Abstract
Periodontal disease has been linked to poor glycemic control among individuals with type 2 diabetes. Using integrated dental, medical, and pharmacy commercial claims from Truven MarketScan® Research Databases, we implement inverse probability weighting and doubly robust methods to estimate a relationship between a periodontal intervention and healthcare costs and utilization. Among individuals newly diagnosed with type 2 diabetes, we find that a periodontal intervention is associated with lower total healthcare costs (−$1799), lower total medical costs excluding pharmacy costs (−$1577), and lower total type 2 diabetes‐related healthcare costs (−$408). © 2016 The Authors. Health Economics Published by John Wiley & Sons Ltd.
Keywords: periodontal intervention, type 2 diabetes, healthcare costs and utilization, inverse probability weighting, doubly robust estimation
1. Introduction
A bidirectional relationship has been established between type 2 diabetes and periodontitis, otherwise known as periodontal disease (Taylor et al., 1996; Chee et al., 2013). However, the evidence is mixed as to whether periodontal interventions in individuals with type 2 diabetes lead to improvements in glycemic control and other health outcomes (Simpson et al., 2015; Engebretson and Kocher, 2013). These studies sought to determine whether periodontal interventions for individuals with type 2 diabetes result in reduced inflammatory markers in the body. Reduced inflammation could lead to fewer diabetes‐related complications, which would lower the probability of hospitalizations and emergency room visits and subsequently reduce total healthcare costs. We evaluate whether a periodontal intervention in the 2 years after initial diagnosis of type 2 diabetes is associated with lower healthcare costs and utilization in years 3 and 4. We also perform a subgroup analysis among individuals who start prescription drug treatment for type 2 diabetes after initial diagnosis. Our objective here is to determine whether a periodontal intervention is associated with lower healthcare costs among individuals who are managing their type 2 diabetes with prescription drug treatment.
2. Study Data and Methods
2.1. Data source
We use integrated medical, pharmacy, and dental claims from Truven Health MarketScan® Research Databases. For more information, please see Appendix A1 in the Supporting Information.
2.2. Study sample and sample selection
In this study, we selected adults aged 18 through 64 who were newly diagnosed for type 2 diabetes anytime from January 1, 2006 to December 31, 2007. An individual has to have one inpatient or two outpatient medical claims for type 2 diabetes with a corresponding ICD‐9 code (American Medical Association, 2003). For individuals without an inpatient type 2 diabetes medical claim, we required two outpatient type 2 diabetes medical claims to reduce the chance of false positives. Individuals have to be continuously enrolled in a medical plan for 12 months prior to and 48 months after the initial medical claim for type 2 diabetes. Figure 1 outlines the sample selection criterion, and Figure 2 shows the longitudinal design of the study. After incorporating these restrictions, 15,002 individuals were included in the analysis. We also stratified our sample into two subgroups: initiators of diabetes medication (7367 individuals) and non‐initiators of diabetes medication (7635 individuals) in the first 2 years after diagnosis. We assessed the impact of a periodontal intervention on healthcare outcomes in these two subgroups.
Figure 1.
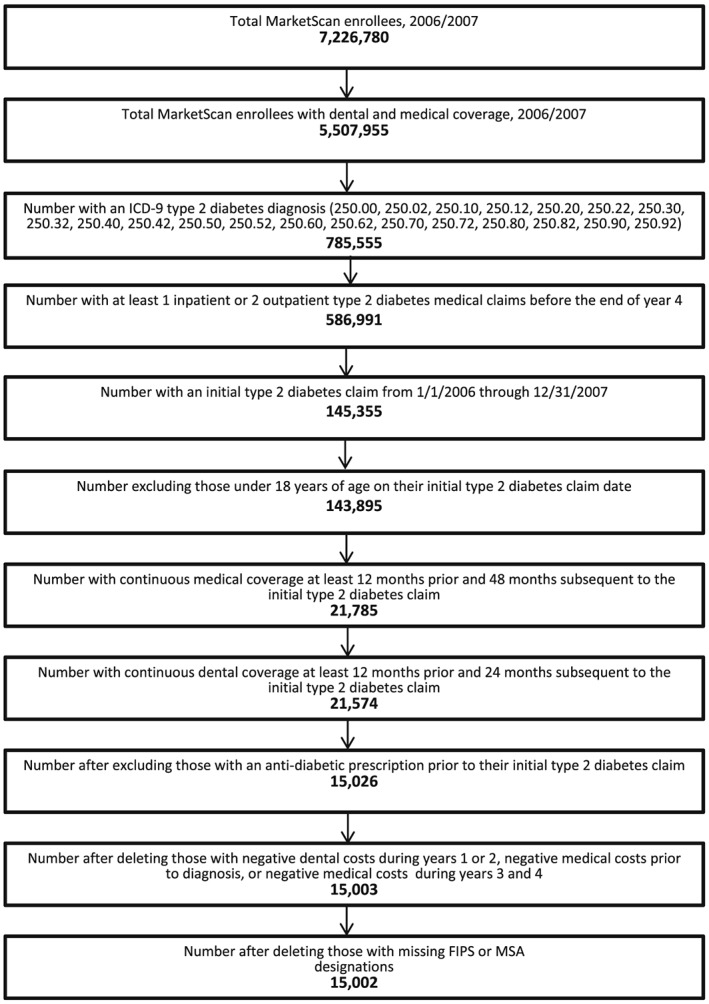
Study flow. Sample selection criterion for individuals with type 2 diabetes
Figure 2.
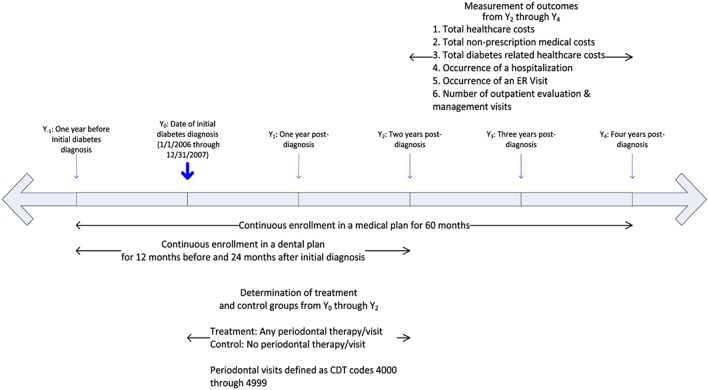
Study timeline. Determination of treatment and control groups along with outcome measures. [Colour figure can be viewed at wileyonlinelibrary.com]
2.3. Treatment and control groups
We grouped individuals into treatment and control groups. Over the 2‐year period after diagnosis, patients were categorized as periodontal therapy users if they had any periodontal therapy visit. We used Codes on Dental Procedures and Nomenclature (CDT) (American Dental Association, 2014) D4000 through D4999 to enumerate periodontal therapy visits. Individuals with type 2 diabetes who had no periodontal therapy visits in the 2 years after initial diagnosis were placed in the control group. We classify 2310 individuals as users of periodontal therapy.
2.4. Outcomes
We analyzed the impact of a periodontal intervention on total all‐cause healthcare costs (sum of total inpatient medical costs, outpatient medical costs, and prescription drug costs), total all‐cause medical costs (sum of total inpatient and outpatient medical costs), and total type 2 diabetes‐related healthcare costs. The claims associated with type 2 diabetes‐related medical costs have a corresponding type 2 diabetes ICD‐9 diagnosis code, while the claims associated with diabetes‐related prescription drug costs are paired with a diabetes prescription. We generated two binary variables capturing the incidence of a hospitalization and emergency room visit and a count variable measuring total outpatient physician visits. All cost and utilization outcomes were measured over years 3 and 4 after the initial type 2 diabetes medical claim.
2.5. Confounders
In evaluating potential confounders, we assessed factors that might influence a patient newly diagnosed with type 2 diabetes to have a periodontal intervention. Factors such as age, gender, area of residence (census region or rural inhabitant), prior medical or dental care utilization, the comorbid medical conditions a patient presents, and accumulated healthcare costs prior to diagnosis could impact a patient's likelihood to seek a periodontal intervention and their overall health. These factors are included in the model. Patient comorbidity is measured by a Charlson score (Charlson et al., 1987), an index value capturing the number of chronic medical conditions a person has. We calculated the Charlson index using ICD‐9 diagnosis codes over 12‐month periods in the year prior to and the 2 years after the initial type 2 diabetes medical claim. These three index values were then averaged to generate an average Charlson comorbidity score for each individual.
2.6. Methodology
We estimate the average treatment effect (ATE) (Rubin, 1974) of a periodontal intervention on healthcare costs and utilization. Each subject in our study has potential outcomes (Y 1, Y 0), where Y 1 is the outcome the subject would have if he received a periodontal intervention and Y 0 is the outcome the subject would have if he did not receive an intervention. We do not observe (Y 1, Y 0) for all subjects in our study but instead only observe Y, which is defined as
| (1) |
where w is the intervention indicator. Given the vector of observed covariates, X, which have been previously specified in Section 2.4, we assume ignorability of treatment otherwise known as ‘selection on observables’ (Rosenbaum and Rubin, 1983). By ignorability, we assume that conditional on X, w and (Y 1, Y 0) are independent. We make the strong assumption that unobservable factors do not confound the relationship between a periodontal intervention and healthcare outcomes. We also assume overlap, 0 < p (w = 1|X) < 1, in the distribution of the propensity score in the treatment and control groups. Given these assumptions, we estimate an ATE.
| (2) |
For each observation, m 1(X) and m 0(X) are estimates of the potential outcomes (Y 1, Y 0). We average the differences in these estimates across the entire sample in order to estimate an ATE. After using a logit model to estimate the propensity score, ,
we estimate ATEs of a periodontal intervention on years 3 and 4 costs and utilization using inverse‐probability weighting and doubly robust (DR) methods (Wooldridge, 2007). The estimated propensity score is used in the inverse weights used to compute the ATEs (Appendix A2 in the Supporting Information). In order to account for the fact that costs and number of physician visits are nonnegative responses with continuous and discrete properties respectively, we use an exponential mean function with the Poisson quasi‐log likelihood (QLL) weighted by the inverse of the propensity score to estimate a DR estimator (Robins and Rotnitzky, 1995; Robins, Rotnitzky, and Zhao, 1995; van der Laan and Robins, 2003; Wooldridge, 2007). Without having to make strong distributional assumptions, as long as either the propensity score model or the conditional mean outcome model is correctly specified, double robustness holds, and the ATE is consistently estimated. The DR property holds when estimating a Poisson QLL for nonnegative outcome responses and a logistic function with the Bernoulli QLL for a binary outcome response (Wooldridge, 2007). We used a Bernoulli QLL when estimating the DR ATE of a periodontal intervention on binary outcome variables. Standardized differences on the reweighted sample are calculated to assess balance in observed covariates across the treatment and control groups. For comparison, we also estimate average treatment effects on the treated (ATET). For more information on ATETs, please see Appendix A2 in the Supporting Information.
2.7. Limitations
There are a number of limitations in this study. First, because diagnostic coding is not used in dentistry, we cannot identify periodontal disease status. Ideally, we would select patients with type 2 diabetes and periodontitis and split them into treatment and control groups depending on whether they received a periodontal intervention. Unfortunately, we can only identify whether a person newly diagnosed with type 2 diabetes had a periodontal visit, which is identified using CDT codes. As a result, we expect that some portion of newly diagnosed individuals with type 2 diabetes not receiving a periodontal intervention actually do not have periodontal disease. Among this subgroup, there is likely no medical cost savings associated with a periodontal intervention. Hence, we believe that our estimates of medical costs savings associated with periodontal interventions are biased downward because of this limitation.
Second, we cannot observe other possible confounders, such as a patient's motivation to seek care or ability to self‐manage their health. We also do not have access to variables measuring household income or race/ethnicity, factors that could influence one's ability to seek a periodontal intervention and subsequent healthcare outcomes. We proxy for possible unobserved confounders by including pre‐diagnosis healthcare costs and medical and dental care use in our models. Despite the inclusion of these factors in our models, we cannot rule out unobservable confounding.
Although we attempt to control for confounding bias using propensity score methods, we may not be able to mitigate the impact of selection bias on our treatment effect estimates (Haneuse, 2013). Our sample falls from 143,895 individuals to 21,785 when we impose a continuous insurance enrollment restriction. However, in order to identify individuals who are newly diagnosed for type 2 diabetes, we need to make sure that no individual had a drug or medical claim for the disease in the year prior to diagnosis. In order to assess the long‐term impact of a periodontal intervention on healthcare costs and utilization, we also require that an individual be enrolled in a healthcare plan. Because of the nature of the association between type 2 diabetes and periodontitis, a sufficient follow‐up period is necessary in order to pick up any effect of a periodontal intervention on healthcare outcomes. Unfortunately, our continuous enrollment criteria may induce sample selection bias in our study and compromise external validity. We also do not have variables in our claims data that would allow us to determine the potential factors that would cause an enrollee to drop out of a healthcare plan and allow for us to build a selection model. For example, an individual's employment status may determine whether someone is enrolled in a healthcare plan. As acknowledged by Haneuse (2013), resolving selection bias using claims data is an ‘open methodologic problem’.
Furthermore, we restricted our sample to only individuals newly diagnosed with diabetes in order to minimize patient heterogeneity between the treatment and control groups. Patients with established diabetes and higher HbA1c levels may be more likely to self‐select into periodontal treatment. Ideally, we would have individuals in the treatment and control groups with roughly the same HbA1c levels. Unfortunately, we do not have access to lab records in our claims data. Hence, diabetes disease progression and HbA1c levels are potential unobserved confounders in our models.
3. Results
In Appendix Table I in the Supporting Information, we calculate descriptive statistics. Standardized differences on the un‐weighted data indicate some imbalance in the potential confounders. The treatment group is older, more urban, less concentrated in the south, and more likely to have a dental visit prior to initial diabetes diagnosis. After the data are re‐weighted based on the estimated propensity score, the standardized differences in the confounders across the treatment and control groups declined significantly. This indicates good balance between the two groups (Appendix Table II in the Supporting Information). The distribution of the propensity score for the treatment and control groups also suggests good overlap (Appendix Figure 1 in the Supporting Information).
Table 1 shows the DR ATE estimates of a periodontal intervention on costs and utilization. Individuals newly diagnosed with type 2 diabetes who have undergone a periodontal intervention have total healthcare costs that are $1799 lower on average over years 3 and 4 compared with those who have not had a periodontal intervention. Total medical costs are $1577 less on average for the treatment group. Total diabetes‐related healthcare costs are $408 lower on average for the treatment group. There are not statistically significant differences in total outpatient physician visits, probability of a hospitalization, or the occurrence of an emergency room visit between the treatment and control groups. For visual comparison of all ATE estimates, please see Appendix Figures 2–7 in the Supporting Information. DR ATETs estimates measuring the relationship between a periodontal intervention and healthcare costs and utilization were very similar to DR ATE estimates (Table 2).
Table 1.
Cost and utilization outcomes
| Outcome | Simple difference | Un‐weighted regression adjustment (Poisson) | Un‐weighted regression adjustment (Gamma) | Un‐weighted regression adjustment (Logit) | IPW estimate | Doubly robusta |
|---|---|---|---|---|---|---|
| Total healthcare cost | −1485.55** (677.60) | −1744.27*** (665.08) | −1477.59* (785.86) | NA | −1726.01** (702.16) | − 1798.71*** (674.38) |
| Total medical cost | −1422.99** (624.67) | −1541.36** (632.05) | −1229.39 (752.85) | NA | −1525.29** (648.54) | −1576.71** (634.57) |
| Total type 2 diabetes healthcare cost | −449.31** (183.44) | −411.85** (188.46) | −335.76* (192.52) | NA | −401.66** (188.49) | −407.87** (199.65) |
| Any hospitalization | −0.017** (0.008) | NA | NA | −0.013* (0.008) | −0.012 (0.008) | −0.012 (0.009) |
| Any emergency room visit | −0.026*** (0.010) | NA | NA | −0.012 (0.010) | −0.011 (0.011) | −0.011 (0.011) |
| Total outpatient physician visits | 0.023 (0.227) | −0.179 (0.209) | NA | NA | −0.111 (0.232) | −0.142 (0.205) |
| Number of observations | 15,002 | |||||
| Periodontal treatment group observed | 2310 | |||||
| Periodontal control group observed | 12,692 |
ATE estimates. All type 2 diabetes individuals.
Standard errors are in parentheses. Cost and utilization outcomes measured in years 3 and 4 after initial diabetes diagnosis. Ordinary least squares used to estimate simple differences. Robust standard errors used in simple difference, pooled Poisson, pooled Gamma, and inverse‐probability weighting (IPW) estimation. Truven MarketScan® Research Databases.
Bootstrapped standard errors using 400 replications.
Significant at 10% level;
Significant at 5% level;
Significant at 1% level.
Table 2.
Cost and utilization outcomes
| Outcome | ATETa |
|---|---|
| Total healthcare cost | −1829.50*** (676.38) |
| Total medical cost | −1580.87** (640.80) |
| Total type 2 diabetes healthcare cost | −439.22** (193.66) |
| Any hospitalization | −0.012 (0.008) |
| Any emergency room visit | −0.013 (0.010) |
| Total outpatient physician visits | −0.242 (0.209) |
Average treatment effect on the treated (ATET) doubly robust estimates. All individuals with type 2 diabetes
Standard errors are in parentheses. Cost and utilization outcomes measured in years 3 and 4 after initial diabetes diagnosis. Truven MarketScan® Research Databases.
Bootstrapped standard errors using 400 replications.
Significant at 10% level;
Significant at 5% level;
Significant at 1% level.
3.1. Subgroup analysis
In Appendix Table III in the Supporting Information, we estimate the DR ATE of a periodontal intervention among individuals who did not receive any diabetes‐related medication in years 1 and 2 after initial diagnosis. Users of periodontal therapy have total healthcare costs that are $2042 lower on average over years 3 and 4. Total medical costs are $1609 lower on average. Type 2 diabetes‐related healthcare costs are $411 lower on average. A periodontal intervention does not have a statistically significant association with emergency room usage or the number of outpatient physician visits. The un‐weighted logit regression estimate suggests that a periodontal intervention is associated with a 2.1 percentage point lower probability of a hospitalization (odds ratio: 0.829; 95% CI [0.684, 1.00]).
A periodontal intervention does not have a statistically significant association with healthcare costs or utilization among individuals who initiated drug treatment (Appendix Table IV in the Supporting Information). The DR ATETs estimates were very similar to DR ATE estimates (Appendix Tables V and VI in the Supporting Information).
4. Summary
We found a statistically significant association between a periodontal intervention and lower healthcare costs. However, this association is only noted among individuals who did not initiate diabetes prescription drug therapy after diagnosis. Among individuals who have initiated diabetes prescription drug therapy, a periodontal intervention appears to have no impact on costs. This may be a sign of a heterogeneous treatment effect. For example, a periodontal intervention may provide little incremental benefit when type 2 diabetes is being managed through prescription drug treatment. Or, there could be unobservable confounding factors. For example, prescription drug treatment could be a marker for disease severity. Patients with higher HbA1C levels may be more likely to be on a prescription drug regimen. As stated previously, we do not have access to patient lab records in our data. As for patients who did not initiate prescription drug treatment after diagnosis, we hypothesize that their HbA1c levels are sufficiently controlled that their physicians do not deem drug treatment necessary. Hence, there may be a modest benefit from a periodontal intervention in this subgroup.
After factoring in the cost of periodontal therapy in the first 2 years after diagnosis ($471) and subsequent total healthcare cost savings in years 3 and 4 among users of a periodontal intervention ($1799), net savings are $1328 over 2 years. If one only accounts for total type 2 diabetes‐related healthcare cost savings in years 3 and 4 ($408), there is a small net cost of $63 associated with a periodontal intervention. Compared with the simple difference estimates, our DR ATE estimates were similar. This may suggest that observable factors do not significantly confound the estimates. However, we were unable to fully capture factors, such as patient motivation, that potentially confound the relationship between a periodontal intervention and healthcare outcomes.
Disclosure Statement
There are no further disclosures or disclaimers.
Supporting information
Supporting info item
Acknowledgements
The views expressed by the authors do not necessarily reflect those of the American Dental Association. Mr. Barry Grau provided research assistance in helping to gather and program the data. Research for this article is based partly upon claims data compiled and maintained by Truven Health MarketScan® Research Databases. The authors are responsible for the research and conclusions reflected in this article. Truven Health MarketScan® is not responsible for the conduct of the research or for any of the opinions expressed in this article.
Nasseh, K. , Vujicic, M. , and Glick, M. (2017) The Relationship between Periodontal Interventions and Healthcare Costs and Utilization. Evidence from an Integrated Dental, Medical, and Pharmacy Commercial Claims Database. Health Econ., 26: 519–527. doi: 10.1002/hec.3316.
The copyright line for this article was changed on 2 April 2016 after original online publication
References
- American Dental Association . 2014. Codes on Dental Procedures and Nomenclature. American Dental Association: Chicago, IL. [Google Scholar]
- American Medical Association . 2003. International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). American Medical Association: Chicago, IL. [Google Scholar]
- Charlson ME, Pompei P, Ales KL, Mackenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal Chronic Diseases 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- Chee B, Park B, Barthold PM. 2013. Periodontitis and type II diabetes: a two‐way relationship. International Journal of Evidence‐based Healthcare 11(4): 317–329. [DOI] [PubMed] [Google Scholar]
- Engebretson S, Kocher T. 2013. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta‐analysis. Journal of Periodontology 84(4 Suppl): S153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneuse S. 2013. Distinguishing selection bias and confounding bias in comparative effectiveness research. Medical Care[epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan MJ, Robins JM. 2003. Unified Methods for Censored Longitudinal Data and Causality. Springer: New York, NY. [Google Scholar]
- Robins JM, Rotnitzky A. 1995. Semiparametric efficiency in multivariate regression models. Journal of the American Statistical Association 90: 122–129. [Google Scholar]
- Robins JM, Rotnitzky A, Zhao L. 1995. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. Journal of the American Statistical Association 90: 106–121. [Google Scholar]
- Rosenbaum PR, Rubin DB. 1983. The central role of the propensity score in observational studies for causal effects. Biometrika 70: 41–55. [Google Scholar]
- Rubin DB. 1974. Estimating causal effects of treatments in randomized and nonrandomized studies. Journal of Educational Psychology 66: 688–701. [Google Scholar]
- Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor‐Ejiofor Z. 2015. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database of Systematic Reviews 11:CD004714. PMID: 265450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. 1996. Severe periodontitis and risk for poor glycemic control in patients with non‐insulin‐dependent diabetes mellitus. Journal of Periodontology 67(10 Suppl): 1085–1093. [DOI] [PubMed] [Google Scholar]
- Wooldridge JM. 2007. Inverse probability weighted m‐estimation for general missing data problems. Journal of Econometrics 141: 1281–1301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


