghoshal UC. Associations between IL-1RA polymorphisms and small intestinal bacterial overgrowth among patients with irritable bowel syndrome from India. Neurogastroenterol Motil. 2014;26:1408–1416. doi: 10.1111/nmo.12399. [DOI] [PubMed] [Google Scholar]
- 97.Rana SV, Sharma S, Sinha SK, Parsad KK, Malik A, Singh K. Pro-inflammatory and anti-inflammatory cytokine response in diarrhoea-predominant irritable bowel syndrome patients. Trop Gastroenterol. 2012;33:251–256. doi: 10.7869/tg.2012.66. [DOI] [PubMed] [Google Scholar]
- 98.Spinucci G, Guidetti M, Lanzoni E, Pironi L. Endogenous ethanol production in a patient with chronic intestinal pseudo-obstruction and small intestinal bacterial overgrowth. Eur J Gastroenterol Hepatol. 2006;18:799–802. doi: 10.1097/01.meg.0000223906.55245.61. [DOI] [PubMed] [Google Scholar]
- 99.Schmulson M, Bielsa MV, Carmona-Sánchez R, et al. Microbiota, gastrointestinal infections, low-grade inflammation, and antibiotic therapy in irritable bowel syndrome: an evidence-based review. Rev Gastroenterol Mex. 2014;79:96–134. doi: 10.1016/j.rgmx.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 100.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–431. doi: 10.1007/s00535-011-0379-9. [DOI] [PubMed] [Google Scholar]
- 101.Hasler WL. Traditional thoughts on the pathophysiology of irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:21–43. doi: 10.1016/j.gtc.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 102.Patacchini R, Santicioli P, Giuliani S, Maggi CA. Pharmacological investigation of hydrogen sulfide (H2S) contractile activity in rat detrusor muscle. Eur J Pharmacol. 2005;509:171–177. doi: 10.1016/j.ejphar.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 103.Weinstock LB, Klutke CG, Lin HC. Small intestinal bacterial overgrowth in patients with interstitial cystitis and gastrointestinal symptoms. Dig Dis Sci. 2008;53:1246–1251. doi: 10.1007/s10620-007-0022-z. [DOI] [PubMed] [Google Scholar]
- 104.Banik GD, De A, Som S, et al. Hydrogen sulphide in exhaled breath: a potential biomarker for small intestinal bacterial overgrowth in IBS. J Breath Res. 2016;10:026010. doi: 10.1088/1752-7155/10/2/026010. [DOI] [PubMed] [Google Scholar]
- 105.Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450–452. doi: 10.1136/ard.2003.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwon JG, Park KS, Park JH, et al. Guidelines for the treatment of irritable bowel syndrome. Korean J Gastroenterol. 2011;57:82–99. doi: 10.4166/kjg.2011.57.2.82. [DOI] [PubMed] [Google Scholar]
- 107.Neale G, Gompertz D, Schönsby H, Tabaqchali S, Booth CC. The metabolic and nutritional consequences of bacterial overgrowth in the small intestine. Am J Clin Nutr. 1972;25:1409–1417. doi: 10.1093/ajcn/25.12.1409. [DOI] [PubMed] [Google Scholar]
- 108.Scarpellini E, Gabrielli M, Lauritano CE, et al. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25:781–786. doi: 10.1111/j.1365-2036.2007.03259.x. [DOI] [PubMed] [Google Scholar]
- 109.Di Stefano M, Miceli E, Missanelli A, Corazza GR. Treatment of small intestine bacterial overgrowth. Eur Rev Med Pharmacol Sci. 2005;9:217–222. [PubMed] [Google Scholar]
- 110.Quigley EM, Quera R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology. 2006;130(2 Suppl1):S78–S90. doi: 10.1053/j.gastro.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 111.Chedid V, Dhalla S, Clarke JO, et al. Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob Adv Health Med. 2014;3:16–24. doi: 10.7453/gahmj.2014.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lauritano EC, Gabrielli M, Lupascu A, et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;22:31–35. doi: 10.1111/j.1365-2036.2005.02516.x. [DOI] [PubMed] [Google Scholar]
- 113.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 114.Ghoshal UC, Srivastava D, Misra A, Ghoshal U. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28:281–289. doi: 10.1097/MEG.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 115.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 116.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–S26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 117.Lacy BE, Chey WD, Lembo AJ. New and emerging treatment options for irritable bowel syndrome. Gastroenterol Hepatol (N Y) 2015;11(4 Suppl 2):1–19. [PMC free article] [PubMed] [Google Scholar]
- 118.Weinberg DS, Smalley W, Heidelbaugh JJ, Sultan S American Gastroenterological Association. American Gastroenterological Association Institute Guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1146–1148. doi: 10.1053/j.gastro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 119.Attar A, Flourié B, Rambaud JC, Franchisseur C, Ruszniewski P, Bouhnik Y. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: a crossover, randomized trial. Gastroenterology. 1999;117:794–797. doi: 10.1016/S0016-5085(99)70336-7. [DOI] [PubMed] [Google Scholar]
- 120.Gatta L, Scarpignato C. Mo2055 Rifaximin for treatment of small intestine bacterial overgrowth (SIBO): a systematic review and meta-analysis. Gastroenterology. 2013;144(5 Suppl 1):S729. doi: 10.1016/S0016-5085(13)62707-9. [DOI] [Google Scholar]
- 121.Shah SC, Day LW, Somsouk M, Sewell JL. Meta-analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2013;38:925–934. doi: 10.1111/apt.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252–266. doi: 10.5056/jnm.2011.17.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lacy BE, Weiser K, De Lee R. The treatment of irritable bowel syndrome. Therap Adv Gastroenterol. 2009;2:221–238. doi: 10.1177/1756283X09104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 125.Thijssen AY, Jonkers D, Clemens CH, Masclee A. Effect of probiotic treatment on visceral hypersensitivity in irritable bowel syndrome. Gastroenterology. 2011;140(5 Suppl 1):S852. [Google Scholar]
- 126.Lyszkowska M, Popinska K, Idzik M, Ksiazyk P. Probiotics in children with gut failure. J Pediatr Gastroenterol Nutr. 2008;46:543. [Google Scholar]
- 127.Rosania R, Giorgio F, Principi M, et al. Effect of probiotic or prebiotic supplementation on antibiotic therapy in the small intestinal bacterial overgrowth: a comparative evaluation. Curr Clin Pharmacol. 2013;8:169–172. doi: 10.2174/15748847113089990048. [DOI] [PubMed] [Google Scholar]
- 128.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 129.Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 130.Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47:2639–2643. doi: 10.1023/A:1021039032413. [DOI] [PubMed] [Google Scholar]
- 131.Pimentel M, Morales W, Lezcano S, Sun-Chuan D, Low K, Yang J. Low-dose nocturnal tegaserod or erythromycin delays symptom recurrence after treatment of irritable bowel syndrome based on presumed bacterial overgrowth. Gastroenterol Hepatol (N Y) 2009;5:435–442. [PMC free article] [PubMed] [Google Scholar]
- 132.Shepherd SJ, Halmos E, Glance S. The role of FODMAPs in irritable bowel syndrome. Curr Opin Clin Nutr Metab Care. 2014;17:605–609. doi: 10.1097/MCO.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 133.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 134.Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccha-rides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261–268. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (N Y) 2014;10:164–174. [PMC free article] [PubMed] [Google Scholar]
- 136.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 137.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metage-nomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zimmer J, Lange B, Frick JS, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66:53–60. doi: 10.1038/ejcn.2011.141. [DOI] [PubMed] [Google Scholar]
- 140.Ghoshal UC, Singh R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population. J Gastroenterol Hepatol. doi: 10.1111/jgh.13465. Epub 2016 Jun 5. https://doi.org/10.1111/jgh.13465. [DOI] [PubMed] [Google Scholar]
- 141.Drossman DA. Guidelines for use of the multi-dimensional clinical profile. In: Drossman DA, Azpiroz F, Chang L, et al., editors. Multi-dimensional clinical profile (MDCP): for functional gastrointestinal disorders. Raleigh: Rome Foundation; 2015. pp. 7–14. [Google Scholar]
- 142.Lindberg DA. Hydrogen breath testing in adults: what is it and why is it performed? Gastroenterol Nurs. 2009;32:19–24. doi: 10.1097/SGA.0b013e3181965d2d. [DOI] [PubMed] [Google Scholar]
- 143.Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010;8:504–508. doi: 10.1016/j.cgh.2009.12.022. [DOI] [PubMed] [Google Scholar]