Abstract
Nonvalvular atrial fibrillation (AF) is a risk factor for stroke in elderly patients. Although warfarin has been used to prevent AF‐associated stroke for more than 50 years, non–vitamin K antagonist oral anticoagulants (NOACs) including dabigatran, rivaroxaban, apixaban, and edoxaban recently have been developed to overcome the disadvantages of warfarin. Based on the results of NOAC clinical trials, Savelieva and Camm made recommendations regarding selection of NOACs in patients with nonvalvular AF. Recent accumulating evidence indicates that NOACs work differently in Asian and non‐Asian individuals. In this review, we discuss the results of the large, randomized, phase 3 international clinical trials on NOACs, the subanalyses of Asians, and a Japanese phase 3 clinical trial of rivaroxaban to discriminate Japanese patient–specific characteristics with regard to their responses to NOACs and make recommendations. Our analysis revealed that rivaroxaban decreased the incidence of gastrointestinal (GI) bleeding compared with warfarin in Japanese patients. The efficacy results showed that rivaroxaban significantly decreased the incidence of ischemic stroke (hazard ratio: 0.40, 95% confidence interval: 0.17‐0.96) compared with warfarin. The lower incidence of GI bleeding and ischemic stroke may be specific to Japanese patients. Based on the present and previous results, the following recommendations regarding the selection of NOACs are added in the Camm chart for Japanese patients: edoxaban for patients with a high risk of bleeding and those with a previous stroke; and rivaroxaban for patients with a high risk of ischemic stroke and a low bleeding risk, and those with previous GI bleeding.
Keywords: Stroke prevention, Clinical trials
1. Introduction
Nonvalvular atrial fibrillation (AF) is known as a risk factor for stroke. It was reported that approximately 20% of hospitalized patients with ischemic stroke in Japan had AF complications.1 Warfarin has been used to prevent AF‐associated stroke for more than 50 years, but warfarin treatment is a burden for patients and physicians because it requires a dose adjustment achieved by monitoring the prothrombin time–international normalized ratio (PT‐INR). Warfarin also interacts with food and many other drugs.
Non–vitamin K antagonist oral anticoagulants (NOACs) were developed to overcome these disadvantages of warfarin, and the efficacy and safety of NOACs have been tested in comparative studies with warfarin as a control. In recent years, several NOACs have become available. Based on the results of phase 3 clinical trials, Savelieva and Camm presented a chart (the Camm chart) to help physicians choose the best available NOAC in light of each patient's condition.2 However, because the chart was created based on the results of international collaborative clinical trials, it must be used carefully for Japanese patients.
First, the therapeutic range of warfarin's PT‐INR for Japanese patients is different from that for patients in other countries. The range of 1.6 to 2.6 is recommended for Japanese elderly patients (age ≥70 years), whereas a range of 2.0 to 3.0 is recommended in the EU and US guidelines.3 This difference in the therapeutic range in INR is based on the higher incidence of warfarin‐related intracranial hemorrhage (ICH) in Asians, including Japanese.4
Based on the present review, therefore, we propose a method for selecting NOACs for Japanese patients with AF based on the results of subanalyses of Asian patients in the following clinical trials: Randomized Evaluation of Long‐term Anticoagulation Therapy (RE‐LY),5 Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),6 and Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE),7 as well as the Japanese–ROCKET AF (J‐ROCKET AF) study in which Japanese patients participated.8
Edoxaban was approved for stroke prevention in patients with AF (SPAF) in 2014 in Japan, and it is not yet included in the Camm chart. In the present review, we discuss the characteristics, efficacy, and safety of edoxaban and 3 other NOACs: dabigatran, rivaroxaban, and apixaban.
2. Characteristics of the Noacs
The characteristics of the 4 NOACs (edoxaban, dabigatran, rivaroxaban, and apixaban) have been compared with those of warfarin and reported in numerous studies.9, 10, 11, 12, 13, 14, 15, 16 In brief, NOACs have shorter half‐lives and take less time to reach maximum blood concentration than does warfarin. Unlike warfarin, NOACs have a specific target (either thrombin or factor Xa), do not require coagulation monitoring, and can be administered with fixed doses.
3. Evidence From International Clinical Trials
The summary of key patient characteristics and findings from the 4 phase 3 clinical trials of NOACs are described in previous studies,17, 18, 19, 20, 21 and major differences are also summarized in the Table 1. Unlike the other 3 clinical trials, the ROCKET AF study included patients with a moderate to high risk of stroke that resulted in a higher ischemic stroke risk (CHADS2 score), higher proportion of patients with a history of stroke or TIA, and a lower time in therapeutic range (TTR) for INR in the ROCKET AF study (Table 1).17 In the ROCKET AF study, the noninferiority of rivaroxaban compared with warfarin was demonstrated in the incidence of stroke or systemic embolism (SE; hazard ratio [HR]: 0.88, 95% confidence interval [CI]: 0.75‐1.03, P = 0.12) in the intention‐to‐treat population (Table 1).17 The results of the Anti‐Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction 51 (ATLAS ACS2‐TIMI51) study showed that rivaroxaban is effective in reducing the risk of the composite endpoint of death from cardiovascular causes, myocardial infarction, or stroke in patients with recent acute coronary syndrome (ACS).22 The results of the ROCKET AF study also showed that the incidence of major bleeding from a gastrointestinal (GI) site in the rivaroxaban group (3.2%) was significantly higher than that in the warfarin group (2.2%) (P < 0.001). In addition, the incidence of major bleeding in the rivaroxaban group relative to the warfarin group tended to be higher (HR: 1.04, 95% CI: 0.90‐1.20, P = 0.58) compared with the other 3 NOACs.
Table 1.
Key patient characteristics and findings from the phase 3 trials of the non–vitamin K antagonist oral anticoagulants vs warfarin
| RE‐LY19, 20 | ROCKET AF17 | ARISTOTLE21 | ENGAGE AF‐TIMI 4818 | |||
|---|---|---|---|---|---|---|
| NOAC examined | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | ||
| Mean CHADS2 score | 2.1 | 3.5 | 2.1 | 2.8 | ||
| Prior stroke/TIA, % | 20 | 55 | 19 | 28 | ||
| NOAC dosing arm | 110 mg bid | 150 mg bid | 20 mg qd | 5 mg bid | 30 mg qd | 60 mg qd |
| HR (95% CI) for NOAC vs warfarin | ||||||
| Stroke or SE (ITT population) | 0.90 (0.74‐1.10) | 0.65 (0.52‐0.81) | 0.88 (0.75‐1.03) | 0.79 (0.66‐0.96) | 1.13 (0.96‐1.34) | 0.87 (0.73‐1.04) |
| Ischemic stroke | 1.11 (0.89‐1.40) | 0.76 (0.60‐0.98) | 0.94 (0.75‐1.17) | 0.92 (0.74‐1.13) | 1.41 (1.19‐1.67) | 1.00 (0.83‐1.19) |
| Major bleeding | 0.80 (0.70‐0.93) | 0.93 (0.81‐1.07) | 1.04 (0.90‐1.20) | 0.69 (0.60‐0.80) | 0.47 (0.41‐0.55) | 0.80 (0.71‐0.91) |
| ICH | 0.30 (0.19‐0.45) | 0.41 (0.28‐0.60) | 0.67 (0.47‐0.93) | 0.42 (0.30‐0.58) | 0.30 (0.21‐0.43) | 0.47 (0.34‐0.63) |
| GI bleeding | 1.10 (0.86‐1.41) | 1.50 (1.19‐1.89) | Not calculated HR 1 | 0.89 (0.70‐1.15) | 0.67 (0.53‐0.83) | 1.23 (1.02‐1.50) |
| MI | 1.29 (0.96‐1.75) | 1.27 (0.94‐1.71) | 0.81 (0.63‐1.06) | 0.88 (0.66‐1.17) | 1.19 (0.95‐1.49) | 0.94 (0.74‐1.19) |
| Death | 0.91 (0.80‐1.03) | 0.88 (0.77‐1.00) | 0.85 (0.70‐1.02) | 0.89 (0.80‐0.99) | 0.87 (0.79‐0.96) | 0.92 (0.83‐1.01) |
Abbreviations: ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; bid, twice daily; CHADS2, congestive HF, HTN, age ≥75 years, DM, stroke or TIA (2 points); CI, confidence interval; DM, diabetes mellitus; ENGAGE AF‐TIMI 48, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation‐Thrombolysis in Myocardial Infarction 48; GI, gastrointestinal; HF, heart failure; HR, hazard ratio; HTN, hypertension; ICH, intracranial hemorrhage; ITT, intention to treat; MI, myocardial infarction; NOAC, non‐vitamin K antagonist oral anticoagulant; qd, once daily; RE‐LY, Randomized Evaluation of Long‐term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; SE, systemic embolism; TIA, transient ischemic attack; TTR, time in therapeutic range for international normalized ratio.
HRs are for the NOAC group as compared with the warfarin group.
Major bleeding from GI site: 224 bleeding events (3.2%) in the rivaroxaban group, as compared with 154 events in the warfarin group (2.2%; P < 0.001).
In the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF‐TIMI 48) study,18 the noninferiority of edoxaban at 30 mg qd (HR: 1.13, 97.5% CI: 0.96‐1.34, P = 0.10) and 60 mg qd (HR: 0.87, 97.5% CI: 0.73‐1.04, P = 0.08) for decreasing the incidence of stroke or SE compared with warfarin was reported. However, the HR at 30 mg qd was slightly higher than that at 60 mg qd. The results further showed that edoxaban significantly lowered the incidence of major bleeding and ICH at 30 mg qd (major bleeding HR: 0.47, 95% CI: 0.41‐0.55, P < 0.001; ICH HR: 0.30, 95% CI: 0.21‐0.43, P < 0.001) and 60 mg qd (major bleeding HR: 0.80, 95% CI: 0.71‐0.91, P < 0.001; ICH HR: 0.47, 95% CI: 0.34‐0.63, P < 0.001) compared with warfarin. On the other hand, it also significantly increased the incidence of GI bleeding at 60 mg qd (HR: 1.23, 95% CI: 1.02‐1.50, P = 0.03) and that of ischemic stroke at 30 mg qd (HR: 1.41, 95% CI: 1.19‐1.67, P < 0.001).
Dabigatran at 150 mg bid was superior to warfarin in decreasing the incidence of stroke or SE (P < 0.001), and it was noninferior to warfarin at 110 mg bid (HR: 0.90, 95% CI: 0.74‐1.10, P = 0.30).19, 20 Although dabigatran at 150 mg bid significantly lowered the incidence of ischemic stroke compared with warfarin (HR: 0.76, 95% CI: 0.60‐0.98, P = 0.03), dabigatran at 110 mg bid did not (HR: 1.11, 95% CI: 0.89‐1.40, P = 0.35). Overall, dabigatran at 110 mg bid significantly lowered the incidence of major bleeding compared with warfarin (P = 0.003); however, when the bleeding incidence is analyzed by bleeding sites, dabigatran increased the incidence of GI bleeding both at 110 mg bid (HR: 1.10, 95% CI: 0.86‐1.41, P = 0.43) and 150 mg bid (HR: 1.50, 95% CI: 1.19‐1.89, P < 0.001). The incidence of GI bleeding with dabigatran 150 mg bid was highly increased.
The ARISTOTLE study showed that apixaban significantly reduced the incidence of stroke or SE compared with warfarin (P = 0.01).22 Apixaban also significantly reduced the incidence of both major bleeding (HR: 0.69, 95% CI: 0.60‐0.80, P < 0.001) and ICH (HR: 0.42, 95% CI: 0.30‐0.58, P < 0.001). The noninferiority of apixaban to warfarin regarding GI bleeding (HR: 0.89, 95% CI: 0.70‐1.15, P = 0.37) was also shown in the ARISTOTLE study.
4. Findings From Subanalyses of Asians From the International Clinical Trials
The proportion of Asian patients with a history of stroke was higher than that of non‐Asian patients.23 Three NOACs (dabigatran, rivaroxaban, and apixaban) and warfarin showed a higher incidence of stroke in Asians compared with non‐Asians (Figure 1A,B), suggesting that stroke occurs more often in Asians. Warfarin also increased the incidence of major bleeding in Asians compared with non‐Asians, but 3 NOACs reduced major bleeding more in Asians than non‐Asians (Figure 1C,D), indicating that warfarin tends to cause major bleeding in Asians. The incidence of ICH in Asians was higher with any of the drugs tested than that in non‐Asians; however, whereas warfarin increased the ICH incidence 3‐fold, the 3 NOACs doubled the incidence.5, 6, 7 These studies supported the results of previous epidemiological studies, in which an increased incidence of warfarin‐related ICH was shown in Asian populations.4
Figure 1.
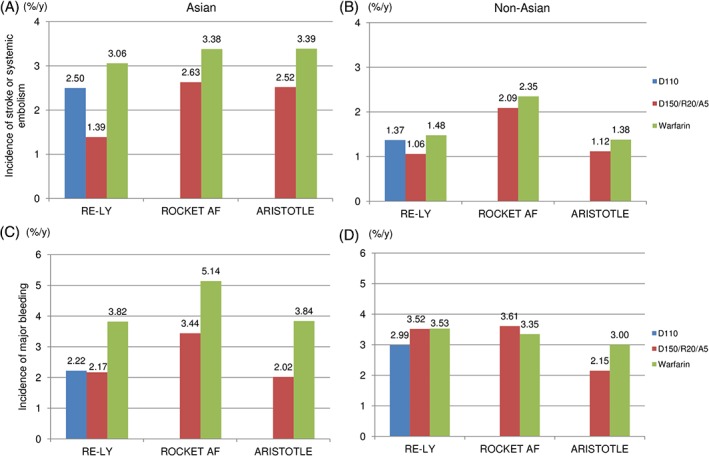
Ethnic differences in the incidence of stroke or systemic embolism and major bleeding.5, 6, 7 (A) Incidence of stroke or systemic embolism in Asian populations. (B) Incidence of stroke or systemic embolism in Non‐Asian populations. (C) Incidence of major bleeding in Asian populations. (D) Incidence of major bleeding in Non‐Asian populations. Abbreviations: ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; bid, twice a day; D110, dabigatran 110 mg bid; D150/R20/A5, dabigatran 150 mg bid/rivaroxaban 20 mg qd/apixaban 5 mg bid; qd, once a day; RE‐LY, Randomized Evaluation of Long‐term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
5. Findings From the Japanese Phase 3 Clinical Trial
There are 2 important differences between the J‐ROCKET AF and ROCKET AF studies.8 First, the rivaroxaban dose in the J‐ROCKET AF study (15 mg qd) was lower than that in the EU and US (20 mg qd), because the pharmacokinetic data (Cmax, area under the curve) of rivaroxaban in Japanese patients who received rivaroxaban at 15 mg qd were observed to be comparable with those of Caucasian patients who received rivaroxaban at 20 mg qd.24 For Japanese patients, the dose is further reduced to 10 mg qd if renal function is impaired (with a creatinine clearance of 30–49 mL/min). Second, in the J‐ROCKET AF study, warfarin with the INR 1.6 to 2.6 is recommended in patients age ≥70 years, as in the Japanese guideline,3 instead of the INR of 2.0 to 3.0 as used in the European Union and United States.
In the J‐ROCKET AF study, the safety data showed that there was no significant difference between rivaroxaban and warfarin in the incidences of major bleeding (HR: 0.85, 95% CI: 0.50‐1.43) or death caused by bleeding (HR: 0.33, 95% CI: 0.03‐3.14).8 However, when the results were analyzed by the bleeding site, several advantages were found in Japanese patients, including that rivaroxaban decreased the incidence of GI bleeding by approximately 50% compared with warfarin. Because the incidence of GI bleeding (P < 0.001) was lower in the warfarin group than in the rivaroxaban group in the global clinical trial ROCKET AF study, the lower GI bleeding in the rivaroxaban group appears to be specific to Japanese patients (Figure 2). Rivaroxaban also decreased the ICH rate in Japanese patients compared with warfarin (rivaroxaban 0.65%/y, warfarin 1.32%/y). It should, however, be noted that the possibility of a lack of robustness of the outcome analysis in the J‐ROCKET AF study could not be excluded because of the small sample size of the Japanese cohort used in the study.8
Figure 2.
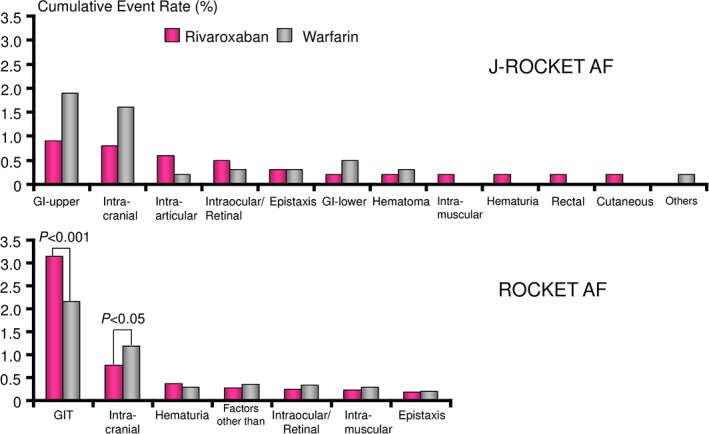
Major bleeding rates by sites in the J‐ROCKET AF and ROCKET AF studies.8, 18 Abbreviations: GI, gastrointestinal; GIT, gastrointestinal tract; J‐ROCKET AF, Japanese ROCKET AF; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
Although difference in the primary efficacy endpoint (all‐cause stroke plus non–central nervous system SE; HR: 0.49, 95% CI: 0.24‐1.00, P = 0.050) was not significant, rivaroxaban significantly decreased the incidence of all‐cause stroke (HR: 0.46, 95% CI: 0.22‐0.98) compared with warfarin.8, 25 Rivaroxaban also significantly decreased the incidence of ischemic stroke (HR: 0.40, 95% CI: 0.17‐0.96) compared with warfarin in Japanese patients, whereas in the global ROCKET AF study, the incidence data were HR: 0.94 and 95% CI: 0.75‐1.17, suggesting that the lower incidence in ischemic stroke with rivaroxaban may be specific to Japanese patients.
6. Recommendations for the Selection of Noacs in Japan
Since edoxaban was approved for SPAF in 2014 in Japan, we reviewed the results of the ENGAGE AF‐TIMI 48 study and the phase 3 Japanese study (J‐ROCKET AF study) to investigate whether there are any Japanese‐specific traits in the Camm chart.2 Based on the results, we propose the following Japanese version of the Camm chart (Figure 3).
Figure 3.
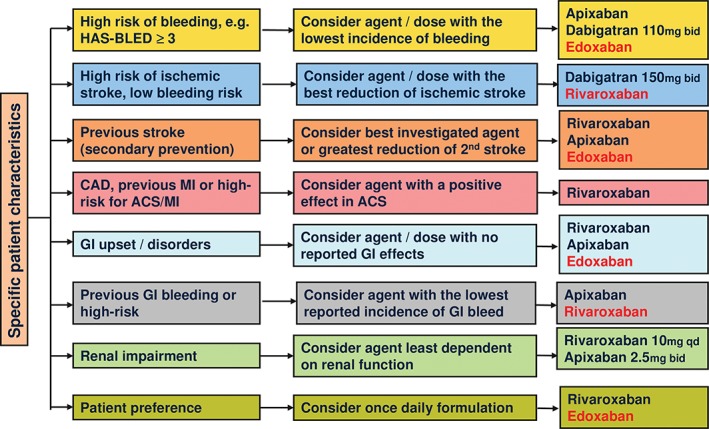
Considerations when selecting a NOAC for Japanese patients. NOACs recommended for Japanese patients but not included in the original Camm chart are highlighted in red. Abbreviations: ACS, acute coronary syndrome; bid, twice a day; CAD, coronary artery disease; GI, gastrointestinal; HAS‐BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age ≥65 years), drugs/alcohol concomitantly; MI, myocardial infarction; NOAC, non‐vitamin K antagonist oral anticoagulant; qd, once a day.
In the Japanese version of the Camm chart, rivaroxaban is excluded from administration to patients with a high risk of bleeding (ie, a HAS‐BLED score ≥3) because it did not significantly decrease the incidence of major bleeding (HR: 1.04, 95% CI: 0.90‐1.20, P = 0.58) compared with warfarin.17 Instead, apixaban (HR: 0.69, 95% CI: 0.60‐0.80, P < 0.001), dabigatran 110 mg bid (HR: 0.80, 95% CI: 0.70‐0.93, P = 0.03) and edoxaban (30 mg qd: HR: 0.47, 95% CI: 0.41‐0.55, P < 0.001; 60 mg qd: HR: 0.80, 95% CI: 0.71‐0.91, P < 0.001) are recommended for Japanese patients with a high risk of bleeding because they significantly reduced the incidence of major bleeding compared with warfarin.18, 19, 20, 21
Dabigatran treatment is recommended for patients with a high risk of ischemic stroke and a low bleeding risk because dabigatran 150 mg bid significantly decreased the incidence of ischemic stroke compared with warfarin in the RE‐LY study (HR: 0.76, 95% CI: 0.60‐0.98, P = 0.03).19 For Japanese patients, rivaroxaban is also included in the Japanese version of the Camm chart because it decreased the incidence of ischemic stroke by 60% compared with warfarin in the J‐ROCKET AF study (HR: 0.40, 95% CI: 0.17‐0.96).8
Based on the results of the ROCKET AF and the ENGAGE AF‐TIMI 48 studies, in which 55% and 28%, respectively, of the participants had a history of stroke,17, 18 rivaroxaban and edoxaban are recommended for patients with a previous stroke. Apixaban is also recommended because it significantly decreased the recurrence of stroke (HR: 0.71, 95% CI: 0.51‐0.97) in the ARISTOTLE subanalysis of stroke recurrence prevention.26
Rivaroxaban is also recommended for patients with coronary artery disease, a previous myocardial infarction (MI), or a high risk for ACS/MI, as the results of the ATLAS ACS2‐TIMI51 study showed that rivaroxaban significantly decreased the rate of death due to cardiovascular events, MI, and stroke compared with placebo (HR: 0.84, 95% CI: 0.74‐0.96, P = 0.008).22 However, rivaroxaban doses tested in ATLAS ACS2‐TIMI 51 were lower than therapeutic doses for prevention of AF‐related stroke (2.5/5.0 mg bid vs 15/20 mg qd) and patients with AF were excluded. Thus, findings from ATLAS ACS2‐TIMI 51 may have the limited relevance to the prevention of stroke in AF patients with high risk for ACS. On the other hand, dabigatran and apixaban are not recommended for these patients because they did not show the benefit in previous randomized controlled studies of dabigatran and apixaban in patients with ACS.27, 28 In addition, the RE‐LY study failed to show the noninferiority of dabigatran to warfarin in MI (110 mg bid: HR: 1.29, 95% CI: 0.96‐1.75; 150 mg bid: HR: 1.27, 95% CI: 0.94‐1.71).19, 20 Edoxaban is also not recommended for patients with coronary artery disease, previous MI, or high risk for ACS/MI because of insufficient evidence.
Dabigatran is not recommended for patients with GI disorders because it causes dyspepsia in 11% to 12% of patients.19 When patients have a history of recent or inadequately managed GI bleeding or are at high risk for GI bleeding, apixaban, which decreased GI bleeding (HR: 0.89, 95% CI: 0.70‐1.15, P = 0.37)21 may be preferred. Edoxaban 30 mg qd significantly decreased GI bleeding in the ENGAGE AF‐TIMI 48 study. However, the recommended dose of edoxaban is 60 mg qd, which is reduced to 30 mg qd depending on renal function or concomitant drugs. Therefore, edoxaban 30 mg qd is not recommended for patients with previous GI bleeding or high risk for GI bleeding. In contrast, for Japanese patients, rivaroxaban is included in the Japanese version of the Camm chart because the results of the J‐ROCKET AF study showed that rivaroxaban decreased GI bleeding by approximately 50% compared with warfarin.8
For patients with renal impairment, apixaban at 2.5 mg bid and rivaroxaban at 10 mg qd would be more appropriate because the respective renal clearances were 27% and 35%.12 At present, edoxaban is not included in the Japanese version of the Camm chart because the relevant data are not yet published.
7. Conclusion
In Japan, 4 NOACs—dabigatran, rivaroxaban, apixaban, and edoxaban—are currently approved for SPAF. It is important to understand the differences between these NOACs and warfarin to maximize the benefit of NOACs in patients with AF. Savelieva and Camm made recommendations on the selection of NOACs (dabigatran, rivaroxaban, and apixaban) depending on patient characteristics.2 However, because studies have found that the stroke incidence and bleeding incidence caused by warfarin and NOACs in Japanese patients differ from those in non‐Japanese patients, a Japanese version of the Camm chart is proposed in this report, based on the clinical trial data. At this time, postmarketing surveillance of rivaroxaban in Japan shows no notable difference in safety and efficacy compared with the findings from the previous clinical trials.29 Currently ongoing Xarelto for Prevention of Stroke in Patients with Atrial Fibrillation (XANTUS) studies in different regions of the world, in which safety and efficacy data on the use of rivaroxaban for stroke prevention in AF in routine clinical practice are collected, are expected to further provide ethnicity‐specific differences.30
Acknowledgments
The authors thank WysiWyg Co., Ltd. for writing assistance and coordination of the manuscript's development.
Conflicts of interest
K.O. has received research funding from Boehringer Ingelheim, Bayer Healthcare, and Daiichi‐Sankyo, and speakers’ bureau/honoraria from Boehringer Ingelheim, Bayer Healthcare, Bristol‐Myers Squibb, Pfizer, and Eisai. M.H. has received honoraria for lectures from Bayer Healthcare and Boehringer Ingelheim. N.T. has received research funding from Boehringer Ingelheim, Daiichi‐Sankyo, Sanofi, and Takeda, and speakers’ bureau/honoraria from Boehringer Ingelheim, Bayer Healthcare, Bristol‐Myers Squibb, Pfizer, and Sanofi. A.J.C. has consulted for Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, and Pfizer‐BMS.
Okumura K, Hori M, Tanahashi N and John Camm A. Special considerations for therapeutic choice of non–vitamin K antagonist oral anticoagulants for Japanese patients with nonvalvular atrial fibrillation, Clin Cardiol, 2017;40(2):126–131.
Funding information Medical writing and editorial support for this article was funded by Bayer Yakuhin, Ltd.
References
- 1. Kimura K, Kazui S, Minematsu K, et al. Analysis of 16 922 patients with acute ischemic stroke and transient ischemic attack in Japan: a hospital‐based prospective registration study. Cerebrovasc Dis. 2004;18:47–56. [DOI] [PubMed] [Google Scholar]
- 2. Savelieva I, Camm AJ. Practical considerations for using novel oral anticoagulants in patients with atrial fibrillation. Clin Cardiol. 2014;37:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. JCS Joint Working Group . Guidelines for pharmacotherapy of atrial fibrillation (JCS2013). Circ J. 2014;78:1997–2021. [DOI] [PubMed] [Google Scholar]
- 4. Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. [DOI] [PubMed] [Google Scholar]
- 5. Hori M, Connolly SJ, Zhu J, et al; RE‐LY Investigators . Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non‐Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. [DOI] [PubMed] [Google Scholar]
- 6. Wong KS, Hu DY, Oomman A, et al; Executive Steering Committee and the ROCKET AF Study Investigators . Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. [DOI] [PubMed] [Google Scholar]
- 7. Goto S, Zhu J, Liu L, et al; ARISTOTLE Investigators . Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Am Heart J. 2014;168:303–309. [DOI] [PubMed] [Google Scholar]
- 8. Hori M, Matsumoto M, Tanahashi N, et al; J‐ROCKET AF Study Investigators . Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation: the J‐ROCKET AF study. Circ J. 2012;76:2104–2111. [DOI] [PubMed] [Google Scholar]
- 9. Ansell J. Warfarin versus new agents: interpreting the data. Hematology Am Soc Hematol Educ Program. 2010;2010:221–228. [DOI] [PubMed] [Google Scholar]
- 10. Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011;62:41–57. [DOI] [PubMed] [Google Scholar]
- 11. Harder S. Renal profiles of anticoagulants. J Clin Pharmacol. 2012;52:964–975. [DOI] [PubMed] [Google Scholar]
- 12. Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with nonvalvular atrial fibrillation. Europace. 2013;15:625–651. [DOI] [PubMed] [Google Scholar]
- 13. Bathala MS, Masumoto H, Oguma T, et al. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40:2250–2255. [DOI] [PubMed] [Google Scholar]
- 14. Matsushima N, Lee F, Sato T, et al. Absolute bioavailability of edoxaban in healthy subjects. AApS J. 2011;13(suppl 2): Abstract T2362. [Google Scholar]
- 15. Mendell J, Zahir H, Matsushima N, et al. Drug‐drug interaction studies of cardiovascular drugs involving P‐glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013;13:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogata K, Mendell‐Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50:743–753. [DOI] [PubMed] [Google Scholar]
- 17. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 18. Giugliano RP, Ruff CT, Braunwald E, et al; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 19. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation [published correction appears in N Engl J Med. 2010;363:1877]. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 20. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE‐LY Investigators . Newly identified events in the RE‐LY trial. N Engl J Med. 2010;363:1875–1876. [DOI] [PubMed] [Google Scholar]
- 21. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 22. Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2‐TIMI 51 Investigators . Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 23. Oldgren J, Healey JS, Ezekowitz M, et al; RE‐LY Atrial Fibrillation Registry Investigators . Variations in cause and management of atrial fibrillation in a prospective registry of 15 ;400 emergency department patients in 46 countries: the RE‐LY Atrial Fibrillation Registry. Circulation. 2014;129:1568–1576. [DOI] [PubMed] [Google Scholar]
- 24. Tanigawa T, Kaneko M, Hashizume K, et al. Model‐based dose selection for phase III rivaroxaban study in Japanese patients with nonvalvular atrial fibrillation. Drug Metab Pharmacokinet. 2013;28:59–70. [DOI] [PubMed] [Google Scholar]
- 25. Tanahashi N, Hori M, Matsumoto M, et al; J‐ROCKET AF Study Investigators . Rivaroxaban versus warfarin in Japanese patients with nonvalvular atrial fibrillation for the secondary prevention of stroke: a subgroup analysis of J‐ROCKET AF. J Stroke Cerebrovasc Dis. 2013;22:1317–1325. [DOI] [PubMed] [Google Scholar]
- 26. Sardar P, Chatterjee S, Wu WC, et al. New oral anticoagulants are not superior to warfarin in secondary prevention of stroke or transient ischemic attacks, but lower the risk of intracranial bleeding: insights from a meta‐analysis and indirect treatment comparisons. PLoS One. 2013;8:e77694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oldgren J, Budaj A, Granger CB, et al; RE‐DEEM Investigators . Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double‐blind, phase II trial. Eur Heart J. 2011;32:2781–2789. [DOI] [PubMed] [Google Scholar]
- 28. Alexander JH, Lopes RD, James S, et al; APPRAISE‐2 Investigators . Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. [DOI] [PubMed] [Google Scholar]
- 29. Ogawa S, Ikeda T, Kitazono T, et al; Rivaroxaban Postmarketing Surveillance Registry Investigators . Present profiles of novel anticoagulant use in Japanese patients with atrial fibrillation: insights from the Rivaroxaban Postmarketing Surveillance Registry. J Stroke Cerebrovasc Dis. 2014;23:2520–2526. [DOI] [PubMed] [Google Scholar]
- 30. Camm AJ, Amarenco P, Haas S, et al. XANTUS: rationale and design of a noninterventional study of rivaroxaban for the prevention of stroke in patients with atrial fibrillation. Vasc Health Risk Manag. 2014;10:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]


