Abstract
Background/Aims
This study aimed to investigate the prevalence and characteristics of small intestinal bacterial overgrowth (SIBO) in patients undergoing abdominal surgeries, such as gastrectomy, cholecystectomy, and hysterectomy.
Methods
One hundred seventy-one patients with surgery (50 hysterectomy, 14 gastrectomy, and 107 cholecystectomy), 665 patients with functional gastrointestinal disease (FGID) and 30 healthy controls undergoing a hydrogen (H2)-methane (CH4) glucose breath test (GBT) were reviewed.
Results
GBT positivity (+) was significantly different among the surgical patients (43.9%), FGID patients (31.9%), and controls (13.3%) (p<0.01). With respect to the patients, 65 (38.0%), four (2.3%), and six (3.5%) surgical patients and 150 (22.6%), 30 (4.5%), and 32 (4.8%) FGID patients were in the GBT (H2)+, (CH4)+ and (mixed)+ groups, respectively (p<0.01). The gastrectomy group had a significantly increased preference in GBT+ (71.4% vs 42.0% or 41.1%, respectively) and GBT (H2)+ (64.3% vs 32.0% or 37.4%, respectively) compared with the hysterectomy or cholecystectomy groups (p<0.01). During GBT, the total H2 was significantly increased in the gastrectomy group compared with the other groups.
Conclusions
SIBO producing H2 is common in abdominal surgical patients. Different features for GBT+ may be a result of the types of abdominal surgery.
Keywords: Glucose breath test, Cholecystectomy, Gastrectomy, Hysterectomy
INTRODUCTION
A sizable number of patients after a previous abdominal surgery experience gastrointestinal symptoms. Abdominal surgery was suggested to have the potential to modify the enteric bacteria which can be associated with intestinal symptoms.1,2 Patients with structural changes or impaired functional factors secondary to abdominal surgeries may exhibit a predisposition to small intestinal bacterial overgrowth (SIBO).
SIBO is a disorder with an increase in the number or alteration in the type of bacteria in small intestine. SIBO results in various gastrointestinal presenting symptoms of abdominal discomfort, bloating and altered bowel habits. Aspiration, or direct culture of the small bowel luminal contents is considered the standard diagnostic test for SIBO, but it is invasive, time-consuming, and expensive. Thus recently, the hydrogen (H2) and methane (CH4) breath test is preferred, because it is simple, safe, and less expensive.3
In recent studies, a history of gastrectomy or cholecystectomy was associated with higher prevalence of SIBO.4,5 Also, a previous study has demonstrated that the prevalence of SIBO in the patients who had undergone abdominal and pelvic surgery was higher than that of controls, but was not statistically significant.6
Thus, it is possible that patients who present with gastrointestinal symptoms after abdominal surgery have SIBO. To our knowledge, there is scanty data on SIBO in patients with a history of previous abdominal surgery. The purpose of this study was to investigate the prevalence of SIBO using glucose breath test (GBT) in patients undergoing abdominal surgery such as hysterectomy, gastrectomy, and cholecystectomy compared with healthy controls with no history of abdominal surgery. We also evaluated the characteristics of breath profiles among patients undergoing different type of surgeries.
MATERIALS AND METHODS
This study was approved by the Ethics Committee and Institutional Review Board of our institution (VC15RISI0209) and adhered to the declaration of Helsinki. This was retrospective study for medical records, therefore informed consents could not be obtained from the patients.
1. Study populations
A collected database was reviewed retrospectively for electrical medical records for the consecutive patients with gastrointestinal symptoms evaluated by GBT at a university hospital, between September 2009 and May 2015. We enrolled the patients who had a functional gastrointestinal disorder (FGID) without any history of abdominal surgery, and the patients who underwent abdominal surgery such as gastrectomy, hysterectomy, or laparoscopic cholecystectomy without any evidence of surgical complications or sequela for leakage, bleeding, adhesion, or perforation. FGID was diagnosed according to the Rome III criteria demonstrating that the symptoms presented for the previous 3 months, begun at least 6 months prior to diagnosis.7,8
The patients with laparoscopic cholecystectomy were included due to acute cholecystitis at least 6 months before, and had no evidence of recurrent or remnant stones at common bile duct of cystic duct by test such as the blood chemistry or abdominal computed tomography.5 The patients with gastrectomy for early gastric cancer have no evidence of recurrence for at least 6 months of follow-up.4 All women undergoing hysterectomy were followed for 12 months.9,10
The patients over 18 years of age were included. Exclusion criteria were previous history of diabetes mellitus, connective tissue disease, chronic pancreatitis, thyroid disease, or other abdominal surgery. The additional exclusion criteria were as followed: taking antisecretory agent such as proton pump inhibitors or histamine (H)2 receptor antagonists, antibiotics, probiotics, laxatives, bulking agents, prokinetics, narcotic use, or antidiarrheal drugs within the previous 4 weeks; having a gastrointestinal disease, renal insufficiency, liver disease, a major psychiatric disease, hearing impairment, and masticatory dysfunction; having undergone colonoscopy within the previous 3 months; or having incomplete data. Controls were 30 consecutive subjects with healthy status for evaluation of standard value of GBT in 2007.4
2. Study design
For each patients enrolled in this study, the electrical medical charts were used to survey the following information such as demographics, the comorbidities, the concurrent use of drugs, history of surgery, and the data for GBT. A GBT was performed with the gas chromatography of equipment (SC breathtracker; Quintron Instrument Co., Milwaukee, WI, USA) after an overnight fast of at least 12 hours. The subjects were asked to follow a strict low residue diet on the day before the examination. Patients were instructed to wash their mouth with 20 mL of 0.05% chlorhexidine, 30 minutes before breath test. Physical exercise and cigarette smoking were bit permitted for 30 minutes before and during the test. The patients then ingested 50 g of glucose (DIASOL-S SOLN; Taejoon Pharm Co., Ltd., Seoul, Korea), and then the duplicate samples of end expired air were collected at baseline, and then at every 10 minutes for 2 hours. The values of breath H2, and CH4 were evaluated with the equipment.
After administration of substrate for GBT, three subtypes were classified according to the predominant gas produced during GBT. The baseline H2 or CH4 by >15 parts per million (ppm), or increase in H2 or CH4 of ≥12 ppm above baseline level within 60 minutes were considered the positivities to GBT for H2 (GBT (H2)+) or CH4 (GBT (CH4)+).5,11,12 The status satisfied with both GBT (H2)+ and GBT (CH4)+ was defined as GBT (mixed)+.
3. Analysis
Statistical analysis was performed using the SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The clinical data included age, gender, and body mass index (BMI). The parameters with GBT including H2 profiles of at the time points, the time point of peak value in H2 concentration, total H2 or CH4 concentration during the GBT, and the positivity to GBT were determined. Continuous data were expressed as means±standard deviations. All the data were compared among each of groups including three subtypes of surgical patients, FGID patents, and controls. For intergroup comparisons, Categorical data were expressed as quantities, and compared by means using chi-square tests or Fisher exact test. Continuous data were analyzed using independent t-test and one way analysis of analysis of variance and Tukey’s multiple comparison test as a post hoc analysis. A p<0.05 was considered significant.
RESULTS
1. Study populations
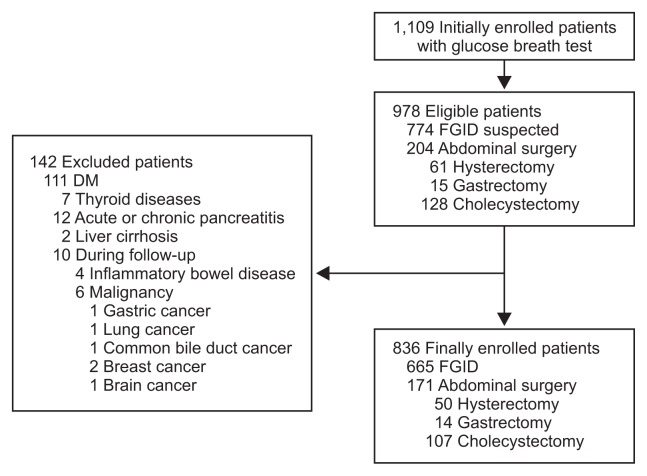
A total of 1,109 patients undergoing the GBT were initially enrolled in the study. Among them, 774 consecutive patients were suspected to have FGID, and the 204 patients had a single history of abdominal surgery including hysterectomy, gastrectomy, and cholecystectomy. One hundred forty-two subjects were excluded owing to a history of diabetes, thyroid diseases, pancreatitis, inflammatory bowel disease, and malignancy (Fig. 1). Finally 171 surgical patients and 665 FGID patients were enrolled. Among the 14 patients with gastrectomy, four (28.6%) underwent Billroth-I (B-I) gastrectomy, eight (57.1%) did Billroth-II (B-II) gastrectomy, and two (14.3%) did total gastrectomy. The mean age was significantly high in surgical patients, FGID patients, and healthy controls, sequentially (Table 1). There were no significant differences in gender and BMI among the subjects.
Fig. 1.
Flow chart of study inclusion.
DM, diabetes mellitus; FGID, functional gastrointestinal disorder.
Table 1.
Demographic Clinical Data of Controls, Patients with Functional Gastrointestinal Disorder and Patients with Abdominal Surgery
| Patients with abdominal surgery (n=171) | Patients with FGID (n=665) | Control (n=30) | p-value* | |
|---|---|---|---|---|
| Age, yr | 54.81±13.62 | 49.86±15.05 | 40.13±16.04 | <0.01 |
| T† | A | A | B | |
| Sex | ||||
| Male | 60 (35.1) | 265 (39.8) | 13 (43.3) | 0.46 |
| Female | 111 (64.9) | 400 (60.2) | 17 (56.7) | |
| BMI, kg/m2 | 23.96±3.76 | 23.07±3.38 | 22.92±2.58 | 0.26 |
| Total H2, ppm | 187.83±202.24 | 148.23±213.35 | 54.83±46.86 | <0.01 |
| T† | A | A | B | |
| Total CH4, ppm | 72.65±78.51 | 80.12±124.99 | 37.77±128.50 | 0.13 |
| Positive GBT | 75 (43.9) | 212 (31.9) | 4 (13.3) | <0.01 |
| H2 | 65 (38.0) | 150 (22.6) | 1 (3.3) | <0.01 |
| CH4 | 4 (2.3) | 30 (4.5) | 2 (6.6) | |
| Mixed | 6 (3.5) | 32 (4.8) | 1 (3.3) | |
Data are presented as the mean±SD or number (%).
FGID, functional gastrointestinal disorder; BMI, body mass index; H2, hydrogen; CH4, methane; GBT, glucose breath test.
Statistical significance among groups was assessed via one-way analyses of variance or chi-square tests;
The same letters indicate nonsignificant differences between the groups based on Tukey’s multiple comparison test.
2. Comparison of the profiles and positivity of GBT in controls, patients with FGID and abdominal surgery
The breath H2 levels were significantly higher in the surgical patients than those in controls at all the time points during the GBT, or than those in patient with FGID at the time points of 40, 50, 60, 70, 80, 90, 100, 110, and 120 minutes intervals. The GBT positivity (+) were significantly high in surgical patients (43.9%, 75/171), FGID patients (31.9%, 212/665), and controls (13.4%, 4/30) (p<0.01), in consequence (Table 1). However, no difference was shown in total H2 concentration between the surgical and FGID groups by post hoc analysis. Among the enrolled patients, 65 (38.0%), four (2.3%), and six (3.5%) of surgical patients, and 150 (22.6%), 30 (4.5%), and 32 (4.8%) of FGID patients were in the GBT (H2)+, (CH4)+, (mixed)+ groups, respectively (p<0.01).
3. Characteristics of the profiles and positivity of GBT according to the subtypes in surgical patients
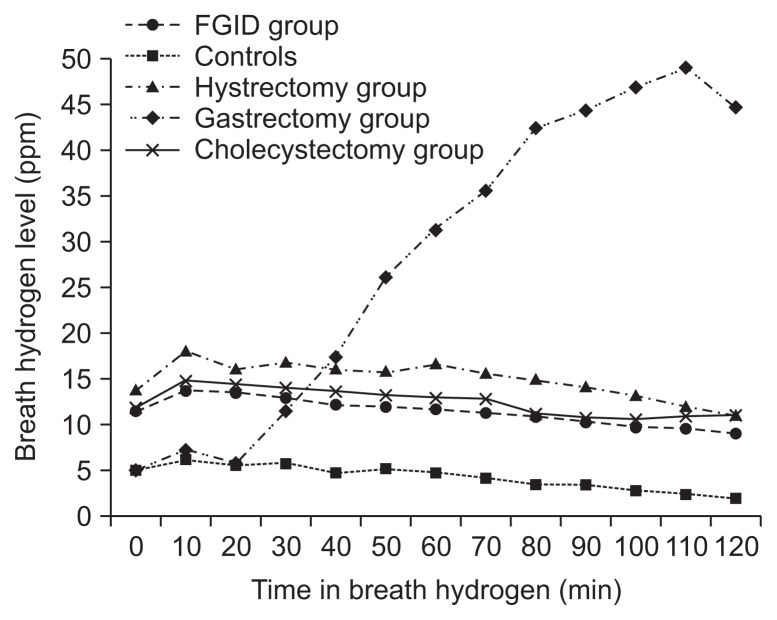
The gastrectomy group had a significant preference of male and high mean age compared to those in other surgical group or FGID patients (Table 2). In the flow of breath H2 profiles during the GBT (Fig. 2), the groups with hysterectomy, cholecystectomy, or FGID had similar trends except the group with gastrectomy. The breath H2 profiles in the gastrectomy group were significantly lower than those in hysterectomy or cholecystectomy groups at the time points of 0, 10, and 20 minutes during the GBT, whereas were significantly higher than those in other surgical groups and FGID patients at the time points of 50, 60, 70, 80, 90, 100, 110, and 120 minutes. The significant differences were not shown in the H2 profiles at all the time points among the patients with FGID, cholecystectomy, hysterectomy. The time point of peak value during the GBT in H2 concentration was 10 minutes in groups with FGID, cholecystectomy and hysterectomy, but was 100 minutes in group with gastrectomy.
Table 2.
Patient Characteristics according to the Types of Abdominal Surgery and the Patients with Functional Gastrointestinal Disorder
| Patients with types of surgery (n=171) | Patients with FGID (n=665) | p-value* | |||
|---|---|---|---|---|---|
|
| |||||
| Hysterectomy (n=50) | Gastrectomy (n=14) | Cholecystectomy (n=107) | |||
| Age, yr | 51.24±9.88 | 61.57±12.21 | 55.59±14.88 | 49.86±15.05 | <0.01 |
| T† | A | B | A,B | A | |
| Sex | |||||
| Male | 0 | 10 (71.4) | 50 (46.7) | 265 (39.8) | <0.01 |
| Female | 50 (100.0) | 4 (28.6) | 57 (53.3) | 400 (60.2) | |
| BMI, kg/m2 | 23.28±3.48 | 22.23±3.63 | 24.40±3.81 | 23.07±3.38 | 0.06 |
| Total H2, ppm | 193.79±210.67 | 367.25±353.34 | 161.57±157.49 | 148.23±213.35 | <0.01 |
| T† | A | B | A | A | |
| Total CH4, ppm | 86.22±107.55 | 102.32±66.03 | 62.42±60.78 | 80.12±124.99 | 0.40 |
| Positive GBT | 21 (42.0) | 10 (71.4) | 44 (41.1) | 212 (31.9) | <0.01 |
| H2 | 16 (32.0) | 9 (64.3) | 40 (37.4) | 150 (22.6) | <0.01 |
| CH4 | 1 (2.0) | 0 | 3 (2.8) | 30 (4.5) | |
| Mixed | 4 (8.0) | 1 (7.1) | 1 (0.9) | 32 (4.8) | |
Data are presented as the mean±SD or number (%).
FGID, functional gastrointestinal disorder; BMI, body mass index; H2, hydrogen; CH4, methane; GBT, glucose breath test.
Statistical significance among groups was assessed via one way analyses of variance or chi-square tests;
The same letters indicate nonsignificant differences between the groups based on Tukey’s multiple comparison test.
Fig. 2.
Flow chart of breath hydrogen (H2) profiles during glucose breath test.
FGID, functional gastrointestinal disorder.
The GBT+ in gastrectomy group was significantly higher than those with FGID patients, hysterectomy group, or those with cholecystectomy group (Table 2). The GBT+ had low tendency in FGID patients (31.9%, 212/665) compared to those with hysterectomy group (vs 42.0%, 21/50; p=0.14), or those with cholecystectomy group (vs 41.1%, 44/107; p=0.06) (Table 2). Among 50 patients with hysterectomy, 28 patients underwent laparoscopic hysterectomy, 18 patients underwent open hysterectomy, and four patients underwent transvaginal hysterectomy. The GBT+ was 39.3% (11/28) in patients with laparoscopic hysterectomy, 50% (9/18) in patients with open hysterectomy, and 25% (1/4) in patients with transvaginal hysterectomy. There was no significant difference in patients with hysterectomy according to the type of hysterectomy (p=0.597). There was no difference in GBT+ between hysterectomy and gastrectomy groups.
The gastrectomy group showed a high total H2 concentration (Table 2) compared to those in other surgical group or FGID patients. Considering the types of gastrectomy, the GBT+ in patients with B-I, B-II, and total gastrectomy were 75% (6/8), 75% (6/8), and 50% (1/2), respectively.
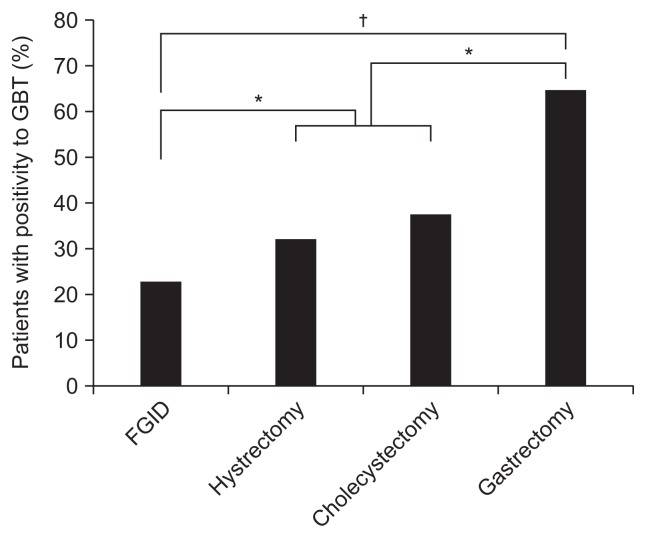
In the subtypes of the positivity to GBT, the GBT (H2)+ was significantly high in gastrectomy group compared to those in hysterectomy or cholecystectomy groups, and each of three surgical groups showed a higher GBT (H2)+ than those with FGID group, respectively (Fig. 3). No significant differences were shown in GBT (CH4)+ or GBT (mixed)+ among the enrolled groups.
Fig. 3.
Positivity to glucose breath test (GBT) via hydrogen (H2) gas measurement.
FGID, functional gastrointestinal disorder. *p<0.05; †p<0.01.
DISCUSSION
To date, this is the largest study on SIBO in patients with abdominal surgery. And we also analyzed the characteristics of breath profiles according to the types of surgery, as well as prevalence of SIBO. Our study indicates that SIBO in patients with abdominal surgery such as hysterectomy, cholecystectomy or gastrectomy is common. Especially the flow curve of H2 levels during the GBT in gastrectomy group was distinctly different from those in other groups (Fig. 2). Our finding suggests that SIBO might be considered in the abdominal surgical patients with intestinal symptoms, and the pathophysiology of SIBO could be different according to the types of abdominal surgery.
The overall GBT+ in patients with abdominal surgery was 43.9% (75/171). The GBT+ in subgroups with gastrectomy, hysterectomy, cholecystectomy and FGID were 71.4% (10/14), 42.0% (21/50), 41.1% (44/107), and 31.9% (212/665), which were consistent with the results in other studies,4,5 despite no reported data for patient with hysterectomy. Although a recent study surveyed the SIBO with abdominal surgery including female reproductive organs without significant results, small numbers of cases were only included without any analysis for breathe gas types.6 SIBO seems to be frequent in patients with abdominal surgery, or FGID. The proposed mechanisms are the impaired defense mechanisms against intestinal bacteria which include reduced gut motility, the loss of immunologic or antibacterial secretions, and the decrease in gastric acid.13,14 The adhesions after surgery could be associated with disordered intestinal peristalsis or intestinal stasis, and contribute to the bacterial overgrowth.
We used the GBT in this study to expect some advantages. The situation with abdominal surgery could be related with altered bowel transit, especially rapid bowel transit in patients with gastrectomy. The lactulose breath test (LBT) has been shown to be inappropriate for detection of SIBO from colonic bacteria in rapid bowel transit.15,16 And the substrate of lactulose itself induces rapid small intestinal transit.17 Moreover, the GBT has lower false positivity and more superior to diagnose SIBO than the LBT has.18,19 Also, we used a low dose of 50 g glucose to reduce false positivity of the GBT. Previous studies demonstrated that the small bowel reserve capacity for absorption of 100 g glucose in normal individuals and 50 g in jejunoileal bypass patients.19 The shapes in flow curve of breath H2 profiles in gastrectomy group were distinctly different from those in other groups including hysterectomy, gastrectomy and FGID which showed similar trends in Fig. 2. The curves of gastrectomy showed lower level of baseline H2, and the H2 levels gradually increased in the process of time for GBT, with highest peak level of H2 in late time of 100 minutes, whereas the trends of other groups marked steady H2 levels with peak values at early time of 10 minutes. Accordingly the H2 profiles in the gastrectomy group were significantly lower than those in hysterectomy or cholecystectomy groups at the time points of 0, 10, and 20 minutes intervals, whereas were significantly higher than those in other surgical groups and FGID patients at the time points between 50 and 120 minutes intervals. The reason why our study found the graphs of H2 in GBT were different between gastrectomy group and other groups is unclear, there is a possibility that the pathophysiology of SIBO is different according to the degree of presence in gastric acid secretion. The representative pathophysiology of SIBO was well known as the loss of intestinal motility and antibacterial secretions such as gastric acid.14 The gastrectomy has the main defect in gastric acid secretion,4 while FGID or cholecystectomy is associated with altered intestinal transit, in which the function of gastric acid is still alive.
A fasting breath H2 is considered a finding for altered intestinal transit. The gas produced by bacterial overgrowth acting on the previous normal meal takes longer to return to baseline, or it shows a delayed presentation with the status of impaired transit.5,13,20 Previous reports in patients with cholecystectomy or FGID showed similar curves of H2 and high fasting H2 levels.5,13 Although there were no reports of GBT in patients with hysterectomy, a literature demonstrated that hysterectomy could affect the slow intestinal transit.21 There is a possibility that hysterectomy is related with SIBO including high level of baseline H2 level during breath test.
Gastric acid might be the powerful defense agent against SIBO in the body. The status of gastrectomy status with the loss of gastric acid is well known for high risk of SIBO. Accordingly, in our study, gastrectomy group had the highest preference, while FGID group showed the lowest value in the GBT (H2)+, or total H2 concentration during GBT among the enrolled groups. There were no significant differences between cholecystectomy and hysterectomy group in GBT characters. Although cholecystectomy might affect the decrease in bile acid pool and the malabsorption of bile acid which could promote bacterial overgrowth,5,22,23 the strong antibacterial secretion of gastric acid may maintain defense mechanism.
The potential limitation of this study was retrospective design. However we used strict criteria based on the electric medical data which were collected consecutively in the common standard protocol for all patients who underwent GBT. Regardless of research purpose, the motility tests including the breath test need cautious standard approach, and survey of demographics, history of drug, or validated ROME questionnaire.5,13 Another limitation was a lack of complained intestinal symptoms in the study. We investigate ROME questionnaire for the suspected patients with FGID, but we used the different questionnaire of Sigstad dumping score in patients with gastrectomy. Therefore this study could not evaluate the clinical significance of intestinal symptoms with SIBO among the patients. However, the positivity to GBT was known to be well related with intestinal symptoms.24 Further study is needed to survey common consensus of the gastrointestinal questionnaire. The other limitation is the small numbers of enrolled surgical subjects, especially patients with gastrectomy.
In conclusion, the SIBO in patients with abdominal surgery was common. Moreover, the characteristics of an increase in H2 concentration during GBT were different according to the types of abdominal surgeries. Further studies will be needed to evaluate the role of SIBO in patients with abdominal surgery by investigating intestinal symptoms or the response to standardized treatment of SIBO (e.g., antibiotics or drug affecting intestinal motility).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Mechetina TA, Il’chenko AA, Lychkova AE. Rifaximin application in the overgrowth bacterial syndrome in the small intestine in patients after cholecystectomy. Eksp Klin Gastroenterol. 2011;(3):93–100. [PubMed] [Google Scholar]
- 2.Lapthorne S, Bines JE, Fouhy F, et al. Changes in the colon microbiota and intestinal cytokine gene expression following minimal intestinal surgery. World J Gastroenterol. 2015;21:4150–4158. doi: 10.3748/wjg.v21.i14.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdogan A, Rao SS, Gulley D, Jacobs C, Lee YY, Badger C. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil. 2015;27:481–489. doi: 10.1111/nmo.12516. [DOI] [PubMed] [Google Scholar]
- 4.Paik CN, Choi MG, Lim CH, et al. The role of small intestinal bacterial overgrowth in postgastrectomy patients. Neurogastroenterol Motil. 2011;23:e191–e196. doi: 10.1111/j.1365-2982.2011.01686.x. [DOI] [PubMed] [Google Scholar]
- 5.Sung HJ, Paik CN, Chung WC, Lee KM, Yang JM, Choi MG. Small intestinal bacterial overgrowth diagnosed by glucose hydrogen breath test in post-cholecystectomy patients. J Neurogastroenterol Motil. 2015;21:545–551. doi: 10.5056/jnm15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrone P, Sarkisyan G, Fernández M, et al. Small intestinal bacterial overgrowth in patients with lower gastrointestinal symptoms and a history of previous abdominal surgery. Arch Surg. 2011;146:444–447. doi: 10.1001/archsurg.2011.55. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Drossman DA. Rome III: the new criteria. Chin J Dig Dis. 2006;7:181–185. doi: 10.1111/j.1443-9573.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 9.Khoshbaten M, Melli MS, Fattahi MJ, Sharifi N, Mostafavi SA, Pourhoseingholi MA. Irritable bowel syndrome in women undergoing hysterectomy and tubular ligation. Gastroenterol Hepatol Bed Bench. 2011;4:138–141. [PMC free article] [PubMed] [Google Scholar]
- 10.Sperber AD, Morris CB, Greemberg L, et al. Development of abdominal pain and IBS following gynecological surgery: a prospective, controlled study. Gastroenterology. 2008;134:75–84. doi: 10.1053/j.gastro.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Park JM, Choi MG, Oh JH, et al. Cross-cultural validation of irritable bowel syndrome quality of life in Korea. Dig Dis Sci. 2006;51:1478–1484. doi: 10.1007/s10620-006-9084-6. [DOI] [PubMed] [Google Scholar]
- 12.Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–988. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim EJ, Paik CN, Chung WC, Lee KM, Yang JM, Choi MG. The characteristics of the positivity to the lactulose breath test in patients with abdominal bloating. Eur J Gastroenterol Hepatol. 2011;23:1144–1149. doi: 10.1097/MEG.0b013e32834b0e5c. [DOI] [PubMed] [Google Scholar]
- 14.Iivonen MK, Ahola TO, Matikainen MJ. Bacterial overgrowth, intestinal transit, and nutrition after total gastrectomy: comparison of a jejunal pouch with Rouxen-Y reconstruction in a prospective random study. Scand J Gastroenterol. 1998;33:63–70. doi: 10.1080/00365529850166220. [DOI] [PubMed] [Google Scholar]
- 15.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–1570. doi: 10.1111/j.1572-0241.2005.40795.x. [DOI] [PubMed] [Google Scholar]
- 16.Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795–1803. [PubMed] [Google Scholar]
- 17.Bond JH, Jr, Levitt MD, Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975;85:546–555. [PubMed] [Google Scholar]
- 18.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellin JH, Hart R. Glucose malabsorption associated with rapid intestinal transit. Am J Gastroenterol. 1992;87:584–589. [PubMed] [Google Scholar]
- 20.Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113–1126. doi: 10.1111/j.1572-0241.2002.05664.x. [DOI] [PubMed] [Google Scholar]
- 21.Possover M, Schneider A. Slow-transit constipation after radical hysterectomy type III. Surg Endosc. 2002;16:847–850. doi: 10.1007/s00464-001-9082-x. [DOI] [PubMed] [Google Scholar]
- 22.Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scand J Gastroenterol. 2010;45:645–664. doi: 10.3109/00365521003702734. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo-Zúñiga V, Bartolí R, Planas R, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551–557. doi: 10.1053/jhep.2003.50116. [DOI] [PubMed] [Google Scholar]
- 24.Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol. 2011;26(Suppl 3):135–138. doi: 10.1111/j.1440-1746.2011.06654.x. [DOI] [PubMed] [Google Scholar]