Abstract
Aim
Periodontitis is a chronic inflammatory disease, characterized by irreversible destruction of tooth‐supporting tissue including alveolar bone. We recently reported mucin 4 (MUC4) and matrix metalloproteinase 7 (MMP7) as highly associated with periodontitis in gingival tissue biopsies. The aim of this study was to further investigate the levels of MUC4 and MMP7 in saliva and gingival crevicular fluid (GCF) samples of patients with periodontitis.
Materials and Methods
Saliva and GCF samples were collected from periodontitis patients and healthy controls. The levels of MUC4, MMP7, and total protein concentrations were analysed using ELISA or Bradford assay.
Results
MUC4 levels were significantly lower in saliva and GCF from periodontitis patients relative to healthy controls. MMP7 levels were significantly higher in saliva and GCF from periodontitis patients. Multivariate analysis revealed that MUC4 was significantly associated with periodontitis after adjusting for age and smoking habits and, moreover, that the combination of MUC4 and MMP7 accurately discriminated periodontitis from healthy controls.
Conclusions
MUC4 and MMP7 may be utilized as possible novel biomarkers for periodontitis.
Keywords: gingival crevicular fluid, matrix metalloproteinase 7, mucin 4, periodontitis, saliva
Periodontitis is an infection‐induced chronic inflammatory disease, which, in its severe form, affects 9–15% of the adult population worldwide (Petersen & Ogawa 2005, Eke et al. 2012, Kassebaum et al. 2014). The disease may lead to irreversible destruction of tooth‐supporting tissue including alveolar bone and, if left untreated, tooth loss. Periodontitis is initiated by a biofilm formation on the teeth, in proximity to the gingival tissue. Products from the biofilm, such as lipopolysaccharides (LPS), get access to the gingival tissue, initiating an immune and inflammatory response. This results in release of inflammatory mediators, including cytokines, prostaglandins, and reactive oxygen species, as well as proteolytic enzymes such as matrix metalloproteinases (MMPs). The production of these proinflammatory mediators collectively, in cascades, contributes to destruction of gingival tissue and alveolar bone surrounding the teeth (Sorsa et al. 2006, Hernandez et al. 2011, Yucel‐Lindberg & Bage 2013).
The first line of defence against microbes is the saliva, which contains various proteins involved in innate and acquired immune activation. Some of these proteins, such as immunoglobulins and chaperokine HSP70/HSPA are involved in both innate and acquired immune activation, while certain salivary proteins, including cationic peptides, lysozyme, amylase, cystatins, proline‐rich proteins, peroxidases, statherins, and mucins are mainly involved in innate immunity (Fabian et al. 2012).
Mucins play a central role in innate immunity by promoting aggregation and clearance of bacteria from the oral cavity. Mucins are the main gel‐forming component of the salivary film that covers the epithelium, functioning as a diffusion membrane against pathogenic substances (Hollingsworth & Swanson 2004, Derrien et al. 2010). The mucin family consists of at least 20 members (Frenkel & Ribbeck 2015), which can be broadly classified into two subgroups, namely secreted and cell surface mucins (Derrien et al. 2010). Secreted mucins act by modulating other proteins in the saliva as well as by interacting with microbes to facilitate their removal and reduce their pathogenicity (Frenkel & Ribbeck 2015).
The activation of proinflammatory cytokines, in response to microbial products, also stimulates up‐regulation of proteolytic enzymes, including MMPs. Structurally related but genetically distinct MMPs are the main proteins responsible for extracellular matrix remodelling and are considered to contribute to the pathogenesis of periodontitis through destruction of periodontal tissue (Sorsa et al. 2006, Silva et al. 2015). Furthermore, they can process various bioactive non‐matrix substrates including cytokines, chemokines, complement components, serum proteins and cell signalling, and serum molecules, thereby modulating immune responses (Sorsa et al. 2006, Hernandez et al. 2011). Several MMPs, including MMP8 and MMP9, have repeatedly been reported as elevated in saliva and gingival crevicular fluid (GCF) samples of periodontitis patients (Mantyla et al. 2003, Pozo et al. 2005, Beklen et al. 2006, Miller et al. 2006, Passoja et al. 2008, Rai et al. 2008, Hernandez Rios et al. 2009, Ramseier et al. 2009, Gursoy et al. 2010, Isaza‐Guzman et al. 2011, Kinane et al. 2011, Rathnayake et al. 2013, Sorsa et al. 2016). The activity of MMPs is controlled at gene expression level, by activation of their proenzymes, and by inhibition via specific inhibitors, such as tissue inhibitors of matrix metalloproteinases (TIMPs) (Folgueras et al. 2004, Sbardella et al. 2012, Silva et al. 2015). These inhibitors are tissue specific endogenous inhibitors of metalloproteinases (Arpino et al. 2015). A disturbed ratio between MMPs and TIMPs causes an imbalance in the tissue breakdown and repair of the extracellular matrix (Al‐Azri et al. 2013). The regulation of MMP activity is crucial for tissue homoeostasis (Al‐Azri et al. 2013, Khokha et al. 2013).
We have recently reported MUC4 and MMP7 as differentially expressed in gingival tissue biopsies from periodontitis patients and healthy controls, analysed by RNA sequencing. The protein products of these genes were also confirmed as differentially expressed in gingival tissue biopsies from periodontitis patients and healthy controls (Lundmark et al. 2015). In this study, we aim to further investigate the levels of MUC4 and MMP7 in saliva and GCF samples from periodontitis patients and healthy controls.
Materials and methods
Ethics statement
This study was performed in accordance with the Declaration of Helsinki and current Swedish legislation. The sample collection was approved by the Ethical Board at the University of Lund with reference number 513/2006 and by the Regional Ethical Review Board in Stockholm with reference number 2014/1588 – 32/3. Written informed consent was obtained from all participants.
Collection and preparation of saliva samples
For analysis of saliva samples, individuals with periodontitis (n = 37) and healthy controls (n = 39) from a population from southern Sweden undergoing dental examination (Lundegren et al. 2012) were included in this study. Anamnestic data including diseases and smoking habits were collected. For the patients diagnosed with periodontitis, gingival inflammation, registered as bleeding on probing (BOP), was more than 30% and all patients had loss of supporting tissues exceeding 1/3 of the root length, determined through radiographical examinations. The majority of these patients (84%) had pocket probing depth (PPD) ≥ 6 mm and six patients (16%) had PPD 4–5 mm. The healthy controls included individuals with no signs of bone loss on radiographs, PPD < 4 mm, and BOP less than 30%.
Stimulated saliva samples were obtained by chewing paraffin tablets for 5 min while the saliva was collected into test tubes. After collection, the saliva samples were immediately frozen at −20°C until further processing. The samples where then thawed and centrifuged at 500 g for 10 min at 4°C. The supernatants were aliquoted into 1.5 ml Eppendorf tubes and stored at −80°C until analysis.
Analysis of salivary levels of total protein, MUC4, and MMPs
The total protein concentrations were measured using the Bradford assay (Bio‐Rad, Hercules, CA, USA) according to the manufacturer's instructions, using bovine serum albumin as standard. The levels of MUC4 and MMP7 were measured using commercially available ELISA kits according to the respective manufacturer's protocols (MUC4: Kamiya Biomedical Company, Seattle, WA, USA, MMP7: Quantikine; R&D Systems, Minneapolis, MN, USA). Before analysis of MUC4 and MMP7, the saliva samples were diluted 1:2 in phosphate buffered saline (PBS) and in calibrator diluent buffer respectively. The sensitivities for the assays used were 0.134 ng/ml for MUC4, 0.084 ng/ml for MMP7, and 0.08 ng/ml for MMP8 (Tuomainen et al. 2007). After optical density readings, all readings that fell below the assay sensitivity were set to the lowest point of the assay sensitivity. The levels of MMP8 were measured by a time‐resolved immunofluorometric assay, using monoclonal MMP8‐antibody 8708 (Medix Biochemica, Kauniainen, Finland) as capture antibody and monoclonal MMP8‐antibody 8706 (Medix Biochemica) labelled with europium‐chelate as tracer antibody. Saliva samples were diluted 1:4 in assay buffer (20 mM Tris‐HCl [pH 7.5], 0.5 M NaCl, 5 mM CaCl2, 50 μM ZnCl2, 0.5% bovine serum albumin, 0.05% sodium azide, and 20 mg/l diethylenetriaminepentaacetic acid) and incubated with the capture antibody for 1 h, followed by incubation for 1 h with the tracer antibody. Enhancement solution was then added and fluorescence was measured after 5 min using a 1234 Delfia Research Fluorometer (Wallac, Turku, Finland) (Hanemaaijer et al. 1997, Gursoy et al. 2010).
Collection and preparation of GCF samples
Gingival crevicular fluid samples were collected from an additional cohort consisting of 20 periodontitis patients, enrolled at a periodontal clinic, and 20 healthy controls. For the patients diagnosed with periodontitis, GCF was collected from sites with PPD ≥ 6 mm. For the healthy controls, GCF was collected from sites with PPD ≤ 3 mm. Before sample collection, supragingival plaque was removed from the tooth surface using a cotton pellet and the surface was gently dried with air. GCF samples were collected from the buccal (mesial and distal) sites from one tooth by inserting two separate paper strips (Periopaper, Proflow Inc., Amityville, NY, USA) into the gingival crevice until slight resistance was felt, and leaving them for 30 s. GCF samples visibly contaminated with blood were discarded. Immediately after GCF collection, the paper strips were frozen at −20°C until further processing. Thereafter, the two paper strips were placed into a vial containing 175 μl PBS with 0.05% Tween‐20, vortexed for 30 s, and stored at −80°C. The levels of MUC4 (diluted 1:2 in PBS buffer) and MMP7 in the GCF solutions were determined using commercial ELISA kits as described above for the saliva samples.
Statistical analysis
Comparisons of patient characteristics were performed with Mann–Whitney U‐test for continuous variables, and cross tables were constructed for dichotomous variables. If all cell values exceeded five, Chi‐square test was used, otherwise Fisher's exact test was used. For boxplots, univariate analyses of protein levels between patients with periodontitis and healthy controls were performed with Mann–Whitney U‐test. Multiple linear regression analyses were used with the concentrations of the proteins as dependent variables. In order to achieve normality, the dependent variables were first log‐transformed. Periodontitis, age, and smoking habits were included as independent variables. In order to evaluate the discriminative power of MUC4 and MMP7, a logistic regression analysis was performed with periodontitis as dependent variable. The ratio of MUC4 over MMP7 related to total protein concentrations, as well as age and smoking habits, was included as independent variables. This was followed by construction of a receiver operating characteristic (ROC) curve and calculation of area under curve (AUC) using the pROC package (Robin et al. 2011). All statistical analyses were performed using software R (R Core Team 2015) and differences were considered significant at a p‐value < 0.05.
Results
Subject characteristics
The characteristics of the study participants including patients with periodontitis (n = 37) and healthy controls (n = 39) from which saliva samples were obtained are presented in Table 1. The mean age of subjects was higher in the periodontitis group compared to the healthy controls (62.0 and 37.6 years, respectively, p < 0.01). In the periodontitis group, the number of sites with PPD 4–5 mm and ≥6 mm were significantly (p < 0.01) higher compared to the periodontally healthy individuals with no sites with PPD 4 mm or more. The majority (84%) of the patients with periodontitis had PPD ≥ 6 mm and six patients (16%) had PPD 4–5 mm. The number of sites with plaque was also significantly (p < 0.01) higher in the periodontitis group than in the control group (mean plaque index 38% and 12% respectively). In addition, the number of sites with BOP was significantly (p < 0.01) higher in the periodontitis group compared to the control group (mean BOP index 54% and 12% respectively). The number of individuals with high blood pressure or heart disease was significantly (p < 0.01) higher in the group of periodontitis patients than in the healthy control group, whereas the number of subjects with diabetes, bowel disease, or muscle and joint diseases were not significantly different between the groups (Table 1).
Table 1.
Characteristics of study participants from whom saliva samples were obtained
| Characteristics | Periodontitis (n = 37) | Healthy controls (n = 39) | p‐value |
|---|---|---|---|
| Age (years, mean ± SD) | 62.0 ± 10.9 | 37.6 ± 13.3 | <0.01a |
| Sex (female/male) | 18/19 | 25/14 | NSb |
| Smokers (n) | 8 | 6 | NSb |
| PPD 4–5 mm (number of sites, mean ± SD) | 23.4 ± 12.2 | 0 | <0.01a |
| PPD ≥ 6 mm (number of sites, mean ± SD) | 5.4 ± 5.1 | 0 | <0.01a |
| Plaque (number of sites, mean ± SD) | 23.0 ± 11.9 | 12.7 ± 0 | <0.01a |
| BOP (number of sites, mean ± SD) | 32.0 ± 10.5 | 13.3 ± 0 | <0.01a |
| High blood pressure/heart disease (n) | 13 | 0 | <0.01c |
| Diabetes (n) | 4 | 0 | NSc |
| Bowel diseases (n) | 2 | 5 | NSc |
| Muscle and joint diseases (n) | 15 | 9 | NSb |
PPD, Pocket probing depth; BOP, Bleeding on probing.
p‐value determined using Mann–Whitney U‐test.
p‐value determined using Chi‐square test.
p‐value determined using Fisher's two‐tailed exact test.
MUC4 and MMP7 levels in saliva samples
The levels of MUC4 and MMP7 in saliva samples from patients with periodontitis and healthy controls are demonstrated in Fig. 1. Salivary concentrations of MUC4 were significantly (p < 0.01) lower in patients with periodontitis relative to healthy controls. MMP7 concentrations, on the other hand, were significantly (p < 0.05) higher in periodontitis patients. In addition, the total protein concentrations were significantly (p < 0.01) higher in patients with periodontitis (Table S1). When relating the levels of MUC4 and MMP7 to the total protein concentrations, MUC4 levels were also significantly (p < 0.01) lower in saliva samples from periodontitis patients. The ratio of MMP7 over total protein concentration was, however, not significantly different in saliva samples obtained from periodontitis patients relative to controls (Fig. 1 and Table S1).
Figure 1.
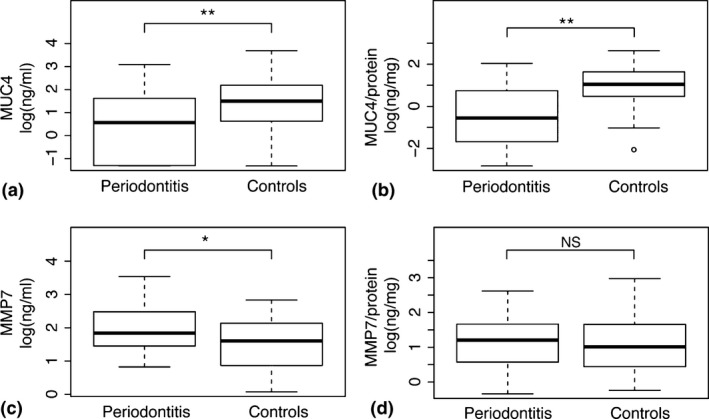
Salivary levels of mucin 4 (MUC4) and matrix metalloproteinase 7 (MMP7). Boxplots demonstrating the concentrations of (a) MUC4 (ng/ml), (b) MUC4/total protein concentrations (ng/mg), (c) MMP7 (ng/ml), and (d) MMP7/total protein concentrations (ng/mg) in saliva samples from periodontitis patients and healthy controls. *p < 0.05, **p < 0.01.
In order to validate our novel biomarkers of salivary proteins for periodontitis, the levels of MMP8 were also determined since it has repeatedly been reported to be up‐regulated in periodontitis compared to healthy controls. In agreement with MMP7, the salivary levels of MMP8 were significantly (p < 0.01) higher in periodontitis patients relative to controls without periodontal disease (Fig. 2 and Table S1). However, the levels of MMP8 related to the total protein concentrations did not significantly differ between the two groups (Fig. 2 and Table S1).
Figure 2.
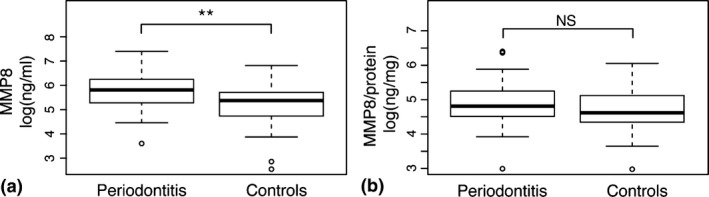
Levels of matrix metalloproteinase 8 (MMP8) in saliva samples. Boxplots demonstrating the concentrations of (a) MMP8 (ng/ml), and (b) MMP8/total protein concentrations (ng/mg) in saliva samples from patients with periodontitis and healthy controls. **p < 0.01.
Regression analyses for salivary levels of MUC4 and MMP7
Multiple linear regression analyses were performed to investigate the impact of periodontitis on the salivary levels of MUC4 and MMP7, adjusted for age and smoking habits (Table 2). The analyses were performed using the total concentrations of MUC4 and MMP7 as well as their concentrations related to the total protein levels. The results revealed that periodontitis contributed significantly (p < 0.05) to the levels of MUC4 in saliva samples, when adjusting for age and smoking. MUC4 remained significant (p < 0.01) also when related to the total protein concentrations in the saliva samples (Table 2). In addition, the analysis revealed that smoking contributed significantly to the levels of MMP7 as well as to MMP7 related to total protein concentrations (Table 2).
Table 2.
Multiple linear regression analyses of the association between salivary protein levels and periodontitis, adjusted for age and smoking
| Dependent variable | Independent variable | Estimate | SE | p‐value | 95% CI |
|---|---|---|---|---|---|
| MUC4 | Periodontitis | −0.92 | 0.43 | 0.03 | −1.77 to −0.07 |
| Age | 0.00 | 0.02 | 0.78 | −0.03 to 0.02 | |
| Smoking | 0.41 | 0.39 | 0.29 | −0.35 to 1.17 | |
| MUC4/total protein | Periodontitis | −1.20 | 0.40 | <0.01 | −2.00 to −0.39 |
| Age | −0.01 | 0.01 | 0.45 | −0.03 to 0.01 | |
| Smoking | 0.34 | 0.37 | 0.36 | −0.38 to 1.06 | |
| MMP7 | Periodontitis | 0.44 | 0.24 | 0.07 | −0.04 to 0.92 |
| Age | 0.00 | 0.01 | 0.89 | −0.01 to 0.01 | |
| Smoking | −0.57 | 0.22 | 0.01 | −1.00 to −0.14 | |
| MMP7/total protein | Periodontitis | 0.16 | 0.25 | 0.53 | −0.34 to 0.65 |
| Age | 0.00 | 0.01 | 0.54 | −0.02 to 0.01 | |
| Smoking | −0.65 | 0.23 | <0.01 | −1.09 to −0.21 |
Statistically significant p‐values (< 0.05) are indicated with boldface.
MUC4, mucin 4; MMP7, matrix metalloproteinase 7.
Furthermore, a logistic regression analysis was performed with periodontitis as dependent variable and the ratio of MUC4 over MMP7 related to total protein concentrations, as well as age and smoking habits as independent variables (Table S2). The results from this analysis revealed that the ratio of MUC4 over MMP7 related to the total protein concentration, as well as age, was significantly associated with periodontitis (p < 0.05 and p < 0.01, respectively, Table S2). The logistic regression analysis was thereafter used to investigate the diagnostic potential of the combination of the novel biomarkers, through construction of a ROC curve. The ROC curve analysis showed that the area under curve was 0.931 (Fig. 3).
Figure 3.
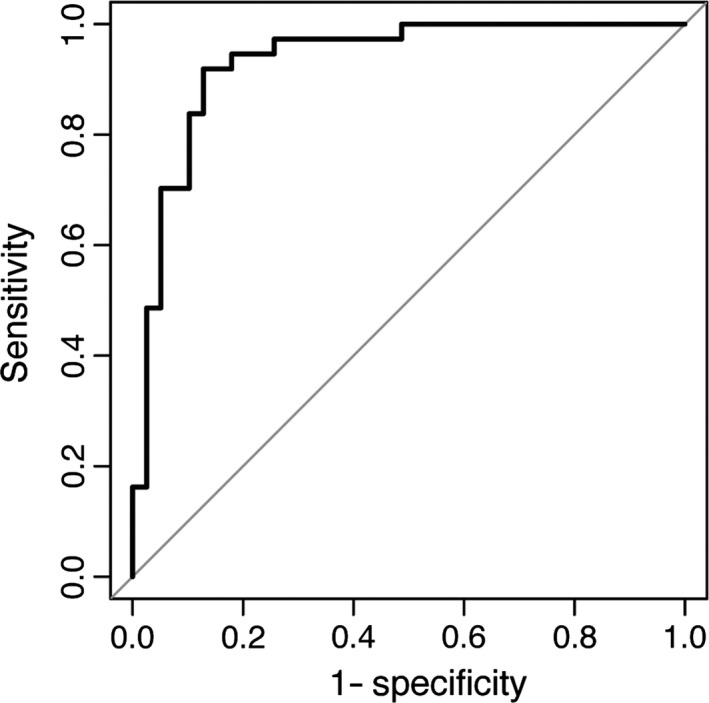
Diagnostic power of mucin 4 (MUC4) and matrix metalloproteinase 7 (MMP7) by receiver operating characteristic curve (ROC curve). The ROC curve is based on a binary logistic regression model with periodontitis as dependent variable and MUC4/MMP7 levels related to the total protein concentrations, age, and smoking habits as independent variables.
MUC4 and MMP7 levels in GCF samples
In addition to saliva samples, the levels of MUC4 and MMP7 were also investigated in GCF samples from a cohort of 40 subjects with periodontitis and healthy controls.
The mean age of subjects was significantly (p < 0.01) higher in the periodontitis group compared to the healthy controls (67.1 ± 9.5 and 38.5 ± 10.9 years respectively). The periodontitis group, comprising 10 females and 10 males, had significantly (p < 0.01) higher PPD (mean 6.9 ± 2.0 mm) and BOP (45%), than the healthy control group, comprising 14 females and six males (PPD ≤ 3 mm, BOP 5%) (Table not shown). In agreement with our findings in saliva, the total concentrations of MUC4 as well as MUC4 related to total protein concentration were significantly (p < 0.05, and p < 0.01 respectively) lower in the GCF from periodontitis patients relative to controls (Fig. 4 and Table S3). Moreover, the levels of MMP7, as well as the total protein concentrations, were significantly (p < 0.01) higher in GCF from periodontitis patients (Fig. 4 and Table S3).
Figure 4.
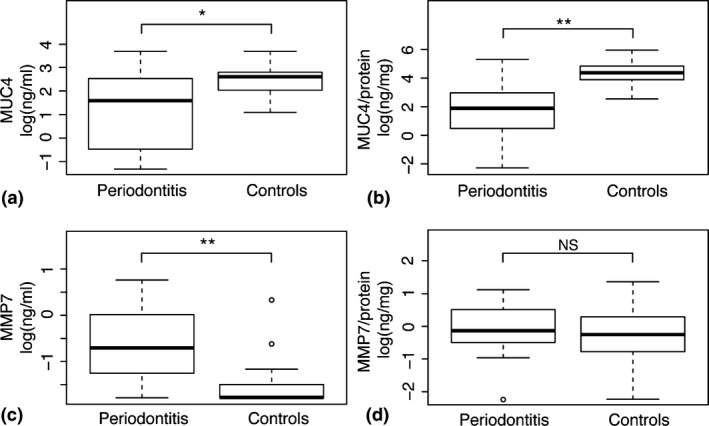
Mucin 4 (MUC4) and matrix metalloproteinase 7 (MMP7) levels in gingival crevicular fluid (GCF) samples. Boxplots demonstrating the concentrations of (a) MUC4 (ng/ml), (b) MUC4/total protein concentrations (ng/mg), (c) MMP7 (ng/ml), and (d) MMP7/total protein concentrations (ng/mg) in GCF samples from periodontitis patients and healthy controls. *p < 0.05, **p < 0.01.
Multiple linear regression analyses were also performed with the total concentrations of MUC4 and MMP7 as well as in relation to protein concentrations as dependent variables, adjusted for age and smoking habits (Table 3). The analyses revealed that periodontitis was significantly (p < 0.01) associated with the GCF levels of MUC4, and MUC4 related to total protein concentrations, as well as with the levels of MMP7 (Table 3).
Table 3.
Multiple linear regression analyses of the association between protein levels of gingival crevicular fluid (GCF) and periodontitis, adjusted for age and smoking
| Dependent variable | Independent variable | Estimate | SE | p‐value | 95% CI |
|---|---|---|---|---|---|
| MUC4 | Periodontitis | −2.00 | 0.73 | <0.01 | −3.49 to −0.52 |
| Age | 0.03 | 0.02 | 0.21 | −0.01 to 0.07 | |
| Smoking | 0.00 | 0.67 | 1.00 | −1.32 to 1.31 | |
| MUC4/total protein | Periodontitis | −3.19 | 0.87 | <0.01 | −4.96 to −1.41 |
| Age | 0.02 | 0.02 | 0.33 | −0.02 to 0.07 | |
| Smoking | −0.09 | 0.80 | 0.91 | −1.67 to 1.48 | |
| MMP7 | Periodontitis | 1.27 | 0.37 | <0.01 | 0.52 to 2.01 |
| Age | −0.01 | 0.01 | 0.46 | −0.03 to 0.01 | |
| Smoking | −0.53 | 0.34 | 0.13 | −1.19 to 0.13 | |
| MMP7/total protein | Periodontitis | 0.53 | 0.49 | 0.28 | −0.45 to 1.52 |
| Age | −0.01 | 0.01 | 0.62 | −0.03 to 0.02 | |
| Smoking | −0.29 | 0.46 | 0.52 | −1.16 to 0.59 |
Statistically significant p‐values (< 0.05) are indicated with boldface.
MUC4, mucin 4; MMP7, matrix metalloproteinase 7.
Discussion
We have recently reported that MUC4 and MMP7 are highly associated with periodontitis, as identified by RNA sequencing analysis of gingival tissue biopsies from periodontitis patients and healthy controls (Lundmark et al. 2015). Here, we report, for the first time, significantly different levels of these two proteins in saliva and GCF samples from patients with periodontitis relative to healthy controls. Furthermore, in this study group, we also show that the combination of the salivary levels of MUC4 and MMP7 has the potential of discriminating between individuals with and without periodontitis.
In this study, protein levels of MUC4, determined by ELISA using specific antibodies, were significantly lower in saliva and GCF samples of patients with periodontitis as compared to healthy controls. MUC4 has previously been implicated in cancer, including pancreatic, breast, and lung (reviewed in Carraway et al. 2009). Regarding periodontitis, previous studies have reported higher levels of mucins in general in saliva samples from periodontitis patients than in healthy subjects (Sanchez et al. 2011, 2013, Acquier et al. 2015). These studies used, however, the Alcian blue method, which stains glycoproteins in general and can therefore not distinguish different mucin family members, up to date 20 members (Frenkel & Ribbeck 2015). At the mRNA level, our previous sequencing study, investigating the whole transcriptome in gingival tissue biopsies from periodontitis patients and healthy controls, identified higher expression of MUC4 in gingival tissue biopsies from patients with periodontitis (Lundmark et al. 2015). The contrasting findings in tissue of gingiva versus oral fluids may be due to the fact that MUC4 exists in both secreted and membrane‐bound form (Hilkens & Buijs 1988, Sheng et al. 1990, Williams et al. 2001, Linden et al. 2008) and that the balance between these two forms may be altered due to reprogramming of signalling pathways of the MUC4 gene (Andrianifahanana et al. 2005) in response to inflammation. As a result of the biofilm formed adjacent to the gingival tissue, the membrane‐bound form of MUC4 may increase in order to prevent the bacteria to access the cell surface and to protect the gingival tissue. This suggestion is in line with our previous results demonstrating increased MUC4 expression in oral epithelial cells stimulated with LPS (Lundmark et al. 2015). Another explanation for the decreased levels of MUC4 in saliva samples might be due to proteolytic degradation, either by enzymes up‐regulated by the host or expressed by microbes (Linden et al. 2008). The decreased levels of MUC4 in saliva might lead to a lower capability to agglutinate and cleanse oral pathogens, allowing biofilm formation on the teeth, leading to sustained inflammatory response in periodontitis patients.
Our findings demonstrating higher levels of MMP7 in saliva and GCF samples from patients with periodontitis relative to healthy controls are in agreement with our RNA sequencing results, which revealed overexpression of MMP7 in gingival tissue biopsies of patients with periodontitis (Lundmark et al. 2015). Levels of MMP7 have, to our knowledge, not previously been reported in saliva of periodontitis patients. With regard to GCF, MMP7 has been reported to be elevated in samples from patients with adult periodontitis relative to GCF samples collected from patients with localized juvenile periodontitis and controls (Tervahartiala et al. 2000). Another study showed no significant differences in levels of total MMP7 in GCF samples from 20 patients with different periodontal diseases and healthy controls. After correcting for the volume of GCF obtained from each site, however, the levels of MMP7 were identified as significantly lower in periodontitis patients than in healthy controls (Emingil et al. 2006). Nonetheless, one limitation with this study as well as our current study is the small number of GCF samples and additional studies should be performed in order to validate these findings. Another limitation with our study is the relatively high age of subjects in the periodontitis group relative to the healthy control group. Nevertheless, our multiple regression analyses were adjusted for age and therefore the results would be applicable to younger patients as well.
In addition to demonstrating that the levels of MUC4 and MMP7 differ significantly between oral fluids of patients with periodontitis and healthy controls, in this study population, we also confirm elevated levels of MMP8 in saliva samples from patients with periodontitis. MMP8 levels have repeatedly been demonstrated to be up‐regulated in periodontitis and the elevated levels of MMP8 in our cohort of saliva samples indicate a concordance of our results with those of previous studies (Miller et al. 2006, Ramseier et al. 2009, Gursoy et al. 2010, Rathnayake et al. 2013). Regarding proinflammatory periodontal tissue destruction cascades, it should be noted that MMP7 can activate pro‐MMP8, thereby potentially promoting periodontal disease progression (Balbin et al. 1998).
Both MUC4 and MMP7 are involved in tissue homoeostasis by promoting cell proliferation and repressing apoptosis (Ii et al. 2006, Carraway et al. 2009). In this study, we also show that the combination of the salivary levels of MUC4 and MMP7 might have a diagnostic application. A logistic regression analysis identified the ratio of MUC4 over MMP7 as significantly associated with periodontitis, and a subsequent ROC curve analysis had a high AUC, indicating that the combination of MUC4 and MMP7 is able to discriminate patients with periodontitis from healthy controls, in this study population. A combination of biomarkers will likely be more specific than a single marker and future studies should focus on panels of biomarkers, including MUC4 and MMP7, for predicting periodontitis.
In conclusion, this study is, to the best of our knowledge, the first to identify significantly different levels of MUC4 and MMP7 in saliva and GCF of patients with periodontitis in comparison to healthy controls, suggesting that MUC4 and MMP7, alone or in combination, might have the potential to act as novel diagnostic markers for periodontitis.
Clinical Relevance.
Scientific rationale for the study: We have recently, through RNA‐sequencing, identified MUC4 and MMP7 as highly associated with periodontitis in gingival tissue biopsies. However, to our knowledge, no study has previously reported these proteins in saliva from patients with periodontitis and healthy controls.
Principal findings: Levels of MUC4 and MMP7 were significantly different in saliva samples from patients with periodontitis relative to healthy controls.
Practical implications: Our novel findings that MUC4 and MMP7 levels differ in saliva samples from patients with periodontitis and healthy controls imply that they might exert the potential to serve as salivary biomarkers for periodontitis.
Supporting information
Table S1. Levels of MUC4, MMP7, MMP8, and total protein in saliva samples from patients with periodontitis and healthy controls.
Table S2. Logistic regression of the saliva samples with periodontitis as dependent variable and MUC4/MMP7 related to total protein concentrations as independent variable, adjusted for age and smoking.
Table S3. Levels of MUC4, MMP7, and total protein in gingival crevicular fluid samples from patients with periodontitis and healthy controls.
Lundmark A, Johannsen G, Eriksson K, Kats A, Jansson L, Tervahartiala T, Rathnayake N, Åkerman S, Klinge B, Sorsa T, Yucel‐Lindberg T. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J Clin Periodontol 2017; 44: 247–254. doi: 10.1111/jcpe.12670.
Conflict of interest and source of funding statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
The study was performed in collaboration with the Regional Board of Dental Public Health in Skåne, Sweden. This work was supported by grants from the Swedish Research Council (project number K2013‐52X‐22194‐01‐3), Stockholm County Council (SOF), the Swedish Patent Revenue Fund (for Research in Preventive Odontology), the Research Foundation of Helsinki University Hospital, Helsinki, Finland, and Karolinska Institutet, Stockholm, Sweden. Professor Timo Sorsa is an inventor of US‐patents 5652223, 5736341, 5866932, and 6143476.
References
- Acquier, A. B. , Pita, A. K. , Busch, L. & Sanchez, G. A. (2015) Comparison of salivary levels of mucin and amylase and their relation with clinical parameters obtained from patients with aggressive and chronic periodontal disease. Journal of Applied Oral Science 23, 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Azri, A. R. , Gibson, R. J. , Keefe, D. M. & Logan, R. M. (2013) Matrix metalloproteinases: do they play a role in mucosal pathology of the oral cavity? Oral Diseases 19, 347–359. [DOI] [PubMed] [Google Scholar]
- Andrianifahanana, M. , Agrawal, A. , Singh, A. P. , Moniaux, N. , van Seuningen, I. , Aubert, J. P. , Meza, J. & Batra, S. K. (2005) Synergistic induction of the MUC4 mucin gene by interferon‐gamma and retinoic acid in human pancreatic tumour cells involves a reprogramming of signalling pathways. Oncogene 24, 6143–6154. [DOI] [PubMed] [Google Scholar]
- Arpino, V. , Brock, M. & Gill, S. E. (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biology 44–46, 247–254. [DOI] [PubMed] [Google Scholar]
- Balbin, M. , Fueyo, A. , Knauper, V. , Pendas, A. M. , Lopez, J. M. , Jimenez, M. G. , Murphy, G. & Lopez‐Otin, C. (1998) Collagenase 2 (MMP‐8) expression in murine tissue‐remodeling processes. Analysis of its potential role in postpartum involution of the uterus. Journal of Biological Chemistry 273, 23959–23968. [DOI] [PubMed] [Google Scholar]
- Beklen, A. , Tuter, G. , Sorsa, T. , Hanemaaijer, R. , Virtanen, I. , Tervahartiala, T. & Konttinen, Y. T. (2006) Gingival tissue and crevicular fluid co‐operation in adult periodontitis. Journal of Dental Research 85, 59–63. [DOI] [PubMed] [Google Scholar]
- Carraway, K. L. , Theodoropoulos, G. , Kozloski, G. A. & Carothers Carraway, C. A. (2009) Muc4/MUC4 functions and regulation in cancer. Future Oncology (London, England) 5, 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien, M. , van Passel, M. W. , van de Bovenkamp, J. H. , Schipper, R. G. , de Vos, W. M. & Dekker, J. (2010) Mucin‐bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 1, 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke, P. I. , Thornton‐Evans, G. , Dye, B. & Genco, R. (2012) Advances in surveillance of periodontitis: the Centers for Disease Control and Prevention periodontal disease surveillance project. Journal of Periodontology 83, 1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emingil, G. , Tervahartiala, T. , Mantyla, P. , Maatta, M. , Sorsa, T. & Atilla, G. (2006) Gingival crevicular fluid matrix metalloproteinase (MMP)‐7, extracellular MMP inducer, and tissue inhibitor of MMP‐1 levels in periodontal disease. Journal of Periodontology 77, 2040–2050. [DOI] [PubMed] [Google Scholar]
- Fabian, T. K. , Hermann, P. , Beck, A. , Fejerdy, P. & Fabian, G. (2012) Salivary defense proteins: their network and role in innate and acquired oral immunity. International Journal of Molecular Sciences 13, 4295–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgueras, A. R. , Pendas, A. M. , Sanchez, L. M. & Lopez‐Otin, C. (2004) Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. International Journal of Developmental Biology 48, 411–424. [DOI] [PubMed] [Google Scholar]
- Frenkel, E. S. & Ribbeck, K. (2015) Salivary mucins in host defense and disease prevention. Journal of Oral Microbiology 7, 29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy, U. K. , Kononen, E. , Pradhan‐Palikhe, P. , Tervahartiala, T. , Pussinen, P. J. , Suominen‐Taipale, L. & Sorsa, T. (2010) Salivary MMP‐8, TIMP‐1, and ICTP as markers of advanced periodontitis. Journal of Clinical Periodontology 37, 487–493. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer, R. , Sorsa, T. , Konttinen, Y. T. , Ding, Y. , Sutinen, M. , Visser, H. , van Hinsbergh, V. W. , Helaakoski, T. , Kainulainen, T. , Ronka, H. , Tschesche, H. & Salo, T. (1997) Matrix metalloproteinase‐8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor‐alpha and doxycycline. Journal of Biological Chemistry 272, 31504–31509. [DOI] [PubMed] [Google Scholar]
- Hernandez, M. , Dutzan, N. , Garcia‐Sesnich, J. , Abusleme, L. , Dezerega, A. , Silva, N. , Gonzalez, F. E. , Vernal, R. , Sorsa, T. & Gamonal, J. (2011) Host‐pathogen interactions in progressive chronic periodontitis. Journal of Dental Research 90, 1164–1170. [DOI] [PubMed] [Google Scholar]
- Hernandez Rios, M. , Sorsa, T. , Obregon, F. , Tervahartiala, T. , Valenzuela, M. A. , Pozo, P. , Dutzan, N. , Lesaffre, E. , Molas, M. & Gamonal, J. (2009) Proteolytic roles of matrix metalloproteinase (MMP)‐13 during progression of chronic periodontitis: initial evidence for MMP‐13/MMP‐9 activation cascade. Journal of Clinical Periodontology 36, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Hilkens, J. & Buijs, F. (1988) Biosynthesis of MAM‐6, an epithelial sialomucin. Evidence for involvement of a rare proteolytic cleavage step in the endoplasmic reticulum. Journal of Biological Chemistry 263, 4215–4222. [PubMed] [Google Scholar]
- Hollingsworth, M. A. & Swanson, B. J. (2004) Mucins in cancer: protection and control of the cell surface. Nature Reviews: Cancer 4, 45–60. [DOI] [PubMed] [Google Scholar]
- Ii, M. , Yamamoto, H. , Adachi, Y. , Maruyama, Y. & Shinomura, Y. (2006) Role of matrix metalloproteinase‐7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Experimental Biology and Medicine (Maywood, N.J.) 231, 20–27. [DOI] [PubMed] [Google Scholar]
- Isaza‐Guzman, D. M. , Arias‐Osorio, C. , Martinez‐Pabon, M. C. & Tobon‐Arroyave, S. I. (2011) Salivary levels of matrix metalloproteinase (MMP)‐9 and tissue inhibitor of matrix metalloproteinase (TIMP)‐1: a pilot study about the relationship with periodontal status and MMP‐9(‐1562C/T) gene promoter polymorphism. Archives of Oral Biology 56, 401–411. [DOI] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Bernabe, E. , Dahiya, M. , Bhandari, B. , Murray, C. J. & Marcenes, W. (2014) Global burden of severe periodontitis in 1990–2010: a systematic review and meta‐regression. Journal of Dental Research 93, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha, R. , Murthy, A. & Weiss, A. (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nature Reviews: Immunology 13, 649–665. [DOI] [PubMed] [Google Scholar]
- Kinane, D. F. , Preshaw, P. M. , Loos, B. G. & Working Group 2 of Seventh European Workshop on Periodontology (2011) Host‐response: understanding the cellular and molecular mechanisms of host‐microbial interactions–consensus of the Seventh European Workshop on Periodontology. Journal of Clinical Periodontology 38(Suppl. 11), 44–48. [DOI] [PubMed] [Google Scholar]
- Linden, S. K. , Sutton, P. , Karlsson, N. G. , Korolik, V. & McGuckin, M. A. (2008) Mucins in the mucosal barrier to infection. Mucosal Immunology 1, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundegren, N. , Axtelius, B. & Akerman, S. (2012) Oral health in the adult population of Skane, Sweden: a clinical study. Acta Odontologica Scandinavica 70, 511–519. [DOI] [PubMed] [Google Scholar]
- Lundmark, A. , Davanian, H. , Bage, T. , Johannsen, G. , Koro, C. , Lundeberg, J. & Yucel‐Lindberg, T. (2015) Transcriptome analysis reveals mucin 4 to be highly associated with periodontitis and identifies pleckstrin as a link to systemic diseases. Scientific Reports 5, 18475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyla, P. , Stenman, M. , Kinane, D. F. , Tikanoja, S. , Luoto, H. , Salo, T. & Sorsa, T. (2003) Gingival crevicular fluid collagenase‐2 (MMP‐8) test stick for chair‐side monitoring of periodontitis. Journal of Periodontal Research 38, 436–439. [DOI] [PubMed] [Google Scholar]
- Miller, C. S. , King, C. P. Jr , Langub, M. C. , Kryscio, R. J. & Thomas, M. V. (2006) Salivary biomarkers of existing periodontal disease: a cross‐sectional study. Journal of the American Dental Association 137, 322–329. [DOI] [PubMed] [Google Scholar]
- Passoja, A. , Ylipalosaari, M. , Tervonen, T. , Raunio, T. & Knuuttila, M. (2008) Matrix metalloproteinase‐8 concentration in shallow crevices associated with the extent of periodontal disease. Journal of Clinical Periodontology 35, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Petersen, P. E. & Ogawa, H. (2005) Strengthening the prevention of periodontal disease: the WHO approach. Journal of Periodontology 76, 2187–2193. [DOI] [PubMed] [Google Scholar]
- Pozo, P. , Valenzuela, M. A. , Melej, C. , Zaldivar, M. , Puente, J. , Martinez, B. & Gamonal, J. (2005) Longitudinal analysis of metalloproteinases, tissue inhibitors of metalloproteinases and clinical parameters in gingival crevicular fluid from periodontitis‐affected patients. Journal of Periodontal Research 40, 199–207. [DOI] [PubMed] [Google Scholar]
- R Core Team (2015) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rai, B. , Kharb, S. , Jain, R. & Anand, S. C. (2008) Biomarkers of periodontitis in oral fluids. Journal of Oral Science 50, 53–56. [DOI] [PubMed] [Google Scholar]
- Ramseier, C. A. , Kinney, J. S. , Herr, A. E. , Braun, T. , Sugai, J. V. , Shelburne, C. A. , Rayburn, L. A. , Tran, H. M. , Singh, A. K. & Giannobile, W. V. (2009) Identification of pathogen and host‐response markers correlated with periodontal disease. Journal of Periodontology 80, 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnayake, N. , Akerman, S. , Klinge, B. , Lundegren, N. , Jansson, H. , Tryselius, Y. , Sorsa, T. & Gustafsson, A. (2013) Salivary biomarkers of oral health: a cross‐sectional study. Journal of Clinical Periodontology 40, 140–147. [DOI] [PubMed] [Google Scholar]
- Robin, X. , Turck, N. , Hainard, A. , Tiberti, N. , Lisacek, F. , Sanchez, J. C. & Muller, M. (2011) pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, G. A. , Miozza, V. A. , Delgado, A. & Busch, L. (2013) Relationship between salivary mucin or amylase and the periodontal status. Oral Diseases 19, 585–591. [DOI] [PubMed] [Google Scholar]
- Sanchez, G. A. , Miozza, V. , Delgado, A. & Busch, L. (2011) Determination of salivary levels of mucin and amylase in chronic periodontitis patients. Journal of Periodontal Research 46, 221–227. [DOI] [PubMed] [Google Scholar]
- Sbardella, D. , Fasciglione, G. F. , Gioia, M. , Ciaccio, C. , Tundo, G. R. , Marini, S. & Coletta, M. (2012) Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Molecular Aspects of Medicine 33, 119–208. [DOI] [PubMed] [Google Scholar]
- Sheng, Z. Q. , Hull, S. R. & Carraway, K. L. (1990) Biosynthesis of the cell surface sialomucin complex of ascites 13762 rat mammary adenocarcinoma cells from a high molecular weight precursor. Journal of Biological Chemistry 265, 8505–8510. [PubMed] [Google Scholar]
- Silva, N. , Abusleme, L. , Bravo, D. , Dutzan, N. , Garcia‐Sesnich, J. , Vernal, R. , Hernandez, M. & Gamonal, J. (2015) Host response mechanisms in periodontal diseases. Journal of Applied Oral Science 23, 329–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsa, T. , Gursoy, U. K. , Nwhator, S. , Hernandez, M. , Tervahartiala, T. , Leppilahti, J. , Gursoy, M. , Kononen, E. , Emingil, G. , Pussinen, P. J. & Mantyla, P. (2016) Analysis of matrix metalloproteinases, especially MMP‐8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology 2000 70, 142–163. [DOI] [PubMed] [Google Scholar]
- Sorsa, T. , Tjaderhane, L. , Konttinen, Y. T. , Lauhio, A. , Salo, T. , Lee, H. M. , Golub, L. M. , Brown, D. L. & Mantyla, P. (2006) Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Annals of Medicine 38, 306–321. [DOI] [PubMed] [Google Scholar]
- Tervahartiala, T. , Pirila, E. , Ceponis, A. , Maisi, P. , Salo, T. , Tuter, G. , Kallio, P. , Tornwall, J. , Srinivas, R. , Konttinen, Y. T. & Sorsa, T. (2000) The in vivo expression of the collagenolytic matrix metalloproteinases (MMP‐2, ‐8, ‐13, and ‐14) and matrilysin (MMP‐7) in adult and localized juvenile periodontitis. Journal of Dental Research 79, 1969–1977. [DOI] [PubMed] [Google Scholar]
- Tuomainen, A. M. , Nyyssonen, K. , Laukkanen, J. A. , Tervahartiala, T. , Tuomainen, T. P. , Salonen, J. T. , Sorsa, T. & Pussinen, P. J. (2007) Serum matrix metalloproteinase‐8 concentrations are associated with cardiovascular outcome in men. Arteriosclerosis, Thrombosis, and Vascular Biology 27, 2722–2728. [DOI] [PubMed] [Google Scholar]
- Williams, S. J. , Wreschner, D. H. , Tran, M. , Eyre, H. J. , Sutherland, G. R. & McGuckin, M. A. (2001) Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. Journal of Biological Chemistry 276, 18327–18336. [DOI] [PubMed] [Google Scholar]
- Yucel‐Lindberg, T. & Bage, T. (2013) Inflammatory mediators in the pathogenesis of periodontitis. Expert Reviews in Molecular Medicine 15, e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Levels of MUC4, MMP7, MMP8, and total protein in saliva samples from patients with periodontitis and healthy controls.
Table S2. Logistic regression of the saliva samples with periodontitis as dependent variable and MUC4/MMP7 related to total protein concentrations as independent variable, adjusted for age and smoking.
Table S3. Levels of MUC4, MMP7, and total protein in gingival crevicular fluid samples from patients with periodontitis and healthy controls.


