Abstract
Aim
Calcium infusion is used after parathyroid surgery for renal hyperparathyroidism to treat postoperative hypocalcaemia. We compared a new infusion regimen to one commonly used in Malaysia based on 2003 K/DOQI guidelines.
Methods
Retrospective data on serum calcium and infusion rates was collected from 2011–2015. The relationship between peak calcium efflux (PER) and time was determined using a scatterplot and linear regression. A comparison between regimens was made based on treatment efficacy (hypocalcaemia duration, total infusion amount and time) and calcium excursions (outside target range, peak and trough calcium) using bar charts and an unpaired t‐test.
Results
Fifty‐one and 34 patients on the original and new regimens respectively were included. Mean PER was lower (2.16 vs 2.56 mmol/h; P = 0.03) and occurred earlier (17.6 vs 23.2 h; P = 0.13) for the new regimen. Both scatterplot and regression showed a large correlation between PER and time (R‐square 0.64, SE 1.53, P < 0.001). The new regimen had shorter period of hypocalcaemia (28.9 vs 66.4 h, P = 0.04), and required less calcium infusion (67.7 vs 127.2 mmol, P = 0.02) for a shorter duration (57.3 vs 102.9 h, P = 0.001). Calcium excursions, peak and trough calcium were not significantly different between regimens. Early postoperative high excursions occurred when the infusion was started in spite of elevated peri‐operative calcium levels.
Conclusion
The new infusion regimen was superior to the original in that it required a shorter treatment period and resulted in less hypocalcaemia. We found that early aggressive calcium replacement is unnecessary and raises the risk of rebound hypercalcemia.
Keywords: calcium gluconate, hypocalcaemia, renal insufficiency, secondary hyperparathyroidism, surgery
Summary at a Glance
The management of hypocalcaemia after parathyroidectomy in renal patients can be difficult and runs a high risk of dangerous hypocalcaemia. This article explores a new calcium infusion protocol in this setting with encouraging results.
Postoperative hypocalcaemia (POH) is a common complication after parathyroid surgery. It tends to be more severe and prolonged in patients with renal hyperparathyroidism (RHP) with rates as high as 36–97%.1, 2
In RHP, there is reduced conversion of vitamin D to calcitriol in the renal parenchyma leading to diminished feedback suppression on the parathyroid glands and hence significantly elevated parathyroid hormone (PTH) levels.3 In addition, long standing chronic kidney disease causes persistent hypocalcaemia from phosphate retention, which leads to a loss of both vitamin D and calcium‐sensing receptors in the parathyroid glands.4
The high turnover state from elevated PTH levels as well as defective mineralization from vitamin D deficiency gives rise to an increase in the amount of osteoid.5 When the PTH level falls abruptly after surgery, the mismatch in bone formation and resorption causes the osteoid to act as a metabolic sink for calcium in a similar way to what occurs in dystrophic calcification, thus resulting in the changes typical of POH.6
The 2003 K/DOQI guidelines had recommended starting an infusion at a rate of 1–2 mg elemental calcium per kilogram body weight per hour once serum calcium falls below 1.8 mmol/L (0.1–0.2 mL/h per kg of 10% calcium gluconate).7 This was to be adjusted to maintain serum calcium within the normal range with monitoring every 4–6 h for the first 48–72 h after surgery. This recommendation was based on expert opinion with no actual titration regimen specified.
Calcium gluconate is preferred over calcium chloride as it is less irritating and less likely to cause tissue necrosis on extravasation. It is thus the drug of choice for haemodynamically stable patients especially with the prolonged infusion regimens which we use in POH.8
In Malaysia, the local consensus is to start the 10% calcium gluconate infusion immediately after surgery at a rate between 5–10 mL/h (1.2–2.3 mmol/h), which is adjusted according to 6‐hourly calcium levels to keep it between 2–3 mmol/L (8–12 mg/dL).9 The rationale for this is that a rapid fall in serum calcium after surgery can cause considerable morbidity and even mortality, hence an aggressive means of calcium replacement is advocated. The shortcomings of this approach are that the original article was based on a limited series of four cases, and furthermore no titration regimen was given.
To address these issues, a subsequent retrospective study formulated a novel regimen for postoperative replacement in RHP, based on a model using calcium fluxes.1 In this regimen, the 10% calcium gluconate infusion is started at 4.5 mL/h (1.0 mmol/h) only when the serum calcium falls below 2 mmol/L (8 mg/dL). This can be increased to 6.5 mL/h (1.5 mmol/h) and subsequently 9 mL/h (2.1 mmol/h) if the calcium level continues to drop. The interval for serum calcium checks varies from 4–6 hourly depending on the current calcium level.
As this regimen had not been practiced or validated yet, our centre trialled it on an exploratory basis with a small modification in that the calcium infusion was started immediately after surgery at 4.5 mL/h (1.0 mmol/h) regardless of the actual calcium level. This was a compromise between existing local practice and the new proposed regimen. The original regimen in our centre is as per the local consensus although we prefer to start the infusion at 5 mL/h (1.2 mmol/h).
This meant that while the initial infusion rates for both the old and new regimens were similar (4.5 vs 5 mL/h, 1.0 vs 1.2 mmol/h), the essential difference was that the new regimen had a structured titration algorithm that was formulated to be closer to the metabolic demands of a patient with POH. In this article, the authors report our experience with the new infusion regimen compared with the original one in terms of both efficacy and the ability to maintain serum calcium within the target range.
Study objectives
The first objective of the study is to characterize calcium flux in patients during the immediate postoperative period so as to verify the findings from the original article using a different group of patients.1 The second and third objectives are to compare the new infusion regimen with the original one looking at both treatment efficacy and calcium excursions outside the target range of 2–3 mmol/L, respectively.
For this study, efficacy is determined by the duration of hypocalcaemia, total amount of calcium gluconate infused, and the time period of infusion. Excursions are measured by the percentage of the treatment period where the calcium level was below, above, or outside the target range, as well as the peak and trough calcium during treatment.
Methods
Inclusion and exclusion criteria
Only patients diagnosed with secondary hyperparathyroidism of renal origin (ICD‐10‐CM Diagnosis Code N25.81) who underwent parathyroidectomy with or without auto‐transplantation were included. In addition, they had to be started on an intravenous calcium gluconate infusion after surgery with either the original or new regimen. Patients younger than 16 years and pregnant women were excluded as physiological differences in skeletal metabolism would make the study data difficult to interpret. Treatment with bisphosphonates, calcitriol and calcium supplementation did not exclude patients from the study. However, usage within the 6 months prior to surgery and during the immediate postoperative period was noted.
Data collection
This study was done with prior ethics approval from the Malaysian Ministry of Health Research Ethics Committee (KKM/NIHSEC/P15‐617) in accordance with current guidelines on Good Clinical Practice and the Declaration of Helsinki, and was registered with the Malaysian National Medical Research Register (NMRR‐15‐554‐24819).
Data were collected retrospectively based on patient records. As this was done anonymously, informed consent was not required by the ethics review board. The period of data collection was from 2011–2015, during which the same surgical team was responsible for the care of all patients. Cases for the period of 2011–2014 were managed using the original infusion regimen while those from 2013–2015 were on the novel regimen. An overlap period of about six months was used to allow the staff to gain confidence in the new regimen.
Standard ALP (reference interval: 0‐129 U/L, Dimension RxL Max, Siemens Healthcare Diagnostics, Newark, DE, USA) was measured without isoenzyme determination, and included only if done in the 6‐month period prior to surgery. The PTH (reference interval: 0.5–4.1 pmol/L, 5–39 pg/mL, immunoradiometric assay, Scantibodies Laboratory, Santee, CA, United States) value used was the most recent reading taken prior to surgery. Serum calcium (reference interval: 2.15–2.55 mmol/L, 8.60–10.20 mg/dL, Dimension RxL Max) was the calcium level corrected for serum albumin using Payne's method.10 The patient age, height, and weight were at operation date, while the adenoma volume was estimated on ultrasonography, or from the histology report of the surgical specimen when available. Symptoms such as peri‐oral numbness, tingling, muscle weakness or cramps, were noted from the clinical records if present concurrent to the periods of biochemical hypocalcaemia.
Analysis
The relevant pre‐operative factors are compared between the two regimens using an independent samples t‐test for continuous variables (age, ALP, PTH, adenoma volume), or a χ2 test with Yates' continuity correction where appropriate for categorical variables (gender, ethnicity, bisphosphonate pre‐treatment, calcitriol pre‐treatment).
-
Objective 1
Calcium flux
The determination of calcium flux follows the methodology detailed in the original study for the infusion regimen.1 Calcium influx (mmol/h) is calculated by multiplying the infusion rate (mL/h) with the conversion factor (0.2325 mmol/mL). The rate of change in the extracellular pool (mmol/h) is calculated by taking the difference in serum calcium (mmol/L) between two consecutive readings divided by the time difference (4 h) and multiplied by total extracellular fluid volume (ECF) (15 L in a normal adult). The calculated efflux (mmol/h) is thus the difference between the rate of change in the extracellular calcium pool and influx rate. The peak efflux rate (PER) and timing are obtained for each subject, ignoring readings immediately after dialysis sessions. The PER was also recalculated using the ECF estimated from Abraham's method and compared with the original PER using linear regression.11
Whenever the calculated PER is greater than 3 mmol/h, this may suggest a sampling error in the calcium readings, most commonly due to prolonged tourniquet application when drawing blood. The actual values are then inspected and the elevated PER associated with isolated high calcium readings are treated as missing values and replaced on a ‘last valid observation carried forward’ basis.
Outliers are detected by plotting Cook's Distance against Centred Leverage Value for the PER‐timing regression, and variances displayed using a residual plot. Outliers are then excluded from the subsequent analysis steps. Normality for PER is determined by a Kolmogorov‐Smirnov test against a normal distribution. A scatterplot of PER against time is charted, along with linear regression to see if a relationship exists between these variables.
In addition, the PER and timing are compared for both regimens using bar charts and an independent samples t‐test. Levene's test is used to check for equality of variances to determine the appropriate type of t‐test to use.
If a significant difference is detected in PER between regimens, a univariate General Linear Model Analysis of Variance (GLM‐ANOVA) is performed with PER as the dependent variable, the type of regimen as the between‐subjects factor, with ALP, PTH, calcitriol pre‐treatment, bisphosphonate pre‐treatment, and total calcium infused as covariates. Oral calcium pre‐treatment is not used as a covariate as every subject received it at our centre. Post‐hoc power is calculated using G*Power 3.1 for each statistical test.12
-
Objective 2
Treatment Efficacy
The duration of hypocalcaemia, total amount of calcium gluconate infused, and the time period of infusion are compared in a similar manner to PER.
-
Objective 3
Calcium Excursions
The percentage of the treatment period where the calcium level was below, above, or outside the target range, as well as the peak and trough calcium during treatment are compared in a similar manner to PER. Subjects were divided into two groups depending on whether they reported symptoms suggestive of hypocalcaemia, and were compared based on PER and trough calcium using an independent samples t‐test.
All computations are performed using SPSS for Windows version 20.0 (IBM Corp., Armonk, NY, USA). Statistical tests are two‐tailed and conducted at 5% level of significance.
Results
Case records for patients with the ICD‐10‐CM N25.81 diagnosis from March 2011 to April 2014 who were on the original infusion regimen were screened, of which 51 satisfied the inclusion and exclusion criteria. A further 34 patients on the new regimen from October 2013 to June 2015 were similarly included. Most of the excluded patients had incomplete data collection.
Patient demographics were as follows: age (21–67, mean 47 years); male (34), female (51); Malay (58), Chinese (20), Indian (6), Others (1). Twenty‐four out of eighty‐five (28%) patients were pre‐treated with bisphosphonates, and none were on cinacalcet. There was no significant difference between bisphosphonate and non‐bisphosphonate groups in the trough calcium (t = ‐1.91, P = 0.06, mean difference = 0.14 mmol/L, post‐hoc power 51.9%) or PER (t = 1.48, P = 0.14, mean difference = 0.31 mmol/h, post‐hoc power 32.3%).
Most patients were on stable doses of calcitriol (0.5–2 µg/day) and calcium carbonate (1–2 g elemental calcium per day) prior to surgery, which was continued during the postoperative period. PTH levels after surgery were not checked in any of the patients. Relevant pre‐operative factors are compared between both regimens and no significant differences were found (Table 1).
-
Objective 1
Calcium flux
Table 1.
Summary comparing a selection of pre‐operative factors using either an independent samples t‐test or a χ2 test (n = 85)
| Factor | t | Significance (p) | Mean | Difference | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Original | New | Lower | Upper | ||||
| Age (years) | 0.052 | 0.959 | 45.53 | 45.39 | 0.14 | −5.15 | 5.42 |
| ALP (U/L) | 0.245 | 0.807 | 569.3 | 548.5 | 20.9 | −148.6 | 190.3 |
| PTH (pmol/L) | 0.933 | 0.354 | 160.6 | 182.3 | −21.7 | −68.0 | 24.6 |
| Adenoma volume (cc) | 1.155 | 0.252 | 2.92 | 3.86 | −0.94 | −2.57 | 0.68 |
| Chi‐Square | Significance (p) | ||||||
| Gender | 3.305* | 0.069 | — | — | — | — | — |
| Ethnicity | 3.601 | 0.165 | — | — | — | — | — |
| Bisphos. Pre‐Rx | 0.900* | 0.343 | — | — | — | — | — |
| Vit. D Pre‐Rx | 0.000* | 1.000 | — | — | — | — | — |
ALP, alkaline phosphatase; Bisphos Pre‐Rx, bisphosphonate pre‐treatment; PTH, parathyroid hormone; Vit. D Pre‐Rx, calcitriol pre‐treatment.
Continuity correction applied for 2×2 contingency tables
The mean PER from the extracellular calcium pool was 2.43 mmol/h (SD 0.87), occurring 30.0 h (SD 47.7) postoperatively. The PER calculated using the ECF estimated from Abraham's method essentially mirrored the PER calculated using a standard 15 L volume (Pearson's correlation coefficient = 0.95). As the weight of the subjects and their estimated ECF can vary significantly as a result of dialysis, a decision was made to only use the PER based on standard extracellular fluid volumes.
A scatterplot of PER against timing of peak efflux suggests a linear relationship between the variables. Four clear outliers (old regimen: 41, 48, 49; new regimen: 73) were identified on a plot of Cook's Distance against Centred Leverage Value.
On reviewing the case records, the infusion regimen for patients 41 and 48 were under‐titrated initially resulting in early hypocalcaemia. Patient 49 had status epilepticus as an adverse reaction to a pethidine injection and was kept in intensive care for an extended period. Patient 73 was over‐supplemented with calcitriol and had persistent hypercalcaemia for several weeks.
A standardized residual plot was charted for PER, confirming both Homoscedasticity and Normality aside from the outliers identified. This was further supported by a non‐significant Kolmogorov‐Smirnov test (Z = 1.04, P = 0.23) against a normal distribution. With outliers excluded, the scatterplot was re‐charted with a regression line through the origin (Fig. S1). The adjusted R‐square was determined to be 0.64 (SE 1.53, P < 0.001 for F change), suggesting a large correlation between PER and timing of peak calcium efflux.13 The regression equation can be represented as PER (mmol/h) = 0.073 (95% CI 0.061–0.085) × peak efflux time (h).
The independent samples t‐test and bar charts showed that PER was lower (2.16 vs 2.56; 0.40 mmol/h difference, P = 0.03, 95% CI 0.03–0.77) and occurred earlier (17.6 vs 23.2; 5.6 h difference, P = 0.13, 95% CI ‐1.7–12.9) for the new regimen (Fig. 1a–b, Table 2). The differences were significant for PER but not for timing as the variability in the latter was fairly high, while the respective post‐hoc powers were 60.4% and 29.3%.
Figure 1.
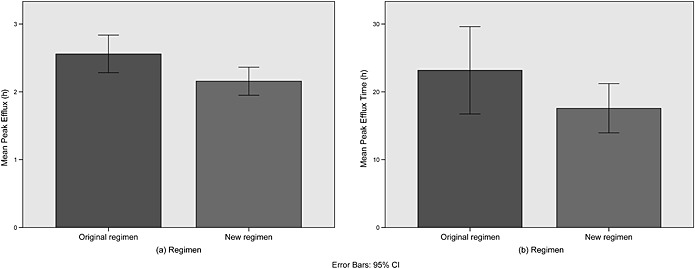
Bar charts comparing the original and new regimens for the (a) peak efflux rate and (b) timing.
Table 2.
Summary results for the independent samples T‐test comparing the original and new regimens for selected parameters
| Levene's Test (P) | t | Significance (P) | Mean | Difference | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Original | New | ||||||
| Peak efflux (mmol/h) | 0.069 | 2.158 | 0.034 | 2.56 | 2.16 | 0.40 | 0.03 – 0.77 |
| Peak efflux time (h) | 0.002 | 1.525 | 0.132 | 23.2 | 17.6 | 5.6 | −1.7–12.9 |
| Duration of hypocalcaemia (h) | 0.001 | 2.132 | 0.036 | 66.3 | 28.9 | 37.5 | 2.4–72.5 |
| Calcium gluconate infused (mmol) | 0.020 | 2.332 | 0.024 | 127.2 | 67.7 | 59.5 | 8.1–110.9 |
| Time period of infusion (h) | <0.001 | 3.423 | 0.001 | 102.9 | 57.3 | 45.6 | 18.9–72.3 |
| Low excursion (%) | 0.689 | 0.097 | 0.923 | 25.75 | 24.99 | 0.76 | −15.1–16.6 |
| High excursion (%) | 0.006 | −1.349 | 0.221 | 7.61 | 14.83 | −7.22 | −20.0–5.6 |
| Total excursion (%) | 0.451 | −0.165 | 0.869 | 21.27 | 22.28 | −1.00 | −13.2–11.2 |
| Peak calcium (mmol/L) | 0.504 | 0.866 | 0.389 | 2.95 | 2.83 | 0.11 | −0.15–0.37 |
| Trough calcium (mmol/L) | 0.316 | −0.469 | 0.640 | 2.06 | 2.09 | −0.03 | −0.14–0.09 |
GLM‐ANOVA showed that the type of infusion regimen had the largest effect on PER (partial eta‐squared = 0.568), while the effect size for total calcium infused was three times that of the remaining four covariates combined (Table 3). The interaction term between the type of infusion regimen and total calcium infused was fairly weak (partial eta‐squared = 0.010).
-
Objective 2
Treatment Efficacy
Table 3.
Summary results for GLM‐ANOVA comparing the original and new regimens against PER with selected covariates
| Type III Sum of Squares | F | Significance (P) | Partial Eta Squared | Observed Power | |
|---|---|---|---|---|---|
| Corrected Model | 433.903 | 158.850 | 0.000 | 0.943 | 1.000 |
| ALP | 0.078 | 0.201 | 0.655 | 0.003 | 0.073 |
| PTH | 0.118 | 0.301 | 0.585 | 0.004 | 0.084 |
| Vit. D Pre‐Rx | 0.655 | 1.677 | 0.200 | 0.024 | 0.248 |
| Bisphos. Pre‐Rx | 0.313 | 0.801 | 0.374 | 0.012 | 0.143 |
| Total Calcium Infused | 3.771 | 9.665 | 0.003 | 0.126 | 0.865 |
| Infusion Regimen | 34.417 | 44.100 | 0.000 | 0.568 | 1.000 |
ALP, alkaline phosphatase; Bisphos. Pre‐Rx, bisphosphonate pre‐treatment; PTH, parathyroid hormone; Vit. D Pre‐Rx, calcitriol pre‐treatment.
The GLM‐ANOVA model is built without intercept as the mean PER difference was already tested in Table 2. The Sum of Squares is a measure of variability analogous to a standard deviation or variance in a population. The Partial Eta Squared is a measure of effect size, which describes the proportion of variability in the PER attributed to that factor. The Observed Power is the probability of correctly rejecting the null hypothesis that the factor has no effect on the PER.
The independent samples t‐test comparing the original and new regimens for the duration of hypocalcaemia, total amount of calcium gluconate infused, and the time period of infusion were all significantly different (Fig. 2a–c, Table 2). The new regimen had a shorter duration of hypocalcaemia (28.9 vs 66.4; 37.5 h difference, P = 0.04, 95% CI 2.4–72.5), required less calcium infusion (67.7 vs 127.2; 59.5 mmol elemental calcium difference, P = 0.02, 95% CI 8.1–110.9) for a shorter duration (57.3 vs 102.9; 45.6 h difference, P = 0.001, 95% CI 18.9–72.3). The post‐hoc power of the three tests were, respectively, 51.0%, 55.2%, and 87.4%.
-
Objective 3
Calcium Excursions
Figure 2.
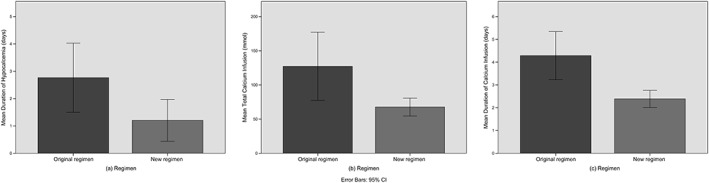
Bar charts comparing the original and new regimens for the (a) duration of hypocalcaemia, (b) total amount of calcium gluconate infused, and (c) the time period of infusion.
Of the 48 patients on the original regimen, 22 (46%) had excursions below 2 mmol/L, 15 (31%) had excursions above 3 mmol/L, and 32 (67%) had excursions outside of the target range of 2–3 mmol/L. Mean duration of low excursions was 25.8% (SD 21.4), mean duration of high excursions was 7.6% (SD 4.7), and mean duration of all excursions was 21.3% (SD 19.8) of the treatment periods. Mean trough calcium was 2.06 mmol/L (SD 0.26), and mean peak calcium was 2.86 mmol/L (SD 0.33). Lowest trough calcium recorded was 1.52 mmol/L and highest peak calcium was 3.77 mmol/L.
Of the 33 patients on the new regimen, 11 (33%) had excursions below 2 mmol/L, 7 (21%) had excursions above 3 mmol/L, and 17 (52%) had excursions outside of the target range of 2–3 mmol/L. Mean duration of low excursions was 25.0% (SD 20.5), mean duration of high excursions was 14.8% (SD 13.8), and mean duration of all excursions was 22.3% (SD 21.0) of the treatment periods. Mean trough calcium was 2.09 mmol/L (SD 0.24), and mean peak calcium was 2.83 mmol/L (SD 0.42). Lowest trough calcium recorded was 1.50 mmol/L and highest peak calcium was 4.39 mmol/L.
The independent samples t‐test comparing the original and new regimens for the percentage of the treatment period where the calcium level was below, above, or outside the target range (Fig. 3a–c), as well as the peak and trough calcium during treatment were all non‐significant (Fig. 4a–b, Table 2). The post‐hoc power of the five tests were, respectively, 5.3%, 86.4%, 5.5%, 14.4%, and 75.5%.
Figure 3.
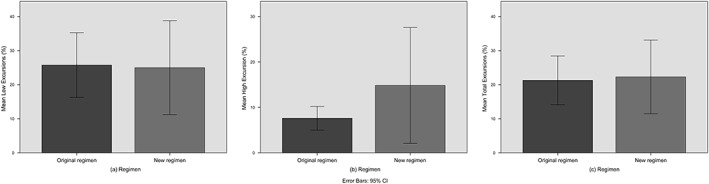
Bar charts comparing the original and new regimens for (a) low, (b) high, and (c) total calcium excursions.
Figure 4.
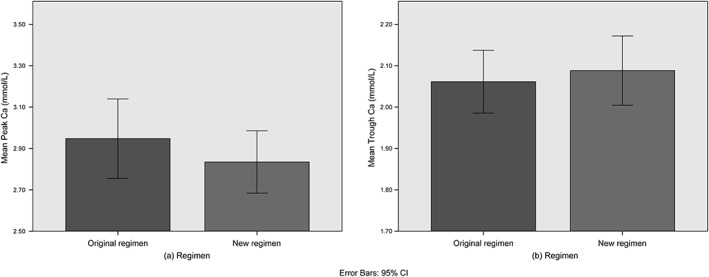
Bar charts comparing the original and new regimens for the (a) peak and (b) trough calcium during treatment.
As the new regimen had a number of high excursions (Fig. 3b), a closer look was taken at the pattern of individual readings to determine the cause (Table S1). Of the eight patients who had high excursions, three of these were due to possible errors in the calcium reading. The remaining five had calcium levels already above the normal range immediately after surgery, and when the infusion was started, this caused the level to rise above the threshold of 3 mmol/L within 12 h.
PER was found to be higher (2.91 vs 2.25 mmol/h, P = 0.015) and trough calcium lower (1.92 vs 2.12 mmol, P = 0.002) in those who reported symptoms suggestive of hypocalcaemia.
Discussion
The model of calcium flux used in the original study simplifies the physiological processes that occur in the postoperative period by considering only the total influx and efflux from the extracellular compartment.1 The results from this study support the findings from the original one in that the peak efflux time was strongly correlated with the PER (adjusted R‐square 0.64, P < 0.001).
The second finding was that the magnitude of PER was significantly lower with the new regimen (0.40 mmol/h difference). In addition, the relatively large effect size for total calcium infused showed that the amount of calcium which is pumped into the patient is far more important in determining the amount of calcium efflux compared to the subject's bone activity (ALP), hormonal levels (PTH), and pre‐treatment with either vitamin D or bisphosphonates. This is particularly interesting given that these factors were previously found to significantly affect POH.14, 15
The weak interaction between total calcium infused and the type of regimen showed that the effect on PER caused by the amount of calcium infused is independent of the regimen used. This in turn suggests that the driving effect of calcium infusion on PER reflects an underlying physiological process rather than an idiosyncrasy of the regimens themselves.
Taken together, we see that the more aggressive the calcium replacement, the greater the efflux from the extracellular compartment and the more prolonged the time course of calcium movements. This means that calcium fluxes after parathyroid surgery for RHP are mostly supply‐driven from the infusion regimen rather than demand‐driven from POH.
Another point to note is that although the mean peak calcium levels for both regimens were comparable (Table 2, Fig. 4), high excursions in the new regimen were solely due to starting the calcium infusion when peri‐operative serum calcium was already above the normal range. Furthermore, the patient who was mistakenly started on 10 mL/h (2.3 mmol/h) immediately after surgery (patient 73, Table Supp1) had a prolonged high excursion of 88.2% of the treatment period indicating that this was far above the demands imposed by the physiological calcium changes after surgery.
We chose a target serum calcium of 2–3 mmol/L (8–12 mg/dL) instead of trying to keep it within the normal range of 2.1–2.6 mmol/L (8.4–10.4 mg/dL) as patients are usually asymptomatic or mildly symptomatic within these bounds.16, 17 Tightening the target range requires more frequent calcium monitoring, and with more aggressive intervention there is always the risk of rebound changes in the opposite direction as observed in this study.
Clinical relevance
When comparing the efficacy of the new regimen against the original, we see that this required significantly less calcium infused over a shorter period, and yet resulted in a reduced duration of hypocalcaemia. This is because a structured titration regimen is superior to an unstructured one in treating POH as the adjustments are often made by the on‐call junior doctor after office hours.
The lower requirement for calcium infusion means that disruption to a patient's metabolic state as reflected by the PER is minimised. This in turn results in an earlier peak efflux time, and a shortening of the period of calcium disturbance. Hence it follows that if we adopt a more conservative infusion regimen, we could reduce morbidity, abbreviate the period of intensive calcium monitoring, shorten hospital stay, and lower overall costs.
The findings from this study strongly suggest that early aggressive calcium replacement as commonly practiced in Malaysia is unnecessary and in some cases may lead to significant hypercalcemia. We recommend that when the new regimen is used, the infusion should not be started immediately after surgery unless the calcium level is below the upper limit of the normal range for the local laboratory (Table 4). This gives a margin of safety as the documented rise in serum calcium did not exceed 0.5 mmol/L (2 mg/dL) in our study (Table Supp1).
Table 4.
Recommended calcium infusion regimen after parathyroidectomy for renal hyperparathyroidism
| Threshold Ca (mmol/L) | Infusion rate (mL/h) | Infusion rate (mmol/h) | Test interval (h) |
|---|---|---|---|
| >2.55 | Nil | Nil | 6 |
| <2.55 | 4.5 | 1.0 | 6 |
| Ca. falls below 2 mmol/L after infusion @ 4.5 | 6.5 | 1.5 | 4 |
| Ca. falls below 2 mmol/L after infusion @ 6.5 | 9.0 | 2.0 | 4 |
Ca, serum calcium. Infusion rate in mL/h is for 10% calcium gluconate.
Pre‐operative oral calcium and calcitriol dose to be maintained. Monitor serum calcium for at least 4 days. If hypocalcaemia persists 6 days after surgery, discontinue infusion and continue monitoring for a further 24 h. The value of 2.55 mmol/L (10.2 mg/dL) can be replaced with the upper limit of the normal range for the local laboratory.
Study considerations and limitations
The main limitation of this study is that data were collected retrospectively, thus precluding any attempt at randomization. Data collection for both regimens occurred at different time periods thus raising the possibility of institutional changes over the years, which can give rise to sampling bias. This was, however, mitigated by the fact that surgery and management of patients at both time periods was under a single primary surgeon at the same hospital.
The treatment of outliers in this study followed the same procedure as in the original study and special care was taken to avoid selective exclusion which can bias the results.1, 18 For each outlier identified on statistical testing, the corresponding case records were inspected to determine whether a valid reason was present to exclude that particular case. Although a number of isolated high calcium readings were detected through their effect on PER, this was not taken as a reason to reject the rest of the data from that patient as previous studies have shown that up to 20% of calcium readings can be erroneous.19
Conclusions
Based on the results from this study, we found that the new calcium infusion regimen was superior to the original one in that it required a shorter treatment period and resulted in less hypocalcaemia. We also found that early aggressive calcium replacement is unnecessary and raises the risk of rebound hypercalcaemia.
Supporting information
Fig. S1 Scatterplot of peak efflux rate against peak efflux time.
Table S1 A selection of calcium readings from patients on the new infusion regimen who had high excursions.
Supporting info item
Supporting info item
Acknowledgements
The authors would like to thank the supporting staff working in the General Surgical unit of Hospital Sultan Ismail for help in data collection. We would also like to extend our gratitude to the Director General of Health Malaysia for allowing the paper to be published.
Tan, J. H. , Tan, H. C. L. , Loke, S. C. , and Arulanantham, S. A/P. (2017) Novel calcium infusion regimen after parathyroidectomy for renal hyperparathyroidism. Nephrology, 22: 308–315. doi: 10.1111/nep.12761.
References
- 1. Loke SC, Kanesvaran R, Yahya R, Fisal L, Wong TW, Loong YY. Efficacy of an intravenous calcium gluconate infusion in controlling serum calcium after parathyroidectomy for secondary hyperparathyroidism. Ann Acad Med Singapore 2009; 38: 1074–80. [PubMed] [Google Scholar]
- 2. Mittendorf EA, Merlino JI, McHenry CR. Post‐parathyroidectomy hypocalcemia: incidence, risk factors, and management. Am Surg 2004; 70: 114–9 discussion 19–20. [PubMed] [Google Scholar]
- 3. Raposo JF, Pires A, Yokota H, Ferreira HG. A mathematical model of calcium and phosphorus metabolism in two forms of hyperparathyroidism. Endocrine 2012; 41: 309–19. [DOI] [PubMed] [Google Scholar]
- 4. Massy ZA, Henaut L, Larsson TE, Vervloet MG. Calcium‐sensing receptor activation in chronic kidney disease: effects beyond parathyroid hormone control. Semin Nephrol 2014; 34: 648–59. [DOI] [PubMed] [Google Scholar]
- 5. Parfitt AM. Renal bone disease: a new conceptual framework for the interpretation of bone histomorphometry. Curr Opin Nephrol Hypertens 2003; 12: 387–403. [DOI] [PubMed] [Google Scholar]
- 6. Loke SC, Leow MK. Calcinosis cutis with siliconomas complicated by hypercalcemia. Endocr Pract 2005; 11: 341–5. [DOI] [PubMed] [Google Scholar]
- 7. National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42: S1–201. [PubMed] [Google Scholar]
- 8. Evans KJ, Greenberg A. Hyperkalemia: a review. J Intensive Care Med 2005; 20: 272–90. [DOI] [PubMed] [Google Scholar]
- 9. Harjit K, Zanariah H, Hisham AN. Hypercalcaemic crisis: immediate parathyroidectomy and intraoperative intravenous calcium infusion improves outcome. Asian J Surg 2007; 30: 173–7. [DOI] [PubMed] [Google Scholar]
- 10. Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J 1973; 4: 643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abraham AG, Munoz A, Furth SL, Warady B, Schwartz GJ. Extracellular volume and glomerular filtration rate in children with chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41: 1149–60. [DOI] [PubMed] [Google Scholar]
- 13. Cohen J. Statistical power analysis for the behavioral sciences, 2nd edn. L. Erlbaum Associates: Hillsdale, N.J., 1988. [Google Scholar]
- 14. Cozzolino M, Gallieni M, Corsi C, Bastagli A, Brancaccio D. Management of calcium refilling post‐parathyroidectomy in end‐stage renal disease. J Nephrol 2004; 17: 3–8. [PubMed] [Google Scholar]
- 15. Loke SC, Tan AW, Dalan R, Leow MK. Pre‐operative serum alkaline phosphatase as a predictor for hypocalcemia post‐parathyroid adenectomy. Int J Med Sci 2012; 9: 611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziegler R. Hypercalcemic crisis. J Am Soc Nephrol 2001; 12 (Suppl 17): S3–9. [PubMed] [Google Scholar]
- 17. Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336: 1298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Winter JC, Dodou D. A surge of p‐values between 0.041 and 0.049 in recent decades (but negative results are increasing rapidly too). Peer J 2015; 3: e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisken RA, Heath DA, Bold AM. Hypercalcaemia‐‐a hospital survey. Q J Med 1980; 49: 405–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Scatterplot of peak efflux rate against peak efflux time.
Table S1 A selection of calcium readings from patients on the new infusion regimen who had high excursions.
Supporting info item
Supporting info item


