Abstract
Research using clinical populations to explore the relationship between hemispheric speech lateralization and handedness has focused on individuals with speech and language disorders, such as dyslexia or specific language impairment (SLI). Such work reveals atypical patterns of cerebral lateralization and handedness in these groups compared to controls. There are few studies that examine this relationship in people with motor coordination impairments but without speech or reading deficits, which is a surprising omission given the prevalence of theories suggesting a common neural network underlying both functions. We use an emerging imaging technique in cognitive neuroscience; functional transcranial Doppler (fTCD) ultrasound, to assess whether individuals with developmental coordination disorder (DCD) display reduced left‐hemisphere lateralization for speech production compared to control participants. Twelve adult control participants and 12 adults with DCD, but no other developmental/cognitive impairments, performed a word‐generation task whilst undergoing fTCD imaging to establish a hemispheric lateralization index for speech production. All participants also completed an electronic peg‐moving task to determine hand skill. As predicted, the DCD group showed a significantly reduced left lateralization pattern for the speech production task compared to controls. Performance on the motor skill task showed a clear preference for the dominant hand across both groups; however, the DCD group mean movement times were significantly higher for the non‐dominant hand. This is the first study of its kind to assess hand skill and speech lateralization in DCD. The results reveal a reduced leftwards asymmetry for speech and a slower motor performance. This fits alongside previous work showing atypical cerebral lateralization in DCD for other cognitive processes (e.g., executive function and short‐term memory) and thus speaks to debates on theories of the links between motor control and language production.
Keywords: developmental coordination disorder, functional transcranial Doppler ultrasound, speech production, cerebral lateralisation, motor control
Background
The relationship between motor control and speech production has long been a focus of neuropsychological research, with theories suggesting a complementary developmental trajectory between the two functions (Iverson, 2010). The majority of neurotypical adults display a common pattern of right‐handedness and left hemispheric dominance for language (Knecht, Deppe et al., 2000). However, evidence suggests that this typical pattern of hemispheric mapping is altered in individuals with neurodevelopmental disorders. Various studies report an increased proportion of left‐handedness in disorders such as dyslexia (Eglinton & Annett, 1994) and autism (Cornish & McManus, 1996) and data from individuals with language and reading impairments, such as specific language impairment (SLI) and dyslexia, reveal reduced left‐hemisphere activation during speech production compared to controls (Illingworth & Bishop, 2009; Whitehouse & Bishop, 2008).
Developmental studies of the relationship between speech and motor function demonstrate differences in fine motor skill abilities in children with speech deficits (Visscher, Houwen, Scherder, Moolenaar, & Hartman, 2007), as well as increases in bilateral cortical activation patterns underlying fine motor control in children with persistent speech disorder (Redle et al., 2014) compared with controls. Furthermore, recent data from epilepsy patients demonstrate that the language‐dominant hemisphere can be identified and predicted by the differential motor performance between the preferred and non‐preferred hand on a peg‐moving task (Flowers & Hudson, 2013).
This convergence of evidence indicates that hemispheric organization of motor and speech functions are related, to the extent that the functional status of one is associated with the cortical representation of the other. This relationship has primarily been examined in cohorts with a predominant language disorder. To date, this relationship has not been examined in individuals with a predominant motor disorder, such as those seen in developmental coordination disorder (DCD), but without co‐occurring language or reading impairments. This is a surprising omission in the light of existing theories of how language and motor systems co‐exist with regard to the neural networks underpinning them (Goldenberg, 2013).
Developmental coordination disorder is a neurodevelopmental condition affecting motor coordination and control often identified in childhood or young adulthood. It is estimated to affect 5–8% of the population (Gillberg, 2003) and is classified in DSM‐V (American Psychiatric Association, 2013) as having difficulties with fine or gross motor coordination such that daily functioning is significantly affected. Importantly, the motor coordination difficulties must not be the result of an underlying medical disorder (such as cerebral palsy). DCD is an idiopathic, stand‐alone neurodevelopmental disorder, although in 25% of cases, there is co‐morbid presentation with other neurodevelopmental disorders, most commonly dyslexia or ADHD (Sugden, 2007). The exact cause of DCD is unknown and despite certain theories on possible neurological underpinnings (for review see Zwicker, Missiuna, Harris, & Boyd, 2012), there has been relatively little neuroimaging research conducted with patients with DCD. Research investigating speech profiles in individuals with DCD suggests that language ability is often reduced in this group, and the co‐occurrence of speech and language disorders in children with a DCD diagnosis is relatively high (see Hill, 2001, for review). The majority of research examining language and motor control disorders focuses on individuals with language impairments who also have motor coordination difficulties, but not necessarily a DCD diagnosis. The authors are not aware of any studies directly assessing the neural organization of language within DCD populations.
The aim of this study was to establish whether there was reduced leftwards hemispheric lateralization for speech production in a group of adult patients with DCD, but without co‐occurring impairments in speech and language. It was predicted that due to the likely overlap between motor sequencing areas involved in fine motor control and in speech and language processes (Flowers & Hudson, 2013), the laterality profile displayed by the DCD participants in a speech production tasks would be significantly less left‐hemisphere dominant. To test this hypothesis, an emerging technique in cognitive neuroscience was used; functional transcranial Doppler (fTCD) ultrasound. fTCD assesses the relative changes in cerebral blood flow volume (CBFV) in each hemisphere, whilst participants undertake a cognitive task and has been shown to reliably detect activation in speech paradigms (Bishop, Watt, & Papadatou‐Pastou, 2009).
Method and materials
Participants
Participants were 12 adults with DCD aged between 18 and 43 years old (4 males; mean age = 25.33 years, SD age = 9.01) and 12 adults without DCD aged between 18 and 28 years old (5 males; mean age = 20 years, SD age = 2.66). All had normal or corrected to normal vision and gave informed consent prior to participating in the study. None of the participants had been diagnosed with a neurological disorder (aside from DCD in the patient group) nor were any taking medications known to affect the central nervous or circulatory systems. None had diagnosed impairments in speech, language, or reading ability. All participants were white British in ethnicity, and all had English as a first and primary language. They had all completed compulsory and further education (which continues until age 18 in the UK) and all were either currently in higher education or full‐time employment. Participants were recruited through adverts placed on social media and around the University and were paid £6.00 for helping with the research. The investigation was approved by the ethics committee of the School of Psychology, University of Lincoln.
Instruments
Sample characterization
All participants in the DCD group had received a diagnosis of DCD within the last 10 years from a clinician in the NHS. These diagnoses were self‐reported by the participants. The speciality of the clinician providing the diagnosis varied between participants, with some having been assessed in primary care via their GP and others being referred to occupational therapists or neurological specialists. For this study, severity of DCD was assessed via the self‐report Adult Developmental Coordination Disorder checklist (ADC; Kirby, Edwards, Sugden, & Rosenblum, 2010). The ADC is a screening tool for identifying DCD characteristics in adults. It is a short self‐report questionnaire with three subscales, which focus on motor and coordination difficulties experienced in childhood and adulthood based around the DSM‐V (American Psychiatric Association, 2013) criteria. The tool has been found to have high internal reliability and has been shown to have high discriminatory power at detecting individuals with DCD from controls (Kirby et al., 2010). All participants in the DCD group met the criteria for significant motor difficulties during childhood, which is necessary for a diagnosis of DCD via this tool. In addition, all DCD participants scored above the borderline threshold on the ADC, meaning that these individuals were in the ‘probable DCD’ category, as opposed to a milder form of the impairment. The ADC has a separate section on self‐reported difficulties as an adult, although this does not focus solely on the motor domain, and again, all DCD participants scored above the diagnostic threshold in this section.
The control groups were selected from the general student and staff population and were not specifically matched to the DCD group for age or gender.
Experimental materials
All participants completed a series of assessments to ascertain their levels of motor, language, and cognitive abilities.
Handedness assessment
Hand usage was measured by a 21‐item handedness questionnaire as described by Flowers and Hudson (2013). In short, respondents are required to indicate their preferred hand for executing 14 unimanual (e.g., hold a toothbrush) and 7 bimanual tasks (e.g., unscrew the lid of a jar). Participants were classified as left‐ or right‐handed if stating consistent hand preference for 90% of the tasks. Scores <90% were classified on the basis of predominant left or right responses as either left or right ambilateral.
Non‐verbal reasoning
A shortened 9‐item version (Bilker et al., 2012) of the Ravens Standard Progressive Matrices (RSPM) test (Raven, Raven, & Court, 2014) was included as a measure of general cognitive ability to ensure comparability between the patient and control groups. Bilker et al. (2012) extensively modelled the 60 items in the original RSPM test and showed that a specific set of nine items correlated highly with scores on the existing 60‐item and 30‐item commercially available versions. The 9‐item version also gave equivalent item and test level characteristics as well as a time saving of 75% against the administration time of the original version. In brief, participants are asked to choose which segment from a choice of six options best completes the pattern shown in a target box above. There are no time restrictions placed on this test, and it does not require high levels of language or reading ability to complete, making it a good indicator of general non‐verbal cognitive ability. For scoring purposes, all items are equally weighted and a proportionate score based on number of correct responses is derived for each participant (see Bilker et al., 2012, for further details).
Language assessment
Phonological processing and speech production abilities were measured using a subset of tests from the York Adult Assessment‐Revised (YAA‐R; Warmington, Stothard, & Snowling, 2008). This test battery has been developed as a screening tool for diagnosing language and reading impairments, such as dyslexia, in students in higher education. Its inclusion here was to ensure comparable ability between patients and control on phonological and speech processing.
Experimental procedures
Speech laterality
Language lateralization was determined by measuring hemispheric changes in CBFV with fTCD during a word‐generation task. Word generation (WG) has been validated in numerous neuroimaging studies as an effective paradigm to elicit speech lateralization (Benson et al., 1999; Bishop et al., 2009; Somers et al., 2007). Within fTCD, it has been used extensively by Knecht et al. (1998, 1996), and the paradigm is described by Knecht et al. (1998). In brief, participants were seated in front of a computer screen with the fTCD headset fitted. Each trial began with a 5‐s period in which participants were prompted to clear their mind (Figure 1). A letter was then presented in the centre of the computer screen for 15 s, during which time participants were required to silently generate as many words as possible that began with the letter displayed. At the onset of the trial, a 500‐ms epoch marker was simultaneously sent to the Doppler. Following the generation phase, to ensure task compliance, participants were requested to report the words aloud within a 5‐s period. The trial concluded with a 35‐s period of relaxation to allow CBFV to return to baseline before the onset of the next trial. The WG paradigm consisted of 23 trials in total. Letter presentation was randomized, and no letter was presented more than once to any given participant. The letters ‘Q’, ‘X’, and ‘Y’ were excluded due to their relatively uncommon occurrence in English. Verbally produced words were recorded by the experimenter, and the number of words per trial was calculated.
Figure 1.
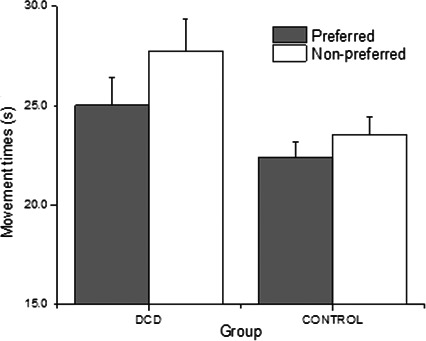
Bar chart showing the mean peg movement times for the preferred and non‐preferred hands across each group.
Motor skill handedness assessment
To determine a more accurate measure of hand skill and motor co‐ordination, and to serve as an additional confirmation of motor difficulties in the DCD group, the participants carried out an electronic version of the peg‐moving task described by Flowers and Hudson (2013). The dimensions of the board and peg movement procedure were identical; however, to improve timing accuracy, the board was constructed to allow detection of peg lifting and placing via an electrical circuit in the board. This was connected to the PC's parallel port, where a Visual Basic programme continuously monitored and recorded the times at which pegs were removed from or inserted into the holes.
Data analysis
Relative changes in CBFV within the left and right middle cerebral arteries (MCAs) were assessed using bilateral fTCD monitoring from a commercially available system (DWL Doppler‐Box™ X: manufacturer, DWL Compumedics Germany GmbH). A 2‐MHz transducer probe attached to an adjustable headset was positioned over each temporal acoustic window bilaterally. PsychoPy Software (Peirce, 2007) controlled the word‐generation experiment and sent marker pulses to the Doppler system to denote the onset of a trial. Data were analysed offline with a MATLAB (Mathworks Inc., Sherborn, MA, USA)‐based software package called dopOSCCI (see Badcock, Holt, Holden, & Bishop, 2012, for a detailed description). dopOSCCI makes a number of computations to summarize the fTCD data and advance the validity of measuring hemispheric differences in CBFV. First, the numbers of samples were reduced by downsampling the data from ~100 to 25 Hz. Second, variations in cardiac cycle which may contaminate task‐related signals were corrected using a cardiac cycle integration technique (Deppe, Knecht, Henningsen, & Ringelstein, 1997). Third, data contaminated by movement or ‘drift’ were removed prior to normalization. Normalized epochs were subsequently screened and excluded as measurement artefacts if activation values exceeded the acceptable range (±40% mean CBFV). Fourth, to control for physiological process that can influence CBFV (e.g., breathing rate, arousal, cardiac cycle), the mean activation of the baseline period was subtracted from each individual epoch. Deviations in left versus right activity were therefore baseline corrected and reflect relative changes in CBFV. A laterality index (LI) was derived for each participant based on the difference between left‐ and right‐sided activity within a 2‐s window, when compared to a baseline rest period of 10 s. The activation window was centralized to the time point at which the left–right deviation was greatest within the period of interest (POI). In the present paradigm, the POI ranged from 3 to 13 s following presentation of the stimulus letter (Bishop et al., 2009). Speech laterality was assumed to be clear in all cases in which the LI deviated by >2 SE from 0 (Knecht et al., 2001). Left‐hemisphere or right‐hemisphere speech dominance was indicated by positive or negative indices, respectively. Cases with an LI <2 SE from 0 were categorized as having bilateral speech representation.
Results
Diagnostic and behavioural assessments
Table 1 shows the characteristics of the two participant groups on the behavioural tests. As expected, the DCD group scored significantly higher than controls on the Adult Developmental Coordination Disorder (ADC) screening tool, t(22) = 10.08, p < .001, effect size reliability; d = .9. Notably, however, there were no significant differences between groups across the phonological processing assessments or the non‐verbal reasoning test. The groups were similarly matched for age and non‐verbal ability, and they did not differ significantly on handedness quotients as derived from the questionnaire; three of the DCD group and one of the control group had a handedness quotient at or below zero, denoting left‐handedness.
Table 1.
Mean (SD), t‐statistic, significance value, and effect size indicator for test scores across developmental coordination disorder (DCD) and control groups
| DCD group (N = 12) | Control group (N = 12) | Statistics | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t | p | d | |
| Age (years) | 25.4 (8.91) | 20 (2.66) | |||
| Handedness quotient | 50.8 (62.2) | 74.6 (46.3) | −1.101 | .283 | .43 |
| ADC score | 79.5 (17.1)a | 20 (8.24)a | 10.08a | .001a | .91 |
| Ravens shortened matrices score | 0.59 (0.27) | 0.58 (0.20) | 0.008 | .993 | .04 |
| YAA‐R subtests | |||||
| Spoonerisms correct | 0.84 (0.23) | 0.93 (0.14) | −1.042 | .309 | .47 |
| Spoonerisms rate | 0.30 (0.19) | 0.29 (0.16) | −0.057 | .955 | .05 |
| Object naming rate | 0.41 (0.30) | 0.56 (0.16) | −1.629 | .117 | .62 |
| Digit naming rate | 0.32 (0.29) | 0.51 (0.24) | −1.878 | .074 | .71 |
Significant difference at p < .001.
Motor skill task
As expected, the DCD group displayed slower mean peg movement times across both hands on the motor skill assessment (Figure 1) Interestingly, this difference between groups resulted specifically from the performance of the non‐preferred hand, t(22) = 2.270, p < .05, d = .92; DCD group mean = 27.7 s, SD = 5.65; control group mean = 23.5 s, SD = 3.11. The effect size for this analysis was shown to be reliable (d = .42). The between‐group difference in the performance of the preferred hand was not significant, t(22) = 1.59, p = .063; DCD group mean = 25.03 s, SD = 4.97; control group mean = 22.43 s, SD = 2.67).
In addition, faster performance on the pegboard task (lower mean movement times) was correlated with higher scores in some of the language assessment components of the YAA. Table 2 shows a breakdown of these correlations.
Table 2.
Pearson's correlations for the Pegboard motor skill task performance and language assessments across the whole sample (N = 24)
| Preferred hand performance | Non‐preferred hand performance | |||
|---|---|---|---|---|
| r | p | r | p | |
| Spoonerisms rate | −.34 | .10 | −.29 | .16 |
| Object naming rate | −.71 | .001a | −.65 | .001a |
| Digit naming rate | −.75 | .001a | −.69 | .001a |
| Mean no. words reported during functional transcranial Doppler paradigm | −.41 | .05a | −.46 | .03a |
Significant correlation.
Speech lateralization
Figure 2 shows a scatter plot of the laterality indices (LI) for the word‐generation paradigm for the DCD and control groups. There was a significant difference, t(22) = −2.2, p < .05, between the groups indicating that the DCD group (Mean LI = 1.89, SD = 2.58) shows less left‐hemisphere lateralization during speech production than controls (Mean LI = 3.77, SD = 1.42). A reliable effect size was found to support this result (d = −.41). This confirms the hypothesis that reduced leftwards lateralization would be seen in the DCD group. In further scrutinizing the spread of mean LI scores (Figure 2), it was observed that one left‐handed participant in the DCD group was more strongly right‐hemisphere lateralized than the remainder of the participants. Although this individual is not statistically an outlier, to check the possibility of this data point driving the interaction, we temporarily removed it from the sample and re‐ran the analysis. Even without this participant, there was a significant difference between the LI scores of the DCD group and controls, t(21) = −1.94, p = .03, one‐tailed, d = .80, confirming the hypothesis that the DCD group (Mean LI = 2.44, SD = 1.87) would be significantly less left‐hemisphere lateralized than controls (Mean LI = 3.77, SD = 1.42).
Figure 2.
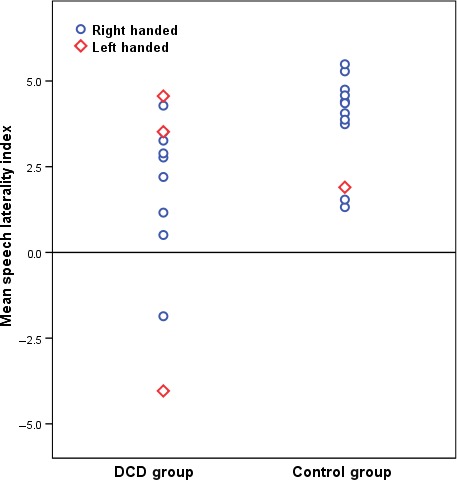
Plot showing distribution of mean speech laterality indices for developmental coordination disorder (DCD) and control groups in the word‐generation task. Negative numbers indicate right‐hemisphere lateralization, and positive numbers indicate left‐hemisphere lateralization.
To ensure high internal reliability of the word‐generation LI scores computed for both groups, split‐half reliability for word‐generation LIs was computed from Pearson correlations for the LIs from odd and even epochs. For the group as a whole, r = .66, p = .001, and specifically for the 12 individuals in the DCD group, r = .79, p = .002. It is clear that the reduced lateralization in the DCD group is not the consequence of unreliability of the LI estimate.
To assess whether the range of ages in the sample contributed to the difference seen in LI score, Pearson correlations of age in years and LI score were conducted across the sample as a whole and also separately for each participant group. None of these correlations were statistically significant: whole sample, r(24) = −.19, p = .38; DCD group, r(12) = .01, p = .97; control group, r(12) = .14, p = .65.
One possibility is that the reduced leftwards lateralization in the DCD group could simply reflect poor ability on the word‐generation task. If the patients are less able to generate words, then they may not engage left‐hemisphere systems as strongly (Illingworth & Bishop, 2009). There was a significant difference between groups in the mean number of words generated per letter: for controls, M = 4.2, SD = 0.66; for DCD patients, M = 3.5, SD = 0.48, t(22) = −3.204, p < .005; with a reliable effect size (d = .51).
Finally, handedness preference as measured by the questionnaire did not correlate significantly with LI score, r(24) = .209, p = .33, and similarly, performance on the motor skill pegboard task also did not significantly correlate with LI score, r(24) = .163, p = .45. This may be due to small sample size reducing power in this instance; however, the relationship between handedness and cerebral language lateralization is considered to be weak and indirect, with inconsistencies in performance and inventory‐based measures being reported in the literature (Groen, Whitehouse, Badcock, & Bishop, 2013).
Discussion
Previous research (Flowers & Hudson, 2013; Illingworth & Bishop, 2009; Whitehouse & Bishop, 2008) suggests that individuals with neurodevelopmental disorders affecting language and/or motor systems may show atypical hemispheric lateralization patterns during speech production due to the common neural systems underpinning both functions. The aim of this study was to assess hemispheric speech lateralization patterns in patients with motor coordination impairments, but with no known speech or language deficits. This was the first study of its kind to employ fTCD to assess speech lateralization in patients with DCD, and the results supported the hypothesis that a reduced leftwards asymmetry would be observed in the DCD group.
One explanation for the link between the hemispheric control of speech and motor systems is that both functions employ sequencing components which are supported by the same neural network, located in the left hemisphere. Haarland, Elsinger, Mayer, Durgerian, and Rao (2004) demonstrated that control of motor actions involving complex sequences are lateralized to left pre‐motor and parietal areas, regardless of the hand used or the handedness of the participants. These regions have been shown to overlap with classic left‐hemisphere speech production areas (e.g., brodmann areas 44 and 46, see Binkofski & Buccino, 2004) meaning that the two tasks in this study may be relying upon the same region in the left hemisphere for their effective execution. Therefore, we suggest that underlying DCD is impairment in motor sequencing, which not only affects the motor coordination abilities, but also the organization of networks controlling speech production. Previous findings showing impaired motor timing and sequencing (unrelated to speech production) in DCD groups provide support for this view (Debrabant, Gheysen, Caeyenberghs, Van Waelvelde, & Vingerhoets, 2013). In support of this explanation is our finding that motor performance is reduced in the DCD group specifically in the non‐preferred hand. Left‐hemisphere control of the non‐preferred hand (usually the left hand) has been demonstrated in previous studies during motor sequencing tasks (Serrien, Ivry, & Swinnen, 2006). The slower non‐preferred hand performance in the DCD group may demonstrate a reduction in the strength of this ipsilateral pathway for complex motor action, to the extent that speech production processes relying on similar networks become atypically organized as well.
It is possible that the difference in laterality scores between the DCD group and the control group could be down to the variances in age within the samples. Previous literature suggests that hemispheric lateralization of speech shifts during development and that younger adults therefore may show a more bilateral speech representation (Holland et al., 2001). However, that view is not supported by this data, as age did not significantly correlate with LI score. This finding is in line with previous fTCD work which shows no difference in laterality scores between children and adults (Groen, Whitehouse, Badcock, & Bishop, 2012; Lohmann, Drager, Muller‐Ehrenberg, Deppe, & Knecht, 2005).
An aspect of this data that needs addressing, which may explain the difference in speech laterality indices found between the groups, is the differences in mean words produced by each group during the speech task. Crucially, this sample of DCD patients displays significantly different patterns of lateralization than controls and yet do not differ significantly from them on tests for phonological and verbal processing or in their non‐verbal cognitive function ability. Therefore, the difference in word production rate could be the result of reduced task engagement by the patient group, thus resulting in a poor representation of speech lateralization.
It is worth considering although why the DCD group reported significantly fewer words, as it may provide insight into the nature of impairments in DCD and why this might impact on speech networks. One possibility is that the specific demands of the word‐generation task were increased for the DCD group, particularly in systems responsible for working memory and executive function, areas shown previously to be impaired in individuals with DCD (Alloway & Archibald, 2008; Pratt, Leonard, Adeyinka, & Hill, 2014). These studies suggest that the motor impairments shown in DCD result from motor plans not being accurately held ‘online’ during the execution of a motor action, thus affecting the efficiency and accuracy with which the eventual motor response is made. The component of the word‐generation task which requires subjects to recall the words they were thinking of occurs after the letter stimulus has disappeared from the screen and so relies heavily on such working memory functions. However, if this task really implicated working memory functions to such an extent, then previous findings (Zwicker, Missiuna, Harris, & Boyd, 2011) would indicate that increases in right‐hemisphere dorso‐lateral prefrontal cortex activation would be associated with a more efficient working memory system, not a poorer one. This perspective would also suggest the DCD participants found the word‐generation task harder than controls, and the reduced leftward activation found could be due to a general slowed processing in this group. Cognitive control systems in speech production tasks have been shown to result in increased right‐hemisphere activation in patient groups compared to healthy controls (Brownsett et al., 2014; Hodgson, Benattayallah, & Hodgson, 2014); however, as no previous imaging research has been conducted into speech production and DCD, it is difficult to extend this finding to our data.
However, whilst it remains a possibility that reduced left‐hemisphere activation is indicative of increased cognitive control processes, the performance data from the motor skill task give support to the idea that it is complex motor actions which have parallels with speech production processes that are organized atypically, thus producing the differences in laterality profiles seen between the groups.
Potential limitations of this study are that it uses a relatively small sample; therefore, this makes it harder to detect specific differences in performance on the motor task and how these may relate to direction of speech lateralization. Furthermore, the data are limited by the lack of participant information on environmental factors such as socio‐economic status and lifestyle, which may impact upon the group differences seen. This study provides a good first step into exploring speech lateralization in DCD, but more extensive studies should now be conducted with larger samples and a cross‐section of differing severity of motor‐impaired individuals.
Conclusions
These data demonstrate that individuals with DCD also present with reduced left hemispheric dominance for speech production despite no behavioural deficits in that function. It is suggested that the two functions involve complex sequencing of movements which use similar neural systems, previously shown to lateralize to the left hemisphere. These results support the perspective that when atypical cerebral lateralization occurs due to developmental impairment in either speech or motor control, this reorganization extends to the related function, but does not disrupt it enough to impact upon the behavioural presentation of that related function. These data have implications for clinical practice as it demonstrates the sensitivity of fTCD to detect neurological differences between populations that are not evident through behavioural testing. This has potential application in the assessment of likely co‐morbidities in individuals with developmental speech and motor impairments, but also extends our knowledge of the impact of neurodevelopmental disorders on brain organization and development.
References
- Alloway, T. , & Archibald, L. (2008). Working memory and learning in children with developmental coordination disorder and specific language impairment. Journal of Learning Disabilities, 41, 251–262. doi:10.1177/0022219408315815 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Badcock, N. A. , Holt, G. , Holden, A. , & Bishop, D. V. (2012). dopOSCCI: A functional transcranial Doppler ultrasonography summary suite for the assessment of cerebral lateralization of cognitive function. Journal of Neuroscience Methods, 204, 383–388. doi:10.1016/j.jneumeth.2011.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, R. R. , FitzGerald, D. , LeSueur, L. , Kennedy, D. , Kwong, K. , Buchbinder, B. , … Logan, W. (1999). Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology, 52, 798. doi:10.1212/WNL.52.4.798 [DOI] [PubMed] [Google Scholar]
- Bilker, W. , Hansen, J. , Brensinger, C. , Richard, J. , Gur, R. E. , & Gur, R. C. (2012). Development of abbreviated nine‐item forms of the Raven's standard progressive matrices test. Assessment, 19, 354–369. doi:10.1177/1073191112446655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski, F. , & Buccino, G. (2004). Motor functions of the Broca's region. Brain and Language, 89, 362–369. doi:10.1016/S0093‐934X(03)00358‐4 [DOI] [PubMed] [Google Scholar]
- Bishop, D. , Watt, H. , & Papadatou‐Pastou, M. (2009). An efficient and reliable method for measuring cerebral lateralization during speech with functional transcranial Doppler ultrasound. Neuropsychologia, 47, 587–590. doi:10.1016/j.neuropsychologia.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsett, S. , Warren, J. , Geranmayeh, F. , Woodhead, Z. , Leech, R. , & Wise, R. (2014). Cognitive control and its impact on recovery from aphasic stroke. Brain, 137, 242–254. doi:10.1093/brain/awt289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish, K. , & McManus, C. (1996). Hand preference and hand skill in children with Autism. Journal of Autism and Developmental Disorders, 26, 597–609. doi:10.1007/BF02172349 [DOI] [PubMed] [Google Scholar]
- Debrabant, J. , Gheysen, F. , Caeyenberghs, K. , Van Waelvelde, H. , & Vingerhoets, G. (2013). Neural underpinnings of impaired predictive motor timing in children with developmental coordination disorder. Research in Developmental Disabilities, 34, 1478–1487. doi:10.1016/j.ridd.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Deppe, M. , Knecht, S. , Henningsen, H. , & Ringelstein, E. (1997). AVERAGE: A windows® program for automated analysis of event related cerebral blood flow. Journal of Neuroscience Methods, 75(2), 147–154. [DOI] [PubMed] [Google Scholar]
- Eglinton, E. , & Annett, M. (1994). Handedness and Dyslexia: A meta analysis. Perceptual and Motor Skills, 79, 1611–1616. doi:10.2466/pms.1994.79.3f.1611 [DOI] [PubMed] [Google Scholar]
- Flowers, K. , & Hudson, J. (2013). Motor laterality as an indicator of speech laterality. Neuropsychology, 27, 256–265. doi:10.1037/a0031664 [DOI] [PubMed] [Google Scholar]
- Gillberg, C. (2003). deficits in attention, motor control and perception: A brief review. Archives of Disease in Childhood, 88, 904–910. doi:10.1136/adc.88.10.904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg, G. (2013). Apraxia: The cognitive side of motor control. Oxford, UK: Oxford University Press. [DOI] [PubMed] [Google Scholar]
- Groen, M. , Whitehouse, A. , Badcock, N. , & Bishop, D. (2012). Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain and Behavior, 2, 256–269. doi:10.1002/brb3.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen, M. A. , Whitehouse, A. J. O. , Badcock, N. A. , & Bishop, D. V. M. (2013). Associations between handedness and cerebral lateralisation for language: A comparison of three measures in children. PLoS One, 8(5), e64876. doi:10.1371/journal.pone.0064876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarland, K. , Elsinger, C. , Mayer, A. , Durgerian, S. , & Rao, S. (2004). Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. Journal of Cognitive Neuroscience, 16, 621–636. doi:10.1162/089892904323057344 [DOI] [PubMed] [Google Scholar]
- Hill, E. L. (2001). Non‐specific nature of specific language impairment: A review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders, 36, 149–171. doi:10.1080/13682820010019874 [DOI] [PubMed] [Google Scholar]
- Hodgson, J. C. , Benattayallah, A. , & Hodgson, T. L. (2014). The role of the dominant versus the non‐dominant hemisphere; An fMRI study of Aphasia recovery following stroke. Aphasiology, 28, 1426–1447. doi:10.1080/02687038.2014.933640 [Google Scholar]
- Holland, S. , Plante, E. , Weber Byars, A. , Strawsburg, R. H. , Schmithorst, V. J. , & Ball, Jr, W. S. (2001). Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage, 14, 837–843. doi:10.1006/nimg.2001.0875 [DOI] [PubMed] [Google Scholar]
- Illingworth, S. , & Bishop, D. (2009). Atypical cerebral lateralisation in adults with compensated developmental dyslexia demonstrated using functional transcranial Doppler ultrasound. Brain and Language, 11, 61–65. doi:10.1016/j.bandl.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson, J. (2010). Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language, 37, 229–261. doi:10.1017/S0305000909990432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby, A. , Edwards, L. , Sugden, D. , & Rosenblum, S. (2010). The development and standardization of the Adult Developmental Co‐ordination Disorders/Dyspraxia Checklist (ADC). Research in Developmental Disabilities, 31, 131–139. doi:10.1016/j.ridd.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Deppe, M. , Dräger, B. , Bobe, L. , Lohmann, H. , Ringelstein, E. , & Henningsen, H. (2000). Language lateralization in healthy right‐handers. Brain, 123, 74–81. doi:10.1093/brain/123.1.74 [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Deppe, M. , Ebner, A. , Henningsen, H. , Huber, T. , Jokeit, H. , & Ringelstein, E. (1998). Noninvasive determination of language lateralization by functional transcranial Doppler sonography: A comparison with the WADA test. Stroke, 29(1), 82–86. doi:10.1161/01.STR.29.1.82 [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Dräger, B. , Flöel, A. , Lohmann, H. , Breitenstein, C. , Deppe, M. , … Ringelstein, E. (2001). Behavioural relevance of atypical language lateralization in healthy subjects. Brain, 124, 1657–1665. doi:10.1093/brain/124.8.1657 [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Henningsen, H. , Deppe, M. , Huber, T. , Ebner, A. , & Ringelstein, E. (1996). Successive activation of both cerebral hemispheres during cued word generation. NeuroReport, 7, 820–824. doi:10.1097/00001756‐199602290‐00033 [DOI] [PubMed] [Google Scholar]
- Lohmann, H. , Drager, B. , Muller‐Ehrenberg, S. , Deppe, M. , & Knecht, S. (2005). Language lateralization in young children assessed by functional transcranial Doppler sonography. NeuroImage, 24, 780–790. doi:10.1016/j.neuroimage.2004.08.053 [DOI] [PubMed] [Google Scholar]
- Peirce, J. W. (2007). PsychoPy – Psychophysics software in python. Journal of Neuroscience Methods, 162(1), 8–13. doi:10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt, M. , Leonard, H. , Adeyinka, H. , & Hill, E. L. (2014). The effect of motor load on planning and inhibition in developmental coordination disorder. Research in Developmental Disabilities, 35, 1579–1587. doi:10.1016/j.ridd.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Raven, J. , Raven, J. C. , & Court, J. H. (2000). Standard progressive matrices. Oxford, UK: Psychology Press. [Google Scholar]
- Redle, E. , Vannest, J. , Maloney, T. , Tsevat, R. , Eikenberry, S. , Lewis, B. , … Holland, S. (2014). Functional MRI evidence for fine motor praxis dysfunction in children with persistent speech disorders. Brain Research, 1597, 47–56. doi:10.1016/j.brainres.2014.11.047 0006‐8993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien, D. , Ivry, R. , & Swinnen, S. (2006). Dynamics of hemispheric specialization and integration in the context of motor control. Nature Reviews Neuroscience, 7, 160–166. doi:10.1038/nrn1849 [DOI] [PubMed] [Google Scholar]
- Somers, M. , Neggers, S. , Diederen, K. , Boks, M. , Kahn, R. , & Sommer, I. (2011). The measurement of language lateralization with functional transcranial Doppler and functional MRI: A critical evaluation. Frontiers in Human Neuroscience, 5(31), 1–8. doi:10.3389/fnhum.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden, D. (2007). Current approaches to intervention in children with developmental coordination disorder. Developmental Medicine and Child Neurology, 49, 467–471. doi:10.1111/j.1469‐8749.2007.00467.x [DOI] [PubMed] [Google Scholar]
- Visscher, C. , Houwen, S. , Scherder, E. , Moolenaar, B. , & Hartman, E. (2007). Motor profile of children with developmental speech and language disorders. Pediatrics, 120, e158–e163. doi:10.1542/peds.2006‐2462 [DOI] [PubMed] [Google Scholar]
- Warmington, M. , Stothard, S. E. , & Snowling, M. J. (2012). Assessing dyslexia in higher education: The York adult assessment battery‐revised. Journal of Research in Special Educational Needs, 13, 48–56. doi:10.1111/j.1471‐3802.2012.01264.x [Google Scholar]
- Whitehouse, A. , & Bishop, D. (2008). Cerebral dominance for language function in adults with specific language impairment or autism. Brain, 131, 3193–3200. doi:10.1093/brain/awn266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker, J. , Missiuna, C. , Harris, S. , & Boyd, L. (2011). Brain activation associated with motor skill practice in children with developmental coordination disorder; an fMRI study. International Journal of Developmental Neuroscience, 29, 145–152. doi:10.1016/j.ijdevneu.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Zwicker, J. , Missiuna, C. , Harris, S. , & Boyd, L. (2012). Developmental coordination disorder: A review and update. European Journal of Paediatric Neurology, 12, 573–581. doi:10.1016/j.ejpn.2012.05.005 [DOI] [PubMed] [Google Scholar]


