Abstract
Background
This study used data from different sources to identify the extent of the unmet need for postprandial glycemic control in patients with type 2 diabetes mellitus (T2DM) after the initiation of basal insulin therapy in Europe, Asia Pacific, the United States, and Latin America.
Methods
Different levels of evidence were used as available for each country/region, with data extracted from seven randomized controlled trials (RCTs), three clinical trial registries (CTRs), and three electronic medical record (EMR) databases. Glycemic status was categorized as “well controlled” (glycated hemoglobin [HbA1c] at target [<7%]), “residual hyperglycemia” (fasting plasma glucose [FPG] but not HbA1c at target [FPG <7.2/7.8 mmol/L, <130/140 mg/dL, depending on country‐specific recommendations]), or “uncontrolled” (both FPG and HbA1c above target). Predictor factors were identified from the RCT data set using logistic regression analysis.
Results
RCT data showed that 16.9% to 28.0%, 42.7% to 54.4%, and 16.9% to 38.1% of patients with T2DM had well‐controlled glycemia, residual hyperglycemia, and uncontrolled hyperglycemia, respectively. In CTRs, respective ranges were 21.8% to 33.6%, 31.5% to 35.6%, and 30.7% to 46.8%, and in EMR databases were 4.4% to 21.0%, 23.9% to 31.8%, and 53.6% to 63.8%. Significant predictor factors of residual hyperglycemia identified from RCT data included high baseline HbA1c (all countries/regions except Brazil), high baseline FPG (United Kingdom/Japan), longer duration of diabetes (Brazil), and female sex (Europe/Latin America).
Conclusions
Irrespective of intrinsic differences between data sources, 24% to 54% of patients with T2DM globally had residual hyperglycemia with HbA1c not at target, despite achieving FPG control, indicating a significant unmet need for postprandial glycemic control.
Keywords: fasting plasma glucose, glycemic control, insulin therapy, postprandial glucose, residual hyperglycemia, type 2 diabetes mellitus
1. INTRODUCTION
In patients with type 2 diabetes mellitus (T2DM), early intervention to achieve and maintain glycemic control is essential to reduce the risk of diabetes‐related vascular disease and associated long‐term complications.1, 2 Current treatment guidelines recommend that patients achieve and maintain their glycated hemoglobin (HbA1c) level below a certain target, often 7%, with the option to increase or decrease this threshold depending on the clinical and demographic characteristics of the individual patient, such as age and gender.3, 4, 5 Moreover, the exact target can vary between different countries/regions, and the target may change for an individual patient during the course of their disease.6
A patient's HbA1c level is a reflection of the sum of blood glucose exposure from the combination of fasting plasma glucose (FPG; when hepatic glucose production predominates) and postprandial plasma glucose (PPG; primarily due to dietary glucose). Both PPG and FPG need to be addressed to achieve sustained glycemic control.4, 7 However, the relative contribution of FPG and PPG to HbA1c can vary considerably depending on factors such as lifestyle, duration of disease, current treatment class,8, 9 and race and ethnicity.10
For patients failing to meet HbA1c targets despite treatment with oral glucose‐lowering therapies (OGLTs), basal insulin (neutral protamine Hagedorn [NPH] insulin and the basal insulin analogs glargine, detemir, or degludec) titrated to target FPG is a recommended treatment option,4, 5 whereas other initial treatments such as glucagon‐like peptide 1 (GLP‐1) receptor agonists may be preferred in certain patients. In the Treat‐to‐Target trial, a simple insulin intensification algorithm with an FPG target ≤5.5 mmol/L (≤100 mg/dL) enabled approximately 60% of patients to achieve HbA1c ≤7% with insulin glargine or NPH, although the proportion of patients who achieved this target without hypoglycemia was higher with insulin glargine.11
For patients who are not successful in achieving target HbA1c with basal insulin despite meeting FPG targets, additional treatment may be required to address residual hyperglycemia associated with PPG excursions.12 A pooled analysis of six similarly designed randomized controlled trials (RCTs) of insulin glargine initiation showed that for patients with HbA1c >7% despite OGLT therapy, basal hyperglycemia dominated glucose exposure; however, after 24 to 28 weeks of basal insulin therapy, postprandial hyperglycemia became predominant.9 In general, the contribution of FPG to HbA1c is greater at higher HbA1c levels, with the contribution of PPG being more consistent across a range of HbA1c values.10, 13, 14 The potential benefits, with respect to glycemic control, of adding an agent that targets PPG excursions to basal insulin have been demonstrated in several recent studies of dipeptidyl peptidase‐4 inhibitors and some GLP‐1 receptor agonists.15, 16, 17
The identification of patients failing to achieve HbA1c targets with basal insulin is important to ensure that treatment can be adjusted promptly. Moreover, monitoring FPG in patients failing to meet target HbA1c can be used to differentiate between individuals who will benefit from further basal insulin titration and patients who require addition of a prandial therapy to address PPG.
The aim of this retrospective analysis was to highlight the global extent of the problem of residual hyperglycemia among patients with T2DM after the initiation of basal insulin, including patients who have failed to achieve glycemic control previously with OGLTs. The analysis uses evidence from RCTs and real‐world data to identify the patients who would most benefit from treatment intensification with a prandial agent. Potential predictive factors that could be used to identify these patients were also evaluated.
2. MATERIALS AND METHODS
This study set out to evaluate glycemic control, as determined by HbA1c and FPG levels in patients with T2DM after treatment with basal insulin, using patient data from different sources. Using recommended treatment targets 5, 18, 19, 20 as the cutoff values for the analysis, patients' glycemic status after basal insulin treatment was categorized as “well controlled” (defined as HbA1c at target, <7%), “residual hyperglycemia” (FPG at target [defined as <7.2 mmol/L, <130 mg/dL, for Asian and Latin American countries, or <7.8 mmol/L, <140 mg/dL, in the rest of the world, according to regional specifications] but HbA1c above target), or “uncontrolled” (defined as both FPG and HbA1c above target).
2.1. Data sources
To gain a broad‐based view, patient data were obtained from three distinct sources: RCTs, observational clinical trial registries (CTRs), and electronic medical record (EMR) databases.
RCTs were identified by a systematic search of the US National Library of Medicine bibliographic database (MEDLINE) using the search string “glargine AND (NPH OR detemir OR degludec) AND trials AND randomized AND type 2 diabetes.” The search was performed in July 2013 and restricted to randomized interventional clinical trials of basal insulin initiation published between 2002 and 2010, with end points reported at a duration of 24 to 36 weeks, and that had been sponsored by Sanofi (to facilitate access to patient‐level data). Studies were required to include at least two treatment arms with insulin glargine versus another basal insulin (NPH insulin, insulin detemir, or insulin degludec).
The literature search based on the aforementioned search string identified a total of 110 publications, of which 63 were excluded on the basis that they were not randomized interventional clinical trials or did not report data. Of the remaining 47 publications, additional exclusions were seven reports of post hoc analyses, 28 studies with a duration longer or shorter than the range mentioned previously, and five reports of studies where access to patient‐level data was not available. Therefore, a total of seven RCTs published between 2003 and 2010 met the criteria and were included in the analysis 21, 22, 23, 24, 25, 26, 27 (Table 1). The comparator to insulin glargine was NPH insulin in five studies, insulin detemir in one study, and premixed 70% human NPH/30% regular insulin (70/30) in one study; the continuation of existing OGLTs was permitted in some studies; only patients receiving basal insulin, including insulin glargine, NPH insulin, and insulin detemir, were included in these post hoc analyses; patients receiving premixed insulin were excluded because of the rapid‐acting insulin component.
Table 1.
Summary of RCTs included in the analysis
| RCTs | Region/country of study | Prestudy treatment | Study treatment | Patient numbers n/N † | Study duration |
|---|---|---|---|---|---|
| Kawamori et al, 2003 (Study 3102) | Asia | OGLTs | GLAR | 158/317 | 28 wk |
| NPH | 159/317 | ||||
| Fritsche et al, 2003 (Study 4001) | Europe | SU ± other OGLTs | Morning GLAR + SU | 236/695 | 24 wk |
| Evening GLAR + SU | 227/695 | ||||
| Evening NPH + SU | 232/695 | ||||
| Pan et al, 2007 (LEAD; Study 4012) | Asia | SU ± other OGLTs | GLAR + SU | 220/443 | 24 wk |
| NPH + SU | 223/443 | ||||
| Eliaschewitz et al, 2006 (Study 4013) | Latin America | SU ± other OGLTs for ≥6 mo | GLAR + SU | 231/481 | 24 wk |
| NPH + SU | 250/481 | ||||
| Janka et al, 2005 (Study 4027)* | Europe | Stable dose of SU and MET for ≥1 mo | GLAR | 177/364 | 24 wk |
| PREMIX: 70% NPH/30% Regular | 187/364 | ||||
| Yki‐Jarvinen et al, 2006 (LANMET; Study 6001) | Finland/United Kingdom | Stable dose of MET ± SU for ≥3 mo | GLAR + MET | 61/110 | 36 wk |
| NPH + MET | 49/110 | ||||
| Swinnen et al, 2010 (L2T3) | Global | Stable dose of OGLTs for ≥3 mo | GLAR | 478/1230 | 24 wk |
| DET | 486/1230 |
Only data from the insulin glargine treatment arm in this study were included in this analysis.
Patients in each arm/total patients in study.
DET, insulin detemir; GLAR, insulin glargine; MET, metformin; NPH, neutral protamine Hagedorn; OGLT, oral glucose‐lowering therapy; SU, sulfonylurea.
The frequency of residual hyperglycemia reported in observational CTRs and EMR databases was also investigated, with inclusion based on access to patient‐level data. Three noninterventional CTR studies of basal insulin were identified and included in this analysis: the Add‐on Lantus® to Oral Hypoglycemic Agents (ALOHA) study, the Cardiovascular Risk Evaluation in People with Type 2 Diabetes on Insulin Therapy (CREDIT) study, and the First Basal Insulin Evaluation (FINE)–Asia study. ALOHA was a 24‐week observational study of 5223 Japanese patients with uncontrolled T2DM (HbA1c ≥7.9% to <12.5%) initiating insulin therapy.28 CREDIT was an observational study of 3031 patients from 12 countries (10 in Europe as well as Canada and Japan) with T2DM initiating insulin therapy, with 4 years of follow‐up.29 FINE‐Asia included 2679 patients from 11 Asian countries with T2DM uncontrolled (HbA1c ≥8%) on OGLTs and initiating basal insulin.30
Data from three different EMR databases representing three regions were used: the German IMS‐Disease Analyzer database, the GE Centricity database in the US, and The Health Improvement Network (THIN) database in the United Kingdom. The IMS‐Disease Analyzer is an IMS Health database of anonymized patient histories over extended periods, categorized by specialist physician groups, that includes patient‐related diagnoses, laboratory data, hospitalizations, transferals, sick notes, and prescriptions issued for statutory health insurance and private health insurance. The GE Centricity database provides access to anonymized patient data from mid‐ to large‐sized group practices across 49 states. THIN database includes anonymized patient records collected from primary care practices throughout the United Kingdom.
Patients with data extracted from CTRs and EMR databases were included in the analysis if they were older than 18 years, were determined as having T2DM via diagnosis code (using the International Classification of Diseases, Ninth/Tenth Revisions, Clinical Modification for the GE Centricity and the German IMS‐Disease Analyzer databases, and Read codes for the United Kingdom THIN database), assisted with a proprietary algorithm to deselect individuals with type 1 diabetes mellitus, and had any basal insulin (insulin glargine, insulin detemir, or human NPH insulin, whichever came first) first issued (defined as index) between January 2008 and December 2011. Patients also had to have at least one measurement for each of HbA1c and FPG values collected at both baseline and follow‐up, a minimum of 12 months of continuous enrollment between baseline and follow‐up, and an HbA1c level of >7% at baseline. Patients diagnosed with gestational diabetes were excluded.
2.2. Statistical analysis
For data from RCTs and CTRs, univariate statistics were used to describe and compare patient demographics and clinical characteristics, as well as efficacy and safety outcome measurements, with P values derived from chi‐square test or analysis of variance, as appropriate. A multivariate logistic regression model was used to evaluate predictors of residual hyperglycemia at trial end point adjusted by patient age, sex, baseline body mass index, diabetes duration, baseline HbA1c, baseline FPG, and baseline basal insulin dose, with a P value of .05 used to determine statistical significance. Sensitivity analyses were performed on RCT data using HbA1c cutoff values of <6.5% and <7.5%, and an FPG cutoff of <6.1 mmol/L (<110 mg/dL) in place of the <7.2‐ or <7.8‐mmol/L (<130 or <140 mg/dL) cutoffs used in the main analysis. Analysis of data was performed by patient, country, and region. Ranges were provided for occurrence of glycemic status as each region was analyzed separately; pooling the regions to give a single value would have weighted the results according to the region with the highest patient enrollment.
For data from EMR databases, the number of people meeting or not achieving target HbA1c and the FPG cutoff were summarized based on the glycemic values collected at a 1‐year follow‐up. To identify the potential underlying factors associated with patients who did not achieve glycemic targets, both univariate (t test for continuous variables and Cochran‐Mantel‐Haenszel test for categorical variables) and multivariate logistic regression analyses were used. The demographics and baseline characteristics examined included age and sex, specialist visit, diabetes complications (micro‐ and macrovascular, foot examination, and lower extremity amputation), comorbidities including Charlson Comorbidity Index,31 body mass index, antiglycemia and nonantiglycemia medications (lipid, hypertension, antiplatelet, and obesity), tobacco use, and the last values collected at baseline for HbA1c and FPG.
3. RESULTS
3.1. Residual hyperglycemia in RCTs
The glycemic status of patients included in the seven trials at each study's respective end point is summarized according to region (Europe, Latin America, and Asia Pacific) in Figure 1. Overall, 42.7% to 54.4% of patients across the three regions had achieved the FPG target with basal insulin but still had residual hyperglycemia, with HbA1c levels remaining above target. A total of 16.9% to 28.0% of patients were well controlled with HbA1c levels below target, whereas 16.9% to 38.1% of patients were uncontrolled with both FPG and HbA1c levels above target.
Figure 1.
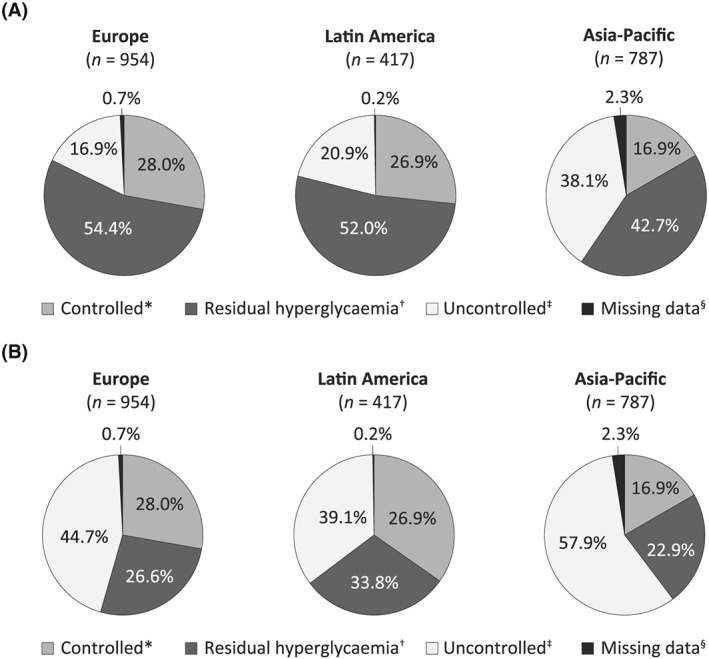
Glycemic control at study end point relative to a target HbA1c <7.0% and a target FPG of A, <7.2/7.8 mmol/L (<130/140 mg/dL) and B, <6.1 mmol/L (<110 mg/dL) in randomized clinical trials published from 2003 to 2010. *HbA1c at target. †HbA1c above target despite FPG at target. ‡Neither HbA1c nor FPG at target. §Data not available at study end point. Residual hyperglycemia identifies a subgroup of patients who may benefit from the addition of prandial therapies to lower PPG to assist in achieving target HbA1c. The difference in observed levels of residual hyperglycemia between the <7.2/7.8‐mmol/L (<130/140 mg/dL) FPG target and the <6.1‐mmol/L (<110 mg/dL) FPG target identifies a subgroup of patients who may benefit from further titration of basal insulin to achieve the FPG treatment target, which may also help them to meet target HbA1c. FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; PPG, postprandial plasma glucose
Baseline parameters that were significantly predictive of residual hyperglycemia in these trials (identified by logistic regression) are summarized in Figure 2. Baseline HbA1c was a predictor of residual hyperglycemia across Europe, Latin America, and Asia Pacific, with a higher baseline value indicating a greater likelihood of failing to achieve target HbA1c despite FPG being controlled, and female sex was predictive of residual hyperglycemia in two regions (Europe and Latin America) and two individual countries (China and Germany). Statistically significant associations were also identified between residual hyperglycemia and higher baseline FPG levels in patients from Japan (odds ratio [OR], 0.9770; 95% confidence interval [CI], 0.9590–0.9960; P = .0169) and the United Kingdom (OR, 0.9850; 95% CI, 0.9730–0.9970; P = .0121), and between residual hyperglycemia and longer duration of diabetes in patients from Brazil (OR, 1.092; 95% CI, 1.006–1.185; P = .0365).
Figure 2.
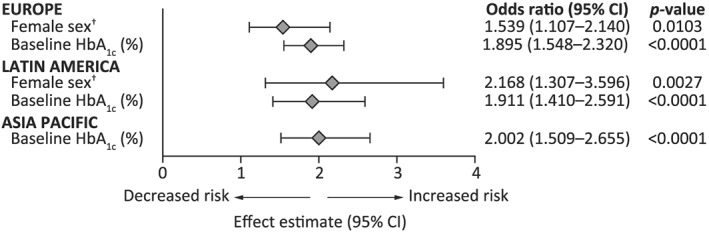
Odds ratio summary for significant predictors of residual hyperglycemia by region, based on data from published RCTs. Residual hyperglycemia was defined as end point HbA1c not at the target of <7% despite end point FPG being at target (<7.2/7.8 mmol/L [<130/140 mg/dL]). CI, confidence interval; HbA1c, glycated hemoglobin
Sensitivity analyses were performed using HbA1c cutoffs of <6.5% and <7.5%, to better reflect real‐world clinical targets, and with an FPG cutoff value of <6.1 mmol/L (<110 mg/dL; Table 2). When the more stringent HbA1c target of <6.5% was applied, the proportion of patients classed as having residual hyperglycemia increased substantially to 52.2% to 69.3%. Levels of residual hyperglycemia remained relatively high (31.3%–37.3%), even when the less stringent HbA1c target of <7.5% was used. However, the proportion of patients with uncontrolled hyperglycemia remained relatively consistent across all target HbA1c values assessed.
Table 2.
Sensitivity analysis: residual hyperglycemia across RCTs data by region
| Target cutoff value | Controlled | Residual hyperglycemia | Uncontrolled | Missing | |||
|---|---|---|---|---|---|---|---|
| Europe (N = 954) | 0.7% | ||||||
| HbA1c target <6.5% | 12.3% | 69.3% | 17.6% | ||||
| HbA1c target <7.0% | 28.0% | 28.0%* | 54.4% | 26.6%* | 16.9% | 44.7%* | |
| HbA1c target <7.5% | 47.2% | 37.3% | 14.9% | ||||
| Latin America (N = 417) | 0.2% | ||||||
| HbA1c target <6.5% | 14.6% | 62.1% | 23.0% | ||||
| HbA1c target <7.0% | 26.9% | 26.9%* | 52.0% | 33.8%* | 20.9% | 39.1%* | |
| HbA1c target <7.5% | 46.5% | 35.5% | 17.8% | ||||
| Asia Pacific (N = 787) | 2.3% | ||||||
| HbA1c target <6.5% | 5.2% | 52.2% | 39.9% | ||||
| HbA1c target <7.0% | 16.9% | 16.9%* | 42.7% | 22.9%* | 38.1% | 57.9%* | |
| HbA1c target <7.5% | 34.7% | 31.3% | 32.0% | ||||
Sensitivity analysis performed with FPG target <110 mg/dL (<6.1 mmol/L); refer to Figure 1B for additional information.
Patients were categorized as controlled (end point HbA1c at target), residual hyperglycemia (end point HbA1c not at target despite end point FPG at target), or uncontrolled (both end point HbA1c and end point FPG above target). Unless stated otherwise, HbA1c target was <7%. FPG target was <7.2 mmol/L (<130 mg/dL) in Latin America and Asia Pacific and <7.8 mmol/L (<140 mg/dL) in Europe.
FPG, fasting plasma glucose; HbA1c, glycated hemoglobin.
When a more stringent FPG target of <6.1 mmol/L (<110 mg/dL) was used in a sensitivity analysis, the proportion of patients classed as having uncontrolled hyperglycemia increased from 16.9%–38.1% to 39.1%–57.9%, and the proportion of patients with residual hyperglycemia decreased from 42.7%–54.4% to 22.9%–33.8%.
3.2. Residual hyperglycemia in CTRs
The glycemic status of patients in the ALOHA, CREDIT, and FINE‐Asia studies who were included in this analysis is summarized in Figure 3. The proportion of patients with residual hyperglycemia ranged from 31.5% in the CREDIT study at 4 years to 35.6% in the ALOHA study after 6 months of treatment. Across the CTRs, 21.8% to 33.6% of patients were well controlled, with the highest proportion in the FINE‐Asia study, whereas 30.7% to 46.8% of patients were uncontrolled, with the lowest proportion in the FINE‐Asia study. No regression analysis for predictors of residual hyperglycemia was performed on the data from CTRs, as preliminary analyses for the ALOHA and FINE‐Asia registries indicated sample sizes that were too small to allow for the effective identification of predictors.
Figure 3.

Distribution of glycemic control relative to country‐recommended targets in CTRs. *HbA1c at target (HbA1c <7%). †HbA1c above target despite FPG at target (FPG <7.2/7.8 mmol/L [<130/140 mg/dL] depending on country‐specific recommendations). ‡Neither HbA1c nor FPG at target. §Data not available at study end point. ALOHA, Add‐on Lantus® to Oral Hypoglycemic Agents study; CREDIT, Cardiovascular Risk Evaluation in People with Type 2 Diabetes on Insulin Therapy study; FINE‐Asia, First Basal Insulin Evaluation–Asia study; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin
3.3. Residual hyperglycemia in EMR databases
The glycemic status of eligible patients in the three EMR databases at 1‐year follow‐up on basal insulin therapy is summarized in Figure 4. The proportion of patients with residual hyperglycemia was 25.4% for German patients, 23.9% for US patients, and 31.8% for UK patients. The proportion of well‐controlled patients was low across all three databases, being highest in Germany at 21.0% and lowest in the United Kingdom at 4.4%. Conversely, uncontrolled hyperglycemia was more common, with the highest rates reported in the United Kingdom and the US (63.8% for both) and the lowest rates in Germany (53.6%).
Figure 4.

Distribution of glycemic control at 1 year relative to country‐recommended targets in EMR databases (basal insulin initiators from January 2007 to December 2011). *HbA1c at target (HbA1c <7%). †HbA1c above target despite FPG at target (FPG <7.2/7.8 mmol/L [<130/140 mg/dL] depending on country‐specific recommendations). ‡Neither HbA1c nor FPG at target. §Data not available at study end point. FPG, fasting plasma glucose; HbA1c, glycated hemoglobin
No consistent global predictors of residual hyperglycemia could be identified reliably, although there was a trend toward significance for baseline HbA1c levels in some countries.
4. DISCUSSION
Data from this analysis reveal a high prevalence of patients on basal insulin with residual hyperglycemia, as defined by failure to achieve HbA1c targets despite effective control of FPG. Residual hyperglycemia was common in RCTs, where high rates were consistent across countries and regions, as also seen in real‐world clinical practice, based on CTR and EMR data. These results indicate an unmet need for additional antihyperglycemic agents to control PPG in a substantial percentage of patients receiving basal insulin treatment and support the need for a more comprehensive and proactive strategy for the treatment of T2DM that takes both FPG and PPG into consideration.
Residual hyperglycemia was somewhat more frequent in RCTs compared with real‐world settings, although the prevalence of patients with uncontrolled hyperglycemia, where both HbA1c and FPG were above target, was lower in RCTs, perhaps reflecting better intensification of basal insulin to meet FPG targets. The selection criteria for inclusion of patients into RCTs may also have influenced outcomes, with a much more narrowly defined population than might be expected in real‐world clinical practice. In addition, there are important treatment differences between patients in these data sets to consider. First, insulin in clinical practice is usually titrated to HbA1c targets with the target FPG adjusted as appropriate to meet this, as recommended in the guidelines.5 By contrast, titration in RCTs is frequently conducted to a set FPG target defined by the study protocol. Second, physicians in an RCT are restricted by the treatment protocol, whereas patients in the real world are in a position to intensify or change treatment as appropriate. Therefore, the comparatively high levels of uncontrolled hyperglycemia seen in the EMR data suggest that these patients may be undermanaged with respect to glycemic control.
High baseline HbA1c was a consistent predictor of residual hyperglycemia in RCTs, with high baseline FPG, female sex, and longer diabetes duration also predictive in some populations. Higher HbA1c or FPG at baseline and longer duration of diabetes can all be indicative of poorer β‐cell function.32, 33 Although the relative contribution of FPG to hyperglycemia may be greater at higher HbA1c levels, the absolute contribution of PPG to HbA1c is relatively consistent across a wide range of HbA1c levels.9 Thus, the proportion of HbA1c attributable to PPG will increase as HbA1c decreases, and the relative benefit of increased intensity of treatments to improve FPG could be expected to decrease as target HbA1c is approached. This would suggest that patients with high HbA1c should experience the most benefit from the improved FPG control associated with initiation of basal insulin. However, pragmatically, patients with high baseline HbA1c would need to achieve an improvement that was both proportionally and absolutely greater to achieve glycemic targets, and this could explain the results seen in this analysis.
Previous studies of patients with T2DM have identified a link between female sex and poor glycemic control.34, 35, 36 However, it should be noted that other studies have identified little or no difference between male and female patients with T2DM with respect to glycemic outcomes.37
Sensitivity analyses indicate that a significant proportion (31%–37%) of patients would still be classified as having residual hyperglycemia even if less stringent HbA1c targets were used. Analysis using a lower FPG target value (<6.1 mmol/L [<110 mg/dL]) resulted in a large increase in the proportion of patients categorized as having uncontrolled hyperglycemia. However, it also identified a considerable proportion (23%–34%) of patients who still failed to meet HbA1c targets, despite achieving the more stringent FPG target. The difference observed in rates of residual hyperglycemia when using the (<6.1 mmol/L [<110 mg/dL]) versus the <7.2/7.8‐mmol/L (<130/140 mg/dL) FPG cutoff value is indicative of a proportion of patients (27.8%, 19.8%, and 18.2% in Europe, Asia Pacific, and Latin America, respectively) who could benefit from additional basal insulin titration to achieve lower FPG and, as a consequence, help attain target HbA1c, before moving on to treatment intensification with a prandial agent if still required.
It is important to note that the FPG targets used in this study were based on general clinical use, and that the use of these instead of the lower treatment targets used in RCTs (<4.0–5.6 mmol/L [<72–101 mg/dL]) as the cutoff for the analysis would have resulted in an underestimation of the occurrence of uncontrolled hyperglycemia, with a corresponding increase in the levels of residual hyperglycemia seen. However, results from the sensitivity analysis using a lower FPG target cutoff of <6.1 mmol/L (<110 mg/dL) indicated a significant proportion of patients with residual hyperglycemia who could benefit from the addition of prandial therapies to reduce PPG and assist in achieving target HbA1c without increasing the risk of hypoglycemic events or body weight gain. The difference in observed levels of residual hyperglycemia between the <7.2/7.8‐mmol/L (<130/140 mg/dL) FPG target and the <6.1‐mmol/L (<110 mg/dL) FPG target identifies a subgroup of patients who may benefit from further titration of basal insulin to achieve target FPG.
The trajectory of change in FPG and HbA1c when initiating and titrating basal insulin, along with the duration of observation, are important clinical considerations. Nevertheless, it is interesting to note that the prevalence of residual hyperglycemia at 4 years in the CREDIT registry was numerically lower but similar to the rate of residual hyperglycemia seen in the same registry at 1 year and in studies with shorter follow‐up, suggesting that the duration of basal insulin treatment may not be a major determinant of residual hyperglycemia. As such, the observed differences in the proportions of patients with residual hyperglycemia are more likely to be due to differences in patient glycemic management in RCTs versus real‐world situations, as discussed previously. However, because the duration of treatment was not factored into the analysis, such a contribution cannot be ruled out.
Residual hyperglycemia while receiving basal insulin can be addressed by PPG management with rapid‐acting insulin given as a basal‐plus or basal‐bolus regimen.38, 39 PPG management can also be achieved through the initiation of OGLTs, such as acarbose, dipeptidyl peptidase‐4 inhibitors, sulfonylureas and glinides, or subcutaneous therapies, including prandial GLP‐1 receptor agonists. However, current guidelines do not distinguish between short‐ and long‐acting GLP‐1 receptor agonists, which differ significantly in their respective mechanisms of action. Long‐acting GLP‐1 receptor agonists provide robust reductions in FPG and modest reductions in PPG through the stimulation of insulin secretion and the inhibition of glucagon secretion 40, 41, 42, 43, whereas short‐acting, prandial GLP‐1 receptor agonists have comparatively lesser effects on insulin secretion but strongly inhibit postprandial glucagon secretion and have a substantial delaying effect on gastric emptying, resulting in a pronounced effect on PPG.44, 45, 46, 47, 48
The different sources from which the data were obtained—RCTs, CTRs, and EMR databases—each have inherent strengths and limitations. RCTs provide a population of patients with T2DM defined by the study inclusion criteria, with fewer potential confounding external factors, increased likelihood of a greater level of general medical care than might be expected in a real‐world clinical population, and a limited pool of treatment options. By contrast, the patient data obtained from EMR databases covered a longer duration of treatment and a less narrowly defined patient population, with a wider range of treatment options, as seen in real‐world clinical situations. Furthermore, in real‐world situations as captured in the EMRs and CTRs, background treatment with OGLTs may change or be stopped on initiation of basal insulin, as per local guidelines, potentially resulting in a relative increase in PPG. Indeed, the differences in the level of residual hyperglycemia seen across RCTs, CTRs, and EMRs will be influenced by differences in treatment options, treatment target values used, and patient populations included, as discussed previously, and would also reflect the difficulties of translating success with therapies in clinical studies to a real‐world setting. By performing the analysis using data from both RCT and real‐world situations, our findings should be more robust and generally applicable than if the levels of residual hyperglycemia seen had been identified from one type of data source alone. In addition, although the analysis would have been simplified by using a single cutoff point to determine treatment success or failure, such as the target FPG between 4.4 and 7.2 mmol/L (80–130 mg/dL) as recommended by the ADA, it is important to remember that the data analyzed here were gathered between 1999 and 2012. During this period, various professional organizations updated their guidance regarding the threshold value to use at different times. In addition, there are also differences in national and regional guidelines to consider. Thus, using a cutoff value based on the regional guidelines at the time the studies were conducted may better reflect the real‐world unmet need for each region.
Owing to a need for access to patient‐level data, only RCTs conducted by the study sponsor were included in the analysis. A consequence of this is that all RCTs that met the specified inclusion criteria had insulin glargine as the comparator. Patients receiving other basal insulins were included in the analysis (NPH insulin in five studies and insulin detemir in one study) but only where the comparator was insulin glargine.
No attempt was made to account for previous background therapies or concomitant medication, including OGLTs, in this analysis. However, the continuation of existing OGLTs when receiving basal insulin is an established treatment paradigm in T2DM,5 and many patients treated with basal insulin will continue to receive background OGLT therapies during clinical trials. Patients who are initiating basal insulin treatment—the population of interest in our analysis—have T2DM that has progressed beyond the point where it can be controlled effectively with OGLTs. Therefore, it is common practice not to stratify clinical trial results according to patients' background OGLT medication. In the present analysis, which included real‐world data, the emphasis was on the unmet medical needs of a diverse group of patients, who were all receiving basal insulin therapy. Thus, from the perspective of clinicians and payers, the current analysis was designed appropriately to illustrate the generalized unmet medical need in the basal insulin‐treated patient population, without additionally controlling for background therapies.
It is important to remember that hyperglycemia is driven by a combination of basal hyperglycemia and postprandial hyperglycemia, and the difference between the two may be important when selecting agents for treatment intensification.49, 50 In this study, a single FPG value was used to identify patients who failed to achieve HbA1c targets despite adequate FPG control, and thereby to implicate PPG as the cause of the residual hyperglycemia. However, it should be remembered that FPG is not identical to basal hyperglycemia. The reduction of FPG to normal or near‐normal levels does not mean that basal hyperglycemia has been eradicated entirely, and monitoring of premeal FPG levels at breakfast, lunch, and dinner would be needed to assess if basal hyperglycemia is still present in these patients.
The results of this study identify a sizeable proportion of patients who are receiving basal insulin but have residual hyperglycemia, with HbA1c levels above target despite having adequately controlled FPG levels. These findings support the need for a more comprehensive strategy for the treatment of T2DM that takes both FPG and PPG into consideration at an earlier stage of the disease. Monitoring blood glucose levels throughout the day in patients treated with basal insulin could identify whether the intensification of FPG control is appropriate or if additional treatment to address postprandial hyperglycemia is required. Large, prospective, real‐world studies to determine risk factors for residual hyperglycemia are needed so that high‐risk patients initiating basal insulin can be identified early on in their treatment and intervention with prandial therapies can be initiated.
CONFLICTS OF INTEREST
DR has served on an advisory panel and as an author for Bristol‐Myers Squibb, Eli Lilly, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi and has served on an advisory panel and as speaker for AstraZeneca, Eli Lilly, Janssen, Novartis, Novo Nordisk, and Sanofi. SC has served on an advisory panel and as an author for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Roche, Sanofi, Servier, and Takeda, and on the speakers bureau and as an author for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, Roche, Sanofi, Servier, and Takeda. FL has served on an advisory panel and as an author for Boehringer Ingelheim, Bristol‐Myers Squibb/AstraZeneca, Eli Lilly, Janssen Pharmaceuticals, Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Takeda; has served on the speakers bureau and as an author for Boehringer Ingelheim, Bristol‐Myers Squibb/AstraZeneca, Eli Lilly, Janssen, Merck, Novo Nordisk, Pfizer, Sanofi, and Silanes; and has received grants/research support from Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Pfizer, and Sanofi. AM has served on an advisory panel and as an author for Eli Lilly, Novo Nordisk, Roche, Sanofi, Servier, and Takeda and has received grants/research support from Eli Lilly, Novartis, Sanofi, and Servier. NT has served on scientific advisory boards and received honoraria from companies involved in development of medications for the treatment of type 2 diabetes, including AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Novo Nordisk, and Sanofi, and has received grants/research support from Eli Lilly, Novartis, Pfizer, and Sanofi. MJD has served on an advisory panel and as an author for Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi and as a consultant for Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; has served on the speakers bureau and as an author for Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novo Nordisk, and Sanofi and as a speaker for Mitsubishi Tanabe Pharma Corporation; and has received grants/research support from Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. EC is an employee of Sanofi. EN is a consultant for Sanofi. ZG and JV have no conflicts to declare.
ACKNOWLEDGMENTS
DR and MD contributed to the concept and design of the study. All authors contributed to the data analysis and interpretation and were involved in the drafting of the publication and critical revisions. All authors approved the final draft for submission.
This study was sponsored by Sanofi. Assistance with the statistical analysis was provided by Jay Lin of Novosys Health and Hsing‐wen Chung and was funded by Sanofi. Editorial support for this manuscript was provided by Andy Shepherd of Caudex Medical and was funded by Sanofi.
Raccah D, Chou E, Colagiuri S, et al. A global study of the unmet need for glycemic control and predictor factors among patients with type 2 diabetes mellitus who have achieved optimal fasting plasma glucose control on basal insulin. Diabetes Metab Res Rev. 2017;33:e2858 https://doi.org/10.1002/dmrr.2858
REFERENCES
- 1. UK Prospective Diabetes Study Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation Guideline Development Group . Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Standards of care: approaches to glycemic treatment. Diabetes Care. 2015;38(suppl 1):S41–S47. [DOI] [PubMed] [Google Scholar]
- 5. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Clinical Excellence . Primary Care Quality and Outcomes Framework Indicator Advisory Committee recommendations. http://www.nice.org.uk/guidance/cg87/resources/guidance‐type‐2‐diabetes‐pdf. Accessed February 16, 2015.
- 7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract. 2013;19(3):536–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881–885. [DOI] [PubMed] [Google Scholar]
- 9. Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care. 2011;34(12):2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27(1):79–84. [DOI] [PubMed] [Google Scholar]
- 11. Riddle MC, Rosenstock J, Gerich J. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. [DOI] [PubMed] [Google Scholar]
- 12. Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough—what next? Diabetes Metab Res Rev. 2007;23(4):257–264. [DOI] [PubMed] [Google Scholar]
- 13. Peter R, Dunseath G, Luzio SD, Owens DR. Estimates of the relative and absolute diurnal contributions of fasting and post‐prandial plasma glucose over a range of hyperglycaemia in type 2 diabetes. Diabetes Metab. 2013;39(4):337–342. [DOI] [PubMed] [Google Scholar]
- 14. Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77(2):280–285. [DOI] [PubMed] [Google Scholar]
- 15. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof‐of‐concept study. Diabetes Care. 2010;33(7):1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care. 2013;36(9):2497–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice‐daily exenatide in basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–112. [DOI] [PubMed] [Google Scholar]
- 18. American Diabetes Association . Clinical practice recommendations 1995. Diabetes Care. 1995;18(suppl 1):S1–S96. [PubMed] [Google Scholar]
- 19. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Diabetes Association Standards of Medical Care in Diabetes—2009 . Diabetes Care. 2009;32(Suppl 1):S13–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eliaschewitz FG, Calvo C, Valbuena H, et al. Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch Med Res. 2006;37(4):495–501. [DOI] [PubMed] [Google Scholar]
- 22. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138(12):952–959. [DOI] [PubMed] [Google Scholar]
- 23. Janka HU, Plewe G, Riddle MC, Kliebe‐Frisch C, Schweitzer MA, Yki‐Jarvinen H. Comparison of basal insulin added to oral agents versus twice‐daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005;28(2):254–259. [DOI] [PubMed] [Google Scholar]
- 24. Pan CY, Sinnassamy P, Chung KD, Kim KW. Insulin glargine versus NPH insulin therapy in Asian Type 2 diabetes patients. Diabetes Res Clin Pract. 2007;76(1):111–118. [DOI] [PubMed] [Google Scholar]
- 25. Swinnen SG, Dain MP, Aronson R, et al. A 24‐week, randomized, treat‐to‐target trial comparing initiation of insulin glargine once‐daily with insulin detemir twice‐daily in patients with type 2 diabetes inadequately controlled on oral glucose‐lowering drugs. Diabetes Care. 2010;33(6):1176–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yki‐Jarvinen H, Kauppinen‐Makelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49(3):442–451. [DOI] [PubMed] [Google Scholar]
- 27. Kawamori R. Efficacy and safety of insulin glargine in concurrent use with oral hypoglycaemic agents for the treatment of type 2 diabetes patients. Rinsho Iyaku. 2003;19(1):445–464. [Google Scholar]
- 28. Ohtani T, Ito T. Safety and effectiveness of BOT (basal supported oral therapy) using insulin glargine in Japanese patients with type 2 diabetes—results from postmarketing surveillance of insulin glargine (ALOHA study) Shinyaku to Rinsho. J New Rem Clin. 2011;6:458–475. [Google Scholar]
- 29. Freemantle N, Evans M, Christensen T, Wolden ML, Bjorner JB. A comparison of health‐related quality of life (health utility) between insulin degludec and insulin glargine: a meta‐analysis of phase 3 trials. Diabetes Obes Metab. 2013;15(6):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsai ST, Pathan F, Ji L, et al. First insulinization with basal insulin in patients with type 2 diabetes in a real‐world setting in Asia. J Diabetes. 2011;3(3):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 32. Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta‐cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10‐year follow‐up of the Belfast Diet Study. Diabet Med. 1998;15(4):290–296. [DOI] [PubMed] [Google Scholar]
- 33. Ostgren CJ, Lindblad U, Ranstam J, Melander A, Rastam L. Glycaemic control, disease duration and beta‐cell function in patients with type 2 diabetes in a Swedish community. Skaraborg Hypertension and Diabetes Project. Diabet Med. 2002;19(2):125–129. [DOI] [PubMed] [Google Scholar]
- 34. Pound N, Sturrock ND, Jeffcoate WJ. Age related changes in glycated haemoglobin in patients with insulin‐dependent diabetes mellitus. Diabet Med. 1996;13(6):510–513. [DOI] [PubMed] [Google Scholar]
- 35. Valle T, Koivisto VA, Reunanen A, Kangas T, Rissanen A. Glycemic control in patients with diabetes in Finland. Diabetes Care. 1999;22(4):575–579. [DOI] [PubMed] [Google Scholar]
- 36. McGill JB, Vlajnic A, Knutsen PG, Recklein C, Rimler M, Fisher SJ. Effect of gender on treatment outcomes in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013;102(3):167–174. [DOI] [PubMed] [Google Scholar]
- 37. Ewenighi CO, Uchechukwu D, Adejumo BI, et al. Responses to glycemic control therapy according to age, gender, level of adiposity, and duration of diabetes in type 2 diabetic patients. Indian J Med Sci. 2013;67(3‐4):61–69. [PubMed] [Google Scholar]
- 38. Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single‐dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10(12):1178–1185. [DOI] [PubMed] [Google Scholar]
- 39. Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17(3):395–403. [DOI] [PubMed] [Google Scholar]
- 40. Owens DR, Luzio SD, Sert‐Langeron C, Riddle MC. Effects of initiation and titration of a single pre‐prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6‐month ‘proof‐of‐concept’ study. Diabetes Obes Metab. 2011;13(11):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374(9683):39–47. [DOI] [PubMed] [Google Scholar]
- 42. Degn KB, Juhl CB, Sturis J, et al. One week's treatment with the long‐acting glucagon‐like peptide 1 derivative liraglutide (NN2211) markedly improves 24‐h glycemia and alpha‐ and beta‐cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53(5):1187–1194. [DOI] [PubMed] [Google Scholar]
- 43. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. [DOI] [PubMed] [Google Scholar]
- 44. Ahren B, Gautier JF, Berria R, Stager W, Aronson R, Bailey CJ. Pronounced reduction of postprandial glucagon by lixisenatide: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(9):861–868. [DOI] [PubMed] [Google Scholar]
- 45. Edwards CM, Stanley SA, Davis R, et al. Exendin‐4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281(1):E155–E161. [DOI] [PubMed] [Google Scholar]
- 46. Lorenz M, Pfeiffer C, Steinstrasser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes—relationship to postprandial glycemia. Regul Pept. 2013;185:C1–C8. [DOI] [PubMed] [Google Scholar]
- 47. Meier JJ, Yabe D, Wang E, Lin J, Rosenstock J, Ahrén B. Efficacy of lixisenatide in patients with different levels of beta cell function as assessed by C‐peptide/glucose ratio. Diabetologia. 2013;56(suppl 1):S1–S566. (Abstract 896) [Google Scholar]
- 48. Meier JJ, Rosenstock J, Hincelin‐Mery A, et al. Effect of lixisenatide vs liraglutide on glycemic control, gastric emptying, and safety parameters in optimized insulin glargine T2DM ± metformin. Presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, USA, 13–17 June 2014, 1017‐P.
- 49. Monnier L, Colette C, Dejager S, Owens D. Residual dysglycemia when at target HbA1c of 7% (53 mmol/mol) in persons with type 2 diabetes. Diabetes Res Clin Pract. 2014;104(3):370–375. [DOI] [PubMed] [Google Scholar]
- 50. Monnier L, Colette C, Dejager S, Owens D. “Mild dysglycemia” in type 2 diabetes: to be neglected or not? J Diabetes Complications. 2015;29(3):451–458. [DOI] [PubMed] [Google Scholar]


