Abstract
Objectives
The objective of this prospective comparative cohort study was to establish the effectiveness and safety of Erbium:YAG (Er:YAG) laser treatment for genitourinary syndrome of menopause and to compare it with an established topical estriol treatment.
Methods
Fifty patients with genitourinary syndrome of menopause were divided into two groups. The estriol group received a treatment of 0.5 mg estriol ovules for 8 weeks and the laser group was first treated for 2 weeks with 0.5 mg estriol ovules 3 times per week to hydrate the mucosa and then received three sessions with 2,940 nm Er:YAG laser in non‐ablative mode. Biopsies were taken before and at 1, 3, 6, and 12 months post‐treatment. Maturation index, maturation value and pH where recorded up to 12‐months post‐treatment, while the VAS analysis of symptoms was recorded up to 18 months post‐treatment.
Results
Statistically significant (P < 0.05), reduction of all assessed symptoms was observed in the laser group at all follow‐ups up to 18 months post‐treatment. Significant improvement in maturation value and a decrease of pH in the laser group was detected up to 12 months after treatment. The improvement in all endpoints was more pronounced and longer lasting in the laser group. Histological examination showed changes in the tropism of the vaginal mucosa and also angiogenesis, congestion, and restructuring of the lamina propria in the laser group. Side effects were minimal and of transient nature in both groups, affecting 4% of patients in the laser group and 12% of patients in the estriol group.
Conclusions
Our results show that Er:YAG laser treatment successfully relieves symptoms of genitourinary syndrome of menopause and that the results are more pronounced and longer lasting compared to topical estriol treatment. Lasers Surg. Med. 49:160–168, 2017. © 2016 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: Erbium:YAG laser, genitourinary syndrome of menopause, topical estriol, vaginal atrophy
INTRODUCTION
Vulvovaginal atrophy can be progressive and unlikely to resolve without intervention. It can have a significant effect on woman's sexual health and quality of life (QOL) 1. It is estimated that 10–45 percent of postmenopausal women have symptoms of vulvovaginal atrophy 1, 2. Despite the prevalence of symptoms, only 20–25 percent of symptomatic women seek medical attention 3.
In 2014, genitourinary syndrome of menopause (GSM) has been accepted as a consensus new terminology for vulvovaginal atrophy and defined as a collection of symptoms and signs associated with a decrease in estrogen and other sex steroids syndrome, including but not limited to genital symptoms of dryness, burning, and irritation; sexual symptoms of lack of lubrication, discomfort or pain, and impaired function; and urinary symptoms of urgency, dysuria, and recurrent urinary tract infections 4.
Before menopause, in the presence of endogenous estrogen levels, the vagina is characterized by a thickened rugated vaginal surface, increased vaginal blood flow, and vaginal lubrication. Estrogen is a dominant regulator of vaginal physiology, its effects including increased blood flow, improved epithelial thickness, reduced pH, and increased secretions. GSM is most commonly associated with the diminished estrogen levels that accompany spontaneous or induced menopause and aging 1. Estrogen plays an essential role in maintaining the elasticity and health of genital tissues. Declining levels of estrogen during menopause result with increased tissue fragility and higher risk of vaginal and urinary infections, irritation, dryness, urogenital pain, and vaginal tissue trauma. GSM is characterized by changes in the quantity and quality of vaginal secretions, loss of collagen, adipose‐ and water‐retaining ability. The vaginal walls become thinner, less elastic, and pale with loss of rugation; the vaginal surface becomes friable with petechiae, ulcerations, and bleeding often occurring after minimal trauma 1, 5. The prevalence of urogenital symptoms in postmenopausal women includes vaginal dryness in 29% of patients, burning or irritation in 21.3%, nocturia/pollakiuria in 16.4%, urinary incontinence in 15.2%, dyspareunia in 14%, chronic leukorrhea in 13.5%, and dysuria in 7.2% of patients 5. The goals of GSM management are to alleviate symptoms and to reverse atrophic anatomic changes. Treatment with exogenous estrogen preparations delivered either systemically or locally is the therapeutic standard for prescription therapies. Even though systemic estrogen therapy (e.g., oral, transdermal) is an effective treatment, there are some drawbacks to be considered. Women with a previous medical history of hormone‐dependent cancer must be evaluated and informed in relation to the risks/benefits 6. There is a high rate of patient abandonment of hormone replacement therapy (HRT) and not all patients wish to receive HRT; many have valid concerns to its long‐term use. There is also speculation that recurrences of certain hormone‐dependent cancers are dose dependent. In the study by Von Schoultz 7, researchers established that different doses of estrogen and progesterone and treatment regimens for menopausal hormone therapy may be associated with the recurrence of breast cancer. Local estrogen preparations in the form of tablets, rings, or creams are often prescribed as they are perceived to have a low systemic absorptionand have been shown to result in significant symptomatic benefit 1, 8.
However, there are some limitations to these therapies, such as the fact that the beneficial effect is evident only for the time of the therapy and in general its effect stops when the treatment is discontinued, and also that these changes affect mostly the surface of the vagina 6. Changes during vaginal aging affect the whole vaginal wall, not only the epithelium, which is significantly reduced in thickness, as well as the glycogenic load 8. The lamina propria of the atrophic vagina has decreased extracellular matrix components and reduced vascularization and water‐retaining capacity 9. Local estrogen treatments have a high recurrence rate once they are discontinued; symptoms tend to reappear after the treatment has been completed, with systemic effects being reported during the use of some vaginal estrogens. On the other hand, patients are increasingly expressing high concern over cancer‐related consequences, and there is a lack of long‐term documented studies on the safety of these absorbed estrogens.
This is the reason why in recent times new treatments that work on the long‐term and also on the level of connective tissue and vascularization are being developed, some of which represent important alternatives. Thermal laser treatment is one of the newer treatments. The medical effects of lasers are well established in terms of biochemical, ablative, and thermal effects. Depending on the laser energy delivered and the time during which it is delivered, the tissue effect ranges from more a destructive one (e.g., tissue ablation) to a thermal only effect (e.g., coagulation, photo chemical reactions). The special SMOOTH mode produces a fast sequence of low‐fluence laser pulses inside and overall super‐long pulse allowing the heat to dissipate and distribute approximately 200 μm deep into the mucosa 10. In this way, a controlled deep thermal effect is achieved, without causing ablation. There have been many reports that thermal energy from the laser source, especially in a moist environment, enhances the collagen component and the vascularization 11, 12, 13]. Collagen remodeling and new collagen synthesis has been suggested as a mechanism of laser induced skin resurfacing and remodeling of vaginal connective tissue 10, 13, 14. Minimally invasive, non‐ablative thermal Erbium:YAG (Er:YAG) laser treatment for gynecological applications has been previously described as a safe and effective option for the treatment of stress urinary incontinence 15. Recently, non‐ablative thermal Er:YAG laser therapy has demonstrated improvement of GSM symptoms in a pilot study of 45 postmenopausal women 16.
The objective of this study was to establish safety and effectiveness of non‐ablative Er:YAG laser (RenovaLase®) treatment (3 sessions over 8 weeks) and to compare the effectiveness and durability of the new treatment to the standard topical estriol treatment administered during the same time period (8 weeks). In order to assess and compare the durability of the effect after treatment has been stopped, 8 week duration was chosen for both treatment regimes.
METHODS
Fifty post‐menopausal patients were recruited in this pilot comparative cohort study conducted at the Gynecology Department of the Faculty of Medicine at Mendoza University in Mendoza, Argentina, in the period from March 2012 till November 2012. Inclusion criteria were: measured estradiol level of ≤20 pg /ml and experience of more than one of the following symptoms of GSM: dyspareunia, vaginal dryness, vaginal burning or irritation, and chronic leukorrhea. Exclusion criteria included pelvic organ prolapse greater than stage I, previous vaginal surgery, smoking, subjection to any hormonal therapy in the 6 months prior to inclusion into the study and infectious leukorrhea. Patients’ BMIs ranged from 24 to 28.
The active control group of 25 patients (estriol group) received an 8‐week treatment of 0.5 mg of estriol ovules. The ovules were administered daily during the first 2 weeks, three times a week in weeks 2–4, then twice a week in weeks 4–8.
The laser treatment group of 25 patients first received a 2 week pre‐treatment with 0.5 mg estriol ovules (three ovules/week) and then received 3 sessions of laser therapy during the course of 8 weeks. The rationale for the pre‐treatment comes from the fact that the Erbium laser has high absorption in water and the methods of the thermal‐only Er:YAG mucosal treatment requires a highly moist environment. Because of that, in order to improve the hydro retention of the tissue, a pre‐treatment was done by applying 0.5 mg of estriol ovules three times per week for 2 weeks. Then on the 3rd week, the first laser treatment was performed with a 2,940 nm Er:YAG laser (XS Dynamis, Fotona, Slovenia) using a special modality, SMOOTH mode, which delivers laser energy in a non‐ablative, thermal‐only technique based on the manufacturer's proprietary pulsing sequence. A total of three treatments, once every 3 weeks, were performed. In the treatment protocol the laser irradiation was applied in two steps, firstly to the whole vaginal canal and then to the introitus area. A special laser speculum was introduced into the patient's vagina to serve as a guide for the laser beam delivery system, which is an Er:YAG handpiece with a 360° circular adapter. Several longitudinal passes using a step‐by‐step retraction of the handpiece were performed, delivering cumulative energy between 1,000 and 1,500 J to the whole vaginal canal. In the second step of the procedure, laser energy was delivered to the entrance of the vaginal canal, the vestibule, and introitus area. No anesthesia was used before or during the session. Patients were instructed to avoid sexual intercourse and activities that can increase intra‐abdominal pressure during the initial post‐op period of 7 days after the intervention.
During the laser intervention, patient discomfort, and treatment tolerability, as well as potential adverse events were monitored.
Biopsies for histological examination from six patients from each group were taken with Tischler biopsy forceps before treatment and 1, 3, 6, and 12 months post treatment. The biopsy location was at the junction of the mid third with the distal third of the anterior vaginal wall. Lidocaine anesthesia was applied before procedure.
The maturation index (MI), which shows increased ratios of intermediate and parabasal cells compared to superficial cells, was established by cytological examination using light microscopy. The data of MI were then used to calculate the maturation value (MV) 17. For normally menstruating women, the MV range is between 50 and 95; for women with various degrees of GSM, the MV is below 50. MI was measured before intervention and at 1, 3, 6, and 12 months after starting the treatment.
Vaginal pH testing, used to evaluate estrogen deprivation, was performed before and 3 and 12 months post treatment.
VAS (Visual Analog Scale) analysis was performed for assessment of the severity of the following GSM symptoms: dyspareunia, dryness, irritation, and leukorrhea. Patients assessed the severity of the symptoms as: severe—3, moderate—2, mild—1, and none—0. Assessments were made before intervention and at 1, 3, 6, 12, and 18 months after starting the treatment.
Results were statistically analyzed using Student's t‐test, values below 0.05 were considered statistically significant.
RESULTS
Fifty post‐menopausal patients with typical GSM symptoms were recruited in this study in the period from March 2012 till November 2012. Patient's demographic data are included in Table 1.
Table 1.
Patient Data
| Er:YAG laser group (n = 25) | Estriol group (n = 25) | P‐value | |
|---|---|---|---|
| Age (years ± SD) | 55.0 ± 6.7 | 53.5 ± 5.7 | 0.3929 |
| Menopause entry a | 49.0 ± 4.0 | 48.6 ± 2.8 | 0.7128 |
| Parity | 2.1 ± 1.5 | 2.6 ± 1.7 | 0.3395 |
| BMI (kg/m2) | 26.2 ± 1.4 | 25.9 ± 1.3 | 0.4335 |
| Estradiol (pg/ml) | 14.1 ± 3.1 | 13.6 ± 3.2 | 0.5233 |
| POP stage I | 9/25 (36%) | 14/25 (56%) | / |
| GSM symptoms | |||
| Dyspareunia | 25/25 (100%) | 25/25 (100%) | |
| Irritation | 25/25 (100%) | 25/25 (100%) | |
| Dryness | 25/25 (100%) | 25/25 (100%) | |
| Chronic leukorrhea | 18/25 (72%) | 21/25 (84%) | |
Menopause duration was not more than 7 years.
There were no statistically significant differences between the groups’ demographic data (P < 0.05). The estriol group had a higher baseline percentage of stage I POP, which did not seem to impact the symptoms of GSM or estradiol levels, which were very similar in both groups.
Symptoms of dyspareunia, irritation, dryness, chronic leukorrhea (Table 2 and Fig. 1a–d) were assessed on the 0–3 VAS scale. There was a statistically significant (P < 0.05) reduction of all the symptoms in both groups up to the 6 month follow‐up; however, the relief of symptoms was more prominent in the laser group at all follow‐ups. More importantly, the effect of the laser treatment remained statistically significant at the 12 and 18‐month follow‐up, while the effects of the estriol treatment were diminished at the 12‐month follow‐up and at 18 month follow‐up some were even significantly worse than before treatment (Table 2 and Fig. 1a–d).
Table 2.
Severity of GSM Symptoms Before and at 1, 3, 6, 12 and 18 Month Follow‐Ups
| Er:YAG laser group (n = 25) | ||||||
|---|---|---|---|---|---|---|
| Before | 1 month | 3 months | 6 months | 12 months | 18 months | |
| Dyspareunia | 2.44 ± 0.65 | 1.52 ± 0.77 * (P < 0.001) | 1.00 ± 0.96 * (P < 0.001) | 1.08 ± 1.00 * (P < 0.001) | 0.96 ± 0.98 * (P < 0.001) | 1.60 ± 0.65 * (P < 0.001) |
| Irritation | 2.04 ± 0.79 | 1.16 ± 0.55 * (P < 0.001) | 0.88 ± 0.88 * (P < 0.001) | 0.96 ± 0.93 * (P < 0.001) | 1.48 ± 0.82 * (P < 0.05) | 1.56 ± 0.58 * (P < 0.01) |
| Dryness | 2.24 ± 0.60 | 1.12 ± 0.78 * (P < 0.001) | 0.80 ± 0.82 * (P < 0.001) | 1.04 ± 0.89 * (P < 0.001) | 1.04 ± 0.73 * (P < 0.001) | 1.52 ± 0.71 * (P < 0.001) |
| Chronic leukorrhea | 2.28 ± 0.83 | 0.84 ± 0.90 * (P < 0.001) | 0.84 ± 0.90 * (P < 0.001) | 0.60 ± 0.71 * (P < 0.001) | 1.00 ± 0.71 * (P < 0.001) | 1.28 ± 0.74 (P = 0.054) |
| Estriol group (n = 25) | ||||||
|---|---|---|---|---|---|---|
| Dyspareunia | 2.28 ± 0.74 | 1.80 ± 0.82 * (P < 0.001) | 1.60 ± 0.96 * (P < 0.001) | 1.88 ± 0.83 * (P < 0.01) | 2.24 ± 0.72 | 2.36 ± 0.70 |
| Irritation | 2.00 ± 0.65 | 1.48 ± 0.59 * (P < 0.001) | 1.20 ± 0.82 * (P < 0.001) | 1.32 ± 0.85 * (P < 0.01) | 2.12 ± 0.53 | 2.28 ± 0.61x (P < 0.01) |
| Dryness | 2.28 ± 0.46 | 1.32 ± 0.85 * (P < 0.001) | 1.20 ± 0.96 * (P < 0.001) | 1.68 ± 0.80 * (P < 0.01) | 2.36 ± 0.49 | 2.40 ± 0.50x (P < 0.05) |
| Chronic leukorrhea | 1.71 ± 0.64 | 0.95 ± 0.74 * (P < 0.001) | 0.62 ± 0.74 * (P < 0.001) | 0.86 ± 0.96 * (P < 0.01) | 1.48 ± 0.82 | 1.76 ± 0.83x (P < 0.01) |
The values present mean ± standard deviation. Statistical significance was determined using student's t‐test. P values <0.05 were considered statistically significant. Bold values represent statistically significant mean as compared to mean before treatment.
indicates statistically significant improvement. x indicates statistically significant worsening.
Figure 1.
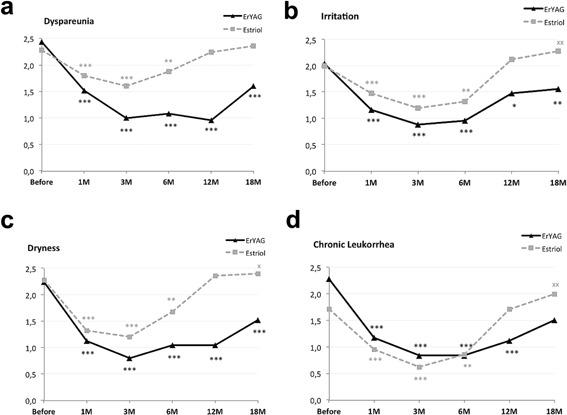
Assessment of the following symptoms of GSM: (a) dyspareunia, (b) irritation, (c) dryness, (d) chronic leukorrhea—on a 0–3 VAS scale in the laser group and in the estriol group before the treatment and after 1, 3, 6,12, and 18 months. *Indicates level of statistically significant improvement—***P < 0.001; **P < 0.01; *P < 0.05; x denotes statistically significant worsening ‐xx ‐ P < 0.01; x ‐ P < 0.05.
Side effects included a sensation of warmth and related mild‐to‐moderate pain in 4% of patients, as well as slight transient edema. One patient also developed transitory pain after laser treatment and one patient experienced spotting. In the estriol group, 8% of patients experienced spotting, 4% mastodynia, and 12% abdominal pain. Overall, side effects in both groups were minimal and of a transient nature.
The MV in the estriol group improved significantly at 1 and 3 month follow‐up and then it started declining, with still significant, although borderline improvement (22.5–25 points) after 12 months. In comparison to this, the laser group improvement was much higher, from 20.8 to 52.2 points after 12 months and was highly statistically significant (P< 0.001) (Table 3 and Fig. 2a).
Table 3.
Results of the Maturation Value and pH for Both Groups
| Before | 1 month | 3 months | 6 months | 12 months | |
|---|---|---|---|---|---|
| Erbium:YAG laser group (n = 25) | |||||
| Maturation value | 20.8 ± 5.4 | 33.3 ± 13.3 * (P < 0.001) | 39.7 ± 11.5 * (P < 0.001) | 47.9 ± 12.7 * (P < 0.001) | 52.2 ± 8.5 * (P < 0.001) |
| pH value | 5.0 ± 0.4 | / | 4.1 ± 0.4 * (P < 0.001) | / | 4.4 ± 0.6 * (P < 0.001) |
| Estriol group (n = 25) | |||||
| Maturation value | 22.5 ± 7.1 | 26.8 ± 7.1 * (P < 0.001) | 32.6 ± 13.9 * (P < 0.001) | 24.7 ± 13.2 | 25 ± 6.37 * (P < 0.05) |
| pH value | 4.9 ± 0.6 | / | 4.5 ± 0.7 * (P < 0.01) | / | 4.9 ± 0.6 |
Results for the maturation value were measured before and after 1, 3, 6, and 12 months, and for the pH value before and after 3 and 12 months. The values present mean ± standard deviation. Statistical significance was determined using student's t‐test. P values <0.05 were considered statistically significant. Bold values represent statistically significant values as compared to values before treatment.
indicates statistically significant improvement.
Figure 2.
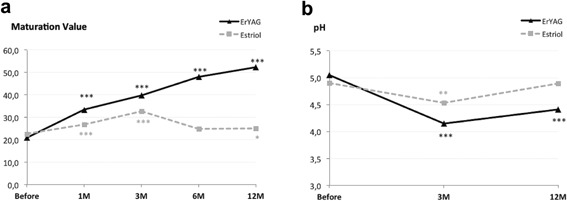
(a) Maturation value before and at 1, 3, 6, and 12 months post treatment, and (b) pH before and at 3 and 12 months follow‐up. *Indicates level of statistical significance—***P < 0.001; **P < 0.01; *P < 0.05.
The pH value was measured before and after 3 and 12 months and also shows a statistically significant decrease of pH value even at the 12‐month follow‐up, while the decrease at the estriol group was not as pronounced and has diminished at the 12‐month follow‐up (Table 3 and Fig. 2b).
Histological examination (Fig. 3), which was done in six patients before and 1, 3, 6, and 12 months after the laser treatment, showed changes in the tropism of the vaginal mucosa. Changes in the epithelial tissue included parakeratosis and acanthosis (thickness). Changes in the lamina propria included an initial vasodilation with pericapillary oedema due to the photothermal effect, with major congestion and improvement in vascularization. On the other hand, there was an increase in the amount of blasts and the fibrilar components of the extracellular matrix (which could possibly be the result of increased collagenesis). Finally, a marked angiogenesis could be seen at all follow‐ups months with a noticeable papillomatosis.
Figure 3.
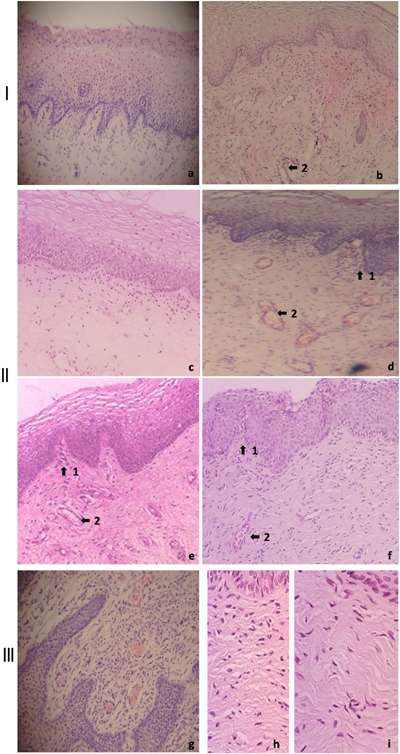
Histological examination (hematoxylin‐eosin staining) of the vaginal mucosa of patients with GSM symptoms before and after Er:YAG laser treatment. Panel I shows images before treatment (a) and 1 month after laser treatment (b). New vessel formation can be observed (2). Panel II shows images before treatment (c) and 3, 6, and 12 months after laser treatment (d–f, respectively). Increased cellularity and neo‐angiogenesis can be observed (2) after Er:YAG laser treatment, increase in papillomatosis (1), basal cells hyperplasia, and a complete restorative reaction at the level of the lamina propria. Panel III shows images at 6 months after laser treatment, displaying increased angiogenesis (g) and restorative reaction at the level of the extracellular matrix (h and i). Photomicrographs at 4× (a–g) and 10× (h and i) magnification are presented.
DISCUSSION
The primary goal of GSM treatment is to restore normal physiological conditions and thus to relieve GSM symptoms. Our results have shown that novel Er:YAG laser therapy is more efficient and longer lasting than topical estriol treatment, which has shown transient relief of symptoms that started to diminish after the treatment stopped. In this study, we aimed at comparing the effect of a novel treatment to topical estriol treatment in terms of the durability of the effect after treatment has been stopped. For this purpose, an equal duration of treatment regimen has been chosen as the most appropriate, that is, laser treatment over 8 weeks compared to 8 week of estriol.
Vaginal mucosa has several functions: absorption, secretion, protection, and response to stimuli. These four functions directly depend on the integrity and normal function and morphology of the mucosa, which also depends on estrogen levels 18. Physiological changes in GSM are the results of aging and diminished estrogen concentrations during menopause. These changes present histologically as changes in vaginal wall structures—thinning and decreased thickness of the epithelium, and clinically as dryness, increased pH, painful intercourse, irritation of the vulva with burning and itching, paleness of tissue, and loss of elasticity. Improvement in the symptoms of GSM can be achieved either by increasing the local estrogen levels or the blood flow in the affected area 6.
It is well known that therapy with local estrogen in the form of estradiol, estriol or conjugated estrogens, restores vaginal pH and increases the thickness of the epithelium, and vaginal secretions 1. Recently, it has been shown that local estrogens are also able to induce collagen synthesis and reorganization in animal models as well as clinically 19, 20. However, to the best of our knowledge local estrogen therapy does not seem to have a significant effect on angiogenesis. In the present study, estriol was used as a comparison to laser therapy. Estriol is a lower‐potency estrogen that has recently been shown to improve symptoms of GSM 21. Local estrogen preparations affect mostly the surface of the vaginal mucosa and the improvement is transient—lasting only as long as the therapy is administered 6.
In contrast, Er:YAG laser therapy with non‐ablative SMOOTH mode acts by producing pulse sequences of low fluence pulses that are absorbed at the tissue surface and cause transient heat increase of the mucosa, inducing restructuring of the lamina propria, but also microvascularization and new vessel formation. This tissue response on mild heat pulsing has been confirmed in prior studies 10, 22, 23. The consequence of this mechanism of action is long term improvement of GSM symptoms.
The histology of the inspected samples prior to the treatment showed typical consequences of lack of estrogen, which also resulted with macroscopic findings, such as diminished humidity, rugation, elasticity, lubrication, and secretions 1.
In histological samples taken from patients in the laser group at 1, 3, 6, and 12 months after the treatment, an improvement was observed in terms of basal cell hyperplasia, parakeratosis, and papillomatosis. Changes in the lamina propria included an initial vasodilation effect and an increase in the cellularity of the extracellular matrix with papillomatosis. Changes in the lamina propria could explain the immediate restorative reaction that the heat has on the mucosal tissue: the improvement in vascularization could result in an increase in oxygen and nutrients supply to the treated area thus favoring a restorative process.
The MV, which indicates the degree of maturation attained by the vaginal epithelium and serves as an objective means of evaluating hormonal response 17 and atrophy, was significantly increased in the laser group even after having completed the laser therapy, indicating the long‐term effect of laser regeneration. In contrast, the effect in the estriol group was less pronounced and has shown a trend of decrease after the end of the hormonal treatment 6.
It can be speculated that histological changes seen in the laser group ultimately result with an improvement in the assessed symptoms of GSM (irritation, dyspareunia, dryness, and chronic leukorrhea). Assessing the symptoms of dyspareunia, dryness, irritation, and chronic leukorrhea, as well as the MV and pH, there was greater reduction of symptoms and greater improvement in the MV and pH in the laser group as compared to the estriol‐only group.
It is important to highlight that improvements in the estriol group were observed during the time that the therapy was administered and remained significantly improved up to the 6 month follow‐up (4 months after therapy was stopped), while in the laser group they were sustained after the laser treatment was concluded, up to 18 months after 1st treatment (and 16 months after therapy was stopped), showing a much longer lasting effect in comparison to estriol. The effects of estriol on the symptoms of GSM showed improvement up to the 6‐month follow up, with results diminishing to the level before treatment at the 12‐month follow up, and some even showing significantly worse symptoms at the 18‐month follow‐up compared to baseline. This transient effect of estriol has also been reported in previous studies 6.
The pH value decreased in both groups, the effect of the laser being more pronounced. It can be hypothesized that the decrease is due to an increase in superficial cells (evidenced by an increased MV) and the thickness in the epithelium and consequently glycogen production. Glycogen in the mucous layer serves as a substrate for vaginal colonization by Doderlein's lactobacillus which produce lactic acid which in turn regulates the low vaginal pH 24.
The effect of laser treatment on GSM symptoms has been previously described only in a few studies. In one of the studies by Gaspar et al. 25, a fractional CO2 laser was used for the treatment of vaginal rejuvenation. It showed beneficial effects in the three layers of the vaginal tissue and in sexual function. A study by Salvatore et al. 26, has shown in a 3‐month follow‐up study that treatment with a fractional CO2 laser improves symptoms of GSM. Histological analysis of vaginal tissue at 1 and 2 months after treatment revealed thicker epithelium with increased glycogenic store, papillae formation, and increased content of blood vessel in the connective tissue, all suggestive of a metabolic reactivation of the connective components of the vaginal mucosa 27. Similar histological changes were observed in biopsies taken up to 12 months post treatment in our study. However, the major difference between CO2 laser and the Er:YAG laser method used in the present study is in the ablative characteristics of the CO2 laser, which works by vaporizing columns of tissue. Tissue vaporization of epithelial layer surface is necessary to expose deeper, underlying connective tissue, which is more abundant in water, to the thermal effects of the CO2 laser pulse 27 for achieving the desired photobiomodulatory effect. In contrast, Er:YAG laser with SMOOTH mode creates heat pulses without damaging the mucosa. The temporal distribution of energy delivered with the special SMOOTH mode allows the heat to slowly dissipate to depths of approximately 200 μm 10, thus achieving the same biological effects than the thermal effects of a CO2 laser pulse, with the additional benefit of avoiding mucosal damage. Consequently, the risk of infection, necrosis, scarring, and other side effects is minimized compared to CO2.
The beneficial effects of non‐ablative Er:YAG laser treatments in gynecology were previously described by Ogrinc et al. 15 and Gambacciani et al. 16. In a study by Ogrinc et al. 15, the results confirmed that a minimally invasive, non‐ablative laser treatment is an effective, safe, and comfortable treatment alternative with at least 1 year lasting positive effects in patients with stress urinary incontinence. A positive effect on the symptoms of stress urinary incontinence was ascribed to neocollagenesis and collagen remodeling in the vaginal wall. In the study, a special sub‐ablative sequence of laser pulses was used to achieve an optimal temperature to stimulate collagen tightening and new collagen formation in the vaginal connective tissue.
A similar pulsing sequence, which delivers laser energy in a non‐ablative mode (SMOOTH mode), was used in our case to achieve moderate homogeneous heating within a several hundred micron thick superficial layer, without any ablation and with a controlled temperature deposition 28.
Gambacciani et al. 16, have recently published a study where the same type of laser—Er:YAG with SMOOTH mode, was used for the treatment of GSM. The study reported significant improvement of GSM symptoms, which lasted up to 6 months post‐treatment. The treatment was equally or more effective than local estriol gel.
In the present study, we show that the results are reproducible and can be sustained up to 18 months after treatment. Based on examination of vaginal wall histology before and after treatment the effects of non‐ablative Er:YAG laser treatment can be suggested.
The positive effect on the epithelium and lamina propria is presumably due to the stimulation of cell proliferation via heat shock protein activation, an increase of collagen production as well as anti‐inflammatory action 29, 30, 31.
It is also very important to acknowledge that laser therapy has most probably a different mechanism of action than estriol, resulting in induced vessel formation, reconstitution of the lamina propria, and consequent regeneration of the mucosa lasting for an extended period of time even after therapy has been ceased. Having all the above considerations in mind, we believe that the Er:YAG laser treatment in combination with a low‐dose/short‐term estriol mucosa preparation phase could be considered a preferred treatment with respect to effectiveness and safety. In patients that cannot receive estrogen therapy due to previous or existing estrogen responsive cancers, mucosa could be prepared differently (i.e., using platelet‐rich plasma or just by using moisturizing gel during the treatment), making the laser treatment a completely estrogen‐free, non‐invasive option for the treatment of GSM symptoms. The safety profile of the treatment has also been shown to be favorable, consisting of only mild and transient side effects of local nature. It has to be emphasized, that a long‐term safety profile has not yet been established; however, based on the transient nature of the observed side effects and considering the mode of action of Er:YAG laser treatment, no long‐term safety issues are to be expected. In conclusion, the major benefit of the laser treatment in comparison to the 8‐week local estriol treatment is that the laser treatment produces a long lasting effect characterized by an improved vascularization and increased extracellular matrix component, while estriol‐only treatment increases the glycogen level in the vaginal epithelium and its turnover 1, with less effect and only transient effect on vascularization and changes in the lamina propria, requiring maintenance treatment for the effects to be sustained.
Due to these enhanced effects, the laser treatment provides great improvement in the signs and symptoms of GSM, which remains evident also after the treatment is concluded. We have observed a trend of diminishing effect at the 18‐month follow‐up, although the improvement was still highly significant. Since the treatment is safe and non‐invasive, it could be repeated once the patients feel the return of symptoms, and in that way sustain the beneficial mucosal state.
Although, this is a pilot study and longer follow‐ups with greater numbers of patients would be needed to better determine the effectiveness and long‐term safety of this new treatment option, our results indicate that Er:YAG laser promises to be an efficient and safe treatment alternative for GSM.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
REFERENCES
- 1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the north american menopause society. Menopause 2013; 20(9):888–902; quiz 903–904. [DOI] [PubMed] [Google Scholar]
- 2. Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med 2009; 6(8):2133–2142. [DOI] [PubMed] [Google Scholar]
- 3. Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000; 61(10):3090–3096. [PubMed] [Google Scholar]
- 4. Portman DJ, Gass ML. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Menopause 2014; 21(10):1063–1068. [DOI] [PubMed] [Google Scholar]
- 5. Iosif CS, Bekassy Z. Prevalence of genito‐urinary symptoms in the late menopause. Acta Obstet Gynecol Scand 1984; 63(3):257–260. [DOI] [PubMed] [Google Scholar]
- 6. Johnston SL, Farrell SA, Bouchard C, Farrell SA, Beckerson LA, Comeau M, Johnston SL, Lefebvre G, Papaioannou A; SOGC Joint Committee‐Clinical Practice Gynaecology and Urogynaecology . The detection and management of vaginal atrophy. J Obstet Gynaecol Can 2004; 26(5):503–508. [DOI] [PubMed] [Google Scholar]
- 7. Von Schoultz E, Rutqvist LE. Menopausal hormone therapy after breast cancer: The Stockholm randomized trial. J Natl Cancer Inst 2005; 97(7):533–535. [DOI] [PubMed] [Google Scholar]
- 8. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane database Syst Rev 2006;(4):CD001500. [DOI] [PubMed] [Google Scholar]
- 9. Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta‐analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: Second report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol 1998; 92(4 Pt 2):722–727. [DOI] [PubMed] [Google Scholar]
- 10. Drnovsek‐Olup B, Beltram M, Pizem J. Repetitive Er:YAG laser irradiation of human skin: A histological evaluation. Lasers Surg Med 2004; 35(2):146–151. [DOI] [PubMed] [Google Scholar]
- 11. Kunzi‐Rapp K, Dierickx CC, Cambier B, Drosner M. Minimally invasive skin rejuvenation with erbium: YAG laser used in thermal mode. Lasers Surg Med 2006; 38:899–907. [DOI] [PubMed] [Google Scholar]
- 12. Bodendorf MO, Willenberg A, Anderegg U, Grunewald S, Simon JC, Paasch U. Connective tissue response to fractionated thermo‐ablative Erbium: YAG skin laser treatment. Int J Cosmet Sci 2010; 32(6):435–445. [DOI] [PubMed] [Google Scholar]
- 13. Salvatore S, Leone Roberti Maggiore U, Athanasiou S, Origoni M, Candiani M, Calligaro A, Zerbinati N. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: An ex vivo study. Menopause 2015; 22(8):845–849. [DOI] [PubMed] [Google Scholar]
- 14. Berlin AL, Hussain M, Phelps R, Goldberg DJ. A prospective study of fractional scanned nonsequential carbon dioxide laser resurfacing: A clinical and histopathologic evaluation. Dermatol Surg 2009; 35(2):222–228. [DOI] [PubMed] [Google Scholar]
- 15. Ogrinc UB, Senčar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med 2015; 47:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: The second‐generation thermotherapy for the genitourinary syndrome of menopause. Climacteric 2015; 18:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meisels A. The maturation value. Acta Cytol 1967; 11(4):249. [PubMed] [Google Scholar]
- 18. Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rahn DD, Good MM, Roshanravan SM, Shi H, Schaffer JI, Singh RJ, Word RA. Effects of preoperative local estrogen in postmenopausal women with prolapse: A randomized trial. J Clin Endocrinol Metab 2014; 99(10):3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montoya TI, Maldonado PA, Acevedo JF, Word RA. Effect of vaginal or systemic estrogen on dynamics of collagen assembly in the rat vaginal wall. Biol Reprod Feb 2015; 92(2):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cano A, Estévez J, Usandizaga R, Gallo JL, Guinot M, Delgado JL, Castellanos E, Moral E, Nieto C, del Prado JM, Ferrer J. The therapeutic effect of a new ultra low concentration estriol gel formulation (0.005% estriol vaginal gel) on symptoms and signs of postmenopausal vaginal atrophy: Results from a pivotal phase III study. Menopause 2012; 19(10):1130–1139. [DOI] [PubMed] [Google Scholar]
- 22. Dams SD, de Liefde‐van Beest M, Nuijs AM, Oomens CWJ, Baaijens FPT. Heat shocks enhance procollagen type I and III expression in fibroblasts in ex vivo human skin. Skin Res Technol 2011; 17(2):167–180. [DOI] [PubMed] [Google Scholar]
- 23. Romanos GE, Gladkova ND, Feldchtein FI, Karabut MM, Kiseleva EB, Snopova LB, Fomina YV. Oral mucosa response to laser patterned microcoagulation (LPM) treatment. An animal study. Lasers Med Sci 2013; 28(1):25–31. [DOI] [PubMed] [Google Scholar]
- 24. Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 2010; 13(6):509–522. [DOI] [PubMed] [Google Scholar]
- 25. Gaspar A, Addamo G, Brandi H. Vaginal fractional CO 2 laser: A minimally invasive option for vaginal rejuvenation. Am J Cosmet Surg 2011; 28(3):156–162. [Google Scholar]
- 26. Salvatore S, Nappi RE, Zerbinati N, et al. A 12‐week treatment with fractional CO2 laser for vulvovaginal atrophy: A pilot study. Climacteric 2014; 17(4):363–369. [DOI] [PubMed] [Google Scholar]
- 27. Zerbinati N, Serati M, Origoni M, Candiani M, Iannitti T, Salvatore S, Marotta F, Calligaro A. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci 2015; 30(1):429–436. [DOI] [PubMed] [Google Scholar]
- 28. Vizintin Z, Rivera M, Fistonić I, Saracoglu F, Guimares P, Gaviria J, Garcia V, Lukac M, Perhavec T, Marini L. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad 2012; 1:46–58. [Google Scholar]
- 29. Thomsen S. Pathologic analysis of photothermal and photomechanical effects of laser‐tissue interactions. Photochem Photobiol 1991; 53(6):825–835. [DOI] [PubMed] [Google Scholar]
- 30. Melo de VA, Anjos dos DCS, Albuquerque Júnior R, Melo DB, Carvalho FUR. Effect of low level laser on sutured wound healing in rats. Acta Cir Bras 2011; 26(2):129–134. [DOI] [PubMed] [Google Scholar]
- 31. Kao B, Kelly KM, Majaron B, Nelson JS. Novel model for evaluation of epidermal preservation and dermal collagen remodeling following photorejuvenation of human skin. Lasers Surg Med 2003; 32(2):115–119. [DOI] [PubMed] [Google Scholar]


