Abstract
Marital status correlates with health. Our goal was to examine the impact of marital status on the survival outcomes of patients with colorectal neuroendocrine neoplasms (NENs). The Surveillance, Epidemiology and End Results program was used to identify 1,289 eligible patients diagnosed between 2004 and 2010 with colorectal NENs. Statistical analyses were performed using Chi-square, Kaplan–Meier, and Cox regression proportional hazards methods. Patients in the widowed group had the highest proportion of larger tumor (>2cm), and higher ratio of poor grade (Grade III and IV) and more tumors at advanced stage (P<0.05). The 5-year cause specific survival (CSS) was 76% in the married group, 51% in the widowed group, 73% in the single group, and 72% in the divorced/separated group, which manifest statistically significant difference in the univariate log-rank test and Cox regression model (P<0.05). Furthermore, marital status was an independent prognostic factor only in Distant stage (P<0.001). In conclusion, patients in widowed group were at greater risk of cancer specific mortality from colorectal NENs and social support may lead to improved outcomes for patients with NENs.
Keywords: colorectal neuroendocrine neoplasms, marital status, prognostic analysis
BACKGROUND
Neuroendocrine neoplasms (NENs) originated from neuroendocrine cells present throughout the body and are capable of producing a variety of biogenic amines. NENs of the large intestine account for 20% of all NENs and are most commonly found in the rectum [1]. The data from Surveillance, Epidemiology, and End Results (SEER) database indicated that the age-adjusted incidence of NENSs rose from 1.9 to 5.25 cases per 100,000 people between 1973 and 2004 [2]. Diagnosed incidence of NENs is predicted to continue rising at a faster rate than other malignant neoplasms [3]. Although with advances in surgical therapy and the introduction of new treatment strategies, survival outcomes of NENs remain unchanged in the past 30-year [4]. This may be due to late diagnosis and lack of effective prognostic biomarkers.
In a variety of malignancies, the effect of marital status is being increasingly recognized as a determinant of stage at diagnosis, as well as a determinant of the prognosis of treated cancer [5–11]. A study based on SEER database demonstrated that patients of unmarried are always accompanied with higher risk of advanced tumor stage, undertreatment, and cancer related death in top ten causes of cancer-related death [5]. However, to our knowledge, the correlation between marital status and survival of patients with NENs has not been previously studied. In the present study, we used data from the SEER database of patients diagnosed between 2004 and 2010 to investigate what aspects of marital status affect survival of patients with colorectal NENs in detail.
RESULTS
The characteristic of patients in SEER database
We identified 1,289 eligible patients during the 7-year study period, including 617 male and 672 female patients. Of these, 808 (62.7%) were married, 119 (9.2%) were widowed, and 226 (17.5%) had never been married. The 9 (0.7%) patients in separated subgroup and 127 (9.9%) in divorced subgroup were incorporated into the divorced/separated group in the present study. Patients in the widowed group had the highest proportion of female, more common of colon as primary site, more prevalence of elderly patients (>50 years), higher percentage of larger tumor size (>2cm), higher ratio of poor grade (Grade III and IV) and more tumors at advanced stage (Distant and Regional) (P<0.001). The percentage of surgery performed was comparable between the married and widowed groups (P=0.367). The characteristics of patients are summarized in Table 1.
Table 1. Baseline demographic and tumor characteristics of patients in SEER database.
| Characteristic | Married | Windowed | Single | Divorced/separated | Total | Chi-square(χ2) | P-value | |
|---|---|---|---|---|---|---|---|---|
| n=808 N (%) |
n=119 N (%) |
n=226 N (%) |
n=136 N (%) |
n=1289 N (%) |
||||
| Sex | 55.037 | <0.001 | ||||||
| Male | 438(54.20) | 26(21.8) | 106(46.90) | 47(34.60) | 617(47.90) | |||
| Female | 370(45.80) | 93(78.20) | 120(53.10) | 89(65.40) | 672(52.10) | |||
| Age | 89.456 | <0.001 | ||||||
| <=50 | 216(26.70) | 2(1.70) | 110(48.70) | 35(25.70) | 363(28.20) | |||
| >50 | 592(73.30) | 117(98.30) | 116(51.30) | 101(74.30) | 926(71.80) | |||
| Race | 44.221 | <0.001 | ||||||
| White | 606(76.70) | 97(81.50) | 160(71.40) | 95(70.40) | 958(75.60) | |||
| Black | 87(11.00) | 14(11.80) | 53(23.70) | 31(23.00) | 185(14.60) | |||
| Other | 97(12.3) | 8(6.70) | 11(4.90) | 9(6.70) | 125(9.90) | |||
| Primary site | 24.592 | <0.001 | ||||||
| Colon | 437(54.10) | 89(74.80) | 117(51.80) | 89(65.40) | 732(56.80) | |||
| Rectum | 371(45.90) | 30(25.20) | 109(48.20) | 47(34.60) | 557(43.20) | |||
| Grade | 55.811 | <0.001 | ||||||
| Grade I | 494(61.10) | 44(37.00) | 137(60.60) | 77(56.60) | 752(58.30) | |||
| Grade II | 121(15.00) | 13(10.90) | 36(15.90) | 14(10.30) | 184(14.30) | |||
| Grade III | 160(19.80) | 51(42.90) | 36(15.90) | 31(22.80) | 278(21.60) | |||
| Grade IV | 33(4.10%) | 11(9.20) | 17(7.50) | 14(10.30) | 75(5.80) | |||
| Tumor size | 26.463 | <0.001 | ||||||
| <=2cm | 438(54.20) | 38(31.90) | 126(55.80) | 58(42.60) | 660(51.20) | |||
| >2cm | 370(45.80) | 81(68.10) | 100(44.20) | 78(57.40) | 629(48.80) | |||
| Histological stage | 27.481 | <0.001 | ||||||
| Localized | 406(50.20) | 37(31.10) | 129(57.10) | 56(41.20) | 628(48.70) | |||
| Distant | 185(22.90) | 43(36.10) | 52(23.00) | 38(27.90) | 318(24.70) | |||
| Regional | 217(26.90) | 39(32.80) | 45(19.90) | 42(30.90) | 343(26.60) | |||
| Surgery | 3.165 | 0.367 | ||||||
| Not performed | 67(8.30) | 11(9.20) | 26(11.50) | 9(6.60) | 113(8.80) | |||
| Performed | 741(91.70) | 108(90.80) | 200(88.50) | 127(93.40) | 1176(91.20) | |||
| CSS status | 33.460 | <0.001 | ||||||
| Live | 640(79.21) | 66(55.46) | 178(78.76) | 105(77.21) | 989(76.73) | |||
| Dead | 168(20.79) | 53(44.53) | 48(21.24) | 31(22.79) | 300(23.23) | |||
Impact of marital status on CSS in NENs
Of all, 300 patients died of NENs and 69 (5.35%) patients who died of other reasons were censored for analysis. The overall 5-year CSS was 76% in the married group, 73% in the single group, 72% in the divorced/separated group, and 51% in the widowed group (P<0.05). Patients in the widowed group always had the lowest survival rate when compared with patients in other groups. Patients in widowed group had a 24% reduction in 5-year CSS compared with married patients (P<0.001), and the difference between the never married and divorced/separated group was not of significance (Table 2) (Figure 1). Additionally, in univariate analysis, elderly patients (P<0.001), primary site at colon (P<0.001), White race (P=0.004), poor or undifferentiated tumor grade(Grade III/IV) (P< 0.001), tumor size above 2cm (P<0.001), having distant metastasis or lymph node metastasis(P<0.001) and no surgery performed (P<0.001) were negatively correlated with survival outcome, while there is no significant difference in sex (P=0.328)(Table 2). Factors with significance in univariate analysis were incorporated into multivariate Cox regression analysis and six variables were verified as independent predictors, including Grade [grade II, hazard ratio (HR) 3.026, 95% confidence interval (CI) 1.786-5.127; grade III, HR 9.761, 95% CI 6.5-14.658; grade IV, HR 13.062, 95% CI 8.204-20.797), tumor size (tumor size above 2cm, HR 1.84, 95% CI 1.17-2.892), histological stage (distant, HR 12.954, 95% CI 7.07-23.737; regional, HR 3.496, 95% CI 1.88-6.5), primary site (rectum, HR 0.661, 95% CI 0.469-0.931), surgery (surgery performed, HR 0.637, 95% CI 0.452-0.896), and marital status (widowed, HR 1.734, 95%CI 1.259-2.39; single HR 1.365, 95% CI 0.972-1.917; divorced/separated, HR 0.694, 95% CI 0.467-1.032) (Table 3).
Table 2. Univariate analysis of cause specific survival in patients with colorectal neuroendocrine neoplasms.
| Variable | N | 5-year CCS (%) | Log rank test (χ2) |
P-value |
|---|---|---|---|---|
| Sex | 0.955 | 0.328 | ||
| Male | 617 | 72 | ||
| Female | 672 | 74 | ||
| Age | 23.294 | <0.001 | ||
| <=50 | 363 | 82 | ||
| >50 | 926 | 70 | ||
| Primary site | 120.706 | <0.001 | ||
| Colon | 732 | 60 | ||
| Rectum | 557 | 90 | ||
| Race | 10.918 | 0.004 | ||
| White | 958 | 70 | ||
| Black | 185 | 82 | ||
| Other | 125 | 80 | ||
| Marital status | 43.411 | <0.001 | ||
| Married | 808 | 76 | ||
| Windowed | 119 | 51 | ||
| Single | 226 | 73 | ||
| Divorced/separated | 136 | 72 | ||
| Grade | 768.64 | <0.001 | ||
| Grade I | 752 | 94 | ||
| Grade II | 184 | 82 | ||
| Grade III | 278 | 28 | ||
| Grade IV | 75 | 14 | ||
| Tumor size | 297.924 | <0.001 | ||
| <=2cm | 660 | 95 | <0.001 | |
| >2cm | 629 | 51 | ||
| Histological stage | 652.227 | <0.001 | ||
| Localized | 628 | 98 | ||
| Distant | 318 | 23 | ||
| Regional | 343 | 75 | ||
| Surgery | 37.949 | <0.001 | ||
| Not performed | 113 | 52 | ||
| Performed | 1176 | 75 |
Figure 1. Survival curves of patients with colorectal neuroendocrine tumor according to marital status.
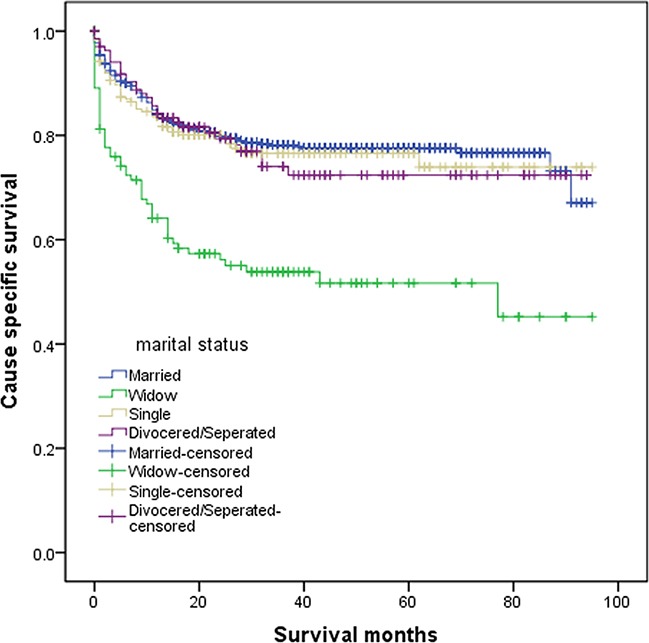
The overall 5-year CSS was 76% in the married group, 51% in the widowed group, 73% in the single group, and 72% in the divorced/separated group, the difference of which was significantly different according to the univariate log-rank test (P<0.001).
Table 3. Multivariate analysis of cause specific survival in patients with colorectal neuroendocrine neoplasms.
| Variables | Hazard Ratio | 95%CI | P-value |
|---|---|---|---|
| Race | 0.786 | ||
| White | 1 | reference | |
| Black | 1.085 | 0.732-1.609 | 0.684 |
| Other | 1.144 | 0.733-1.787 | 0.553 |
| Grade | <0.001 | ||
| Grade I | 1 | reference | |
| Grade II | 3.026 | 1.786-5.127 | <0.001 |
| Grade III | 9.761 | 6.5-14.658 | <0.001 |
| Grade IV | 13.062 | 8.204-20.797 | <0.001 |
| Marital status | <0.001 | ||
| Married | 1 | reference | |
| Widowed | 1.734 | 1.259-2.39 | 0.001 |
| Single | 1.365 | 0.972-1.917 | 0.072 |
| Divorced/separated | 0.694 | 0.467-1.032 | 0.071 |
| Age | 0.596 | ||
| <=50 | 1 | reference | |
| >50 | 1.09 | 0.792-1.502 | 0.596 |
| Tumor size | 0.008 | ||
| <=2cm | 1 | reference | |
| >2cm | 1.84 | 1.17-2.892 | 0.008 |
| Histological stage | <0.001 | ||
| Localized | 1 | reference | <0.001 |
| Distant | 12.954 | 7.07-23.737 | <0.001 |
| Regional | 3.496 | 1.88-6.5 | <0.001 |
| Primary site | 0.018 | ||
| Colon | 1 | reference | |
| Rectum | 0.661 | 0.469-0.931 | 0.018 |
| Surgery | 0.01 | ||
| Not performed | 1 | reference | |
| Performed | 0.637 | 0.452-0.896 | 0.01 |
The C-index of the Cox regression model is 0.869 (95%CI: 0.851-0.886) and the calibration plot indicated perfect calibration (Figure 2).
Figure 2. Calibration plot for Table 3.
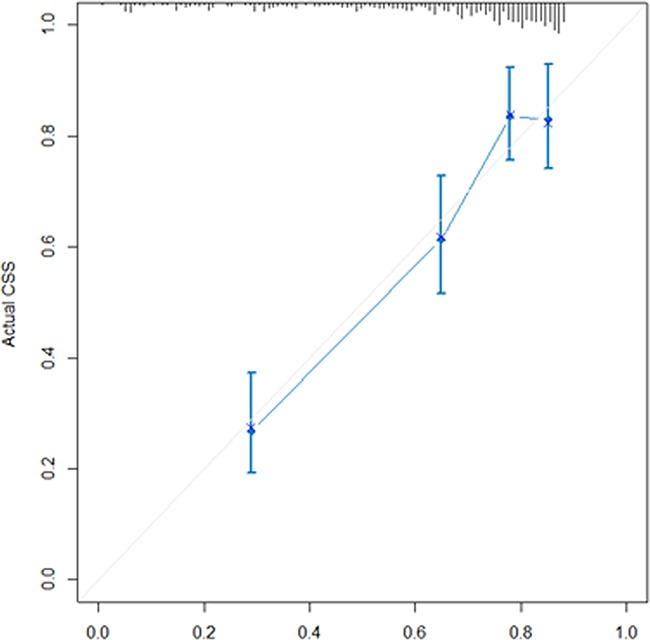
The reference line is 45 degree and indicates perfect calibration.
DISCUSSION
The conclusion in the present study demonstrates the first available research of the impact of marital status on survival of patients with colorectal NENs. The impact of marital status is independent of age, grade, histological stage, tumor size, primary site and surgical treatment. These results suggest that patients in unmarried group are always accompanied with metastatic cancer and high incidence of death from colorectal NENs. Besides, widowed patients always had worse survival than other patients, which was verified as an independent predictor in NENs.
An important aspect of the present study is making detailed analysis of unmarried patients and identifying widowed patients as a subgroup who have the poorest survival outcomes. Although the impact of marriage has been studied in many cancers, fewer of them pay attention to the heterogeneity of unmarried patients. In the present study, we found unmarried patients with NENs was a heterogeneous group. Single and divorced/separated patients have similar 5-year CSS with married patients, and only widowed patients had significantly worse survival outcome than patients in other groups.
Two hypotheses have always been used to explain the relationship between health and marital status. First, unmarried patients are often diagnosed with more advanced tumor stage, and they are also more likely to receive insufficient treatment. In the present study, widowed patients have highest proportion of distant histological stage while with lowest percentage of localized disease. In addition, widowed patients are more prevalence of poor tumor differentiation (Grade III/IV). Conversely, widowed patients are more common of White in race. Previous studies showed that patients in White race always have good insurance status and relatively better survival outcomes than others [12–14], which suggests that marital status may play a critical role in survival outcomes of patients with colorectal NENs.
The other hypothesis for the impact of marital status on CSS considered marriage as a means of social support. Cancer diagnosis and treatment induce acute and chronic stress, which may cause poor adherence to medical interventions [15]. Meta-analyses confirmed that patients with depression have an increased death rates with range from 19% to 39% [16, 17]. A behavioral research suggests that cognitive, behavioral and social factors are of great importance in promoting the customization to active treatment and throughout cancer survivorship, which forms the fundamentals for the application of many psychological and social supportive interventions in patients with cancer [18]. The loss of social support or the inability to cope with stress in the widowed group seems very apparent, which may lead to excess mortality [11].
There are several limitations of our present study. First, we did not consider changes of marital status that may occurred during follow-up, which may influence long time survival rate. Thus, our findings may underestimate the protective effect of marriage on colorectal NENs outcomes [19]. Second, SEER database lacks information of education, income status, life style, insurance status, socioeconomic status and quality of marriage, which might have impacts on the survival outcomes and confound the multivariate Cox regression analysis. For example, marital distress has long term immune consequences and enhances the risk of a variety of health problems [20]. Third, SEER database lacks the information of adjuvant therapy. Fourth, it is likely that some individuals cohabitated without marriage and were categorized as unmarried in SEER database. Actually, patients with such partnership are likely to have better survival outcomes than the unmarried patients, which may bias our results [5].
In conclusion, our study found that widowed patients are associated with higher risk for diagnosis with a later stage of colorectal NENs and for worse cancer related survival outcomes than others. This finding indicated social support may greatly amend traditional therapy and may improve outcomes in widowed patients.
MATERIALS AND METHODS
Patient selection in the SEER database
The SEER database is a population-based cancer registry across several disparate geographic regions. The SEER research data includes cancer incidence as well as age, sex, race/ethnicity, year of diagnosis, marital status, Tumor-Node-Metastasis (TNM) stage, and tumor grade. It contains no identifiers and is widely used for studies of the relationship between marital status and survival outcomes of patients with cancer [5, 11, 21]. The exact dataset we used for this analysis was SEER Program (www.seer.cancer.gov) Research Data (2004–2010)
The National Cancer Institute's SEER*Stat software (Surveillance Research Program, National Cancer Institute SEER*Stat software, www.seer.cancer.gov/seerstat)(Version 8.1.2) was used to identify patients whose pathological diagnosis was colorectal NENs between 2004 and 2010. We identified colorectal neoplasms cases using ICD-O-3 histology codes for NENs (8240, 8241, 8246, 8249) and selected patients with primary site labeled as C18.0-C19.9 (colon) and C20.9 (rectum). Race was categorized into White, Black, and other (American Indian, AK Native, Asian and et al) as provided by the SEER data. Patients were excluded if age at diagnosis was less than 18 years, or if they had undefined marital status or tumor grade, of if they had missed therapy information (surgery performed or not), unknown cause of death or unknown survival months.
Ethics statement
This study was based on the free public SEER database (www.seer.cancer.gov/seerstat). We have got permission to access the research data file in SEER program and the reference number was 11756-Nov2013. The study was approved by the Review Board of The Second Medical School of Yangzhou University, Yangzhou, China.
Statistical analysis
Detailed information regarding patient (age, sex, race, marital status) -, tumor- related variables (tumor size, histological stage, primary site, and tumor grade), therapy information (surgery performed or not), and survival information [SEER cause cancer specific survival (CSS)] was retrieved from SEER database. The patients were divided into two age groups: ≤50 years (young) and >50 years (old). Marital status is coded as married, divorced, widowed, separated, and never married at the time of diagnosis. Individuals in the separated and divorced group were clustered together as the divorced/separated group in the present study. According to the SEER staging system, tumors that remain in situ or confined to the organ are regarded as localized, while those that locally invade or metastasize to regional lymph nodes are considered to be regional, and those that travel to distant organs are categorized as distant. Furthermore, there is no accepted uniform grading system for malignant NENs to now. Pathologists in the United States before 2010 typically used the term“carcinoid tumor” to denote well-differentiated NENs (GI) and the term “atypical carcinoid”to describe a moderately differentiated carcinoid (GII). Poorly differentiated tumors are generally classified as GIII tumors, and undifferentiated anaplastic tumors are classified as GIV tumors [22].
Chi-square tests were used to examine the association between marital status and other variables. The Kaplan-Meier method was used to estimate survival curves. Differences between the curves were analyzed by log-rank test. Multivariable Cox regression models were built for analysis of risk factors for survival outcomes. Exact 95% CIs for proportions were calculated. The primary endpoint of this study was CSS, which was calculated from the date of diagnosis to the date of cancer specific death. Deaths attributed to colorectal NENs were treated as events and deaths from other causes were treated as censored observations. The 5-year CSS rate was estimated from Kaplan-Meier curves. All of the statistical analyses were done using the statistical software package SPSS for Windows, version 17 (Chicago: SPSS Inc, USA). Statistical significance was set at two-sided (P < 0.05).
Acknowledgments
The authors would like to thank SEER for open access to the database.
Footnotes
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to declare.
Author contributions
QGL, LCX, JDT, and ZW conceived of and designed the study. YW and FH performed the analyses. QGL, LCX, JDT and ZW prepared all figures and tables. QGL and LCX wrote the main manuscript. All authors reviewed the manuscript.
REFERENCES
- 1.Starzynska T, Deptala A, Krolicki L, Kunikowska J, Londzin-Olesik M, Nasierowska-Guttmejer A, Ruchala M, Strzelczyk J, Szawlowski A, Zgliczynski W, Kos-Kudla B, Consensus C. Colorectal neuroendocrine neoplasms - management guidelines (recommended by the Polish Network of Neuroendocrine Tumours) Endokrynol Pol. 2013;64:494–504. doi: 10.5603/EP.2013.0032. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Modlin IM, Moss SF, Chung DC, Jensen RT, Snyderwine E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst. 2008;100:1282–1289. doi: 10.1093/jnci/djn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 5.Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Hu JC, Nguyen PL. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denberg TD, Beaty BL, Kim FJ, Steiner JF. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer. 2005;103:1819–1825. doi: 10.1002/cncr.20982. [DOI] [PubMed] [Google Scholar]
- 7.Torssander J, Erikson R. Marital partner and mortality: the effects of the social positions of both spouses. J Epidemiol Community Health. 2009;63:992–998. doi: 10.1136/jech.2009.089623. [DOI] [PubMed] [Google Scholar]
- 8.Nelles JL, Joseph SA, Konety BR. The impact of marriage on bladder cancer mortality. Urol Oncol. 2009;27:263–267. doi: 10.1016/j.urolonc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Johansen C, Schou G, Soll-Johanning H, Mellemgaard A, Lynge E. Influence of marital status on survival from colon and rectal cancer in Denmark. Br J Cancer. 1996;74:985–988. doi: 10.1038/bjc.1996.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Wilson SE, Stewart DB, Hollenbeak CS. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011;35:417–422. doi: 10.1016/j.canep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Gan L, Liang L, Li X, Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6:7339–7347. doi: 10.18632/oncotarget.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitzkorski JR, Willis AI, Nick D, Zhu F, Farma JM, Sigurdson ER. Association of race and socioeconomic status and outcomes of patients with rectal cancer. Ann Surg Oncol. 2013;20:1142–1147. doi: 10.1245/s10434-012-2837-x. [DOI] [PubMed] [Google Scholar]
- 13.Kong AL, Yen TW, Pezzin LE, Miao H, Sparapani RA, Laud PW, Nattinger AB. Socioeconomic and racial differences in treatment for breast cancer at a low-volume hospital. Ann Surg Oncol. 2011;18:3220–3227. doi: 10.1245/s10434-011-2001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Zhuo C, Liang L, Zheng H, Li D, Cai S. Lymph node count after preoperative radiotherapy is an independently prognostic factor for pathologically lymph node-negative patients with rectal cancer. Medicine (Baltimore) 2015;94:e395. doi: 10.1097/MD.0000000000000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 16.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 17.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav Immun. 2013;30:S88–98. doi: 10.1016/j.bbi.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 20.Jaremka LM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology. 2013;38:2713–2719. doi: 10.1016/j.psyneuen.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804. doi: 10.1186/1471-2458-11-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]


