Summary
Background
Several studies have shown that patients with non‐erosive reflux disease (NERD) are less responsive to proton pump inhibitors (PPIs) than those with erosive disease as they belong to different subgroups, in whom factors other than acid can trigger symptoms.
Aim
To evaluate whether combined therapy (mucosal protection plus acid suppression) would improve symptom relief compared to PPI treatment alone.
Methods
In a multicenter, randomised, double‐blind trial, 154 patients with NERD were randomised to receive Esoxx (Alfa Wassermann, Bologna, Italy), a hyaluronic acid‐chondroitin sulphate based bioadhesive formulation, or placebo, in addition to acid suppression with standard dose PPIs for 2 weeks. Symptoms (heartburn, acid regurgitation, retrosternal pain and acid taste in the mouth) and health‐related quality of life (HRQL) were evaluated before and after treatment. The primary endpoint was the proportion of patients with at least a 3‐point reduction in the total symptom score.
Results
At the end of treatment, the primary endpoint was reached by 52.6% of patients taking Esoxx compared to 32.1% of those given placebo (P < 0.01). The same was true also for HRQL, evaluated by means of the Short Form‐36 questionnaire, which improved with both treatments, but some items were significantly better after Esoxx plus PPI therapy.
Conclusion
The synergistic effect of Essox with PPI treatment suggests that mucosal protection added to acid suppression could improve symptoms and HRQL in NERD patients.
Introduction
Gastro‐oesophageal reflux disease (GERD) is a highly prevalent disorder in Western countries, as its predominant symptom, heartburn, can occur once a week in up to 26% of the general population.1 Despite geographical variations, the prevalence of GERD is increasing worldwide.
Over the past decade, it has been realised that there are two different phenotypes of the disease. Some patients present oesophageal mucosal lesions (i.e. erosive oesophagitis), but the majority (up to 70%) have a macroscopically normal mucosa at endoscopy. Such patients are usually considered to have non‐erosive reflux disease (NERD).2, 3, 4
Proton pump inhibitors (PPIs) represent the first choice medical treatment for GERD,5 in that they are able to provide an 80–85% healing rate for oesophageal lesions, including ulcers, and also reduce the incidence of complications. Pooled analyses6, 7 have shown that in 56–76% of cases, symptom relief can also be achieved, even though this benefit seems to be reduced in patients with NERD. According to a widely quoted systematic review,7 compared to patients with erosive oesophagitis, patients with NERD display a reduced symptom relief with PPIs, with about 20% reduction of therapeutic gain. A large AGA survey8 found that – despite PPI use – over 55% of subjects with GERD symptoms in the general population (where non‐erosive and erosive diseases are obviously mixed) report continued disruption of their quality of life.
Recent investigations have shown that not only acidic, but also non‐acidic refluxes are able to induce the histo‐pathological alterations, which have been clearly documented by electron and light microscopy in the majority of NERD patients.9, 10, 11 In particular, the dilation of intercellular spaces between adjacent cells of the oesophageal epithelium represents a feature that has become the hallmark of microscopic oesophagitis. This intercellular gap leads to increased permeability that favours the penetration of hydrogen ions and other substances (including pepsin and bile) into oesophageal sub‐mucosa, thus reaching nerve fibres, whose stimulation generates the typical symptom heartburn. Several studies12 suggested a synergistic action between acid and duodeno‐gastric reflux in inducing lesions. The important role of pepsin in the pathogenesis of extra‐oesophageal manifestations of GERD is increasingly being appreciated.13
An ideal therapy for NERD patients should – in addition to acid secretion – address all the above‐mentioned pathophysiologic features, that is provide a barrier to (and/or bind) the residual aggressive components of the refluxate (i.e. weakly acidic content and pepsin) while stimulating mucosal repair. To achieve these goals, a class III medical device, Esoxx (Alfa Wassermann, Bologna, Italy), was specifically designed and developed.14, 15 It consists of a mixture (1:2.5 ratio) of low molecular weight (80–100 kDa) hyaluronic acid and low molecular weight (10–20 kDa) chondroitin sulphate, dispersed in a bioadhesive carrier (poloxamer 407) to form a macromolecular complex, coating the oesophageal mucosa and acting as a mechanical barrier against the noxious components of the refluxate. Transit time of liquids through the oesophagus is very short (less than 16 s), even in a supine subject.16 A viscous liquid formulation that adheres to and coat the mucosa will limit the contact of refluxed acid and pepsin with the epithelial surface17 and can act as a vehicle to deliver drugs for local action within the oesophagus.18
The components of Esoxx are two well‐known physiologic substances. Hyaluronic acid is a widespread, biologically active substance, which regulates cellular function through interaction with specific receptors.19 It is a multifunctional, high molecular weight glycosaminoglycan, component of the majority of extracellular matrices and involved in several key physiologic processes, including wound repair and regeneration, morphogenesis and matrix organisation.20 The biological roles of hyaluronic acid are in part dependent on its hydrophilic and hydrodynamic properties, which allow it to retain water and play a structural role. Indeed, hydrogels (cross‐linked hydrophilic polymers) have been used as scaffolds to allow tissue repair or regeneration at sites of injury, being degraded by tissue enzymes after repair is completed.19 Low molecular weight hyaluronic acid is pro‐angiogenic, induces the formation of new blood vessels and activates a signal transduction pathway leading to endothelial cell proliferation and migration. In contrast, native high molecular weight hyaluronic acid is anti‐angiogenic and will inhibit blood vessel formation.19 Topic hyaluronic acid formulations are employed to treat recurrent aphthous ulceration of the oral mucosa21, 22 with fast symptom relief, to which the dose‐dependent anti‐inflammatory activity of the compound23 may also contribute.
Chondroitin sulphate is a natural glycosaminoglycan, present in the extracellular matrix surrounding cells, especially in the cartilage, skin, blood vessels, ligaments and tendons, where it forms an essential component of proteoglycans.24 Current evidence shows that chondroitin sulphate fulfils important biological functions in inflammation, cell proliferation, differentiation, migration, tissue morphogenesis, organogenesis, infection and wound repair.25 These effects are related to the capacity of chondroitin sulphate to interact with a wide variety of molecules including (but not limited to) matrix molecules, growth factors, protease inhibitors, cytokines, chemokines and adhesion molecules via nonspecific/specific saccharide domains within the chains.25 The compound is endowed with immune‐modulatory,26 anti‐inflammatory25, 26 and antioxidant27 properties. Along with nonspecific interactions, chondroitin sulphate may display specific binding to bioactive molecules, such as pepsin. Peptic activity is indeed reduced both in vitro 28 and in vivo 29, 30, and treatment of peptic ulcer with chondroitin sulphate has been attempted in the past.31
Poloxamer 407 (ethylene oxide and propylene oxide blocks) is a hydrophilic non‐ionic surfactant, which shows thermo‐reversible properties of the utmost interest in optimising drug formulation (fluid state at room temperature, facilitating administration and gel state above sol–gel transition temperature at body temperature, promoting prolonged release of pharmacological agents).32 Poloxamer 407 formulations lead to enhanced solubilisation of poorly water‐soluble drugs and prolonged release profile for many galenic applications.33 The poloxamer 407 adhesive properties are used to lengthen residence time of agents in the gastrointestinal tract. Good adhesion in the oesophagus with efficient diffusion of the drug into the mucosa was observed in the mouse, by means of an optical fibre spectrofluorimetric method.32
According to European Council Directive 93/42/EEC,34 the National Health Institute in Rome classified this bioadhesive formulation as class III medical device, intended for use in human beings for the purpose of treatment or alleviation of disease. Typically, the medical device function is achieved by physical means (including mechanical action, physical barrier, replacement of or support to organs or body functions).
An ex vivo experimental study on a swine model showed that perfusion of the oesophageal lumen with this medical device is able to prevent the increase in mucosal permeability induced by acid and/or pepsin.35 With these data at hand, two double‐blind, placebo‐controlled studies demonstrated that short‐term Esoxx treatment achieves a significant and quick symptom relief both in patients with erosive36 or non‐erosive reflux disease.37
In this prospective, double‐blind, placebo‐controlled trial the efficacy and safety of Esoxx, combined to acid suppression, vs. acid suppression alone, was evaluated in patients with NERD, diagnosed merely as endoscopy‐negative reflux disease. This was selected to mirror the clinical practice, outside the referral centres, where advanced investigations are not available.
Patients and methods
Non‐erosive reflux disease patients with typical reflux symptoms were enrolled in the study. They were of both sexes, and age ranged from 18 to 75 years. Two of the following symptoms, for example heartburn, acid regurgitation, retrosternal pain and acid taste in the mouth, should have been present from at least 3 months and at least three times per week in the month preceding the study screening visit. The diagnosis of NERD was based on the absence of macroscopic lesions of distal oesophageal mucosa at endoscopy,3, 4 performed within 6 months from the screening visit, and by the positivity of a validated questionnaire (Reflux Disease Questionnaire, RDQ),38 that is an RDQ score ≥8.39 In accordance with the NICE Guidelines40 and to avoid interference with the rapid urease test,41 routinely performed during endoscopy, patients were free from anti‐secretory medication (either a PPI or an H2RA) for at least 2 weeks.
Exclusion criteria were the presence of erosive oesophagitis or Barrett's oesophagus, gastric or duodenal ulcer, previous gastric or major GI surgery, atopy or food intolerance, thyroid diseases, diabetes or metabolic syndrome. Moreover, pregnant, lactating or fertile women (without contraception) were also excluded.
Study design
The study was multicenter, randomised, double‐blind, placebo‐controlled with parallel groups. Sixteen Italian hospitals were involved, and each of them obtained the approval of the respective ethical committee.
The trial was performed according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), guidelines for Good Clinical Practice (GCP)42 and the Declaration of Helsinki (1996 version, amended October 2000).43
Patients eligible for the study gave informed and written consent and were asked to start a 15‐day (±2 days) run in/wash out period, during which any (prescription or OTC) therapy was discontinued (visit 1). The only medications permitted were antacids or alginate‐containing formulations in case of symptom occurrence. At visit 2, patients were randomised – according to a computer‐generated sequence – to receive one standard dose of a PPI (30 min before breakfast) + 10 mL (1 stick) of Esoxx One (single dose stick formulation) or placebo (with the same taste and viscosity, packed in identical, sequentially numbered, containers) q.d.s., that is 1 h after each daily meal, and at bedtime for a 14‐day treatment period. The allocation sequence was generated by LB Research (contract research organisation), while enrolment and assignment of participants to a given treatment were performed by the principal investigator of the study centres (see Appendix 1). During the study period, daily symptom diaries, recording the presence or absence of each symptom during the day and the night, were filled by each patient. Before (visit 2) and after this short course of therapy (visit 3), frequency and severity of NERD symptoms were evaluated using the same RDQ questionnaire. Health‐related quality of life (HRQL) was also assessed using the SF‐36 questionnaire.44 Both questionnaires were administered by physicians, who were unaware of the treatment given. Results of each item were compared with those of published data for the Italian normative sample.45 Pre‐ and post‐treatment results for each item were also compared. The study design and the detailed assessment schedule can be found in the Table S1.
Safety and tolerability were assessed by recording all the adverse events, defined as any unfavourable or unintended symptom and/or sign, considered to be casually related to the drug(s) used in the study. The palatability was evaluated after each drug administration, according to a 4‐item scale (excellent, good, irrelevant and bad). Hence, there were 4 per day × 15 days evaluations for each patient.
Finally, patients’ compliance was defined as the percentage of the test drug used, obtained by counting the returned medications at visit 3. A treatment compliance of 80–120% was considered acceptable.
The European Clinical Trials Database (EudraCT), launched by the European Medicines Agency (EMA), does not accept clinical trials investigating medical devices, but refers to the procedures in place in the Country, where the clinical trial is conducted. Accordingly, the Clinical Trial Protocol was registered (Protocol code: Esoxx‐NERD/001/2012) at the Italian Ministry of Health, and the beginning of the trial (i.e. the inclusion of the first patient), as well as the end of the trial (i.e. the last evaluation of the last included patient), was notified to the regulatory authorities.
Statistical analysis
The primary endpoint was the treatment efficacy analysis, which was calculated as the proportion of patients with at least 3‐point reduction of the total symptom score (TSS). This was calculated by collecting and computing the intensity of each patient's symptom (on the basis of the RDQ questionnaire at the final visit) and comparing it with the baseline values, obtained at the end of the run in/wash out period (visit 2). Typical symptoms were evaluated according to a 5‐degree Likert scale46: 0 = no symptom, 1 = poorly troublesome symptoms, 2 = troublesome symptoms, 3 = very troublesome symptoms, interfering with daily activities, 4 = intolerable symptoms, not permitting any daily activity.
There were four different secondary endpoints: (i) number of patients with 50% reduction of TSS at final visit, (ii) number of patients with TSS reduction at the final visit, (iii) change TSS after treatment and (iv) HRQL physical and mental items according to the SF‐36 questionnaire, which were calculated via a web‐based program47 and presented as radar plots or spidergrams.48 Changes in the severity and frequency of each symptom (heartburn, acid regurgitation, retrosternal pain, acid taste in the mouth) were also evaluated.
The intention‐to‐treat (ITT) population included all randomised patients, who took at least one dose of medication while per protocol (PP) analysis was performed on all randomised patients, who concluded the treatment, with an adequate compliance rate and without any protocol violation. The former analysis was used to evaluate the primary endpoint and the latter for both primary and secondary endpoints. The safety population included all randomised patients, who took at least one dose of the study drugs.
Chi‐squared and Fisher's exact test, two tails, were used to compare percentages of values for primary and secondary endpoints, while arithmetic means and frequencies were assessed by means of 95% confidence intervals (CIs).49 All the calculations were performed using the PRISM 6.0 software (GraphPad, San Diego, CA, USA), running on a MAC.
The sample size was calculated on the basis of the reduction of NERD TSS by 3 points at final visit and assuming a rate of 10% improvement in the placebo group and 30% in the Esoxx arm. A power level of 80% with a significance value ≤0.05 (two‐sided Fisher's exact test) required a sample size of 70 patients for each group. Taking into account a 12% of non‐evaluable patients, the sample was raised to 80 patients. The estimation was made, using the stata (Version 13, StataCorp LP, College Station, TX, USA) for MAC.
Results
In the 16 centres involved in the study, 172 NERD patients were screened and 154 out of them were randomised to treatment, 76 in the Esoxx group and 78 in the placebo one. Among them, 18 patients were considered dropouts for various reasons: eight for adverse events, two for treatment failure and eight denied consent (Figure 1).
Figure 1.
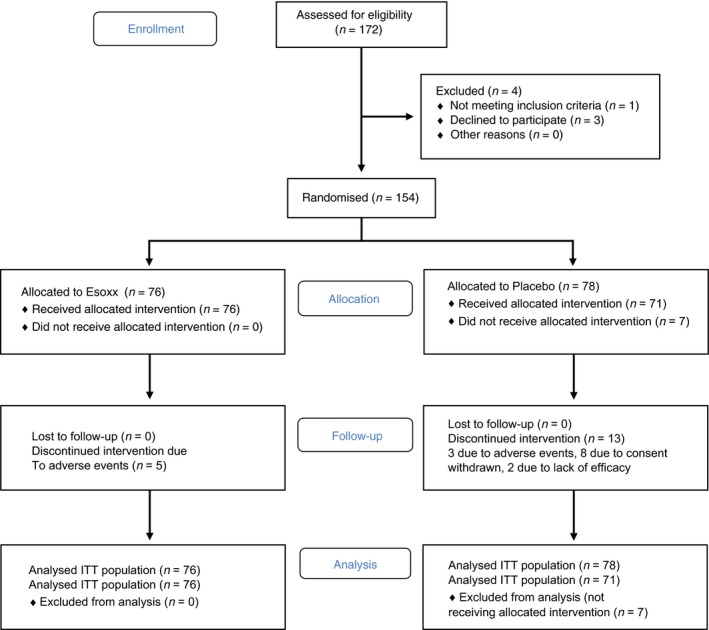
Consort 2010 flow diagram.
Table 1 shows the baseline demographics and the clinical characteristics of the groups studied, in the ITT population. There were no statistical differences among the different characteristics of the recruited patients in the two arms.
Table 1.
Baseline characteristics of NERD patients receiving Esoxx or placebo, combined with PPIs
| Esoxx (n = 76) | Placebo (n = 78) | P value | |
|---|---|---|---|
| Female, N (%) | 48 (63.2%) | 46 (59.0%) | NS |
| Age (years), mean ± s.d. | 45.45 ± 14.98 | 45.51 ± 13.37 | NS |
| Range (min–max) | 18–81 | 24–75 | |
| BMI (kg/m2) | 23.87 ± 3.10 | 23.77 ± 3.23 | NS |
| GERD total symptom score | 7.30 ± 2.4 | 7.19 ± 2.6 | NS |
| Proportion of patients with ≥3 GERD symptoms (%) | 44.0 | 41.0 | NS |
| Heartburn | 84.2 | 85.9 | |
| Retrosternal pain | 53.9 | 49.3 | |
| Acid regurgitation | 69.7 | 66.2 | |
| Acid taste in the mouth | 60.5 | 59.2 | |
| Past treatment with PPIs (%) | 56.6 | 64.8 | NS |
| Past treatment with other anti‐GERD therapies (%) | 23.7 | 29.6 | NS |
The compliance, defined as mean number (±s.d.) of sticks taken, was similar (P = NS) in the two arms of treatment, that is 90.9 ± 22.9 vs. 90.2 ± 20.7 in the Esoxx and placebo groups, respectively.
As regards the primary endpoint, Table 2 (ITT analysis) shows that the proportion of patients with TSS reduction of at least 3 points at final visit was higher in the Esoxx than in the placebo group and the difference was always significant. Also the proportion of patients with 50% TSS reduction at visit 3, as secondary endpoint, resulted to be significantly higher (P < 0.042) in the Esoxx (38.2%) than in the placebo (23.1%) group (Table 2). In addition, number of patients with TSS reduction at the final visit was significantly higher in Esoxx than in placebo arm (P < 0.026). Finally, TSS after treatment improved more with Esoxx than with placebo treatment (P < 0.011). Similar results were obtained in the PP population (Table S2). As shown in Table 3, all the symptoms evaluated subsided with both treatments, but the amelioration of heartburn and especially regurgitation was more marked with Esoxx combined with PPIs. The therapeutic gain with Esoxx was 20.5%, 15.3% and 10.2% for TSS (symptom severity), heartburn and regurgitation incidence, respectively.
Table 2.
Effect of Esoxx, combined with PPI therapy, on primary and secondary endpoints in patients with NERD: ITT analysis
| Trial endpoints | PPI + Esoxx | PPI + Placebo | P value | ||
|---|---|---|---|---|---|
| n/N | % | n/N | % | ||
| Primary | |||||
| No of patients with TSS reduction of at least 3 points | 40/76 | 52.6 | 25/78 | 32.1 | 0.01 |
| Secondary | |||||
| No of patients with 50% reduction of TSS | 29/76 | 38.2 | 18/78 | 23.1 | 0.042 |
| No of patients with TSS reduction at final visit | 60/76 | 78.9 | 44/78 | 56.4 | 0.003 |
| TSS (±s.d.) before and after treatment | Before | After | Before | After | |
| 8.53 ± 2.6 | 5.42 ± 2.1 | 8.03 ± 2.7 | 6.49 ± 2.6 | ||
| Change (±s.d.) in TSS | −3.11 ± 3.1 | −1.54 ± 3.0 | 0.002 | ||
TSS, total symptom (heartburn, retrosternal pain, regurgitation, acid taste) score.
Table 3.
Effect of Esoxx, combined with PPI therapy, on (a) severity and (b) frequency of GERD symptoms in patients with NERD: ITT analysis
| Symptom | PPI + Esoxx, mean score ± s.d. | Adjusted mean change (95% CI) | PPI + placebo, mean score ± s.d. | Adjusted mean change (95% CI) | P value Esoxx vs. placebo | ||
|---|---|---|---|---|---|---|---|
| Before therapy | After therapy | ||||||
| Before therapy | After therapy | ||||||
| (a) | |||||||
| Heartburn | 1.80 ± 1.1 | 0.72 ± 0.8 | −1.131 (−1.340 to −0.922) | 1.99 ± 1.0 | 1.09 ± 1.0 | −0.836 (−1.034 to −0.638) | 0.0319 |
| Regurgitation | 1.84 ± 1.1 | 0.64 ± 0.8 | −1.095 (−1.280 to −0.911) | 1.53 ± 1.1 | 0.94 ± 1.0 | −0.685 (−0.861 to −0.509) | 0.0009 |
| Retrosternal pain | 1.36 ± 1.2 | 0.42 ± 0.7 | −0.852 (−1.023 to −0.682) | 1.15 ± 1.2 | 0.59 ± 0.8 | −0.612 (−0.775 to −0.449) | 0.0323 |
| Acid taste in the mouth | 1.53 ± 1.1 | 0.63 ± 0.8 | −0.754 (−0.968 to 0.541) | 1.3 ± 1.1 | 0.8 ± 1.0 | −0.494 (−0.696 to −0.291) | 0.0623 |
| (b) | |||||||
| Heartburn | 3.08 ± 1.7 | 1.38 ± 1.5 | −1.719 (−2.083 to −1.354) | 3.23 ± 1.5 | 1.94 ± 1.6 | −1.229 (−1.578 to −0.883) | 0.0408 |
| Regurgitation | 2.92 ± 1.7 | 1.23 ± 1.5 | −1.562 (−1.892 to −1.233) | 2.60 ± 1.8 | 1.63 ± 1.7 | −1.021 (−1.332 to −0.710) | 0.0128 |
| Retrosternal pain | 2.14 ± 1.8 | 0.82 ± 1.3 | −1.232 (−1.511 to −0.952) | 1.86 ± 1.7 | 1.03 ± 1.3 | −0.896 (−1.163 to −0.630) | 0.0676 |
| Acid taste in the mouth | 2.57 ± 1.7 | 1.16 ± 1.5 | −1.285 (−1.640 to 0.930) | 2.38 ± 1.8 | 1.53 ± 1.7 | −0.876 (−1.213 to −0.540) | 0.0790 |
Finally, the quality of life, evaluated by means of the SF‐36 items, improved with both treatments (Figure 2). Indeed, 2 weeks after therapy the SF‐36 items become closer to those of the Italian normative sample.45 However, the improvements in General Health Perception and the Social Function items were significantly (P < 0.01 and P < 0.02, respectively) better after Esoxx plus PPI therapy.
Figure 2.
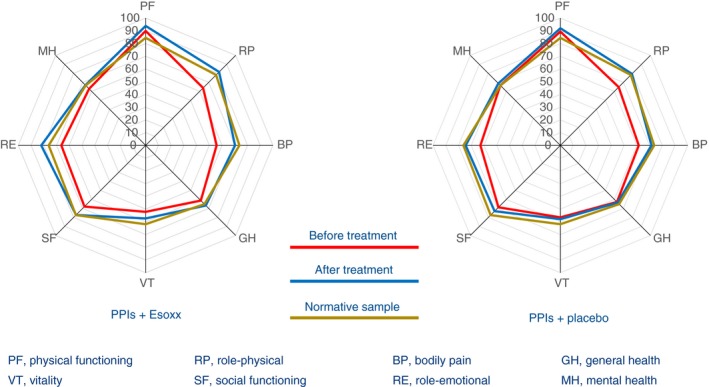
HRQL measured in NERD patients before and after 2‐week treatment with Esoxx or Placebo combined to PPIs. Note that, after treatment, the SF‐36 items are close to those of the Italian normative sample.
The safety of Esoxx was very good, as the total number of adverse events was similar to that of placebo and there were no serious adverse events in any treatment arms (Table 4). The most frequent manifestations pertained to the gastrointestinal tract (nausea, flatulence, bloating, dyspepsia, etc.) and respiratory organs (cough, rhinitis, pharyngeal disorders) (Table 5).
Table 4.
Adverse events in NERD patients, included in the ITT analysis, receiving PPI + Esoxx or PPI + placebo
| Esoxx (n = 76) | Placebo (n = 71) | P value | |
|---|---|---|---|
| Total number of unique AEs | 32 | 14 | NS |
| Total number of AEs | 35 | 20 | NS |
| Total number of patients with at least one AE | 18 (23.7) | 11 (15.5) | NS |
| Total number of unique drug‐related AEs | 23 | 13 | NS |
| Total number of drug‐related AEs | 24 | 19 | NS |
| Total number of patients with at least one related AEs | 13 (17.1) | 10 (14.1) | NS |
| Total number of serious AEs | 0 | 0 | NS |
| Total number of patients with at least one AE leading to discontinuation | 5 (6.6) | 3 (3.8) | NS |
Values within parenthesis are expressed as percentage. AE, adverse event.
Table 5.
Patients, treated with PPI combined with Esoxx or placebo, with at least one TEAEs classified for system organ class (SOC) – safety analysis
| SOC | Esoxx (n = 76) | Placebo (n = 71) |
|---|---|---|
| Patients with at least one TEAE | 18 (23.7) | 11 (15.5) |
| Gastrointestinal disorders | 13 (17.1) | 7 (9.9) |
| Respiratory, thoracic, mediastinal disorders (cough, rhinitis, throat irritation, pharyngeal disorders) | 4 (5.3) | 1 (1.4) |
| Nervous system disorders (dysgeusia, headache, migraine) | 3 (3.9) | – |
| Cardiac disorders (palpitations, tachycardia) | 1 (1.3) | 1 (1.4) |
| Ear and labyrinth (vertigo) | 1 (1.3) | – |
| General disorders (hypertension) | 1 (1.3) | – |
| Infections and infestations | 1 (1.3) | 3 (4.2) |
Values within parenthesis are expressed as percentage.
On the basis of the total number of evaluations collected (n = 7230), in 92% of Esoxx administrations, palatability was considered acceptable, independently of the intake time (be it during the day or at bedtime) while the same held true for 90% of placebo administrations (P = NS). The distribution of these evaluations is shown in Figure 3.
Figure 3.
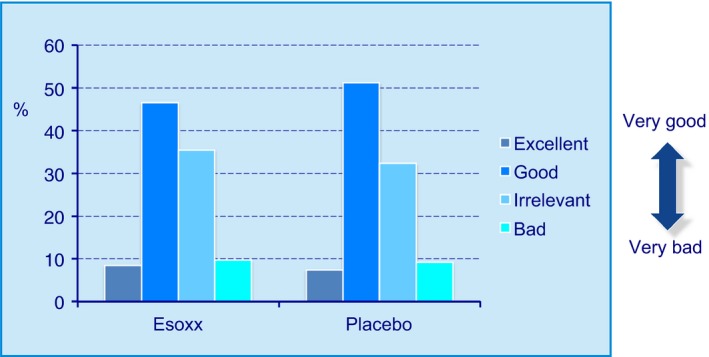
Palatability assessment of Esoxx or placebo formulations, used in the present study. Distribution of the 60 evaluations for each patient.
Discussion
The results of this study show that, when mucosal protection is added to acid suppression, a significantly higher number of NERD patients obtained symptom relief with combination therapy. Indeed, both the primary and secondary endpoints were achieved in a larger proportion of subjects.
Although PPIs are effective in obtaining symptom relief in both erosive and NERD,50 their efficacy for the relief of regurgitation is modest, and considerably lower than that achieved for heartburn.51 In addition, although not as frequent as previously suggested,7 PPI‐refractory heartburn, occurring more commonly in NERD than in erosive disease, does exist. Some 20% (range 15–27%) of correctly diagnosed and appropriately treated patients do not respond to PPI therapy at standard doses.52
Various underlying mechanisms have been shown to contribute to the failure of PPI treatment. They include patient‐related (e.g. lack of compliance), physician‐related (e.g. misdiagnosis) and drug‐related (e.g. short duration of action) mechanisms.53, 54 At the present time, much current research is focused on weakly acidic reflux55 and oesophageal hypersensitivity.56 The pH‐impedance technique has been increasingly used to explore the underlying pathophysiology in PPI‐resistant patients. Several groups of investigators have indeed shown that weakly acidic reflux plays a major role in PPI‐resistant erosive and non‐erosive disease.57pH‐impedance monitoring has also allowed identification of a previously unknown subgroup of patients, namely those with normal oesophageal pH‐impedance recording, but a positive association between symptoms and non‐acidic reflux episodes, that is patients who are hypersensitive to non‐acidic reflux.57 Finally, this methodology has enabled a better differentiation between patients with NERD and those with functional heartburn.58 It is therefore evident that different patient subgroups belong to NERD, which is indeed an umbrella term. Among them, only patients with true NERD or acid hypersensitive oesophagus (now called reflux hypersensitivity, according to the Rome IV criteria59) are expected to display a satisfactory symptomatic response to acid suppression therapy with a PPI. On the contrary, subjects hypersensitive to non‐acid reflux or those with functional heartburn (which – together with reflux hypersensitivity – does not pertain anymore to the realm of GERD) will obviously be nonresponsive to anti‐secretory drugs.60
In patients with NERD, who are refractory to a correctly performed PPI therapy, the lack of symptom relief could be due to persistence of microscopic mucosal alterations induced by weakly acidic reflux,61 by pepsin or other components of the refluxate62 and underlined by an impaired mucosal integrity.63 Current pharmacologic approaches to address this clinically challenging condition are limited. Reflux inhibitors represent a promise unfulfilled,64 effective prokinetics are lacking 65 and anti‐depressants, despite being effective in selected patients,66 give rise to adverse events in up to 32% of patients.67
A better approach to patients with NERD should be therefore making a more precise diagnosis, by adding a functional evaluation (e.g. pH or pH‐impedance recording) to negative endoscopy. When this has been done, the estimated complete symptom response rate after PPI therapy appeared comparable to that observed in patients with GERD.68 Including biopsy (and subsequent histology) of the ‘macroscopically normal’ mucosa during endoscopic examination61 would be ideal. It is evident, however, that this approach, being time‐consuming and costly, is not achievable in the everyday clinical practice.
An alternative, easier, approach could be combination therapy, that is adding drugs with different mechanism(s) of action to PPIs. Up to now, only irsogladine (a mucosal protective compound)69 and alginate‐containing formulations70, 71 – given as add‐on medications – proved to be capable of improving symptom control in NERD patients. The addition of mosapride (a prokinetic compound) to PPIs does not add any benefit72, 73 unless NERD patients display a delay in gastric emptying.74
The mucosal protective device, Esoxx, was shown to be capable of achieving a significant and quick symptom relief in patients with NERD in this and a previous trial.37 Its amelioration of regurgitation severity and frequency is of clinical interest, taking into account the negligible effect PPIs have on this cardinal symptom of reflux disease.51, 63 As shown by a small study,36 this formulation may well be effective also in patients with erosive disease, in whom its protective and reparative properties would favour healing of oesophageal mucosal lesions.
The synergistic effect of Esoxx with PPIs, shown in this study, suggests that mucosal protection, routinely added to acid suppression, could extend to a larger number of patients with NERD both symptom relief and improvement of HRQL, thus reducing the incidence of treatment failures. PPIs achieve a symptom relief, which increases over time both in erosive and non‐erosive disease. This has been further shown by the studies comparing PPIs (namely esomeprazole) with P‐CABs (namely linaprazan).75, 76 It may well be that this combined approach achieves at 2 weeks the same symptom relief, obtained with PPIs at 4 weeks. However, for those patients asking for quick symptom relief, this time‐dependent therapeutic gain could be worthwhile from their own perspective.
The present study has intrinsic limitations. As functional investigation (i.e. pH‐impedance recording) was not performed, the population studied included patients with functional heartburn and reflux hypersensitivity. In addition, although adequately powered to show a significant effect, this was a relatively small trial. A larger study in patients with PPI‐resistant NERD as well as a trial in patients with extra‐oesophageal symptoms is worthwhile.
Despite recent research has established the sites and mechanisms underlying oesophageal mucosal defence, its enhancement is very rarely pursued in clinical practice. Drugs able to strengthen mucosal defence do exist, but they have not been studied in well designed clinical trials.63 Due their high efficacy in reflux disease, it is unlikely that these drugs represent a real alternative to PPIs. However, their use in less severe disease or as add‐on medications to PPIs could be useful. Furthermore, used in the long term, these mucosal protective compounds might prolong remission and delay relapse.
Authorship
Guarantor of the article: C. Scarpignato.
Author contributions: According to the International Committee of Medical Journal Editors (JCMJE), Carmelo Scarpignato and Vincenzo had full access to all of the data in the study and take the responsibility for the integrity of the data. Carmelo Scarpignato, Vincenzo Savarino performed study concept and study design and drafted the manuscript; Carmelo Scarpignato, Vincenzo Savarino and Fabio Pace analysed and interpreted the data and critically revised the manuscript for important intellectual content; LB Research (Claudio Iannacone) and Carmelo Scarpignato performed statistical analysis; Carmelo Scarpignato obtained funding; LB Research (Sara Bellasio) performed administrative, technical or material support; and Antonella Ferrieri performed study supervision. All authors approved the final version of the manuscript.
Supporting information
Table S1. Study design and assessment schedule.
Table S2. Effect of Esoxx, combined with PPI therapy, on primary and secondary endpoints in patients with NERD: PP analysis.
Acknowledgements
The company did not have any role in the execution of the study or interpretation of data. The terms of the financial support included freedom for the authors to reach their own conclusions, and an absolute right to publish the results of their work, irrespective of any conclusions reached.
Declaration of personal interests: Vincenzo Savarino is member of the Speakers’ Bureau of Alfa Wassermann, the manufacturer of Esoxx. Fabio Pace has no conflict of interests to disclose. Carmelo Scarpignato is member of the Speakers’ Bureau and of the Advisory Board of Alfa Wassermann.
Declaration of funding interests: This study was supported by Alfa Wassermann SpA (Bologna, Italy), which involved the contract research organisation, LB Research SRL (Cantú, Como, Italy) to deal with the logistic aspects of the study.
Appendix 1.
Members of the Esoxx Study Group
Marco Astegiano, MD, Department of Gastroenterology & Digestive Endoscopy, University Hospital, Turin; Carlo Calabrese, MD, PhD, Department of Medical & Surgical Sciences, S. Orsola University Hospital, Bologna; Michele Cicala, MD, PhD, Department of Gastroenterology & Digestive Endoscopy, Campus Biomedico University Hospital, Rome; Enrico Ciliberto, MD, Division of Gastroenterology & Digestive Endoscopy, San Giovanni di Dio Hospital, Crotone; Rita Conigliaro, MD, Division of Digestive Endoscopy, Baggiovara Teaching Hospital, Modena; Guido Costamagna, MD, Department of Surgical Digestive Endoscopy, Gemelli University Hospital, Rome; Rosario Cuomo, MD, PhD, Department of Gastroenterology & Digestive Oncology, Federico II University Hospital, Naples; Alfredo Di Leo, MD, PhD, Department of Gastroenterology & Digestive Endoscopy, Giovanni XXII University Hospital, Bari; Massimo Pierluigi Di Simone, MD, Department of Medical & Surgical Sciences, S. Orsola University Hospital, Bologna; Pasquale Esposito, MD, Department of Gastroenterology & Digestive Endoscopy, University Hospital, Naples; Marzia Groppo, MD, Department of Gastroenterology & Digestive Endoscopy, Teaching Hospital, Treviso; Santino Marchi, MD, Department of Gastroenterology & Digestive Endoscopy, Cisanello University Hospital, Pisa; Matteo Neri, MD, PhD, Department of Internal Medicine and Ageing, SS. Annunziata University Hospital, Chieti; Fabio Pace, MD, Division of Gastroenterology & Digestive Endoscopy, Teaching Hospital, Seriate, Milan; Vincenzo Savarino, MD, PhD, Department of Gastroenterology & Digestive Endoscopy, S. Martino University Hospital, Genoa; Sergio Segato, MD, Division of Gastroenterology & Digestive Endoscopy, Circolo Hospital, Varese.
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer review.
References
- 1. El‐Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 2014; 63: 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fass R, Ofman JJ. Gastroesophageal reflux disease – should we adopt a new conceptual framework? Am J Gastroenterol 2002; 97: 1901–9. [DOI] [PubMed] [Google Scholar]
- 3. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus G . The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. Am J Gastroenterol 2006; 101: 1900–20; quiz 1943. [DOI] [PubMed] [Google Scholar]
- 4. Modlin IM, Hunt RH, Malfertheiner P, et al Diagnosis and management of non‐erosive reflux disease – the vevey NERD consensus group. Digestion 2009; 80: 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savarino V, Di Mario F, Scarpignato C. Proton pump inhibitors in GORD: an overview of their pharmacology, efficacy and safety. Pharmacol Res 2009; 59: 135–53. [DOI] [PubMed] [Google Scholar]
- 6. Tack J, Fass R. Review article: approaches to endoscopic‐negative reflux disease – part of the GERD spectrum or a unique acid‐related disorder? Aliment Pharmacol Ther 2004; 19(Suppl. 1): 28–34. [DOI] [PubMed] [Google Scholar]
- 7. Katz PO, Scheiman JM, Barkun AN. Review article: acid‐related disease – what are the unmet clinical needs? Aliment Pharmacol Ther 2006; 23(Suppl. 2): 9–22. [DOI] [PubMed] [Google Scholar]
- 8. Gupta N, Inadomi JM, Sharma P. Perception about gastroesophageal reflux disease (GERD) and its impact on daily life in the general population: results from a large population based AGA survey. Gastroenterology 2012; 142: S411. [Google Scholar]
- 9. Caviglia R, Ribolsi M, Maggiano N, et al Dilated intercellular spaces of esophageal epithelium in nonerosive reflux disease patients with physiological esophageal acid exposure. Am J Gastroenterol 2005; 100: 543–8. [DOI] [PubMed] [Google Scholar]
- 10. Zentilin P, Savarino V, Mastracci L, et al Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group. Am J Gastroenterol 2005; 100: 2299–306. [DOI] [PubMed] [Google Scholar]
- 11. Savarino E, Zentilin P, Mastracci L, et al Microscopic esophagitis distinguishes patients with non‐erosive reflux disease from those with functional heartburn. J Gastroenterol 2013; 48: 473–82. [DOI] [PubMed] [Google Scholar]
- 12. Tack J. Review article: role of pepsin and bile in gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 2005; 22(Suppl. 1): 48–54. [DOI] [PubMed] [Google Scholar]
- 13. Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: advancing the role of pepsin. Int J Otolaryngol 2012; 2012: 646901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pizzoni A. (Inventor). Glycosaminoglycan oral use and compositions. EU Patent 2581090 A1. 2013.
- 15. Pizzoni A. (Inventor). Glycosaminoglycan oral use and composition. US Patent 0107064 A1. 2014.
- 16. Blackshaw LA, Bordin DS, Brock C, et al Pharmacologic treatments for esophageal disorders. Ann N Y Acad Sci 2014; 1325: 23–39. [DOI] [PubMed] [Google Scholar]
- 17. Tang M, Dettmar P, Batchelor H. Bioadhesive oesophageal bandages: protection against acid and pepsin injury. Int J Pharm 2005; 292: 169–77. [DOI] [PubMed] [Google Scholar]
- 18. Batchelor HK, Tang M, Dettmar PW, Hampson FC, Jolliffe IG, Craig DQ. Feasibility of a bioadhesive drug delivery system targeted to oesophageal tissue. Eur J Pharm Biopharm 2004; 57: 295–8. [DOI] [PubMed] [Google Scholar]
- 19. Gaffney J, Matou‐Nasri S, Grau‐Olivares M, Slevin M. Therapeutic applications of hyaluronan. Mol BioSyst 2010; 6: 437–43. [DOI] [PubMed] [Google Scholar]
- 20. Volpi N, Schiller J, Stern R, Soltes L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem 2009; 16: 1718–45. [DOI] [PubMed] [Google Scholar]
- 21. Nolan A, Baillie C, Badminton J, Rudralingham M, Seymour RA. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med 2006; 35: 461–5. [DOI] [PubMed] [Google Scholar]
- 22. Kapoor P, Sachdeva S, Sachdeva S. Topical hyaluronic acid in the management of oral ulcers. Indian J Dermatol 2011; 56: 300–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ialenti A, Di Rosa M. Hyaluronic acid modulates acute and chronic inflammation. Agents Actions 1994; 43: 44–7. [DOI] [PubMed] [Google Scholar]
- 24. Lauder RM. Chondroitin sulphate: a complex molecule with potential impacts on a wide range of biological systems. Complement Ther Med 2009; 17: 56–62. [DOI] [PubMed] [Google Scholar]
- 25. Volpi N. Anti‐inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule. Inflammopharmacology 2011; 19: 299–306. [DOI] [PubMed] [Google Scholar]
- 26. du Souich P, Garcia AG, Verges J, Montell E. Immunomodulatory and anti‐inflammatory effects of chondroitin sulphate. J Cell Mol Med 2009; 13: 1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campo GM, Avenoso A, Campo S, Ferlazzo AM, Calatroni A. Chondroitin sulphate: antioxidant properties and beneficial effects. Mini Rev Med Chem 2006; 6: 1311–20. [DOI] [PubMed] [Google Scholar]
- 28. Bonfils S, Dubrasquet M, Lambling A. The inhibition of peptic proteolysis by various polysaccharides. Rev Fr Etud Clin Biol 1960; 5: 71–4. [PubMed] [Google Scholar]
- 29. Galzigna L, Previerocoletti MA. Action of sodium chondroitin sulfate on the enzymatic activity of pepsin. Gazz Med Ital 1965; 124: 65–7. [PubMed] [Google Scholar]
- 30. Lenzi G, Rapino P, Ferri S. On the behavior of gastric hydrochloric and peptic activity after administration of sodium chondroitin sulfate. Minerva Med 1963; 54: 3421–4. [PubMed] [Google Scholar]
- 31. Baldini E, Tincani GP. Treatment of gastroduodenal ulcer with sodium chondroitin sulfate. Minerva Gastroenterol 1963; 9: 25–9. [PubMed] [Google Scholar]
- 32. Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res 2006; 23: 2709–28. [DOI] [PubMed] [Google Scholar]
- 33. Ramya Dev D, Sandhya P, Vedha Hari BN. Poloxamer: a novel functional molecule for drug delivery and gene therapy. J Pharm Sci Res 2013; 5: 159–65. [Google Scholar]
- 34. Parliament E Council Directive 93/42/EEC concerning medical devices. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:en:PDF (accessed on 20 November 2016).
- 35. Di Simone MP, Baldi F, Vasina V, et al Barrier effect of Esoxx((R)) on esophageal mucosal damage: experimental study on ex‐vivo swine model. Clin Exp Gastroenterol 2012; 5: 103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palmieri B, Corbascio D, Capone S, Lodi D. Preliminary clinical experience with a new natural compound in the treatment of oesophagitis and gastritis: symptomatic effect. Trends Med 2009; 9: 219–25. [Google Scholar]
- 37. Palmieri B, Merighi A, Corbascio D, Rottigni V, Fistetto G, Esposito A. Fixed combination of hyaluronic acid and chondroitin‐sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non‐erosive gastroesophageal reflux. Eur Rev Med Pharmacol Sci 2013; 17: 3272–8. [PubMed] [Google Scholar]
- 38. Pace F, Scarlata P, Casini V, Sarzi‐Puttini P, Porro GB. Validation of the reflux disease questionnaire for an Italian population of patients with gastroesophageal reflux disease. Eur J Gastroenterol Hepatol 2008; 20: 187–90. [DOI] [PubMed] [Google Scholar]
- 39. Pace F, Molteni P, Casini V, Pallotta S, Bianchi Porro G. Assessment of gastro‐oesophageal reflux symptoms in Italian physicians – a survey by reflux disease questionnaire. Dig Liver Dis 2008; 40: 235–9. [DOI] [PubMed] [Google Scholar]
- 40. NICE . Dyspepsia and gastro‐oesophageal reflux disease: investigation and management of dyspepsia, symptoms suggestive of gastro‐oesophageal reflux disease, or both. Available at: https://wwwniceorguk/guidance/cg184/documents/dyspepsiagord-nice-guideline2, 2014. [PubMed]
- 41. Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med 2015; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. (ICH) ICoH . Guidelines for Good Clinical Practice. http://wwwichorg/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guidelinepdf, 1996.
- 43. (WMA) WMA . Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. http://wwwwmanet/en/30publications/10policies/b3/17cpdf, 2008. [PubMed]
- 44. Apolone G, Mosconi P. The Italian SF‐36 Health Survey: translation, validation and norming. J Clin Epidemiol 1998; 51: 1025–36. [DOI] [PubMed] [Google Scholar]
- 45. Apolone G, Mosconi P, Ware JE. Il questionario sullo stato di salute SF‐36: manuale d'uso e quida ll'interpretazione dei risultati. Milan: Guerini Editori Associati, 1997. [Google Scholar]
- 46. Bytzer P. Assessment of reflux symptom severity: methodological options and their attributes. Gut 2004; 53 Suppl. 4: iv28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Negri IdRFM . Questionario Sullo Stato di Salute SF‐36 (V1). Available at: http://crc.marionegri.it/sf36/sf36v1ita.htm.
- 48. Strand V, Crawford B, Singh J, Choy E, Smolen JS, Khanna D. Use of “spydergrams” to present and interpret SF‐36 health‐related quality of life data across rheumatic diseases. Ann Rheum Dis 2009; 68: 1800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Motulsky H. Intuitive Biostatistics. A Nonmathematical Guide to Statistical Thinking. New York: Oxford University Press, 2013: 1–576. [Google Scholar]
- 50. Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short‐term treatment with proton pump inhibitors, H2‐receptor antagonists and prokinetics for gastro‐oesophageal reflux disease‐like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2013; 5: CD002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011; 106: 1419–25; quiz 1426. [DOI] [PubMed] [Google Scholar]
- 52. Bytzer P, van Zanten SV, Mattsson H, Wernersson B. Partial symptom‐response to proton pump inhibitors in patients with non‐erosive reflux disease or reflux oesophagitis – a post hoc analysis of 5796 patients. Aliment Pharmacol Ther 2012; 36: 635–43. [DOI] [PubMed] [Google Scholar]
- 53. Bredenoord AJ, Smout AJ. Refractory gastrooesophageal reflux disease. Eur J Gastroenterol Hepatol 2008; 20: 217–23. [DOI] [PubMed] [Google Scholar]
- 54. Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009; 58: 295–309. [DOI] [PubMed] [Google Scholar]
- 55. Tsoukali E, Sifrim D. The role of weakly acidic reflux in proton pump inhibitor failure, has dust settled? J Neurogastroenterol Motil 2010; 16: 258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Knowles CH, Aziz Q. Visceral hypersensitivity in non‐erosive reflux disease. Gut 2008; 57: 674–83. [DOI] [PubMed] [Google Scholar]
- 57. Savarino E, Zentilin P, Savarino V. NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol 2013; 10: 371–80. [DOI] [PubMed] [Google Scholar]
- 58. Savarino E, Marabotto E, Zentilin P, et al The added value of impedance‐pH monitoring to Rome III criteria in distinguishing functional heartburn from non‐erosive reflux disease. Dig Liver Dis 2011; 43: 542–7. [DOI] [PubMed] [Google Scholar]
- 59. Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional esophageal disorders. Gastroenterology 2016. doi: 10.1053/j.gastro.2016.02.012 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 60. Scarpignato C. Poor effectiveness of proton pump inhibitors in non‐erosive reflux disease: the truth in the end!. Neurogastroenterol Motil 2012; 24: 697–704. [DOI] [PubMed] [Google Scholar]
- 61. Farre R, Fornari F, Blondeau K, et al Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non‐exposed human oesophagus. Gut 2010; 59: 164–9. [DOI] [PubMed] [Google Scholar]
- 62. Farre R, Blondeau K, Clement D, et al Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut 2011; 60: 885–92. [DOI] [PubMed] [Google Scholar]
- 63. Farre R. Pathophysiology of gastro‐esophageal reflux disease: a role for mucosa integrity? Neurogastroenterol Motil 2013; 25: 783–99. [DOI] [PubMed] [Google Scholar]
- 64. Kahrilas PJ, Boeckxstaens G. Failure of reflux inhibitors in clinical trials: bad drugs or wrong patients? Gut 2012; 61: 1501–9. [DOI] [PubMed] [Google Scholar]
- 65. Looijer‐van Langen M, Veldhuyzen van Zanten S. Does the evidence show that prokinetic agents are effective in healing esophagitis and improving symptoms of GERD? Open Med 2007; 1: e181–3. [PMC free article] [PubMed] [Google Scholar]
- 66. Weijenborg PW, de Schepper HS, Smout AJ, Bredenoord AJ. Effects of antidepressants in patients with functional esophageal disorders or gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol 2015; 13: 251–9 e251. [DOI] [PubMed] [Google Scholar]
- 67. Ford AC, Quigley EM, Lacy BE, et al Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta‐analysis. Am J Gastroenterol 2014; 109: 1350–65; quiz 1366. [DOI] [PubMed] [Google Scholar]
- 68. Weijenborg PW, Cremonini F, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well‐defined non‐erosive reflux disease and in reflux esophagitis: a meta‐analysis. Neurogastroenterol Motil 2012; 24: 747–57 e350. [DOI] [PubMed] [Google Scholar]
- 69. Suzuki T, Matsushima M, Masui A, et al Irsogladine maleate and rabeprazole in non‐erosive reflux disease: a double‐blind, placebo‐controlled study. World J Gastroenterol 2015; 21: 5023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Manabe N, Haruma K, Ito M, et al Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: a randomized clinical trial. Dis Esophagus 2012; 25: 373–80. [DOI] [PubMed] [Google Scholar]
- 71. Reimer C, Lodrup AB, Smith G, Wilkinson J, Bytzer P. Randomised clinical trial: alginate (Gaviscon Advance) vs. placebo as add‐on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment Pharmacol Ther 2016; 43: 899–909. [DOI] [PubMed] [Google Scholar]
- 72. Madan K, Ahuja V, Kashyap PC, Sharma MP. Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastroesophageal reflux disease: a randomized trial. Dis Esophagus 2004; 17: 274–8. [DOI] [PubMed] [Google Scholar]
- 73. Miwa H, Inoue K, Ashida K, et al Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non‐erosive reflux disease – a double‐blind, placebo‐controlled study. Aliment Pharmacol Ther 2011; 33: 323–32. [DOI] [PubMed] [Google Scholar]
- 74. Futagami S, Iwakiri K, Shindo T, et al The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI‐resistant NERD patients with delayed gastric emptying. J Gastroenterol 2010; 45: 413–21. [DOI] [PubMed] [Google Scholar]
- 75. Kahrilas PJ, Dent J, Lauritsen K, et al A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol 2007; 5: 1385–91. [DOI] [PubMed] [Google Scholar]
- 76. Dent J, Kahrilas PJ, Hatlebakk J, et al A randomized, comparative trial of a potassium‐competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol 2008; 103: 20–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study design and assessment schedule.
Table S2. Effect of Esoxx, combined with PPI therapy, on primary and secondary endpoints in patients with NERD: PP analysis.


