Abstract
Aim
We aimed to investigate the non‐inferiority of PA21 (sucroferric oxyhydroxide) to sevelamer hydrochloride (sevelamer) in terms of efficacy and safety in Japanese haemodialysis patients with hyperphosphataemia.
Methods
In this Phase III, open‐label, multicentre study, 213 haemodialysis patients with hyperphosphataemia were randomized to PA21 or sevelamer treatment for 12 weeks. The primary outcome was adjusted serum phosphorus concentration at the end of treatment; the non‐inferiority of PA21 was confirmed if the upper limit of the two‐sided 95% confidence interval (CI) is ≤0.32 mmol/L. Secondary outcomes were corrected serum calcium and intact‐parathyroid hormone concentrations. Adverse events (AEs) and adverse drug reactions (ADRs) were evaluated.
Results
The adjusted mean serum phosphorus concentration at the end of treatment confirmed the non‐inferiority of PA21 for lowering serum phosphorus compared with sevelamer (1.62 vs 1.72 mmol/L; difference, −0.11 mmol/L; 95% CI, −0.20 to −0.02 mmol/L). The mean daily tablet intake was 5.6 ± 2.6 and 18.7 ± 7.1 tablets in the PA21 and sevelamer groups, respectively. The incidences of AEs and ADRs were not significantly different between the two groups.
Conclusion
The non‐inferiority of PA21 to sevelamer was confirmed for the treatment of Japanese haemodialysis patients with hyperphosphataemia. PA21 was effective, safe, and well tolerated, while having a considerably lower pill burden than sevelamer.
Keywords: haemodialysis, hyperphosphataemia, PA21 compound, sevelamer, sucroferric oxyhydroxide
Summary at a Glance
This short‐term randomized trial of PA21 (sucroferric oxyhydroxide) performed in Japanese haemodialysis patients was able to demonstrate non‐inferiority for phosphorus lowering with a significantly lower pill burden.
Hyperphosphataemia occurs in patients with chronic kidney disease (CKD) as a result of phosphorus accumulation in the body from dietary phosphate intake and decreasing excretion of phosphorus into the urine. Hyperphosphataemia has been shown to be an independent prognostic factor for mortality.1, 2 For the management of hyperphosphataemia in chronic haemodialysis patients, dietary restriction and the use of phosphate binding agents are recommended in CKD management guidelines both in Japan and internationally.3, 4
The use of phosphate binders is necessary to inhibit the absorption of dietary phosphate from the gastrointestinal tract, because dietary restriction and haemodialysis are not enough to eliminate the phosphorus excess. Four non‐calcium‐containing phosphate binders have been marketed in Japan, because the calcium load during administration of calcium carbonate is of concern.4, 5 Although the use of sevelamer in combination with calcium carbonate was recommended in the first Japanese Mineral and Bone Disorder guideline,6 sevelamer is not widely used in Japan because of frequent gastrointestinal adverse reactions, including constipation and flatulence. In fact, many Japanese haemodialysis patients complain of constipation, in general. Bixalomer and lanthanum were launched after sevelamer; however, these phosphate binders also cause concerns about constipation and lanthanum accumulation with long‐term use, respectively.3, 7, 8 Ferric citrate, an iron‐based phosphate binder, was approved in Japan in 2014.9 Dialysis patients often need to take several medications for the treatment of comorbidities; therefore, a phosphate binder with strong phosphorus absorption potency is required to reduce the tablet intake in dialysis patients. PA21, also known as sucroferric oxyhydroxide, is a novel non‐calcium‐, iron‐based phosphate binder that has already been launched in Europe, the US, and other countries.
The aim of the present study was to investigate the non‐inferiority of PA21 to sevelamer in terms of efficacy and safety after 12 weeks of treatment in Japanese haemodialysis patients with hyperphosphataemia.
Methods
Patients
The inclusion criteria were: patients with chronic renal failure undergoing stable maintenance haemodialysis three times weekly, for 12 weeks or more, before the initiation of the washout period (Week−3), and with planned continued haemodialysis during the treatment period; patients with no change in their phosphate binder agent dose, for 4 weeks or more before the initiation of the washout period; patients whose predialysis serum phosphorus concentration was >1.94 mmol/L and ≤3.23 mmol/L at Week −1; patients with no change, at least 4 weeks before the initiation of the washout period, in the dose of any vitamin D receptor activator, calcimimetic, or osteoporosis drug that they may have been receiving; patients considered able to discontinue their current therapy for hyperphosphataemia for the 3‐week washout period; and patients aged 20 or older, at the time their consent was obtained, regardless of sex.
The main exclusion criteria were: patients whose corrected serum calcium concentration was ≤1.88 mmol/L or >2.75 mmol/L, at Week −1; patients whose intact‐parathyroid hormone (PTH) concentration was >800 ng/L at the beginning of the washout period; patients with a history of haemochromatosis, or any other iron accumulation disorder, or patients whose serum ferritin was >1797.60 pmol/L or whose transferrin saturation (TSAT) was >50% at the beginning of the washout period; patients with severe gastrointestinal disorders based on the investigator's diagnosis; and patients with a history of a severe digestive tract procedure based on the investigator's diagnosis.
Study design
This was a Phase III, parallel‐group, randomized, open‐label, active‐controlled, multicentre study performed in haemodialysis patients with hyperphosphataemia. The study was conducted in 31 centres in Japan.
This study consisted of a washout period and a treatment period. The washout period consisted of 3 weeks for discontinuing any phosphate binders that were taken before the study start, and the treatment period lasted 12 weeks. Patients were randomized to treatment with PA21 or sevelamer. The randomization of subjects was carried out by an interactive web response system with subjects randomized in a 1:1 ratio to the PA21 group or the sevelamer group.
The initiation dosage and administration methods for each drug were as follows: PA21 was orally administered at 250 mg/dose three times per day (750 mg/day) immediately before every meal; sevelamer was orally administered at 1000 mg/dose or 2000 mg/dose if the serum phosphorus concentration before dialysis at Week −1 was <2.58 mmol/L or ≥2.58 mmol/L, respectively. Sevelamer was administered three times per day, immediately before every meal. During Week 2 to Week 8, based on the serum phosphorus concentration from the previous week, the investigator decided to maintain, increase, or decrease the dose of each drug using the following criteria for dose adjustment. If the serum phosphorus concentration at the beginning of the previous week was >1.94 mmol/L, the dose of PA21 was increased by 750 mg/day and that of sevelamer was increased by 750 or 1500 mg/day; if it was 1.13–1.94 mmol/L, both PA21 and sevelamer doses were maintained; and if it was <1.13 mmol/L, the dose of PA21 was reduced by 750 mg/day and that of sevelamer was reduced by 750 or 1500 mg/day. The maximum allowed dose of PA21 was 1000 mg/dose three times per day (3000 mg/day) and that of sevelamer was 3000 mg/dose three times per day (9000 mg/day). The dose was maintained from Week 8 to Week 12. Each PA21 tablet contained 250 mg of iron and each sevelamer tablet contained 250 mg of sevelamer hydrochloride. Patients recorded in a diary the number of tablets taken, and compliance was checked by the investigator before each week's first dialysis session (at weeks 1 to 12, or at discontinuation) during the treatment period.
Concomitant use of the following drugs was prohibited during the study period: other phosphate binders, drugs with a phosphate binding action, oral iron agents, drugs having an effect on serum phosphorus concentrations, and any study drugs other than PA21. The use of intravenous iron was permitted if the investigator considered it necessary. The discontinuation criteria for individual subjects were as follows: the development of any adverse event (AE) that would make study continuation difficult; any serum phosphorus concentration <0.97 mmol/L or >3.23 mmol/L, twice consecutively; and any corrected serum calcium concentration or serum ferritin concentration ≤1.88 mmol/L or >1797.60 pmol/L, respectively. The use of vitamin D receptor activators and calcimimetics was allowed as long as the subjects were receiving it for 4 weeks or more before the start of the observation period, and the dose was not to be changed during the study period. Any patient not using vitamin D receptor activators or calcimimetics at the study start was not allowed to begin using them during the study period. There were no between‐group differences in the use of these drugs. Pre‐specified diet therapies were not to be changed during the study (observation and treatment) periods.
Measurements of parameters were standardized at a central laboratory. Laboratory tests were processed collectively at an assay facility with the same measurement methods throughout the study period. Tests at each evaluation point were conducted on the week's first dialysis day. Because the visiting time was different for each patient, laboratory tests were not standardized to the same time of day among all patients; however, each individual patient visited the participating centre at the same time and under the same fasting condition throughout their individual treatment.
Ethical approval was obtained from the institutional review boards of each participating centre, and the study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki as revised in 2013. All patients provided written informed consent prior to their inclusion in the study.
Efficacy outcomes and additional evaluations
The primary efficacy outcome was the adjusted serum phosphorus concentration at the end of treatment. Additional evaluations included serum phosphorus concentration at each time point, the change in serum phosphorus concentration from baseline to end of treatment, and the achievement rates for target serum phosphorus concentrations of both 1.13–1.94 mmol/L and 1.13–1.78 mmol/L. The target serum phosphorus concentrations of ≥1.13 mmol/L and ≤1.94 mmol/L were based on the target range of the Japanese Society for Dialysis Therapy (JSDT) guidelines,3 whereas the target serum phosphorus concentrations of ≥1.13 mmol/L and ≤1.78 mmol/L were based on the target range from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines.4 To assess the adequacy of dialysis treatment, the protein catabolic rate and urea clearance (Kt/V) were calculated.
Secondary efficacy outcomes were corrected serum calcium concentrations and serum intact‐PTH concentrations.
Safety and tolerability
Safety outcomes were AEs, ADRs, and laboratory tests including TSAT, ferritin, haemoglobin, and bicarbonate levels.
Statistical analyses
From Phase III studies of sevelamer and similar drugs, a standard deviation of 0.48–0.65 mmol/L for serum phosphorus concentration range, and a non‐inferiority margin of 0.32 mmol/L were assumed. In a dose‐response study in Japanese haemodialysis patients with hyperphosphataemia, standard deviations in a PA21 group were 0.37–0.51 mmol/L. Thus, the standard deviation for PA21 and sevelamer in the present study was set at 0.55 mmol/L. The difference between PA21 and sevelamer was set at 0 mmol/L, and the non‐inferiority margin was established at 0.32 mmol/L. Sixty‐two subjects would be needed per group, for a two‐sided significance level of 5%, and a detection power of 90%. Therefore, a target number of 100 subjects per group was established. To compare demographic and baseline characteristics between the two treatment groups, two‐sample t‐test, Fisher's exact test, and two‐sample Wilcoxon test were used, and statistical significance was set at 15%.
For the adjusted serum phosphorus concentration at the end of treatment (primary efficacy outcome), analyses of covariance (ANCOVA) were performed with the groups as fixed effects, and serum phosphorus concentrations at Week 0 as covariates, and the non‐inferiority of PA21 versus sevelamer was confirmed. Adjusted intergroup means and their two‐sided 95% confidence intervals were calculated. If the upper limit of the two‐sided 95% confidence interval did not exceed 0.32 mmol/L, the non‐inferiority of PA21 was defined as confirmed.
Serum phosphorus concentrations at each time‐point, summary statistics for each of the secondary endpoint measures by group at each evaluation time, and two‐sided 95% confidence intervals for means were calculated. The achievement rates of target serum phosphorus concentrations at each time‐point per group were calculated.
For safety outcomes, the AEs and ADRs did not include discolouration events, such as discoloured faeces and discoloured tongue, caused by the iron contained in PA21. The number of events, the number of patients with events, and event incidence were calculated in each group. Between‐group comparisons were performed using Fisher's exact test. The level of significance was set at 5% (two‐sided). AEs and ADRs were classified by MedDRA Primary System Organ Class and Preferred Term. For laboratory test parameters, summary statistics of measurements at each evaluation point were derived for each group. All statistical analyses were performed using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA).
Results
Patients
The disposition of patients is shown in Figure 1. A total of 321 patients were screened in this study, and 213 eligible patients were randomised to treatment with PA21 (n = 108) or sevelamer (n = 105). A total of 209 (PA21 group, 106; sevelamer group, 103), 192 (PA21 group, 100; sevelamer group, 92), and 213 (PA21 group, 108; sevelamer group, 105) patients were included in the full analysis set, per protocol set, and safety set, respectively.
Figure 1.
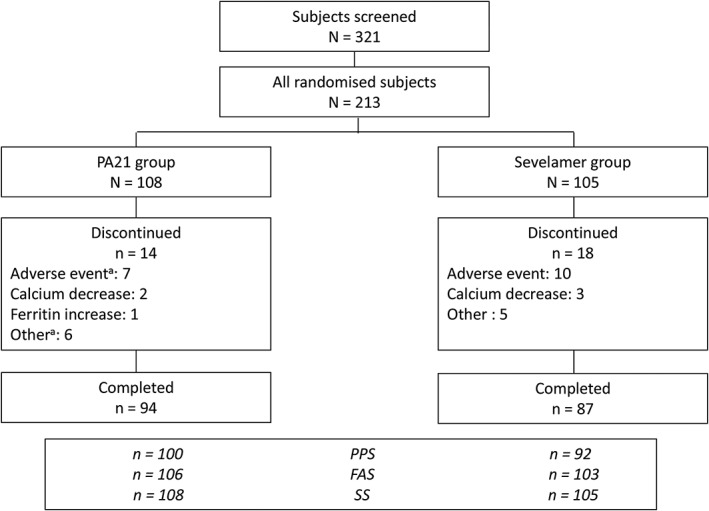
Patient disposition. aSome subjects had more than one reason for discontinuation. FAS, full analysis set; PPS, per protocol set; SS, safety set.
In the PA21 and sevelamer groups, respectively, 14 patients (13.0%) and 18 patients (17.1%) discontinued the study. A total of 94 (87.0%) and 87 (82.9%) of the patients in the PA21 and sevelamer group completed the study, respectively. No imbalance was seen in the discontinuation rate between the PA21 and sevelamer groups.
The background characteristics of patients are shown in Table 1. There were no statistically significant differences between the two groups except for sex and intact‐PTH. Adjusted analyses for sex and intact‐PTH were performed, and the analysis indicated that the study results were unaffected by these imbalanced baseline characteristics.
Table 1.
Patient demographic and clinical characteristics (per protocol set)
| PA21 group n = 100 | Sevelamer group n = 92 | P‐value | |
|---|---|---|---|
| Age (years), mean ± SD | 61.0 ± 11.7 | 60.8 ± 12.0 | NS† |
| Sex, n (%) | 0.048‡ | ||
| Male | 72 (72.0) | 53 (57.6) | |
| Female | 28 (28.0) | 39 (42.4) | |
| Prior use of phosphate binders, n (%) | – | ||
| Calcium carbonate | 71 (71.0) | 73 (79.3) | |
| Sevelamer hydrochloride | 33 (33.0) | 30 (32.6) | |
| Lanthanum carbonate | 47 (47.0) | 43 (46.7) | |
| Bixalomer | 8 (8.0) | 8 (8.7) | |
| Prior use of iron preparation, n (%) | 5 (5.0) | 4 (4.4) | – |
| Prior use of erythropoiesis stimulating agent, n (%) | 88 (88.0) | 86 (93.5) | – |
| Primary disease, n | – | ||
| Diabetic nephropathy | 32 | 20 | |
| Chronic glomerulonephritis | 43 | 42 | |
| Nephrosclerosis | 13 | 9 | |
| Polycystic kidney disease | 3 | 5 | |
| Others | 4 | 4 | |
| Unknown | 8 | 12 | |
| Mode of dialysis, n (%) | – | ||
| Haemodialysis | 87 (87.0) | 82 (89.1) | |
| Haemodiafiltration | 13 (13.0) | 10 (10.9) | |
| Dialysis vintage (months), mean ± SD | 104.9 ± 79.7 | 102.4 ± 90.6 | – |
| Serum phosphorus (mmol/L), mean ± SD | 2.51 ± 0.45 | 2.45 ± 0.39 | NS† |
| Corrected serum calcium (mmol/L), mean ± SD | 2.24 ± 0.15 | 2.22 ± 0.14 | NS† |
| Intact parathyroid hormone (ng/L), median (interquartile range) | 235 (176–339) | 282 (174–386) | 0.082†† |
Two‐sample t‐test;
Fisher's exact test;
Two‐sample Wilcoxon test; NS, not significant
Efficacy outcomes and additional evaluations
The adjusted mean serum phosphorus concentration at the end of treatment was 1.62 mmol/L in the PA21 group and 1.72 mmol/L in the sevelamer group, with a difference of −0.11 mmol/L (95% confidence interval [CI], −0.20 to −0.02 mmol/L) in the per protocol set (Fig. 2). The non‐inferiority of PA21 to sevelamer was confirmed. Furthermore, the mean serum phosphorus concentration at the end of treatment was significantly lower in the PA21 group compared with that in the sevelamer group (ANCOVA, P = 0.020). The non‐inferiority of PA21 to sevelamer was also confirmed in the full analysis set. Drug adherence was maintained well throughout the treatment in both groups. Furthermore, in both groups, no subject showed deviation from the dose adjustment criteria.
Figure 2.
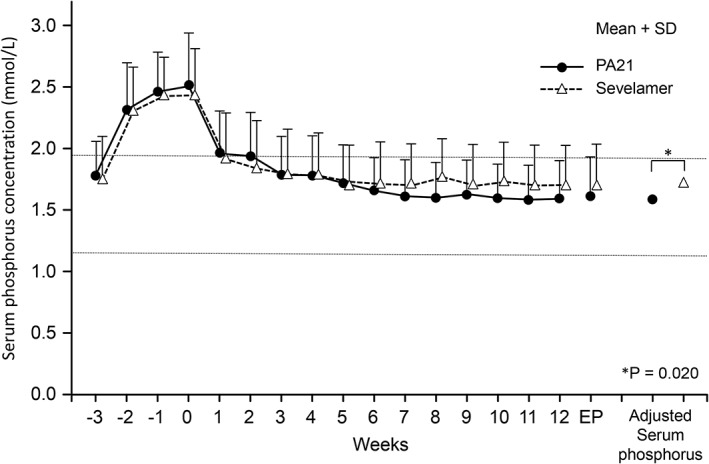
Serum phosphorus concentrations (mean + SD) (per protocol set). The adjusted mean serum phosphorus concentration at the end of treatment confirmed the non‐inferiority of PA21 (sucroferric oxyhydroxide) to sevelamer (1.62 vs 1.72 mmol/L; difference, −0.11 mmol/L; 95% CI, −0.20 to −0.02 mmol/L; ANCOVA, P = 0.020). A notable decrease in serum phosphorus concentrations was shown at Week 1 in both groups and was maintained until Week 12. The horizontal lines indicate the target range of serum phosphorus concentration in the JSDT guideline. ANCOVA, analysis of covariance; CI, confidence interval; EP, endpoint; SD, standard deviation.
A notable decrease in serum phosphorus concentrations was shown at Week 1 in both groups and was maintained until Week 12 (Fig. 2).
The mean daily tablet intake at the end of treatment was 5.6 ± 2.6 tablets (mean daily dose, 1403 ± 654 mg) in the PA21 group and 18.7 ± 7.1 tablets (mean daily dose, 4671 ± 1776 mg) in the sevelamer group. The mean number of tablets for daily dose in each group at the end of treatment is shown in Figure 3.
Figure 3.
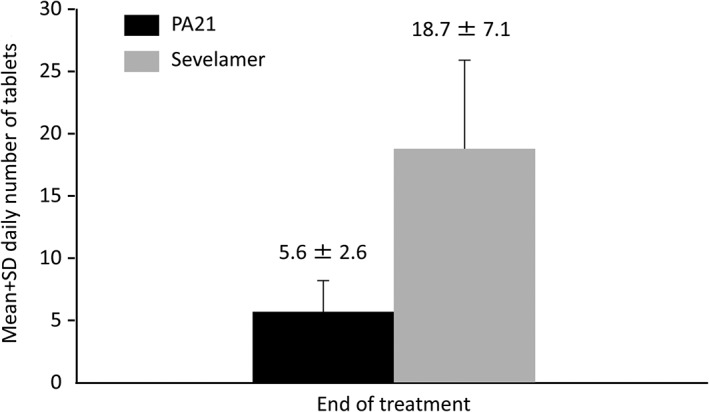
Mean daily number of tablets for daily dose of PA21 (sucroferric oxyhydroxide) and sevelamer (per protocol set). The mean number of tablets for daily dose of PA21 was notably lower than that of sevelamer.
At the end of treatment, the achievement rate of target serum phosphorus concentration (1.13–1.94 mmol/L) was 82.0% in the PA21 group and 67.4% in the sevelamer group. The rates were over 80% in the PA21 group after Week 6; meanwhile, the rates were significantly higher in the PA21 group than in the sevelamer group after Week 8 (Fig. 4). The achievement rate of serum phosphorus concentration (1.13–1.78 mmol/L) was 63.0% in the PA21 group and 52.2% in the sevelamer group at the end of treatment.
Figure 4.
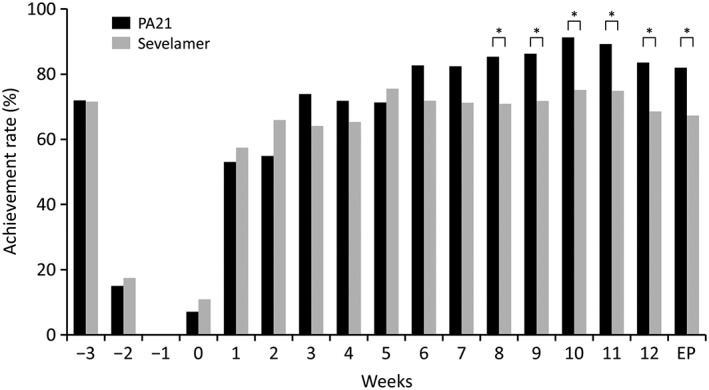
Achievement rates of target serum phosphorus concentration (per protocol set). The achievement rates of target serum phosphorus concentration (1.13–1.94 mmol/L) at the end of treatment were 82.0% and 67.4% in the PA21 (sucroferric oxyhydroxide) and sevelamer groups, respectively. The rates were >80% in the PA21 group after Week 6. Meanwhile, the rates were significantly higher in the PA21 group than in the sevelamer group after Week 8. EP, endpoint. *P < 0.05.
No change was seen in protein catabolic rate and Kt/V from baseline to end of treatment in both groups (Table 2), which indicated that dietary protein intake and dialysis treatment were adequate in both groups.
Table 2.
Changes in serum phosphorus concentrations, corrected calcium levels, intact‐PTH levels, protein catabolic rate, and Kt/V (per protocol set)
| PA21 group n = 100 | Sevelamer group n = 92 | |
|---|---|---|
| Serum phosphorus concentration (mmol/L), mean ± SD | ||
| Week −3 | 1.80 ± 0.27 | 1.76 ± 0.36 |
| Week 0 | 2.51 ± 0.45 | 2.45 ± 0.39 |
| End of treatment | 1.62 ± 0.33 | 1.72 ± 0.33 |
| Change from Week 0 | −0.90 ± 0.53 | −0.73 ± 0.45 |
| Corrected serum calcium level (mmol/L), mean ± SD | ||
| Week −3 | 2.34 ± 0.13 | 2.32 ± 0.14 |
| Week 0 | 2.24 ± 0.15 | 2.22 ± 0.14 |
| End of treatment | 2.29 ± 0.17 | 2.23 ± 0.18 |
| Change from Week 0 | 0.05 ± 0.13 | 0.01 ± 0.15 |
| Serum intact‐PTH level (ng/L), median (quartiles: 25% and 75%) | ||
| Week −3 | 162 (95, 263) | 192 (116, 271) |
| Week 0 | 235 (176, 339) | 282 (174, 386) |
| End of treatment | 190 (123, 259) | 228 (123, 348) |
| Change from Week 0 | −52 (−118, −8) | −49 (−87, −5) |
| Protein catabolic rate (g/kg per day), mean ± SD | ||
| Week 0 | 0.889 ± 0.126 | 0.917 ± 0.151 |
| End of treatment | 0.872 ± 0.160 | 0.888 ± 0.152 |
| Kt/V, mean ± SD | ||
| Week 0 | 1.541 ± 0.297 | 1.557 ± 0.293 |
| End of treatment | 1.507 ± 0.299 | 1.533 ± 0.271 |
Kt/V, urea clearance; PTH, parathyroid hormone.
The changes in corrected serum calcium levels and intact‐PTH levels are shown in Table 2. No notable changes were observed in the corrected serum calcium levels. Although the intact‐PTH levels increased during the phosphate binder washout period, the levels decreased after treatment with PA21 or sevelamer.
Safety and tolerability
The incidence of AEs (excluding discolouration events caused by the iron contained in PA21) was 75.0% (81/108) and 66.7% (70/105), and the incidence of ADRs was 26.9% (29/108) and 26.7% (28/105) in the PA21 group and the sevelamer group, respectively (Table 3). No significant difference in the incidence of either AEs or ADRs was seen between the two groups. The most frequently reported ADR was diarrhoea (PA21, 21.3%; sevelamer, 1.0%) in the PA21 group and constipation (PA21, 0.0%; sevelamer, 18.1%), abdominal discomfort (PA21, 0.0%; sevelamer, 2.9%), and abdominal distension (PA21, 0.0%; sevelamer, 2.9%) in the sevelamer group. Most cases of diarrhoea were mild, transient, and developed early in the treatment. No death was observed in this study. There were two cases of serious ADRs in the PA21 group (congestive cardiac failure and acute pulmonary oedema) and one case in the sevelamer group (diverticulitis).
Table 3.
Adverse events that occurred at an incidence of ≥5%, adverse drug reactions that occurred at an incidence of ≥2% and adverse events that led to withdrawal at an incidence of ≥2% (excluding discolouration events caused by the iron contained in PA21) (safety set)
| PA21 group n = 108 | Sevelamer group n = 105 | |
|---|---|---|
| Adverse events, n (%) | 81 (75.0) | 70 (66.7) |
| Nasopharyngitis | 24 (22.2) | 24 (22.9) |
| Diarrhoea | 27 (25.0) | 3 (2.9) |
| Constipation | 2 (1.9) | 19 (18.1) |
| Adverse drug reactions, n (%) | 29 (26.9) | 28 (26.7) |
| Diarrhoea | 23 (21.3) | 1 (1.0) |
| Constipation | 0 (0.0) | 19 (18.1) |
| Abdominal discomfort | 0 (0.0) | 3 (2.9) |
| Abdominal distension | 0 (0.0) | 3 (2.9) |
| Adverse events that led to withdrawal, n (%) | 7 (6.5) | 10 (9.5) |
| Diarrhoea | 4 (3.7) | 1 (1.0) |
| Constipation | 0 (0.0) | 3 (2.9) |
Iron parameters, such as serum ferritin, transferrin saturation, and haemoglobin, were prone to increase in the PA21 group. No bicarbonate decrease was seen in the PA21 group (Table 4).
Table 4.
Laboratory parameters (ferritin levels, transferrin saturation, haemoglobin, and bicarbonate) (safety set)
| PA21 group | Sevelamer group | |
|---|---|---|
| Ferritin levels (pmol/L), median (quartiles: 25% and 75%) (n) | ||
| Week 0 | 95.50 (43.37, 255.03) (108) | 126.06 (44.94, 289.86) (105) |
| End of treatment | 202.23 (106.06, 312.33) (108) | 118.64 (55.73, 231.44) (105) |
| Change from Week 0 | 75.50 (11.68, 152.35) (108) | −5.62 (−57.97, 32.58) (105) |
| Serum transferrin saturation (%), mean ± SD (n) | ||
| Week 0 | 22.89 ± 9.50 (108) | 23.31 ± 10.20 (105) |
| End of treatment | 29.86 ± 13.51 (108) | 22.09 ± 9.96 (105) |
| Change from Week 0 | 6.97 ± 14.41 (108) | −1.22 ± 8.38 (105) |
| Haemoglobin (g/L), mean ± SD (n) | ||
| Week 0 | 105.50 ± 9.70 (108) | 106.40 ± 9.70 (104) |
| End of treatment | 114.10 ± 12.50 (108) | 103.10 ± 8.90 (105) |
| Change from Week 0 | 8.60 ± 11.50 (108) | −3.30 ± 9.40 (104) |
| Bicarbonate (mmol/L), mean ± SD (n) | ||
| Week 0 | 15.21 ± 2.17 (108) | 15.51 ± 2.07 (105) |
| End of treatment | 17.06 ± 2.24 (107) | 14.76 ± 2.18 (103) |
| Change from Week 0 | 1.89 ± 1.84 (107) | −0.71 ± 2.12 (103) |
Discussion
The adjusted mean serum phosphorus concentrations at the end of treatment confirmed the non‐inferiority of PA21 to sevelamer, and a significant decrease was observed in the PA21 group compared with the sevelamer group. This significant difference in serum phosphorus concentration between both groups was not caused by differences in dietary protein intake or the adequacy of dialysis treatment. Our findings are consistent with those of the Phase III study by Floege et al. that showed the non‐inferiority of PA21 to sevelamer.10 The efficacy of both PA21 and sevelamer in decreasing the serum phosphorus concentrations was evident at Week 1 of treatment and this was maintained until the end of the treatment period. The phosphorus‐lowering effect in the present study was comparable to that of the previous study.10
The changes in serum phosphorus concentration from baseline to the end of treatment were comparable in both treatment groups and similar to the findings in the previous studies.9, 10 In both groups, the serum phosphorus concentrations at the end of treatment were within the range of the control target values (between 1.13 and 1.94 mmol/L). Furthermore, high achievement rates within the target range were maintained in the PA21 group. Because a U‐shaped curve association between serum phosphorus and mortality in haemodialysis patients was reported,2 this result suggests that PA21, which can maintain serum phosphorus concentration within the target range in more patients, demonstrates a clinical benefit.
The mean daily tablet intake was markedly lower in the PA21 group than in the sevelamer group. PA21 showed an equivalent efficacy to sevelamer in lowering serum phosphorus concentration with a notably smaller number of tablets. The necessary number of tablets and the daily dose in the PA21 group (5.6 tablets and 1403 mg/day) was approximately one‐third that in the sevelamer group (18.7 tablets and 4671 mg/day). A similar result was shown in the previous Phase III study of PA2110 in which the number of tablets for daily dose for the first 12 weeks was 2.8 tablets in the PA21 group and 7.6 tablets in sevelamer group (ratio, 1:4.3 when converted to daily dose). A high pill burden in patients undergoing dialysis was affirmed in a survey reporting that haemodialysis patients take a median of 19 tablets per day,11 and it has also been reported that a higher pill burden in dialysis patients is associated with poorer health‐related quality of life.11 In the meantime, higher adherence and good control of phosphorus concentrations are achieved with a lower pill burden.12 PA21 is considered a clinically useful drug in CKD patients because it can control phosphorus concentrations with a smaller number of tablets than sevelamer, which is considered to have a high pill burden.
The changes in corrected serum calcium levels and serum intact‐PTH levels in the PA21 group were comparable to those of the sevelamer group. No increasing trend was seen during treatment in either group.
In terms of safety, although diarrhoea was frequently observed with the PA21 group, most cases were mild and transient. Only four patients withdrew from the treatment because of diarrhoea; however, other patients who developed diarrhoea were able to continue the treatment. All of the patients recovered from diarrhoea soon after discontinuation of the treatment. The onset and course, such as the incidence, severity, duration, and withdrawal, of diarrhoea were comparable to those of the previous Phase III study.10 The incidence of constipation in the PA21 group was low; therefore, dialysis patients could benefit from PA21 treatment because it is known that many CKD patients undergoing dialysis suffer from constipation due to water restriction.13 The incidence of serious ADR was similar between the PA21 and sevelamer groups.
Regarding the clinical laboratory tests, increasing trends in serum ferritin, TSAT, and haemoglobin levels were observed in the PA21 group as well as the previous Phase III study.10 However, the previous non‐clinical14 and clinical studies15 of PA21 confirmed that the iron absorption level was consistently low and no iron accumulation was indicated in the previous long‐term study.16 When comparing the current study and the previous Phase III study, the absolute ferritin values and fluctuation range of ferritin were higher in the previous study. These differences may have been caused by the more frequent use of intravenous iron in the US than in Japan. In fact, the Dialysis Outcomes and Practice Patterns Study surveys indicated that among several countries, Japan used the lowest monthly intravenous iron dose and subsequently showed the lowest mean serum ferritin.17, 18 It is known that dialysis patients often develop metabolic acidosis resulting from a low bicarbonate condition due to renal dysfunction.19 In this study, the bicarbonate ion concentration decreased in the sevelamer group; meanwhile, no notable change was seen in the PA21 group.
Regarding the study limitations, our study results may have been biased by the open‐label design. Although a 12‐week study period would not be long enough to evaluate a therapy for chronic diseases, we have conducted a 52‐week long‐term study to evaluate the safety and efficacy of PA21 in Japanese haemodialysis patients, the results of which will be published separately. Another limitation is that, because not all the patients visited the site at the same time of day and under the same fasting conditions, laboratory tests could not be standardized to the time of day or fasting vs non‐fasting. Finally, although the study protocol did not allow diet therapies to be changed throughout the observation and treatment periods, dietary phosphate intake was not standardized across all study centres.
In conclusion, treatment with PA21 was non‐inferior to that with sevelamer in Japanese haemodialysis patients with hyperphosphataemia. Treatment with PA21 was effective, safe, and well tolerated in this patient population, while having a considerably lower pill burden than sevelamer. The effectiveness of PA21 in this current Japanese study is comparable to that of previous overseas studies; therefore, PA21 represents a new treatment alternative for haemodialysis patients with hyperphosphataemia.
Acknowledgements
Fumihiko Koiwa, Keitaro Yokoyama, Masafumi Fukagawa, and Tadao Akizawa were medical advisors for this clinical study and have received consulting fees from Kissei Pharmaceutical Co, Ltd. Akira Terao was a statistical advisor for this clinical study and has received consulting fees from Kissei Pharmaceutical Co, Ltd. This study was funded by Kissei Pharmaceutical Co, Ltd. We would like to thank the following investigators who contributed to this study: Tomohito Matsunaga, Eijinkai Hospital; Takayuki Toda, Tsuchiura Kyodo General Hospital; Akira Ohishi, Ohishi Naika Clinic; Hiromi Kurosawa, Sumiyoshi Clinic; Kazue Ueki, Toho Hospital; Haruyuki Ogura, Kurosawa Hospital; Joji Ooshima, Kubojima Clinic; Naofumi Ikeda, Sayama Jin Clinic; Keita Kimura, Shin Kashiwa Clinic; Masashi Kaneko, Naganuma Clinic; Toshiaki Suzuki, Asagaya Suzuki Clinic; Toshimasa Takahashi, Bousei Shinjuku Minamiguchi Clinic; Kanji Shishido, Kawasaki Clinic; Shigeru Miyazaki, Shinrakuen Hospital; Masaki Nagasawa, Shinonoi General Hospital; Hiroshi Kasahara, Suwa Red Cross Hospital; Naoki Tachibana, Suwa Red Cross Hospital; Hiroaki Shimosaka, Tajimi Clinic; Takeshi Onogi, Hekikai Kyoritsu Clinic; Akikazu Yamamoto, Hakuyoukai Hospital; Hirotake Kasuga, Kaikoukai Central Clinic; Takashi Sato, Meiko Kyoritsu Clinic; Masaru Kato, Kamiiida Clinic; Hajime Inoue, Ama Kyoritsu Clinic; Tetsuro Yoshioka, Kyoto Station Takeda Dialysis Clinic; Yoji Akagaki, Akagaki Clinic; Mitsuru Yoshimoto, Ohno Memorial Hospital; Tsutomu Tabata, Inoue Hospital; Fukushima Masaki, Shigei Medical Research Hospital; Jun Minaguchi, Kawashima Hospital; Koji Mitsuiki, Japanese Red Cross Fukuoka Hospital; and Satoru Fujimi, Fukuoka Renal Clinic. We thank Dr. Michelle Belanger of Edanz Group Ltd. for providing medical writing assistance. This trial was registered on ClinicalTrials.gov (NCT01850602).
Koiwa, F. , Yokoyama, K. , Fukagawa, M. , Terao, A. , and Akizawa, T. (2017) Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphataemia: A randomized, open‐label, multicentre, 12‐week phase III study. Nephrology, 22: 293–300. doi: 10.1111/nep.12891.
References
- 1. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004; 15: 2208–2218. [DOI] [PubMed] [Google Scholar]
- 2. Kalantar‐Zadeh K, Kuwae N, Regidor DL et al. Survival predictability of time‐varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006; 70: 771–780. [DOI] [PubMed] [Google Scholar]
- 3. Fukagawa M, Yokoyama K, Koiwa F et al. Clinical practice guideline for the management of chronic kidney disease‐mineral and bone disorder. Ther. Apher. Dial. 2013; 17: 247–288. [DOI] [PubMed] [Google Scholar]
- 4. National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003; 42: S1–S201. [PubMed] [Google Scholar]
- 5. Bleyer AJ, Burke SK, Dillon M et al. A comparison of the calcium‐free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am. J. Kidney Dis. 1999; 33: 694–701. [DOI] [PubMed] [Google Scholar]
- 6. Guideline Working Group, Japanese Society for Dialysis Therapy . Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther. Apher. Dial. 2008; 12: 514–525. [DOI] [PubMed] [Google Scholar]
- 7. Akizawa T, Origasa H, Kameoka C, Kaneko Y, Kawasaki S, Bixalomer Study Group . Randomized controlled trial of bixalomer versus sevelamer hydrochloride in hemodialysis patients with hyperphosphatemia. Ther. Apher. Dial. 2014; 18: 122–131. [DOI] [PubMed] [Google Scholar]
- 8. Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N. Engl. J. Med. 2010; 362: 1312–1324. [DOI] [PubMed] [Google Scholar]
- 9. Yokoyama K, Akiba T, Fukagawa M et al. A randomized trial of JTT‐751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol. Dial. Transplant. 2014; 29: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 10. Floege J, Covic AC, Ketteler M et al. A phase III study of the efficacy and safety of a novel iron‐based phosphate binder in dialysis patients. Kidney Int. 2014; 86: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin. J. Am. Soc. Nephrol. 2009; 4: 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol. Dial. Transplant. 2014; 29: 2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong R, Guo ZY, Ding JR, Zhou YY, Wu H. Gastrointestinal symptoms: a comparison between patients undergoing peritoneal dialysis and hemodialysis. World J. Gastroenterol. 2014; 20: 11370–11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cozzolino M, Funk F, Rakov V, Phan O, Teitelbaum I. Preclinical Pharmacokinetics, Pharmacodynamics and Safety of Sucroferric Oxyhydroxide. Curr. Drug Metab. 2014; 15: 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geisser P, Philipp E. PA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clin. Nephrol. 2010; 74: 4–11. [DOI] [PubMed] [Google Scholar]
- 16. Floege J, Covic AC, Ketteler M et al. Long‐term effects of the iron‐based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol. Dial. Transplant. 2015; 30: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bailie GR, Larkina M, Goodkin DA et al. Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2013; 28: 2570–2579. [DOI] [PubMed] [Google Scholar]
- 18. Bailie GR, Larkina M, Goodkin DA et al. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015; 87: 162–168. [DOI] [PubMed] [Google Scholar]
- 19. Kraut JA, Nagami GT. The use and interpretation of serum bicarbonate concentration in dialysis patients. Semin. Dial. 2014; 27: 577–579. [DOI] [PubMed] [Google Scholar]


