Abstract
Aims
Biomarkers can be used for diagnosis, risk stratification, or management of patients with heart failure (HF). Knowledge about the biological variation is needed for proper interpretation of serial measurements. Therefore, we aimed to determine and compare the biological variation of a large panel of biomarkers in healthy subjects and in patients with chronic HF.
Methods and results
The biological variability of established biomarkers [NT‐proBNP and high‐sensitivity troponin T (hsTnT)], novel biomarkers [galectin‐3, suppression of tumorigenicity 2 (ST2), and growth differentiation factor 15 (GDF‐15)], and renal/neurohormonal biomarkers (aldosterone, phosphate, parathyroid hormone, plasma renin concentration, and creatinine) was determined in 28 healthy subjects and 83 HF patients, over a period of 4 months and 6 weeks, respectively. The analytical (CVa), intraindividual (CVi), and interindividual (CVg) variations were calculated, as well as the reference change value (RCV), which reflects the percentage of change that may indicate a ‘relevant’ change. All crude biomarker levels were significantly increased or decreased in HF patients compared with controls (all P < 0.01). Variation indices were comparable in healthy individuals and HF patients. CVi was not influenced by the individual levels of the biomarker itself. NT‐proBNP and GDF‐15 had relatively high CVi (21.8% and 16.6%) and RCV (61.7% and 64.3%), whereas ST2 (CVi, 15.0; RCV, 42.9%), hsTnT (CVi, 11.1; RCV, 31.4%), and galectin‐3 (CVi, 8.1; RCV, 25.0%) had lower indices of variation.
Conclusion
Biological variation indices are comparable between healthy subjects and HF patients for a broad spectrum of biomarkers. NT‐proBNP and GDF‐15 have substantial variation, with lower variation for ST2, hsTnT, and galectin‐3. These data are instrumental in proper interpretation of biomarker levels in HF patients.
Keywords: Heart failure, Biomarkers, NT‐proBNP, Biological variation, Management programme
Introduction
Biomarkers are helpful for diagnosis, risk stratification, and management of patients. Incorporation of biomarkers in heart failure (HF) management is therefore recommended by the European and American guidelines, with different levels of evidence for various biomarkers.1, 2
For prognostic purposes, serial measures have been shown to provide superior power over a single measurement.3, 4, 5, 6, 7 Also, for disease monitoring, serial test results proved to be specifically useful.8, 9 Ideally, changes over time should reflect clinical improvement or disease progression. However, proper interpretation as to whether these changes over time are clinically relevant is complex. There are two factors that influence the variability of a biomarker: (i) the analytical variability (imprecision of the test); and (ii) the biological variability (expected variability within a subject over time).10
Although the concept of biological variation has been established in the 1980s,11 it is still evolving.12 One of the key parameters is the so‐called reference change value (RCV), which is the percentage change in a biomarker within an individual that is necessary to reflect a ‘true’ change. This technique was recently employed to define the significance of changes in high‐sensitivity troponin values in dialysis patients and manifested potent prognostic efficacy.13
However, studies on biological variation are relatively scarce, and most have been conducted in healthy subjects.14, 15, 16 Additionally, it remains questionable if biological variation in healthy subjects is identical to biological variation in disease patients. To date, few studies have therefore reported on biological variation in patients with HF.17, 18
It is currently unknown whether the variation in a diseased population is comparable, and for several old and new HF biomarkers, including aldosterone, renin, suppression of tumorigenicity 2 (ST2), and growth differentiation factor 15 (GDF‐15), biological variation is unknown.
Therefore, our aim was to assess the conjoint analytical and biological variation of the established clinical biomarkers NT‐proBNP and high‐sensitivity troponin T (hsTnT), the novel biomarkers, galectin‐3, ST2, and GDF‐15, the renal/neurohormonal biomarkers including creatinine, aldosterone, plasma renin concentration (PRC), phosphate, and parathyroid hormone (PTH), and the tightly regulated electrolyte calcium, in healthy subjects and in chronic HF patients. We determined which increases/decreases over time would be of clinical relevance and whether these results differ between healthy subjects and chronic HF patients.
Methods
Healthy subjects
The healthy subjects were enrolled locally at the University Medical Center Groningen (UMCG). They had no medical history of cardiac disease and reported to be healthy. Nobody received medication and all had normal estimated glomerular filtration rate (eGFR) and normal values of NT‐proBNP. We aimed to include 50% female subjects. A total of 30 subjects were included in this study to assess biological variation. Blood was drawn at five different time points each 4 weeks apart. A complete biomarker set was available in 28 subjects who were included in our analyses. This study was approved by the local Medical Ethics Committee (METC 2011.296).
Chronic heart failure patients
The HF patient cohort has been described in detail previously (Clinical trial identifier NCT01092130).19, 20 In brief, 101 HF patients ≥18 years of age with an LVEF <45% were included, who received optimal HF medication [i.e. ACE inhibitor or ARB), beta‐blocker, and mineralocorticoid receptor antagonist (MRA), when indicated]. In our substudy, all biomarker measurements were available at three time points (3 weeks apart) for 83 subjects.
Both studies and the current analyses have been performed conforming to the Declaration of Helsinki, and all study subjects provided written informed consent.
Biomarker assays
The markers NT‐proBNP, hsTnT, PTH, calcium, phosphate, and creatinine were measured using the Roche Modular system (Roche, Mannheim, Germany). Galectin‐3 was measured with the BG medicine enzyme‐linked immunosorbent assay (Waltham, MA, USA). Plasma ST2 was measured with the Presage® ST2 Assay (Critical Diagnostics, San Diego, CA, USA). The GDF‐15 concentrations were determined by a quantitative sandwich enzyme immunoassay technique (Quantikine®; R&D Systems, Inc., Minneapolis, MN, USA). The PRC was measured using a radioimmunometric assay kit for the quantitative determination of active renin (Cisbio International, Codolet, France). Plasma aldosterone was measured using a solid phase 125I radioimmunoassay (Siemens Diagnostics, The Netherlands). The same assays were used in both cohorts, and all samples of the same subjects over time were measured at the same time within the same plate.
Mathematical calculations for all biological variation‐associated parameters
Normally distributed, continuous data are expressed as mean values (±SD). In comparisons between groups, differences between mean values of continuous data were calculated using the two‐sample t‐test. Non‐normally distributed continuous data are expressed as median values [interquartile range (IQR)], and differences were calculated using the Mann–Whitney U‐test. Differences in categorical values were calculated using Pearson's χ2 test. We assessed the biological variation by the method of Fraser and Harris.10 The coefficient of variation (CV) is defined as the ratio of the standard deviation (σ) to the mean (μ):
The CV can be assessed for the differences between sample measurements [analytical variability (CVa)] or between subjects in the same cohort (CVg). The within‐individual biological CV (CVi) was calculated from the median CV between different time points of an individual (CVt) adjusted for the analytical imprecision (CVa):
The index of individuality (II), which can determine whether reference ranges or monitoring is appropriate, is calculated as follows:
The II has been used by many to investigate the utility of conventional population‐based reference values. For a high II (>1.4), it has been reported that reference intervals will be more useful than for a low index (<0.6).21
The symmetrical limits of the normal RCV were calculated as follows:
The Z score = 1.96 which corresponds to a 95% confidence level.
With the log‐normal approach, the median normal deviation of the log‐normal distribution (σln) was calculated from the median CVt (as a decimal value), as described by Fokkema et al.:22
The asymmetrical limits for the upward (positive) value for the log‐normal RCV (RCVpos) and for the downward (negative) value for the log‐normal RCV (RCVneg), were determined as:
We performed linear regression analyses to determine whether an association exists between the biomarker value and the variation within an individual.
For all analyses, P‐values <0.05 were considered to denote significant differences. Analyses were performed with Microsoft Excel 2007 and STATA software (version 13.0; Stata Corp, College Station, TX, USA).
Results
Baseline characteristics
Data from 28 healthy subjects were available for the current analyses. The mean (±SD) age was 43 (±13) years, and 14 (50%) subjects were female. Regarding chronic HF patients, 83 subjects had a complete biomarker set and were therefore eligible for this substudy. Most of the patients (92%) were classified as NYHA class II. A full description of the exclusion criteria for the HF cohort is presented on the last page of the Supplementary material online. These criteria were designed in order to enrol stable HF patients. To corroborate further the stability of our HF cohort, we repeated our analyses in those patients without an event (HF rehospitalization or all‐cause mortality) in ∼5 years (Supplementary material online, Table S1). Indeed, no differences were observed between patients with or without an event, supporting the (very) stable situation of the patients. The patients enrolled in this study were randomized to receive 2000 U of 25‐hydroxyvitamin D or no treatment. We observed no differences for CVi and/or RCV between the two study groups (data not shown). Chronic HF patients differed substantially from healthy controls with respect to cardiovascular risk factors such as smoking and diabetes, the use of medication, and biochemical variables. The mean (±SD) age was 64 (±10) years, and 4 (5%) subjects were female. Median (IQR) NT‐proBNP was 377 (223–777) pg/mL. The baseline characteristics of both cohorts are reported in Table 1.
Table 1.
Baseline characteristics of the healthy subjects and the chronic heart failure patients
| Characteristics | Controls (n = 28) | CHF (n = 83) |
|---|---|---|
| Age (years), mean (SD) | 43 (13) | 64 (10) |
| Male, n (%) | 14 (50) | 79 (95) |
| SBP (mm Hg), mean (SD) | 130 (22) | 116 (17) |
| DBP (mm Hg), mean (SD) | 81 (17) | 71 (11) |
| Heart rate, mean (SD) | 72 (12) | 68 (10) |
| BMI (kg/m2), mean (SD) | 24 (4) | 28 (4) |
| Hypertension, n (%) | – | 28 (34) |
| Hypercholesterolaemia, n (%) | – | 45 (54) |
| Diabetes, n (%) | – | 12 (14) |
| Current smoker, n (%) | 0 (0) | 19 (23) |
| HF history | ||
| Ischaemic aetiology | – | 60 (72) |
| NYHA II, n (%) | – | 76 (92) |
| NYHA III, n (%) | – | 7 (8) |
| LVEF (%), mean (SD) | – | 35 (8) |
| Treatment | ||
| Loop diuretic, n (%) | 0 (0) | 42 (51) |
| ACE inhibitor/ARB, n (%) | 0 (0) | 83 (100) |
| Beta‐blocker, n (%) | 0 (0) | 81 (98) |
| MRA, n (%) | 0 (0) | 23 (28) |
| Laboratory measurements | ||
| Creatinine (µmol/L), median (IQR) | 74 (67–83) | 89 (80–98) |
| NT‐proBNP (pg/mL), median (IQR) | 39 (18–57) | 377 (223–777) |
BMI, body mass index; CHF, chronic heart failure; DBP, diastolic blood pressure; HF, heart failure; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure.
Biological variation
All biomarker levels were significantly different between groups (Table 2). Nearly all biomarkers (except for GDF‐15) exhibited a CVa <5%. Although HF patients were stable, the CVg was larger for nearly all biomarkers compared with healthy controls. Only galectin‐3 and calcium showed comparable variation within the group (data not shown). To investigate further the biological variation, we calculated the CVi for both cohorts and observed no significant differences except for aldosterone, phosphate sodium, and PTH (Table 3). Comparing the CVi between all the biomarkers, creatinine had the lowest variation, which was comparable with the tightly regulated electrolytes calcium sodium. Regarding the cardiac markers, galectin‐3 and hsTnT demonstrated the lowest CVi. In healthy individuals, it can clearly be observed that the II was >0.6 for all the renal/neurohormonal markers except for creatinine and PTH, which implicates that population‐derived references can be applied.21 This is not the case for cardiac markers in both cohorts, all showing II <0.6. So, the patient‐specific set point is more important regarding interpretation than the references based upon the population. We calculated the RCV to determine the percentage change in serial measurements that would probably repress a true (statistically significant) rise or fall. Comparably with CVi, no differences in RCV of the biomarkers were observed between HF patients and controls except for aldosterone, phosphate, sodium and PTH. A complete overview of all the biological variation indices is presented in Table 4 (healthy subjects) and Table 5 (HF patients). In addition, besides the CVi, the RCV is also directly compared between healthy controls and HF patients in Table 3.
Table 2.
All biomarker levels compared between healthy subjects and chronic heart failure patients
| Controls (n = 28) | CHF (n = 83) | P‐value | |
|---|---|---|---|
| Established biomarkers | |||
| NT‐proBNP, ng/L | 39 (18–57) | 377 (223–777) | <0.001 |
| hsTnT, pg/mL | 3.2 (3.0–4.2) | 5.8 (3.0–12.9) | 0.001 |
| Novel biomarkers | |||
| Galectin‐3, ng/mL | 10.7 (9.3–12.5) | 16.1 (14.4–18.8) | <0.001 |
| GDF‐15, ng/L | 356 (292–533) | 923 (687–1441) | <0.001 |
| ST2, ng/mL | 22.0 (19.6–27.4) | 27.5 (21.9–33.6) | 0.003 |
| Renal/neurohormonal biomarkers | |||
| Creatinine, µmol/L | 74 (67–83) | 89 (80–98) | <0.001 |
| Plasma renin concentration, ng/L | 17 (13–22) | 74 (18–200) | <0.001 |
| Aldosterone, nmol/L | 0.22 (0.18–0.30) | 0.25 (0.14–0.40) | 0.028 |
| Phosphate, mmol/L | 1.2 (1.1–1.3) | 1.0 (0.9–1.0) | <0.001 |
| PTH, pmol/L | 4.7 (3.2–6.2) | 6.5 (5.0–9.3) | <0.001 |
| Electrolytes | |||
| Calcium, mmol/L | 2.4 (2.4–2.4) | 2.3 (2.2–2.3) | <0.001 |
| Sodium, mmol/L | 145 (143–147) | 141 (140–142) | <0.001 |
Values are median (interquartile range). CHF, chronic heart failure; GDF‐15, growth differentiation factor 15; hsTnT, high‐sensitivity troponin T; PTH, parathyroid hormone; ST2, suppression of tumorigenicity 2.
Table 3.
The within‐individual coefficient of variation and the reference change value compared between healthy controls and chronic heart failure patients
| Within‐individual coefficent of variation | Reference change value | |||||
|---|---|---|---|---|---|---|
| Controls | CHF | P‐value | Controls | CHF | P‐value | |
| Established biomarkers | ||||||
| NT‐proBNP | 25.1 | 21.8 | 0.36 | 70.7 | 61.7 | 0.37 |
| hsTnT | 16.0 | 11.1 | 0.13 | 44.9 | 31.4 | 0.13 |
| Novel biomarkers | ||||||
| Galectin‐3 | 8.1 | 8.1 | 0.97 | 24.6 | 25.0 | 0.92 |
| GDF‐15 | 18.9 | 16.6 | 0.40 | 69.9 | 64.3 | 0.44 |
| ST2 | 10.5 | 15.0 | 0.09 | 31.9 | 42.9 | 0.13 |
| Renal/neurohormonal biomarkers | ||||||
| Creatinine | 4.1 | 5.0 | 0.20 | 12.4 | 15.0 | 0.18 |
| Plasma renin concentration | 30.1 | 32.6 | 0.62 | 83.8 | 90.8 | 0.61 |
| Aldosterone | 36.6 | 27.7 | 0.033 | 104.2 | 80.2 | 0.031 |
| Phosphate | 6.9 | 10.7 | 0.021 | 19.8 | 30.0 | 0.024 |
| PTH | 16.7 | 22.5 | 0.019 | 46.3 | 62.4 | 0.019 |
| Electrolytes | ||||||
| Calcium | 1.7 | 1.6 | 0.45 | 6.6 | 6.3 | 0.53 |
| Sodium | 1.9 | 0.8 | <0.01 | 5.9 | 3.1 | <0.01 |
CHF, chronic heart failure; GDF‐15, growth differentiation factor 15; hsTnT, high‐sensitivity troponin T; PTH, parathyroid hormone; ST2, suppression of tumorigenicity 2.
Table 4.
Biological variation indices for all biomarkers in healthy subjects
| CVa | CVi | CVg | II | RCV (%) | Log normal | ||
|---|---|---|---|---|---|---|---|
| RCV up | RCV down | ||||||
| Established biomarkers | |||||||
| NT‐proBNP | 3.3 | 25.1 | 54.0 | 0.5 | 70.7 | 107.0 | −48.2 |
| hsTnT | 1.5 | 16.0 | 51.2 | 0.3 | 44.9 | 83.4 | −27.0 |
| Novel biomarkers | |||||||
| Galectin‐3 | 3.2 | 8.1 | 21.0 | 0.4 | 24.6 | 28.5 | −21.3 |
| GDF‐15 | 15.2 | 18.9 | 47.6 | 0.5 | 69.9 | 113.3 | −43.1 |
| ST2 | 2.9 | 10.5 | 30.4 | 0.4 | 31.9 | 40.9 | −25.0 |
| Renal/neurohormonal biomarkers | |||||||
| Creatinine | 1.6 | 4.1 | 14.4 | 0.3 | 12.4 | 13.3 | −11.5 |
| Plasma renin concentration | 2.3 | 30.1 | 41.6 | 0.7 | 83.8 | 154.1 | −50.7 |
| Aldosterone | 6.2 | 36.6 | 34.7 | 1.1 | 104.2 | 199.6 | −58.9 |
| Phosphate | 1.3 | 6.9 | 8.2 | 0.9 | 19.8 | 22.0 | −17.6 |
| PTH | 1.1 | 16.7 | 39.8 | 0.4 | 46.3 | 63.5 | −34.8 |
| hsTnT (n = 5) | 4.4 | 19.8 | 56.8 | 0.4 | 57.0 | 78.7 | −41.7 |
| Electrolytes | |||||||
| Calcium | 1.5 | 1.7 | 3.2 | 0.7 | 6.6 | 5.0 | −4.8 |
| Sodium | 0.7 | 1.9 | 2.3 | 1.6 | 5.9 | 4.8 | −4.5 |
CVa, analytical coefficient of variation; CVg, interindividual coefficient of variation; CVi, intraindividual coefficient of variation; GDF‐15, growth differentiation factor 15; hsTnT, high‐sensitivity troponin T; II, index of individuality; PTH, parathyroid hormone; RCV, reference change value; ST2, suppression of tumorigenicity 2.
Table 5.
Biological variation indices for all biomarkers in chronic heart failure patients
| CVa | CVi | CVg | II | RCV (%) | Log normal | ||
|---|---|---|---|---|---|---|---|
| RCV up | RCV down | ||||||
| Established biomarkers | |||||||
| NT‐proBNP | 3.3 | 21.8 | 116.3 | 0.2 | 61.7 | 104.9 | −40.1 |
| hsTnT | 1.5 | 11.1 | 96.6 | 0.1 | 31.4 | 42.6 | −22.1 |
| Novel biomarkers | |||||||
| Galectin‐3 | 3.2 | 8.1 | 21.2 | 0.4 | 25.0 | 30.2 | −20.1 |
| GDF‐15 | 15.2 | 16.6 | 77.1 | 0.3 | 64.3 | 78.2 | −38.3 |
| ST2 | 2.9 | 15.0 | 36.9 | 0.4 | 42.9 | 62.7 | −31.4 |
| Renal/neurohormonal biomarkers | |||||||
| Creatinine | 1.6 | 5.0 | 19.6 | 0.3 | 15.0 | 16.5 | −13.3 |
| Plasma renin concentration | 2.3 | 32.6 | 222.2 | 0.1 | 90.8 | 180.6 | −51.2 |
| Aldosterone | 6.2 | 27.7 | 91.1 | 0.3 | 80.2 | 139.3 | −48.9 |
| Phosphate | 1.3 | 10.7 | 17.4 | 0.6 | 30.0 | 38.7 | −24.3 |
| PTH | 1.1 | 22.5 | 49.2 | 0.5 | 62.4 | 93.0 | −43.3 |
| hsTnT (n = 49) | 4.4 | 13.4 | 70.4 | 0.1 | 37.6 | 52.1 | −28.3 |
| Electrolytes | |||||||
| Calcium | 1.5 | 1.6 | 3.3 | 0.7 | 6.3 | 5.4 | −5.0 |
| Sodium | 0.7 | 0.8 | 1.3 | 0.9 | 3.1 | 2.7 | −2.6 |
CVa, analytical coefficient of variation; CVg, interindividual coefficient of variation; CVi, intraindividual coefficient of variation; GDF‐15, growth differentiation factor 15; hsTnT, high‐sensitivity troponin T; II, index of individuality; PTH, parathyroid hormone; RCV, reference change value; ST2, suppression of tumorigenicity 2.
Biomarker variation over the biomarker spectrum
We assessed whether the degree of variation would be associated with the circulating levels of a given biomarker. Therefore, we performed linear regression analyses, relating the actual baseline biomarker levels to the variation. We observed no clear association(s) between the CVi and the level of the biomarker, as displayed in Figure 1 and in Figure S1 of the Suppplementary material online, except for phosphate and sodium.
Figure 1.
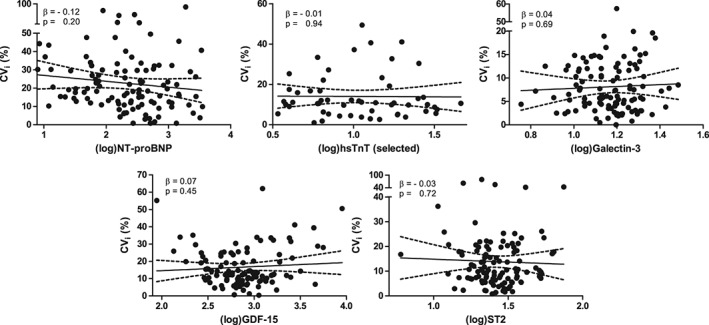
Scatter plot with regression line of the intraindividual coefficient of variation (CVi)i and the log‐transformed biomarker level (established and novel). hsTnT, high‐sensitivity troponin T; GDF‐15, growth differentiation factor 15; ST2, suppression of tumorigenicity 2.
Sample regimen
Timing and frequency of sampling are important factors to be considered when obtaining reliable CVi and RCV. To explore this, we performed a sensitivity analysis with another sampling regimen, comparing CVi and RCV in controls for five sample time points (4 weeks in between) with three sample time points (also 4 weeks in between; Supplementary material online, Figure S2 and Table S2). Collectively, the indices were numerically in the same order of magnitude, and statistical differences between healthy controls and chronic HF patients were comparable regardless of the sample regimen. However, for some markers, there were differences, especially for hsTnT. We conclude, therefore, that biological variation indices may fluctuate with the sample regimen, and this should be addressed in studies such as ours.
Biomarker values and gender differences
Tables S3 and S4 in the Supplementary material online display the levels of all the biomarkers for the complete data set, stratified by sex in both healthy subjects and chronic HF patients. In healthy subjects, NT‐proBNP (P < 0.001), PRC (P = 0.013), and creatinine (P < 0.001) were significantly different between males and females. In the HF cohort, we observed a comparable difference regarding creatinine (P = 0.006). In addition, lower ST2 levels (P = 0.008) and higher phosphate levels (P = 0.01) were observed in females compared with males.
Discussion
Although several biomarkers are incorporated in daily clinical care of HF, and the use of several others may become more common in the coming years, we have insufficient knowledge on biological variation in patients suffering from HF. Such knowledge is critical to ascertain whether a given change in biomarker levels indicates if a HF patient migrates from a stable phase to a vulnerable phase, with higher risk for (acute) admission.
Our study provides the indices of biological variation in healthy subjects and chronic HF patients. We did not limit our study to a single biomarker but measured the biological variation of multiple HF biomarkers which are (i) established; (ii) have great potential; and (iii) are related to the renal/neurohormonal system. Although the crude levels of the biomarkers are significantly different between healthy subjects and stable HF patients, a comparable biological variation was clearly observed. Even the CVi was not influenced by individual levels of the biomarker itself. These findings might be similar regarding other patient groups such as patients with kidney disease or high blood pressure, although future research needs to unravel this further.
Most studies regarding the biological variation of natriuretic peptides have focused on healthy individuals,23, 24 but recently several studies addressed biological variation of HF biomarkers. Bruins et al. 17 focused on natriuretic peptides and demonstrated in 43 HF patients that NT‐proBNP had lower variation than BNP. They observed an even higher RCV of NT‐proBNP compared with our study (98%). Schindler et al. 18 demonstrated in 20 controls and 59 HF patients the biological variation of galectin‐3, BNP, and troponin I. The CVi and RCV of galectin‐3 were comparable with those of our study and they concluded that galectin‐3 could be a useful asset in monitoring HF patients because of its low biological variation indices. Since they measured BNP and troponin I, but neither NT‐proBNP nor hsTnT or any other marker, a direct comparison with our data is not possible. Finally, Wu et al. 16 investigated the biological variation of galectin‐3 and ST2 in 12 healthy subjects, sampling blood every 2 weeks for 8 weeks. In this study, the RCV for ST2 and galectin‐3 was 30% and 60%, respectively, but we could not validate these RCVs. In our HF patients, we demonstrated an RCV for galectin‐3 of 25% (and of 25% in healthy controls). This RCV for HF patients is almost identical to that reported by Schindler et al. (27%),18 but these authors reported an even lower RCV for galectin‐3 in healthy controls (15%). Very recently, Piper et al. 25 demonstrated in 50 patients with chronic HF that variability indices of ST2 are comparable with short‐ and long‐term sample intervals (from hours to 6 months). We report an RCV of 32% in healthy controls, which is close to the RCV reported by Wu et al.,16 whereas for HF patients the RCV was 43%, comparable with that of Piper et al. 25 Solid insights in the RCV of ST2 may be useful, as the use of serial ST2 samples has been advocated to guide drug treatment.26
Further, this study also reports on biological variation of several other neurohormonal and emerging biomarkers. The renin and aldosterone hormones have been studied in HF patients and provide some prognostic information.27, 28 We, however, herein show that the biological variation is very wide and thus only substantial changes (doubling or more) could be considered as relevant. PTH is a marker of parathyroid function but, recently, there has been strong interest in its potential role in HF development.29 We now report that RCV for PTH appears to be in the same range as NT‐proBNP. Markers of renal function such as creatinine are widely used and we show that CVi is low. There are numerous studies that addressed the clinical utility of creatinine and other markers of renal function.30 Finally, GDF‐15 is an emerging biomarker of cardiovascular disease and HF.31, 32 Our results show that CVi and RCV are comparable with NT‐proBNP, suggesting that GDF‐15 may vary considerably within and between individuals, and that only substantial variation hints towards relevant changes.
The size and design of our study also allowed us to conduct several subanalyses. We observed significantly lower NT‐proBNP levels in healthy male subjects compared with female subjects. Costello‐Boerrigter et al. 33 stated that NT‐proBNP levels in healthy subjects are primarily affected by gender and age, and that this should be considered when interpreting values. As in the control group, only few females were present in the chronic HF cohort, but they had significantly lower ST2 levels than males. This was also observed in the Framingham Heart Study.34
From a clinical point of view, a pre‐defined threshold is usually observed when considering serial measurements of HF biomarkers (does it rise above or below the threshold?). The assumption is that this methodology would identify patients who are at high/low risk or, when they go over the threshold, should be reclassified into low/high risk (categorical). This approach neglects, at least to some extent, the individual change within a subject. Clinicians could make use of the RCVs derived from our study and other studies in clinical practice, and this could help to initiate a more tailored clinical approach.
Although some speculate that the interpretation of variation, especially of natriuretic peptides, should only be based upon clinical criteria,35 we argue that when a patient‐specific threshold is passed, this is a sign of either disease improvement or deterioration.
Clinically, there are numerous studies that reported the value of serial biomarker measurements. For instance, serial measurements of NT‐proBNP,7 TnT,36 galectin‐3,37 ST2,5 and GDF‐1538 were performed in the Valsartan Heart Failure Trial (Val‐HeFT). In the relative change analyses, the authors frequently used, amongst other methodology, a cut‐off of 15–30%, but rarely was a clear rationale behind these decisions provided. Thus, it may be that those groups with higher values are enriched with patients whose values will continue to diminish. However, such cut‐off values may not serve individual patients optimally. Van der Velde et al. demonstrated a similar prognostic value of changes in galectin‐3 levels over time in the Coordinating study evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) study.6 Although changes over time of all these biomarkers were reported to have prognostic value, clearly the biological variation profile of these markers is very different, and it would have been logical to study the changes keeping in mind the biomarker‐specific RCVs. In general, for biomarkers with high within‐individual or between‐individual biological variation, it is less clear what a given variation ‘reveals’. In the HABIT trial (Heart Failure assessment with BNP in the Home)39 nearly 7000 BNP values were recorded on a daily basis in 163 acute HF patients after discharge. It was observed that ‘normal’ fluctuation within these patients is hard to predict, and extremely variable BNP values were observed in these more severe and recently unstable HF patients. We argue that studies on variability should be conducted preferably during stable periods, but it should also be considered that there may be differences in variability between measurements performed hour to hour, or week to week, or month to month. Even more difficult, assessment of variability in acute HF patients is also a topic that has not yet been explored in depth. Minor variations in NT‐proBNP and GDF‐15 are difficult to interpret, and they might not be so useful in monitoring individual patients. On the other hand, variations in galectin‐3, hsTnT, and ST2 are more likely to hint towards a true change. A biomarker with less variation may seem to be inferior to pick up changes in clinical status as compared with those biomarkers that have larger variation—at the expense of less accuracy due to this larger variation. Therefore, the RCV should be placed into perspective of the expected changes of the biomarker. If the range of a biomarker is limited, a relatively low RCV could be more relevant than a larger one for a biomarker that has a much larger range.
Strengths and weaknesses
This is the largest study addressing biological variation in HF biomarkers including both healthy subjects and chronic HF patients, testing a broad range of established, novel, and renal/neurohormonal biomarkers. We also describe sex differences for all biomarkers and electrolytes in both healthy subjects and HF patients. We report biological variation of the emerging markers ST2 and GDF‐15 for the first time. Owing to the study design, we could only assess biological variation for a time period of 6 weeks where we obtained three blood samples, and thus cannot provide hourly or daily variation. Because of the observational design of the HF study, we were not able to assess to what extent the calculated RCV would be of prognostic value. Both studies only included Caucasian subjects. Although we attempted to use commonly available, widely used, commercial assays, we are aware that some assays may have advantages over others.40, 41, 42
In addition, we studied by design a well‐treated stable HF cohort. Indeed, studying unstable patients is much more complex since they may manifest disease‐related changes in addition to biological variation. Nonetheless, we would argue that our data provide a reasonable baseline to us even with less stable patients.
Conclusion
We determined the biological variation of a broad spectrum of well‐established and novel biomarkers of HF in 28 healthy subjects and 83 chronic HF patients. Indices of biological variation of this large biomarker panel were comparable for both groups. We confirm a high CVi and RCV of NT‐proBNP and describe this for GDF‐15 as well, whereas other biomarkers showed lower variation: ST2, hs‐TnT, and galectin‐3 (in descending order). A demonstrated lower CVi and RCV renders biomarkers more suitable for patient follow‐up and biomarker targeted strategy programmes, and such indices should be described in studies with serial biomarker measurements.
Supporting information
Figure S1. Scatter plot with regression line of the intraindividual coefficient of variation (CVi) and the log‐transformed biomarker level (renal/neurohormonal and electrolyte).
Figure S2. Outline of timing and frequency of sampling of both cohorts (original study and sensitivity analysis).
Table S1. The intraindividual coefficient of variation (CVi) and the reference change value (RCV) compared between the complete chronic HF cohort and those chronic HF patients without an event (HF rehospitalization and/or all‐cause mortality).
Table S2. (A) The intraindividual coefficient of variation (CVi) of the original study and the sensitivity analysis. (B) The reference change value (RCV) of the original study and the sensitivity analysis.
Table S3. Biomarker values of healthy subjects; all subjects and separately for males and females.
Table S4. Biomarker values of chronic HF patients; all subjects and separately for males and females.
Acknowledgements
The work of Mr J. Koerts in co‐ordinating the samples for the different assays is greatly appreciated.
Conflict of interest: none declared.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 3. deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N‐terminal pro‐B‐type natriuretic peptide testing. J Am Coll Cardiol 2010;55:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schou M, Gustafsson F, Videbaek L, Andersen H, Toft J, Nyvad O, Ryde H, Fog L, Jensen JC, Nielsen OW, Lind‐Rasmussen S, Abdulla J, Hildebrandt PR, Investigators NorthStar. Adding serial N‐terminal pro brain natriuretic peptide measurements to optimal clinical management in outpatients with systolic heart failure: a multicentre randomized clinical trial (NorthStar monitoring study). Eur J Heart Fail 2013;15:818–827. [DOI] [PubMed] [Google Scholar]
- 5. Anand IS, Rector TS, Kuskowski M, Snider J, Cohn JN. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ Heart Fail 2014;7:418–426. [DOI] [PubMed] [Google Scholar]
- 6. van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, Muntendam P, van Veldhuisen DJ, de Boer RA. Prognostic value of changes in galectin‐3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail 2013;6:219–226. [DOI] [PubMed] [Google Scholar]
- 7. Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, Tognoni G, Cohn JN, Val‐HeFT Investigators . Prognostic value of changes in N‐terminal pro‐brain natriuretic peptide in Val‐HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol 2008;52:997–1003. [DOI] [PubMed] [Google Scholar]
- 8. Pascual‐Figal DA, Domingo M, Casas T, Gich I, Ordonez‐Llanos J, Martinez P, Cinca J, Valdes M, Januzzi JL, Bayes‐Genis A. Usefulness of clinical and NT‐proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. Eur Heart J 2008;29:1011–1018. [DOI] [PubMed] [Google Scholar]
- 9. Shah MR, Califf RM, Nohria A, Bhapkar M, Bowers M, Mancini DM, Fiuzat M, Stevenson LW, O'Connor CM. The STARBRITE trial: a randomized, pilot study of B‐type natriuretic peptide‐guided therapy in patients with advanced heart failure. J Card Fail 2011;17:613–621. [DOI] [PubMed] [Google Scholar]
- 10. Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409–437. [DOI] [PubMed] [Google Scholar]
- 11. Harris EK, Yasaka T. On the calculation of a ‘reference change’ for comparing two consecutive measurements. Clin Chem 1983;29:25–30. [PubMed] [Google Scholar]
- 12. Simundic AM, Bartlett WA, Fraser CG. Biological variation: a still evolving facet of laboratory medicine. Ann Clin Biochem 2015;52:189–190. [DOI] [PubMed] [Google Scholar]
- 13. Fahim MA, Hayen AD, Horvath AR, Dimeski G, Coburn A, Tan KS, Johnson DW, Craig JC, Campbell SB, Hawley CM. Biological variation of high sensitivity cardiac troponin‐T in stable dialysis patients: implications for clinical practice. Clin Chem Lab Med 2015;53:715–722. [DOI] [PubMed] [Google Scholar]
- 14. Frankenstein L, Wu AH, Hallermayer K, Wians FH, Jr, Giannitsis E, Katus HA. Biological variation and reference change value of high‐sensitivity troponin T in healthy individuals during short and intermediate follow‐up periods. Clin Chem 2011;57:1068–1071. [DOI] [PubMed] [Google Scholar]
- 15. Wu AH, Smith A. Biological variation of the natriuretic peptides and their role in monitoring patients with heart failure. Eur J Heart Fail 2004;6:355–358. [DOI] [PubMed] [Google Scholar]
- 16. Wu AH, Wians F, Jaffe A. Biological variation of galectin‐3 and soluble ST2 for chronic heart failure: implication on interpretation of test results. Am Heart J 2013;165:995–999. [DOI] [PubMed] [Google Scholar]
- 17. Bruins S, Fokkema MR, Romer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, Muskiet FA. High intraindividual variation of B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in patients with stable chronic heart failure. Clin Chem 2004;50:2052–2058. [DOI] [PubMed] [Google Scholar]
- 18. Schindler EI, Szymanski JJ, Hock KG, Geltman EM, Scott MG. Short‐ and long‐term biologic variability of galectin‐3 and other cardiac biomarkers in patients with stable heart failure and healthy adults. Clin Chem 2015;62:360–366. [DOI] [PubMed] [Google Scholar]
- 19. Schroten NF, Ruifrok WP, Kleijn L, Dokter MM, Sillje HH, Lambers Heerspink HJ, Bakker SJ, Kema IP, van Gilst WH, van Veldhuisen DJ, Hillege HL, de Boer RA. Short‐term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open‐label, blinded end point, randomized prospective trial (VitD‐CHF trial). Am Heart J 2013;166:357–364.e2. [DOI] [PubMed] [Google Scholar]
- 20. Meijers WC, van der Velde AR, Ruifrok WP, Schroten NF, Dokter MM, Damman K, Assa S, Franssen CF, Gansevoort RT, van Gilst WH, Sillje HH, de Boer RA. Renal handling of galectin‐3 in the general population, chronic heart failure, and hemodialysis. J Am Heart Assoc 2014;3:e000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petersen PH, Fraser CG, Sandberg S, Goldschmidt H. The index of individuality is often a misinterpreted quantity characteristic. Clin Chem Lab Med 1999;37:655–661. [DOI] [PubMed] [Google Scholar]
- 22. Fokkema MR, Herrmann Z, Muskiet FA, Moecks J. Reference change values for brain natriuretic peptides revisited. Clin Chem 2006;52:1602–1603. [DOI] [PubMed] [Google Scholar]
- 23. Melzi d'Eril G, Tagnochetti T, Nauti A, Klersy C, Papalia A, Vadacca G, Moratti R, Merlini G. Biological variation of N‐terminal pro‐brain natriuretic peptide in healthy individuals. Clin Chem 2003;49:1554–1555. [DOI] [PubMed] [Google Scholar]
- 24. Klersy C, d'Eril GV, Barassi A, Palladini G, Comelli M, Moratti R, Albertini R, Merlini G. Advantages of the lognormal approach to determining reference change values for N‐terminal propeptide B‐type natriuretic peptide. Clin Chim Acta 2012;413:544–547. [DOI] [PubMed] [Google Scholar]
- 25. Piper S, deCourcey J, Sherwood R, Amin‐Youssef G, McDonagh T. Biologic variability of soluble ST2 in patients with stable chronic heart failure and implications for monitoring. Am J Cardiol 2016;118:95–98. [DOI] [PubMed] [Google Scholar]
- 26. Gaggin HK, Motiwala S, Bhardwaj A, Parks KA, Januzzi JL Jr. Soluble concentrations of the interleukin receptor family member ST2 and beta‐blocker therapy in chronic heart failure. Circ Heart Fail 2013;6:1206–1213. [DOI] [PubMed] [Google Scholar]
- 27. zymanski MK, Damman K, van Veldhuisen DJ, van Gilst WH, Hillege HL, de Boer RA. Prognostic value of renin and prorenin in heart failure patients with decreased kidney function. Am Heart J 2011;162:487–493. [DOI] [PubMed] [Google Scholar]
- 28. Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN, Investigators Val‐HeFT. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val‐HeFT. Eur Heart J 2004;25:292–299. [DOI] [PubMed] [Google Scholar]
- 29. Hagstrom E, Ingelsson E, Sundstrom J, Hellman P, Larsson TE, Berglund L, Melhus H, Held C, Michaelsson K, Lind L, Arnlov J. Plasma parathyroid hormone and risk of congestive heart failure in the community. Eur J Heart Fail 2010;12:1186–1192. [DOI] [PubMed] [Google Scholar]
- 30. van Veldhuisen DJ, Ruilope LM, Maisel AS, Damman K. Biomarkers of renal injury and function: diagnostic, prognostic and therapeutic implications in heart failure. Eur Heart J 2016;37:2577–2785. [DOI] [PubMed] [Google Scholar]
- 31. Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor‐15 in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1054–1060. [DOI] [PubMed] [Google Scholar]
- 32. Chan MM, Santhanakrishnan R, Chong JP, Chen Z, Tai BC, Liew OW, Ng TP, Ling LH, Sim D, Leong KT, Yeo PS, Ong HY, Jaufeerally F, Wong RC, Chai P, Low AF, Richards AM, Lam CS. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2016;18:81–88. [DOI] [PubMed] [Google Scholar]
- 33. Costello‐Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, Jacobsen SJ, Heublein DM, Burnett JC Jr. Amino‐terminal pro‐B‐type natriuretic peptide and B‐type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 2006;47:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, Cheng S, Fradley MG, Kretschman D, Gao W, O'Connor G, Wang TJ, Januzzi JL. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem 2012;58:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clerico A, Zucchelli GC, Pilo A, Emdin M. Clinical relevance of biological variation of B‐type natriuretic peptide. Clin Chem 2005;51:925–926. [DOI] [PubMed] [Google Scholar]
- 36. Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, Maggioni AP, Tavazzi L, Tognoni G, Cohn JN, Latini R, Valsartan Heart Failure Trial (Val‐HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca‐Heart Failure (GISSI‐HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012;125:280–288. [DOI] [PubMed] [Google Scholar]
- 37. Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin‐3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val‐HeFT. Eur J Heart Fail 2013;15:511–518. [DOI] [PubMed] [Google Scholar]
- 38. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth‐differentiation factor‐15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation 2010;122:1387–1395. [DOI] [PubMed] [Google Scholar]
- 39. Maisel A, Barnard D, Jaski B, Frivold G, Marais J, Azer M, Miyamoto MI, Lombardo D, Kelsay D, Borden K, Iqbal N, Taub PR, Kupfer K, Clopton P, Greenberg B. Primary results of the HABIT Trial (heart failure assessment with BNP in the home). J Am Coll Cardiol 2013;61:1726–1735. [DOI] [PubMed] [Google Scholar]
- 40. Meijers WC, van der Velde AR, de Boer RA. The ARCHITECT galectin‐3 assay: comparison with other automated and manual assays for the measurement of circulating galectin‐3 levels in heart failure. Expert Rev Mol Diagn 2014;14:257–266. [DOI] [PubMed] [Google Scholar]
- 41. Dieplinger B, Januzzi JL,Jr, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. Analytical and clinical evaluation of a novel high‐sensitivity assay for measurement of soluble ST2 in human plasma—the Presage ST2 assay. Clin Chim Acta 2009;409:33–40. [DOI] [PubMed] [Google Scholar]
- 42. Kempf T, Horn‐Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC. Circulating concentrations of growth‐differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem 2007;53:284–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scatter plot with regression line of the intraindividual coefficient of variation (CVi) and the log‐transformed biomarker level (renal/neurohormonal and electrolyte).
Figure S2. Outline of timing and frequency of sampling of both cohorts (original study and sensitivity analysis).
Table S1. The intraindividual coefficient of variation (CVi) and the reference change value (RCV) compared between the complete chronic HF cohort and those chronic HF patients without an event (HF rehospitalization and/or all‐cause mortality).
Table S2. (A) The intraindividual coefficient of variation (CVi) of the original study and the sensitivity analysis. (B) The reference change value (RCV) of the original study and the sensitivity analysis.
Table S3. Biomarker values of healthy subjects; all subjects and separately for males and females.
Table S4. Biomarker values of chronic HF patients; all subjects and separately for males and females.


