Abstract
Hyperin is a flavonoid compound derived from Ericaceae, Guttifera, and Celastraceae that has been shown to have various biological effects, such as anti-inflammatory and anti-oxidant effects. However, there is no evidence to show the protective effects of hyperin on lipopolysaccharide (LPS)-induced acute kidney injury (AKI). Therefore, we investigated the protective effects and mechanism of hyperin on LPS-induced AKI in mice. The levels of TNF-α, IL-6, and IL-1β were tested by ELISA. The effects of hyperin on blood urea nitrogen (BUN) and serum creatinine were also detected. In addition, the expression of TLR4, NF-κB, and NLRP3 were detected by western blot analysis. The results showed that hyperin significantly inhibited LPS-induced TNF-α, IL-6, and IL-1β production. The levels of BUN and creatinine were also suppressed by hyperin. Furthermore, LPS-induced TLR4 expression and NF-κB activation were also inhibited by hyperin. In addition, treatment of hyperin dose-dependently inhibited LPS-induced NLRP3 signaling pathway. In conclusion, the results showed that hyperin inhibited LPS-induced inflammatory response by inhibiting TLR4 and NLRP3 signaling pathways. Hyperin has potential application prospects in the treatment of sepsis-induced AKI.
Keywords: hyperin, LPS, kidney injury, TLR4, NLRP3
INTRODUCTION
Acute kidney injury (AKI), as a serious disease, suffered about one in five patients in emergency cases [1]. In hospitalized patients, AKI becomes a common and dangerous factor for death progressively [2, 3]. AKI can be caused by variety factors, such as pharmacologic toxins and sepsis [4, 5]. LPS, the outer membrane component of gram-negative bacteria, has been identified as the major factor that leads to AKI [6]. In the mice model of LPS-induced AKI, LPS significantly induces the release of inflammatory cytokines which promote kidney disease [7]. Studies showed that inflammatory cytokines TNF-α, IL-6,and IL-1β played critical roles in the pathologicprocess of kidney injury [8]. And inhibition of these inflammatory cytokines could attenuate the injury of kidney tissues. Thus, early anti-inflammatory therapy can improve renal function.
Hyperin, a flavonoid compound found in Ericaceae, Guttifera, and Celastraceae, has been reported to have anti-inflammatory effects [9]. Hyperin has been reported to inhibit LPS-induced nitrite production in rat peritoneal macrophages [10]. Hyperin also inhibited LPS-induced acute liver injury in mice [11]. Furthermore, hyperin has been found to protect the heart against ischemia and reperfusion lesions [12]. However, whether hyperin has protective effects against LPS-induced AKI remains unclear. Therefore, in the present study, we investigated the protective effects and mechanism of hyperin on LPS-induced AKI in mice.
RESULTS
Effects of hyperin on LPS-induced kidney histopathologic changes
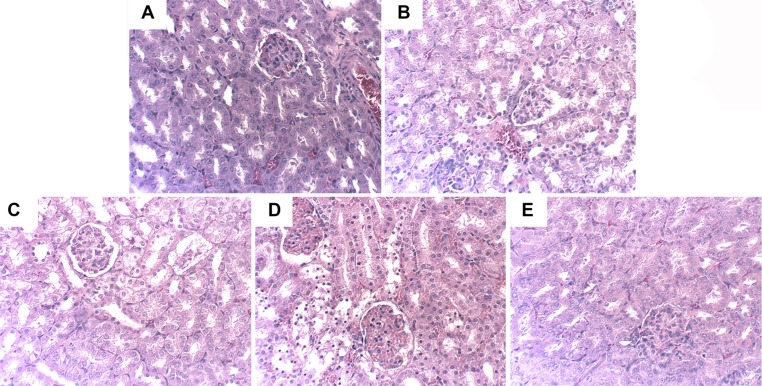
To investigate the protective effects of hyperin, we applied H&E staining to detect the histological changes in renal tissues comparing normal tissues to injured tissues. As shown in Figure 1A, the renal tissues exhibited normal morphology in control group. The renal tissues of LPS group showed severe injury on renal tissues, including tubular cells sloughing, loss of brush, and apoptosis of nephron (Figure 1B). However, the injury was significantly ameliorated by hyperin (Figure 1C, 1D, 1E).
Figure 1. Effects of hyperin on histopathological changes in kidney tissues in LPS-induced AKI mice.
Hhyperin (25, 50, 100 mg/kg) were given intraperitoneally (i.p.) 1 h after LPS treatment. 24 h after LPS challenge, Kidney tissues from each experimental group were processed for histological evaluation. Representative histological changes of kidney obtained from mice of different groups. (A) Control group, (B) LPS group, (C) LPS+ hyperin (25 mg/kg) group, (D) LPS+ hyperin (50 mg/kg) group, (E) LPS + hyperin (100 mg/kg) group (Hematoxylin and eosin staining, magnification 200×).
Hyperin relieves the dysfunction of kidney function of AKI
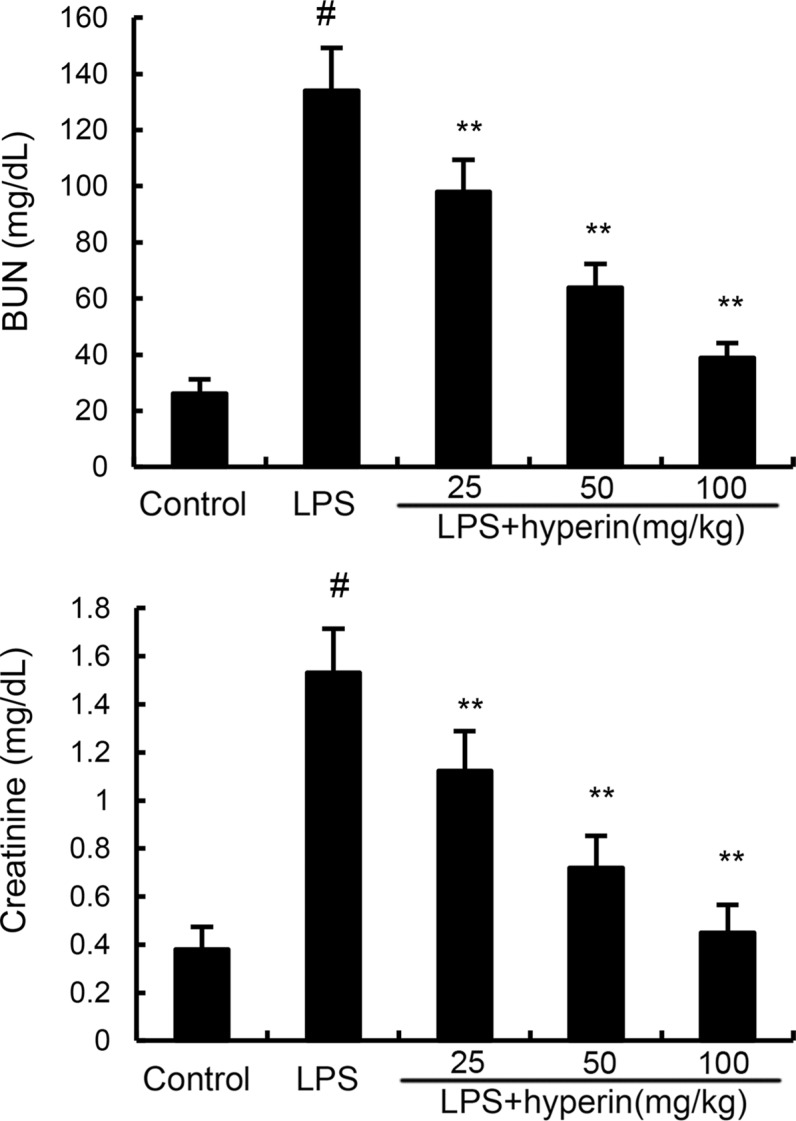
The levels of BUN and creatinine were measured to assess renal function. As shown in Figure 2, compared with the control group, BUN and creatinine levels were found to be dramatically increased in the LPS group. However, the levels of BUN and creatinine induced by LPS were dose-dependently inhibited by hyperin (25, 50, 100 mg/kg).
Figure 2. Effects of hyperin on BUN and creatinine levels in serum.
The values presented are the mean ± SEM (n = 12 in each group). #p < 0.01 vs. control group, *p < 0.05, **p < 0.01 vs. LPS group.
Hyperin inhibits the expression levels of cytokines
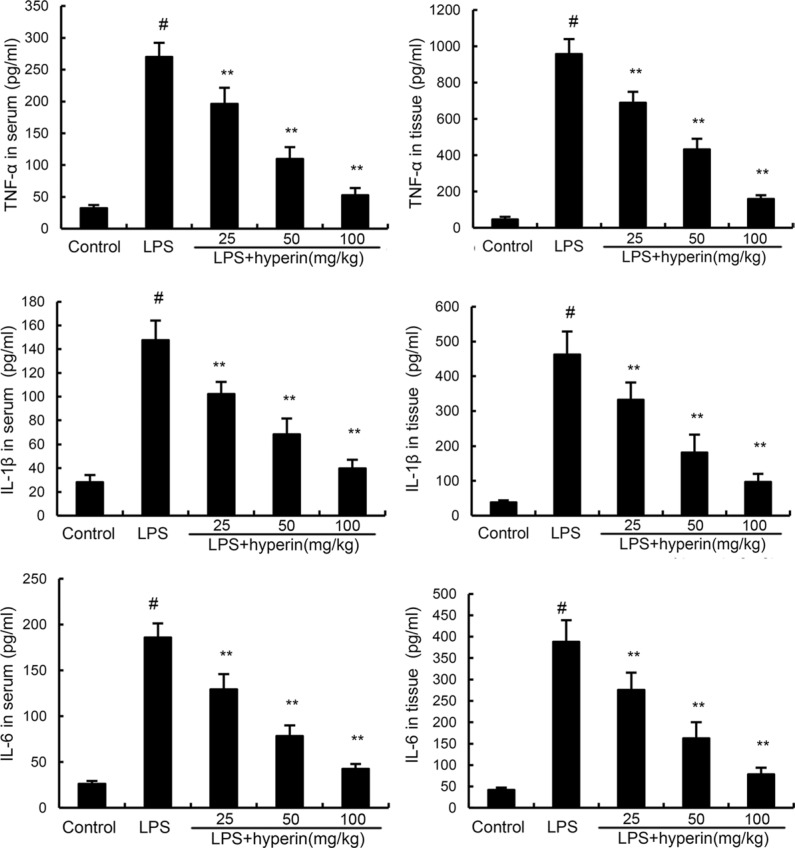
To investigate the anti-inflammatory effects of hyperin, the levels of inflammatory cytokines TNF-α, IL-6, and IL-1β production were detected by ELISA. As shown in Figure 3, compared with the control group, the levels of TNF-α, IL-6, and IL-1β were found to be dramatically increased in the LPS group. However, treatment of hyperin significantly inhibited LPS-induced TNF-α, IL-6, and IL-1β production.
Figure 3. Effects of hyperin on LPS-induced TNF-α, IL-6 and IL-1β in serum and kidney tissues.
The values presented are mean ± SEM (n = 12 in each group). #p < 0.01 vs. control group, *p < 0.05, **p < 0.01 vs. LPS group.
Hyperin inhibits LPS-induced TLR4 expression and NF-κB activation
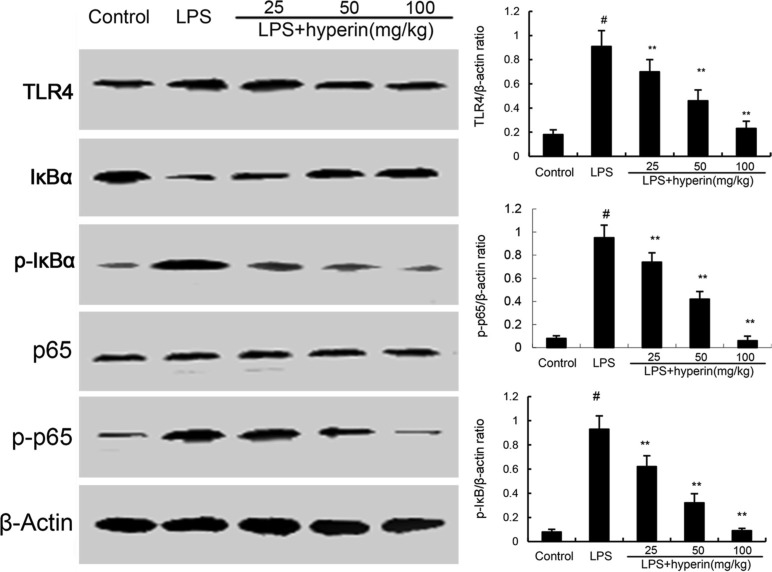
TLR4 plays an important role in LPS-induced acute kidney injury. To investigate the anti-inflammatory mechanism of hyperin, we investigated the effects of hyperin on TLR4 signaling pathway. As shown in Figure 4, LPS challenge significantly up-regulated the expression of TLR4 and activated NF-κB. However, hyperin significantly inhibited LPS-induced TLR4 expression. Furthermore, treatment of hyperin dose-dependently inhibited phosphorylation of NF-κB and IκBα.
Figure 4. Hyperin inhibits LPS-induced TLR4 expression and NF-κB activation.
The values presented are the means ± SEM (n = 12 in each group). #p < 0.01 vs. control group, *p < 0.05 and **p < 0.01 vs. LPS group.
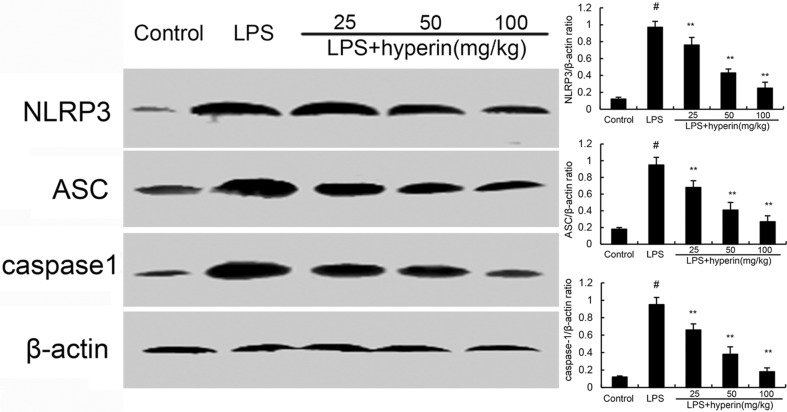
Hyperin inhibits LPS-induced NLRP3 signaling pathway
To further investigate the anti-inflammatory mechanism of hyperin, we examined the effects of hyperin on NLRP3 signaling pathway. As shown in Figure 5, compared with the control group, the expression of NLRP3, ASC, and caspase-1 were found to be dramatically increased in the LPS group. However, hyperin significantly suppressed LPS-induced NLRP3, ASC, and caspase-1 expression.
Figure 5. Hyperin inhibits LPS-induced NLRP3 activation.
The values presented are the means ± SEM (n = 12 in each group). #p < 0.01 vs. control group, *p < 0.05 and **p < 0.01 vs. LPS group.
DISCUSSION
In the present study, we evaluated the protective effects of hyperin on LPS-induced acute kidney injury in mice. The results showed that hyperin inhibited LPS-induced AKI by suppressing the levels of BUN and creatinine, as well as the production of TNF-α, IL-6, and IL-1β. Hyperin protected against LPS-induced AKI by inhibition of TLR4 and NLRP3 signaling pathways.
In this study, an animal model of AKI was established by LPS in mice. In this model, LPS significantly up-regulates the production of inflammatory cytokines, such as TNF-α, IL-6, and IL-1β. These cytokines have been demonstrated to play critical roles in the pathogenesis of LPS-induced AKI [13]. Studies showed that TNF-α and IL-6 were closely related to the extensive tubular damage [8, 14]. IL-1β also plays an important role in the pathogenesis of kidney inflammation and tissue damage [15]. Previous studies showed that inhibition of these inflammatory cytokines could protect against LPS-induced AKI [16]. To investigate the anti-inflammatory effects of hyperin, the effects of hyperin on inflammatory cytokines production were detected in this study. The results showed that hyperin protected against LPS-induced AKI by inhibiting inflammatory cytokines production.
LPS acts via TLR4 signaling pathway, which subsequently induces NF-κB activation and release of inflammatory cytokines [17]. Previous studies showed that TLR4 played an important role in LPS-induced acute kidney injury [18, 19]. NF-κB is a transcriptional factor that regulates a variety of inflammatory gene expression [20]. Studies showed that NF-κB activation is closely associated with the development of kidney injury [21]. Activation of NF-κB has been observed in mice of LPS-induced AKI [22]. To investigate the anti-inflammatory mechanism of hyperin, the effects of hyperin on TLR4 signaling pathway was detected. The results showed that hyperin dose-dependently inhibited LPS-induced TLR4 expression and NF-κB activation. NLRP3, a multiprotein complex, has been reported to activate caspase-1, which leads to the secretion of IL-1β [23]. Studies showed that NLRP3 inflammasome promotes renal inflammation and NLRP3 inflammasome knockout mice are protected against kidney injury [24]. In this study, we observed that treatment of hyperin significantly inhibited NLRP3 activation.
In conclusion, the results of this study provided evidence that hyperin has protective effects against LPS-induced AKI. The mechanism may be through the inhibition of TLR4 and NLRP3 signaling pathways.
MATERIALS AND METHODS
Chemicals and reagents
Hyperin (purity > 98%) was purchased from Preferred (Chengdu, China). LPS (Escherichia coli 055:B5) and Dimethyl sulfoxide (DMSO) was provided from Sigma Chemical Co. (St.Louis, MO, USA). Mouse TNF-α, IL-6 and IL-1β ELISA kits were purchased from Abcam (Cambridge, UK). Mouse NLRP3, ASC, caspase-1, TLR4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). NF-κB p65, NF-κB p-p65, IκB, p-IκB, and β-actin were provided from Cell Signaling Technology Inc. (Beverly, MA). All other chemicals were of reagent grade.
Animals and grouping
Sixty mice (BABL/c mice, 6–8 weeks) in this study were provided by center of Experimental Animals of Baiqiuen Medical College of Shandong University and all the experiments complied with Institutional Animal Care and Use Committee of Shandong University. For the model of AKI, mice were given with 15 mg/kg body weight of LPS in 50μl PBS via intraperitoneal injection. 24 h after LPS challenge, the mice were euthanized and the blood and kidney tissues were collected. Sixty mice were randomly separated into five groups, and each group contained 12 mice. Group 1 was treated as negative control, which received equal amount of PBS. Group 2 was served as LPS group, which received with 15 mg/kg body weight of LPS in 50μl PBS via intraperitoneal injection. Group 3–5 were served as hyperin (25, 50, 100 mg/kg) + LPS groups, which received 25, 50, 100mg/kg 1 h before LPS treatment.
H&E staining of kidney tissues
Kidney tissues were collected and fixed in PBS containing 10% formalin. Then the fixed tissues were trimmed in adapted cube and embedded in paraffin. The paraffin sections (slice thickness is 5 microns) were stained with H&E staining. Finally, the sections were examined by microscope.
Measurement of BUN and creatinine
After 24 hours of the treatment, the blood of each mouse was collected. In this study, AutoAnalyzer was employed to explore the levels of BUN and creatinine. The protocol was conformed to the instruction of the instrument.
ELISA assay
Kidney tissues from different groupwere homogenized with PBS on ice, and then centrifuged at 4°C for 40 min at 12,000 rpm. Subsequently, the supernatants were collected to determine the expression of TNF-α, IL-6, and IL-1β. The levels of inflammatory cytokines were measured by using ELISA kits (R&D) according to the manufacturer's instructions.
Western blot analysis
Kidney tissues were homogenized in protein extraction buffer and centrifuged at 10000 g for 10 min. The supernatants were measured using a BCA protein assay kit to obtain protein concentration. The proteins were separated on 10% SDS-PAGE and transferred to PVDF membranes. After being blocked with 5% fat-free dry milk, the membranes were incubated with the primary antibody at 4°C for 24 h. After washing three times with TBS/Tween20, the membranes were probed with HRP-conjugated secondary antibodies at room temperature for 1 h. Finally, the membranes were visualized with ECL-chemiluminescent kit (ECL-plus, Thermo Scientific, USA).
Statistical analysis
The data are presented as the mean ± SEM. Statistical evaluation of the results were analyzed using one way ANOVA (Dunnett's t-test) and two-tailed Student′s t-test. Statistical significance was accepted at P < 0.05 or P < 0.01.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Hoste EA, Kellum JA. Acute kidney injury: epidemiology and diagnostic criteria. Current opinion in critical care. 2006;12:531–537. doi: 10.1097/MCC.0b013e3280102af7. [DOI] [PubMed] [Google Scholar]
- 2.Kanagasundaram NS. Pathophysiology of ischaemic acute kidney injury. Annals of Clinical Biochemistry. 2015;52:193–205. doi: 10.1177/0004563214556820. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. American Journal of Physiology-Renal Physiology. 2010;298:F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters E, van Elsas A, Heemskerk S, Jonk L, van der Hoeven J, Arend J, Masereeuw R, Pickkers P. Alkaline phosphatase as a treatment of sepsis-associated acute kidney injury. Journal of Pharmacology and Experimental Therapeutics. 2013;344:2–7. doi: 10.1124/jpet.112.198226. [DOI] [PubMed] [Google Scholar]
- 5.Howse ML, Bell GM. Drugs and toxins that damage the kidney. Medicine. 2011;39:356–361. [Google Scholar]
- 6.Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW. Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. American Journal of Physiology-Renal Physiology. 2007;293:F245–F254. doi: 10.1152/ajprenal.00477.2006. [DOI] [PubMed] [Google Scholar]
- 7.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. The Journal of clinical investigation. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramesh G, Reeves WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. The Journal of clinical investigation. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Jung SH, Lee YS, Yamada M, Kim B-K, Ohuchi K, Shin KH. Antiinflammatory activity of hyperin fromAcanthopanax chiisanensis roots. Archives of pharmacal research. 2004;27:628–632. doi: 10.1007/BF02980162. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Park HS, Notsu Y, Ban HS, Kim YP, Ishihara K, Hirasawa N, Jung SH, Lee YS, Lim SS. Effects of hyperin, isoquercitrin and quercetin on lipopolysaccharide-induced nitrite production in rat peritoneal macrophages. Phytotherapy Research. 2008;22:1552–1556. doi: 10.1002/ptr.2529. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Yang Y, Li W-X, Wu X-Q, Li X-F, Ma T-T, Zhang L, Meng X-M, Li J. Hyperin attenuates inflammation by activating PPAR-γ in mice with acute liver injury (ALI) and LPS-induced RAW264. 7 cells. Int Immunopharmacol. 2015;29:440–447. doi: 10.1016/j.intimp.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Wang W-Q, Ma C-G, Xu S-Y. Protective effect of hyperin against myocardial ischemia and reperfusion injury. Zhongguo yao li xue bao. 1996;17:341–344. [PubMed] [Google Scholar]
- 13.Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. The Journal of Immunology. 2002;168:5817–5823. doi: 10.4049/jimmunol.168.11.5817. [DOI] [PubMed] [Google Scholar]
- 14.Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, Cuzzocrea S, Thiemermann C. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. Journal of Pharmacology and Experimental Therapeutics. 2005;312:1170–1178. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 15.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1β mediates leptin induction during inflammation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Chang A, Hack BK, Eadon MT, Alper SL, Cunningham PN. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney international. 2014;85:72–81. doi: 10.1038/ki.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. The Journal of Immunology. 2004;172:2629–2635. doi: 10.4049/jimmunol.172.4.2629. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. Journal of the American Society of Nephrology. 2008;19:923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. The Journal of clinical investigation. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-κB in renal inflammation. Journal of the American Society of Nephrology. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 22.Meyer-Schwesinger C, Dehde S, von Ruffer C, Gatzemeier S, Klug P, Wenzel UO, Stahl RA, Thaiss F, Meyer TN. Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-κB p65 signaling. American Journal of Physiology-Renal Physiology. 2009;296:F1088–F1099. doi: 10.1152/ajprenal.90746.2008. [DOI] [PubMed] [Google Scholar]
- 23.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature Reviews Immunology. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 24.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. Journal of the American Society of Nephrology. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]