Summary
Aim
The aim of this study was to explore factors affecting efficacy of treatment of common cold symptoms with an over‐the‐counter ibuprofen/pseudoephedrine combination product.
Methods
Data from an anonymous survey among 1770 pharmacy customers purchasing the combination product for treatment of own common cold symptoms underwent post‐hoc descriptive analysis. Scores of symptoms typically responsive to ibuprofen (headache, pharyngeal pain, joint pain and fever), typically responsive to pseudoephedrine (congested nose, congested sinus and runny nose), considered non‐specific (sneezing, fatigue, dry cough, cough with expectoration) and comprising all 11 symptoms were analysed. Multiple regression analysis was applied to explore factors associated with greater reduction in symptom intensity or greater probability of experiencing a symptom reduction of at least 50%.
Results
After intake of first dose of medication, typically ibuprofen‐sensitive, pseudoephedrine‐responsive, non‐specific and total symptoms were reduced by 60.0%, 46.3%, 45.4% and 52.8%, respectively. A symptom reduction of at least 50% was reported by 73.6%, 55.1%, 50.9% and 61.6% of participants, respectively. A high baseline score was associated with greater reductions in symptom scores but smaller probability of achieving an improvement of at least 50%. Across both multiple regression approaches, two tablets at first dosing were more effective than one and (except for ibuprofen‐sensitive symptoms) starting treatment later than day 2 of the cold was generally less effective.
Discussion and Conclusions
Efficacy of an ibuprofen/pseudoephedrine combination in the treatment of common cold symptoms was dose‐dependent and greatest when treatment started within the first 2 days after onset of symptoms.
Keywords: common cold, ibuprofen, pseudoephedrine, symptom score, multiple regression analysis
What's known
Ibuprofen and pseudoephedrine are effective and safe to alleviate common cold symptoms. Based on their pharmacological profile, it can be expected that they differentially target symptoms, making use of a combination treatment promising.
What's new
A survey of 1770 pharmacy customers purchasing an ibuprofen/pseudoephedrine combination product showed differential efficacy against ibuprofen‐sensitive, pseudoephedrine‐sensitive, total and non‐specific symptoms. Multiple regression analyses showed that efficacy is dose‐dependent and more pronounced when treatment starts early in the course of a cold episode but largely independent of gender and age of the patient.
1. Introduction
Common cold is a widespread cause of morbidity with an estimated average of six annual episodes in children1 and two in adults.2 The bad news about common cold is that it is very frequent and does not have a cure. The good news is that it is typically self‐limiting and, in contrast to influenza, a benign condition. Nonetheless, common cold symptoms including headache, joint ache, nasal congestion, fever and exhaustion can be very bothersome. While conservative methods such as bed rest and fluid intake can be helpful, many patients seek medical treatment to make symptoms more tolerable and/or shorten the course of symptoms. Drugs used in this regard include analgesics such as acetylsalicylic acid, ibuprofen and paracetamol (acetaminophen) and α‐adrenoceptor agonists such as phenylephrine,3 pseudoephedrine3, 4 and xylometazoline.5 As either of these drug classes is effective only against a subset of common cold symptoms, fixed‐dose combinations of two or more active ingredients are frequently used; specifically, the combination of ibuprofen plus pseudoephedrine has been shown to be more efficacious than either monotherapy.6, 7 Many common cold medications are available without a prescription as over‐the‐counter drugs.
BoxaGrippal® is a common cold medication that has been authorised in Germany for over‐the‐counter sale in 2012. It contains a combination of 200 mg ibuprofen and 30 mg pseudoephedrine hydrochloride per tablet in an immediate‐release formulation; according to the package insert 1 or 2 tablets should be taken with an interval of at least 6 hours between doses, a maximum of six tablets per day and a maximum treatment period of 5 days. The present manuscript uses data from a survey among 1770 pharmacy customers purchasing BoxaGrippal® to explore factors affecting efficacy of treatment of common cold symptoms with this ibuprofen/pseudoephedrine combination product; a focus of this exploration was a comparison of effects on symptoms typically considered to be ibuprofen‐ or pseudoephedrine‐responsive.
2. Methods
Between October 2014 and March 2015, a total of 230 participating pharmacies were asked to invite customers aged 18 years and older who had purchased BoxaGrippal® to participate in an anonymous written survey. Participants received a € 5 coupon for future purchases from an online retailer as compensation for time spent filling the survey. Precondition for participation were the purchase of BoxaGrippal® intended for current treatment of own common cold symptoms, age ≥18 years, willingness and ability of independent, plausible and timely completion of the questionnaire. Participants could return the survey in a sealed envelope either to the pharmacy or send it postage‐free to a contract research organisation. Ethical committee approval was neither required nor recommended for this type of study in Germany at the time it was performed.
The survey captured demographics of the participants. At baseline, it also provided a list of 11 typical cold symptoms; these included nasal congestion/obstructed nasal breathing, congested nasal sinus, runny nose, sneezing, headache, pharyngeal pain, joint pain, fever/increased body temperature, exhaustion/fatigue, dry cough/tussive irritation and cough with expectoration (Table 1). Each subject was asked to identify the four most bothersome symptoms from this list. They were also asked to rate the extent of each symptom on a scale from 0 to 10 (very weak to very strong) prior to intake of first dose of medication. Additional questions were related to time from start of cold to first dose (same day, day 2, 3 or 4, day 5 or later) and to number of tablets (1 or 2) at each dosing on the first, second, third or fourth day of treatment, if applicable.
Table 1.
Participant rating of common cold symptoms. Each participant was asked to list the four most bothersome symptoms from a list of 10 typical symptoms; data are shown as % of subjects naming a given symptom as one of the four. Patients were also asked to rate a given symptom prior to (baseline) and after first intake of medication (irrespective of using 1 or 2 tablets) on a scale of 0 (smallest extent) to 10 (greatest extent); data are shown as mean±SD. All results are based on 1770 responders; missing intensity entries were set to “0”
| % most bothersome | Intensity at baseline | Intensity after first intake | Reduction | |
|---|---|---|---|---|
| Nasal congestion | 64.7 | 6.10±3.25 | 3.06±2.25 | 3.05±2.63 |
| Congested sinus | 43.6 | 4.98±3.55 | 2.56±2.35 | 2.42±2.60 |
| Runny nose | 25.4 | 3.59±3.49 | 1.89±2.18 | 1.70±2.58 |
| Sneezing | 17.9 | 3.18±3.24 | 1.66±2.10 | 1.53±2.53 |
| Headache | 70.6 | 6.33±3.03 | 2.35±2.26 | 3.98±2.96 |
| Pharyngeal pain | 38.6 | 4.28±3.59 | 1.92±2.21 | 2.36±2.78 |
| Joint pain | 49.4 | 5.07±3.41 | 2.04±2.17 | 3.03±2.87 |
| Fever | 24.1 | 2.80±3.24 | 1.18±1.90 | 1.62±2.54 |
| Exhaustion | 50.1 | 5.65±3.40 | 2.64±2.41 | 3.00±2.90 |
| Cough | 30.2 | 2.67±3.29 | 1.69±2.40 | 0.98±2.04 |
| Cough with expectoration | N.R. | 1.83±2.76 | 1.26±2.14 | 0.58±1.66 |
N.R., not reported.
To assess treatment effects, the survey asked participants to report time to onset of symptom relief as 0‐5 minutes, 6‐15 minutes, 16‐30 minutes, 31‐45 minutes, 46‐60 minutes or more than 60 minutes and whether they considered time to onset as very fast, fast, moderately fast or slow. It also asked participants to rate the extent of the 11 symptoms after the first dose of medication on the same scale of 0‐10 and to report duration of symptom relief after the first dose (options: cured, up to 24 hours, up to 12 hours, up to 6 hours, less than 6 hours). At end of treatment, they were asked to report whether four disease‐relevant statements applied: “I can perform daily tasks better again”, “I'm no longer distressed by cold symptoms”, “I can concentrate better again” and “I no longer feel so exhausted and fatigued” (yes and no option for each statement). Finally, they were asked whether they judged the efficacy and the tolerability of BoxaGrippal® as well as overall treatment satisfaction as very good, good, less good and poor.
Data from the survey were analysed in three steps. First, all data were reported descriptively. Second, a total score and three subscores were created based on the 11 cold symptoms. Each symptom was rated on a scale from 0 to 10, at start of treatment and after the first dose of medication. On the basis of the known pharmacodynamic effects of the active pharmaceutical ingredients, we grouped the 11 symptoms into three clusters: typical ibuprofen‐responsive symptoms (TIRS) consisting of headache, pharyngeal pain, joint pain and fever, typical pseudoephedrine‐responsive symptoms (TPRS) consisting of congested nose, congested sinus and runny nose and non‐specific symptoms (NSS) consisting of sneezing, fatigue, dry cough, cough with expectoration. A total symptom score (TSS) summarising all 11 symptoms was also calculated. To allow comparison between the scores, they were normalised based upon the number of included items, that is, divided by 4 for the TIRS and NSS, by 3 for the TPRS and by 11 for the TSS. Data for individual symptoms and scores are reported as absolute values. Moreover, we calculated % of participants reporting an improvement of at least 50% for each of the four scores. In a third step, multiple regression analysis was applied with the difference in scores between the assessments before and after medication as the dependent variable and baseline score, age, gender, body weight, day of first medication intake and number of tablets at first medication intake as potential explanatory variables. Due to the explorative character of our analysis, we concomitantly performed multiple logistic regression analysis with improvement of at least 50% as the dependent binary variable and the same explanatory variables as in the multiple linear models described above.
Data analysis was performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). Except for weight and body mass index (n=1716), quantitative data are based on at least 1750 responders. Data on before and after symptom intensity in Table 1 are based on n=922‐1239, apparently reflecting that participants not experiencing a given symptom may not have entered data for it; in those cases, missing values were set to 0 to avoid overestimation of effect sizes. In line with the exploratory character of the analyses, all statistical analyses and corresponding P‐values are descriptive and should not be considered as confirmatory. Data on categorical variables are reported as % of participants exhibiting a given parameter. Data on quantitative parameters are reported as means±SD or with 95% confidence intervals. The precision of parameter estimates in the regression analyses is reported as estimate ±SEM. Data collection and initial analysis was performed by Winicker Norimed GmbH (Nuremberg, Germany), a contract research organisation. Analyses reported here were performed by the authors.
3. Results
3.1. Descriptive results
A total of 1770 subjects (70.4% women, age 37.9±13.1 years, height 171.6±8.9 cm, body weight 72.1±14.6 kg, BMI 24.3±4.0 kg/m2) participated in the survey. When asked which four from a list of 10 typical cold symptoms they considered most bothersome, headache, nasal congestion, exhaustion and joint pain were named most frequently (Table 1). These four also had the highest rating for extent of symptom (Table 1).
Participants reported to have started use of the ibuprofen/pseudoephedrine combination on the first day of their cold in 23.3%, and on the second, third, fourth or fifth and later day in 49.3%, 21.1%, 2.8% and 2.3%, respectively. At first intake, 28.4% used one tablet, whereas 70.4% used two tablets. This dose selection similarly applied to subjects starting treatment on the first through third day, whereas those starting at a later time exhibited an approximately even split between using one or two tablets.
More than 50% of participants reported time to onset of symptom relief to be 30 minutes or less (Figure 1). Accordingly, subjective assessment for time to onset as very fast in 13.7%, fast in 53.4%, moderately fast in 23.6% and rather slow in 2.3%. Duration of effect of first intake was reported as less than 6 hours in 15.1%, up to 6 hours in 54.4%, up to 12 hours in 22.6% and up to 24 hours in 4.6%; 1% reported full resolution of symptoms after first intake of medication.
Figure 1.
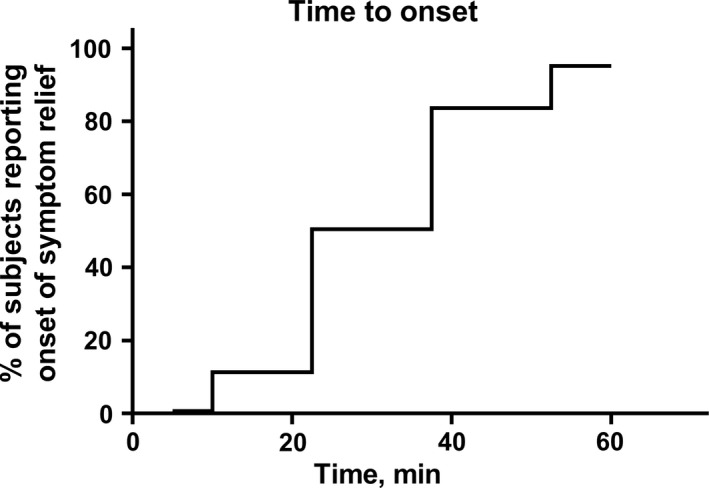
Time to onset of action after ingestion of first dose. Data are shown as cumulative % of survey participants reporting onset of symptom relief after ingestion of combination treatment at a given time point
Table 1 shows intensity of each of the 11 common cold symptoms on a scale from 0 to 10, at start of treatment and after the first dose of medication, as well as intra‐individual reduction upon medication intake.
At end of treatment, participants were asked whether they agreed or disagreed with the following four statements: “I can follow my daily tasks better again” 89.8% agreement; “I no longer feel distressed by cold symptoms” 67.9% agreement; “I can concentrate better again” 76.2% agreement; “I no longer feel so exhausted and fatigued” 81.8% agreement. Accordingly, global efficacy assessment by participants was excellent in 27.5%, good in 65.5%, less good in 6.4% and poor in 0.3%. Global tolerability was rated as excellent by 36.2%, good by 58.9%, less good by 4.4% and poor by 0.2%. Overall treatment satisfaction was rated as very satisfied by 30.3%, satisfied by 61.7%, less satisfied by 6.8% and not satisfied by 0.6%.
3.2. Symptom scores
To better understand the relative roles of each active pharmaceutical ingredient in the combination product, we calculated a TIRS, TPRS, NSS and TSS as described in Methods. The various scores at baseline and after the first dose of medication as well as intra‐individual reductions of scores are shown in Table 2. After normalisation for number of included items, baseline TIRS and TPRS were similarly high, whereas NSS was considerably lower. After the first dose of medication, the TIRS declined by 60.0%, the TPRS by 46.3%, the NSS by 45.4% and the TSS by 52.8%. Thus, the ibuprofen component of the medication appeared to be somewhat more effective against its typical symptoms than the pseudoephedrine component.
Table 2.
Scores for typical ibuprofen‐responsive symptoms (TIRS; consisting of headache, pharyngeal pain, joint pain and fever), typical pseudoephedrine‐responsive symptoms (TPRS; consisting of congested nose, congested sinus and runny nose), non‐specific symptoms (NSS; consisting of sneezing, fatigue, dry cough, cough with expectoration) and total symptoms (TSS; consisting of all 11 symptoms) at baseline and after intake of first dose of study medication (irrespective of using 1 or 2 tablets) as well as intra‐individual change after first Data are mean±SD on a scale of 0 (smallest extent) to 10 (greatest extent) or % change. Note that each score has been normalised for number of included symptoms to facilitate comparison between scores
| Score | Baseline | After first intake | Reduction | % Reduction |
|---|---|---|---|---|
| TIRS | 4.62±2.19 | 1.87±1.69 | 2.75±1.89 | 60.0±33.2 |
| TPRS | 4.89±2.33 | 2.50±1.74 | 2.39±1.93 | 46.3±64.6 |
| NSS | 3.33±2.03 | 1.81±1.58 | 1.52±1.53 | 45.4±41.0 |
| TSS | 4.22±1.65 | 2.02±1.42 | 2.20±1.41 | 52.8±29.7 |
3.3. Multiple regression analysis
In the light of the exploratory nature of our analysis, we performed two types of multiple regression analysis for each of the four scores to enhance robustness of the conclusions. One approach was a general linear model looking at reduction in symptom intensity after the first dosing (Table 3). The other was a logistic regression approach using number of subjects reporting an improvement of a given score by at least 50% (Table 4). This minimum extent of reduction in TIRS, TPRS, NSS and TSS was observed in 73.6%, 55.1%, 50.9% and 61.6% of participants, respectively. Both approaches consistently detected the following associations: A high baseline score was associated with greater reductions in symptom scores but smaller probability of achieving an improvement of at least 50%. Two tablets at first dosing were more effective than one in both analysis approaches. Starting treatment later than day 2 of cold was generally less effective; data on day 5 did not confirm that but, due to small sample sizes, confidence intervals were too wide to detect any effect. Body weight or gender was not associated with the magnitude of reduction or the probability of achieving at least 50% improvement; age exhibited only small and inconsistent associations. Of note, above effects were consistent across all four scores; the notable exception is that TIRS in contrast to the three other scores was not affected by a delayed start of treatment.
Table 3.
Association of explanatory variables with reduction in symptom scores after first drug dose. For purpose of analysis, the reference group was defined as males, starting treatment on day 1 of symptom manifestation with an initial dose of one tablet; data for age and body weight are per 10 year or 10 kg increments, respectively, data for baseline score per point at baseline. Data are shown as parameter estimate±SEM with corresponding P‐value underneath and are relative to mean scores after first intake shown in Table 2. A positive number indicates higher reduction in symptom intensity after dosing, a negative number lower reduction in symptom intensity being associated with the respective explanatory variable
| Explanatory variable | TIRS | TPRS | NSS | TSS |
|---|---|---|---|---|
| Female gender | −0.044±0.091P=.63 | −0.054±0.090P=.55 | −0.095±0.075P=0.21 | −0.060±0.073P=.41 |
| Age | 0.012±0.027P=.65 | 0.061±0.027P=.02 | 0.025±0.023P=.28 | 0.024±0.022P=.27 |
| Body weight | 0.001±0.029P=.99 | −0.023±0.029P=.44 | −0.011±0.024P=.64 | −0.007±0.024P=.76 |
| Baseline score | 0.564±0.016P<.0001 | 0.567±0.015P<.0001 | 0.478±0.014P<.0001 | 0.487±0.017P<.0001 |
| Start of treatment day 2 | −0.081±0.086P=.35 | −0.045±0.086P=.60 | −0.104±0.071P=.15 | −0.080±0.070P=.25 |
| Start of treatment day 3 | −0.153±0.103P=.14 | −0.274±0.103P=.0076 | −0.190±0.085P=.027 | −0.192±0.083P=.02 |
| Start of treatment day 4 | −0.155±0.190P=.42 | −0.566±0.190P=.0029 | −0.285±0.158P=.071 | −0.311±0.154P=.043 |
| Start of treatment day 5 | 0.225±0.240P=.35 | −0.228±0.239P=.34 | 0.296±0.199P=.14 | 0.115±0.194P=.55 |
| Two tablets at 1st dose | 0.273±0.077P=.0004 | 0.276±0.077P=.0003 | 0.243±0.064P=.0001 | 0.284±0.062P<.0001 |
Table 4.
Association of explanatory variables with probability to experience an at least 50% reduction in symptom scores after first drug dose (irrespective of using 1 or 2 tablets). For purpose of analysis, the reference group was defined as males, starting treatment on day 1 of symptom manifestation with an initial dose of one tablet; data for age and body weight are per 10 year or 10 kg increments, respectively, data for baseline score per point at baseline. Data are shown as odds ratio for more than 50% improvement with 95% confidence intervals in square brackets. The percentage of overall participants showing an at least 50% improvement per score is shown in the main text. Odds ratios greater 1 indicate a greater probability of success, odds ratios below 1 a smaller probability being associated with the respective explanatory variable
| Explanatory variable | TIRS | TPRS | NSS | TSS |
|---|---|---|---|---|
| Female gender | 0.856 [0.638–1.145] | 0.970 [0.746–1.261] | 0.828 [0.634–1.082] | 0.949 [0.729–1.235] |
| Age | 1.067 [0.977–1.166] | 1.122 [1.037–1.214] | 1.093 [1.008–1.186] | 1.092 [1.009–1.183] |
| Body weight | 0.944 [0.861–1.036] | 0.920 [0.846–1.001] | 0.934 [0.857–1.018] | 0.954 [0.877–1.039] |
| Baseline score | 0.896 [0.850–0.944] | 1.024 [0.978–1.072] | 0.925 [0.873–0.979] | 0.874 [0.822–0.930] |
| Start of treatment day 2 | 0.785 [0.588–1.042] | 0.904 [0.703–1.161] | 0.725 [0.559–0.939] | 0.823 [0.635–1.062] |
| Start of treatment day 3 | 0.740 [0.529–1.034] | 0.588 [0.436–0.791] | 0.570 [0.418–0.775] | 0.556 [0.411–0.750] |
| Start of treatment day 4 | 0.636 [0.354–1.171] | 0.419 [0.236–0.729] | 0.498 [0.281–0.871] | 0.346 [0.198–0.596] |
| Start of treatment day 5 | 1.436 [0.612–3.949] | 0.587 [0.293–1.164] | 1.150 [0.576–2.354] | 0.867 [0.434–1.790] |
| Two tablets at 1st dose | 1.396 [1.094–1.778] | 1.435 [1.150–1.792] | 1.413 [1.126–1.774] | 1.603 [1.285–2.000] |
4. Discussion
Analgesics and decongestants are established drug classes to alleviate common cold symptoms. As each drug class based on its pharmacodynamic profile preferentially affects a subset of cold symptoms, combination products are often used for more comprehensive symptom control,8, 9, 10, 11, 12, 13 including ibuprofen/pseudoephedrine combinations.6, 7 The present analysis was designed to improve understanding of factors affecting efficacy of treatment of common cold symptoms with a combination product consisting of 200 mg ibuprofen and 30 mg pseudoephedrine (BoxaGrippal®). Specifically, we were interested to explore how the combination product affected symptoms considered ibuprofen‐ or pseudoephedrine‐responsive. As a byproduct, our analysis provides information on symptoms considered most bothersome by common cold sufferers.
4.1. Critique of methods
Common cold is a self‐limiting condition. Therefore, improvement or even resolution of symptoms is expected to occur in at least some patients receiving placebo or even no treatment. To reflect real‐world conditions a patient survey was performed, which excludes a placebo group. This was not deemed necessary as the efficacy of ibuprofen,6 pseudoephedrine3, 6, 7, 8, 10, 14, 15, 16, 17, 18 or their combination6, 7 in the treatment of common cold symptoms has been established in multiple placebo‐controlled studies. Moreover, common cold sufferers had correctly identified whether they were on active treatment or placebo in previous studies,8 questioning the value of a placebo group in this indication. While a placebo‐component is likely to be part of the observed symptom improvements, the differential effects on TIRS and TPRS as well as the observed dose‐dependency (see below) support the view that the reported improvements at least partly reflect pharmacodynamic responses attributable to the active ingredients of the combination product. The self‐limiting nature of the condition is unlikely to have affected responses to the first dose, but may explain why congestive symptoms were improved less when treatment started late, as congestions tends to improve faster than other cold symptoms.
Another relevant limitation is that self‐report of cold symptoms can vary greatly and may skew results. As this may question some of the quantitative outcome measures, the emphasis of our analysis was on the multiple regression approaches to explore factors potentially associated with greater efficacy. As this is an intra‐study analysis, it may be less vulnerable to the limitations of self‐reporting.
For the present analysis, we have operationally defined four symptom scores describing common cold symptoms. The TIRS and the TPRS were based on symptoms assumed to be responsive to ibuprofen and pseudoephedrine, respectively, based on pharmacodynamics of these two drugs and published symptom‐specific study results; of note, some of these studies included objective measurements of nasal airflow.3, 10, 14, 16, 17, 18, 19 Symptoms not clearly linked to one of them were summarised in the NSS; a TSS including all 11 symptoms was also defined to look at the entirety of common cold symptoms. As the four scores ranged from 3 to 11 symptoms, each one was adjusted based on number of included symptoms to make them comparable to each other. However, we did not include weighing of the various symptoms within each score, for instance based on patient rating of bother. However, it should be noted that each of the three subscores included at least one of the four most bothersome symptoms, as reported in Table 1, supporting concept validity of these scores.
The four symptom scores and the multiple regression approaches have been designed after the initial descriptive results of the survey had been obtained. Therefore, all analyses in the present manuscript are explorative and not hypothesis‐testing. Accordingly, in line with recent statistical recommendations,20 reported P‐values are descriptive and not meant to reject prespecified null‐hypotheses. Height and body mass index were not included in the list of potentially explanatory variables in the multiple regression models as neither active ingredient in the combination product is highly lipophilic and, accordingly, expected to have volume of distribution affected by body mass index. These design elements and resulting limitations should be considered in the interpretation of the results.
4.2. Descriptive data and symptom scores
Our analysis shows that from a panel of 10 typical common cold symptoms, survey participants reported headache, nasal congestion, exhaustion and joint pain to be most bothersome, possibly because they were also rated as the four most intense symptoms. Based on the pharmacodynamic profile of ibuprofen and pseudoephedrine as well as based on dedicated studies,10 neither monotherapy is likely to provide relief of all four major symptoms, lending support to the concept of using combination treatment in patients with multiple symptoms. Accordingly, such combination treatment was superior to monotherapy in controlled clinical trials.6 This may explain the high satisfaction with efficacy and overall treatment reported in the present survey.
More than half of all participants reported time to onset of symptom relief to be 30 min or less (Figure 1) and almost two‐thirds of participants considered time of onset to be fast or very fast. While we are not aware of previous studies exploring time to onset of symptom relief with ibuprofen/pseudoephedrine combinations, these values are in line with previous, placebo‐controlled studies on pseudoephedrine monotherapy in common cold patients, being based on subjective symptom relief3, 4, 14 or on objective measurements of nasal airway flow or resistance.4, 10, 14, 15, 18 They are also in line with the pharmacokinetic properties of pseudoephedrine which reaches t max at about 60 minutes with almost maximum plasma concentrations being observed at 30 min (earliest measured time point)6, 16; t max of ibuprofen is also seen after about 60 minutes.6 Therefore, our data based on subjective assessment with an ibuprofen/pseudoephedrine combination product in an open‐label setting together with previous subjective, objective and pharmacokinetic results from placebo‐controlled studies testify a fast time to onset of symptom relief. In contrast, data on duration of symptom relief after the first dose of medication are not easy to explain in the absence of a placebo group in the light of the natural course of the condition. However, rhinomanometry3 as well as pharmacokinetic studies16 support the concept of a dosing interval of 6 hours.
Placebo‐controlled studies have established that treatment of common cold with a single active ingredient improves some but not other symptoms. Specifically, pseudoephedrine was found to reduce subjective or objective measures of congestion3, 8, 10, 16 but has little effect on sneezing,8 on pain10 or on non‐congestion cold symptoms in general.3 In the present survey, the ibuprofen/pseudoephedrine combination product differentially affected the extent of intra‐individual improvement of specific symptoms. Thus, the greatest improvement (63%) was reported for headache, which also was the most bothersome symptom. A greater than 50% improvement was also reported for other forms of pain, joint pain, pharyngeal pain, and for fever and exhaustion (Table 1). In contrast, cough in general and cough with expectoration were improved by 32‐37%, but these two also had the lowest pretreatment values (Table 1). Nasal and sinus congestion were improved by about 50%. Thus, typical ibuprofen‐responsive symptoms were approved to a slightly greater extent than typical pseudoephedrine‐responsive or non‐specific symptoms, which is reflected in the operationally defined symptoms scores used in our analysis. The overall extent of improvement is comparable to values reported in placebo‐controlled trials, for instance for the degree of nasal congestion reduction with a 60 mg dose of pseudoephedrine 16 or the degree of reduction in nasal, throat and systemic symptoms with a 200 mg ibuprofen/60 mg pseudoephedrine combination.7
More than 95% of participants rated global tolerability as excellent or good in our survey; specific adverse events were not reported. This is in line with previously reported safety and tolerability findings in placebo‐controlled studies with an ibuprofen/pseudoephedrine combination.6, 7 Possible adverse effects on vital signs always have to be considered in the use of a sympathomimetic agent, such as pseudoephedrine. Being a survey of pharmacy customers, our study did not capture such data. However, a systematic review and meta‐analysis on possible cardiovascular effects of pseudoephedrine has looked at a total of 24 studies representing 45 treatment arms and 1285 patients; in this meta‐analysis pseudoephedrine did not significantly increase diastolic pressure, increased systolic blood pressures by less than 1 mm Hg and heart rate by less than 3 bpm,21 which are likely to have little consequence with drug used for a few days only.
4.3. Factors associated with greater treatment efficacy
The above descriptive data show that the results from a survey among pharmacy customers are in good agreement with those from placebo‐controlled studies. We also found data collection among pharmacy customers to be a scientifically valuable source of information in a previous airway project.22 The main advantages of such approach are that it can recruit a very broad, representative real life population with little restriction of inclusion/exclusion criteria and much larger numbers of patients than typically can be included in a classic randomised trial. These large numbers allow applying multiple regression approach to identify variables associated with positive treatment outcomes. In the light of the exploratory nature of these analyses, we have attempted to increase robustness by applying two parallel approaches that look at distinct but related outcomes (Tables 3 and 4).
The explanatory variable exhibiting the strongest effect on symptom improvement was baseline symptom intensity. As the general linear model approach used reduction in symptom intensity after intake of medication as dependent variable, this is not surprising as subjects with a high baseline value can reduce absolute symptom intensity to a greater extent. This phenomenon has also been observed in the analysis of databases from observational treatment studies in other indications, for instance arterial hypertension23 or overactive bladder syndrome.24
The combination product tested in this study includes 200 mg ibuprofen and 30 mg pseudoephedrine per tablet. Previous studies on combinations of these two ingredients have shown that the combination is more effective than either monotherapy but have used preparations containing 60 mg pseudoephedrine per dose.6, 7 This study found that for the 200 mg/30 mg combination starting treatment with two tablets is more efficacious than with a single tablet, irrespective of symptom score or of analytical approach being applied. This is in line with findings from previous studies comparing the efficacy of 50‐400 mg ibuprofen25 or in meta‐analyses of ibuprofen studies in analgesic indications, demonstrating an increased efficacy with higher doses in the 200‐800 mg dose range.26 It is also in line with dose‐ranging studies with 15‐180 mg pseudoephedrine effects on nasal airway resistance.19 Despite the greater symptom reduction when starting treatment with two tablets of the 200 mg ibuprofen/30 mg pseudoephedrine combination and the good tolerability of this combination product, we recommend that the higher dose should only be applied when justified by a high degree of symptom intensity and bother prior to treatment.
As common cold is a self‐limiting disease, we were interested in the question whether timing of start of treatment relative to onset of symptoms affects treatment efficacy. Previously reported placebo‐controlled studies using pseudoephedrine‐containing preparations were too small to allow differential analysis of efficacy relative to timing of treatment start. In our survey with much larger patient numbers, timing of treatment start had little effect on the typical ibuprofen‐response symptoms. In contrast, the typical pseudoephedrine‐responsive and the non‐specific symptoms responded less favourably when treatment was started on day 3 or 4 of a cold episode, possibly because nasal congestions tends to already have declined on day 4 or 5 of a cold episode. These findings imply that it is better to start treatment early if the symptom constellation in a patient with common cold justifies inclusion of pseudoephedrine or perhaps another decongestant in the treatment regimen. It would be interesting to see this conclusion confirmed in an independent study.
5. Conclusions
Based on a cohort of 1770 pharmacy customers with common cold purchasing a combination product containing 200 mg ibuprofen and 30 mg pseudoephedrine, we have shown overall efficacy and tolerability data that are comparable to those from placebo‐controlled studies. This and previous studies22 show that surveys among pharmacy customers can be a valuable source of scientific information, particularly for over‐the‐counter products. Treatment effects of the tested combination product are independent of gender and body weight of the patient and similar across age groups. Expectedly, baseline symptom intensity has a large effect on degree of improvement. Efficacy exhibited the expected dose‐dependency and was greater when treatment was started early in the course of a cold episode.
Disclosures
Ludger Klimek has received research grants from and/or has served on the speaker's bureau of ALK‐Abelló (Denmark); Allergopharma (Germany); Bionorica (Germany); Biomay (Austria), Boehringer Ingelheim (Germany), Circassia (USA); Stallergenes (France); HAL (Netherlands); Allergy Therapeutics/Bencard (United Kingdom/Germany); Hartington (Spain); Lofarma (Italy); MEDA (Sweden); MSD (USA); Novartis (Switzerland), Leti (Spain); ROXALL (Germany); GlaxoSmithKline (United Kingdom); Cytos (Switzerland); Curalogic (Denmark). He is a consultant for Bencard (Germany), HAL (Netherlands), MEDA (Germany) and Boehringer Ingelheim (Germany). Tanja Schütt, Heidemarie Gräter and Tobias Mück are employees of Boehringer Ingelheim; Helmut Schumacher and Martin C. Michel are former employees of Boehringer Ingelheim.
Author Contributions
Ludger Klimek: Data analysis/interpretation, Critical revision of article, Approval of article. Helmut Schumacher: Data analysis/interpretation, Critical revision of article, Approval of article, Statistics. Tanja Schütt and Heidemarie Grater: Concept/design, Data analysis/interpretation, Critical revision of article, Approval of article. Tobias Mück: Concept/design, Data analysis/interpretation, Critical revision of article, Approval of article, Funding secured. Martin C. Michel: Data analysis/interpretation, Drafting article, Critical revision of article, Approval of article, Statistics.
Acknowledgements
We thank the staff of the pharmacies and their customers who have participated in the study. Initial descriptive analysis was performed by Winicker Norimed GmbH Medizinische Forschung (Nuremburg, Germany).
References
- 1. Criscione S, Porro E. Infectious agent‐induced rhinitis In: Raeburn D, Giembycz MA, eds. Rhinitis: Immunopathology and Pharmacotherapy. Basel: Birkhäuser Verlag; 1997:147–160. [Google Scholar]
- 2. Garribaldi RA. Epidemiology of community‐acquired respiratory tract infections in adults. Incidence, etiology, and impact. Am J Med. 1985;78:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horak F, Zieglmayer P, Zieglmayer R, et al. A placebo‐controlled study of the nasal decongestant effect of phenylephrine and pseudoephedrine in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol. 2009;102:116–120. [DOI] [PubMed] [Google Scholar]
- 4. Eccles R, Jawad MS, Jawad SSM, et al. Efficacy and safety of single and multiple doses of pseudoephedrine in the treatment of nasal congestion associated with common cold. Am J Rhinol. 2005;19:25–31. [PubMed] [Google Scholar]
- 5. Eccles R, Martensson K, Chen SC. Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold. Curr Med Res Opin. 2010;26:889–899. [DOI] [PubMed] [Google Scholar]
- 6. Oliver SD, Rees TP, Daniel RJE. Pharmacokinetics and clinical activity of a soluble combination of ibuprofen and pseudoephedrine. Eur J Clin Res. 1996;8:269–280. [Google Scholar]
- 7. Sperber SJ, Sorrentino JV, Riker DK, Hayden FG. Evaluation of an alpha agonist alone and in combination with a nonsteroidal antiinflammatory agents in the treatment of experimental rhinovirus colds. Bull N Y Acad Med. 1989;65:145–160. [PMC free article] [PubMed] [Google Scholar]
- 8. Bye CE, Cooper J, Empey DW, et al. Effects of pseudoephedrine and triprolidine, alone and in combination, on symptoms of common cold. Br Med J. 1980;281:189–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loose I, Winkel M. Clinical, double‐blind, placebo‐controlled study investigating the combination of acetylsalicylic acid and pseudoephedrine for the symptomatic treatment of nasal congestion associated with common cold. Arzneimittelforschung. 2004;54:513–521. [DOI] [PubMed] [Google Scholar]
- 10. Eccles R, Jawad M, Jawad S, et al. Efficacy of a paracetamol–pseudoephedrine combination for treatment of nasal congestion and pain‐related symptoms in upper respiratory tract infection. Curr Med Res Opin. 2006;22:2411–2418. [DOI] [PubMed] [Google Scholar]
- 11. Eccles R, Pedersen A, Regberg D, et al. Efficacy and safety of topical combinations of ipratropium and xylometazoline for the treatment of symptoms of runny nose and nasal congestion associated with acute upper respiratory tract infection. Am J Rhinol. 2007;21:40–45. [DOI] [PubMed] [Google Scholar]
- 12. Schachtel BP, Voelker M, Sanner KM, et al. Demonstration of the analgesic efficacy and dose‐response of acetylsalicylic acid with pseudoephedrine. J Clin Pharmacol. 2010;50:1429–1437. [DOI] [PubMed] [Google Scholar]
- 13. Eccles R, Voelker M. Analgesic and decongestant efficacy of the combination of aspirin with pseudoephedrine in patients with symptoms of upper respiratory tract infection. Clin Pharmacol Drug Dev. 2014;3:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taverner D, Danz C, Economos D. The effects of oral pseudoephedrine on nasal patency in the common cold: a double‐blind single‐dose placebo‐controlled trial. Clin Otolaryngol Allied Sci. 1999;24:47–51. [DOI] [PubMed] [Google Scholar]
- 15. Latte J, Taverner D. Clinical trial of 3 days of treatment with oral pseudoephedrine for the common cold in the southern hemisphere. Am J Rhinol. 2007;21:452–455. [DOI] [PubMed] [Google Scholar]
- 16. Roth RP, Cantekin EI, Bluestone CD, et al. Nasal decongestant activity of pseudoephedrine. Ann Otol Rhinol Laryngol. 1977;86:235–242. [DOI] [PubMed] [Google Scholar]
- 17. Jawad SSM, Eccles R. Effect of pseudoephedrine on nasal air flow in patients with nasal congestion associated with common cold. Rhinology. 1998;36:73–76. [PubMed] [Google Scholar]
- 18. Latte J, Taverner D, Slobodian P, Shakib S. A randomized, double‐blind, placebo‐controlled trial of pseudoephedrine in coryza. Clin Exp Pharmacol Physiol. 2004;31:429–432. [DOI] [PubMed] [Google Scholar]
- 19. Empey DW, Young GA, Letley E, et al. Dose‐response study of the nasal decongestant and cardiovascular effects of pseudoephedrine. Br J Clin Pharmacol. 1980;9:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motulsky HJ. Common misconceptions about data analysis and statistics. Naunyn‐Schmiedeberg's Arch Pharmacol. 2014;387:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate: a meta‐analysis. Arch Intern Med. 2005;165:1686–1694. [DOI] [PubMed] [Google Scholar]
- 22. Kardos P, Schütt T, Mück T, et al. Pathophysiological factors in the relationship between chronological age and calculated lung age as detected in a screening setting in community‐dwelling subjects. Frontiers Med. 2016;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michel MC, Bohner H, Köster J, et al. Safety of telmisartan in patients with arterial hypertension. An open‐label observational study. Drug Saf. 2004;27:335–344. [DOI] [PubMed] [Google Scholar]
- 24. Schneider T, Marschall‐Kehrel D, Hanisch JU, Michel MC. Do gender, age or life style factors affect responses to anti‐muscarinic treatment in overactive bladder patients? Int J Clin Pract. 2010;64:1287–1293. [DOI] [PubMed] [Google Scholar]
- 25. Schou S, Nielsen H, Nattestad A, et al. Analgesic dose‐response relationship of ibuprofen 50, 100, 200, and 400 mg after surgical removal of third molards: a single‐dose, randomized, placebo‐controlled, and double‐blind study of 304 patients. J Clin Pharmacol. 1998;38:447–454. [DOI] [PubMed] [Google Scholar]
- 26. McQuay HJ, Moore A. Dose‐response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. Br J Clin Pharmacol. 2007;63:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]


