Abstract
Objectives
The aim of this study was to examine the effect of basic fibroblast growth factor (FGF‐2) on osseointegration of dental implants with low primary stability in a beagle dog model.
Materials and methods
Customized titanium implants that were designed to have low contact with the existing bone were installed into the edentulous mandible of beagle dogs. To degrade the primary stability of the implants, the diameters of the bone sockets exceeded the implant diameters. FGF‐2 (0.3%) plus vehicle (hydroxypropyl cellulose) or vehicle alone was topically applied to the sockets in the FGF‐2 and control groups, respectively. In Study 1, the new bone area and length of new bone‐to‐implant contact (BIC) were evaluated at 4, 8, and 12 weeks after installation using histomorphometry and scanning electron microscopy. In Study 2, the implant stability quotient (ISQ) values were sequentially measured for 16 weeks using an Osstell system.
Results
The histomorphometric analysis revealed that the new bone area and length of BIC in the FGF‐2 group were significantly larger than those in the control group at 4 weeks. Electron microscopic observation showed intimate contact between the mature lamellar bone and the implant surfaces, osseointegration, in both groups. The ISQ values in the FGF‐2 group were significantly increased from 6 to 16 weeks compared with those in the control group.
Conclusions
Taken together, our study demonstrates that FGF‐2 promoted new bone formation around the dental implants and subsequent osseointegration, resulting in promotion of stability of implants with low primary stability.
Keywords: beagle dog, dental implant, fibroblast growth factor‐2, osseointegration, resonance frequency analysis
Implant stability, which consists of primary and secondary stability, is a fundamental prerequisite for successful dental implantations. Primary stability mostly comes from mechanical engagement between the implant surface and the existing cortical bone, while secondary stability arises from not only direct structural connection, but also functional connection between the bone and the implant that is procured by bone regeneration and remodeling (osseointegration) (Branemark et al. 1985; Brunski 1992; Sennerby & Roos 1998; Raghavendra et al. 2005). After installation of an implant, the primary stability is gradually decreased by postoperative bone resorption, while the secondary stability is increased by osseointegration with bone formation (Raghavendra et al. 2005). Thus, the total stability level of the implant is maintained, as long as the primary stability is normally supplemented and/or replaced by the secondary stability (Mall et al. 2011).
However, in cases with poor bone structure, biomechanical overloading, and bone resorption at the interface, the primary stability is insufficient because of gaps between the implant and the bone. As a consequence, the osseointegration process is disturbed and fibrous tissue forms around the implant, resulting in an unstable implant and subsequent clinical failure (Meredith 1998).
To avoid clinical failure induced by low primary stability, a variety of implants with osteoconductive surfaces or scaffolds have been used to support the acquisition of osseointegration. However, even these materials were occasionally unable to promote sufficient bone formation (Salgado et al. 2004). Therefore, we supposed that application of a drug with osteogenic effects would be useful to promote the acquisition of osseointegration and increase the success rate of dental implants.
Basic fibroblast growth factor (FGF‐2) is known to have strong bone‐forming ability. Administration of FGF‐2 at sites of fibula fractures significantly increased the callus bone mineral content, breaking strength, and breaking energy in both healthy rats and streptozotocin‐induced diabetic rats (Kawaguchi et al. 1994). Similarly, locally applied FGF‐2 was reported to promote callus formation in rabbits and dogs with tibia fractures (Kato et al. 1998; Nakamura et al. 1998; Chen et al. 2004). In the dental field, we demonstrated that FGF‐2 enhanced regeneration of the alveolar bone, cementum, and periodontal ligament in artificial periodontal defect models in beagle dogs (Murakami et al. 1999, 2003) and non‐human primates (Takayama et al. 2001). We then conducted clinical trials of 2‐ and 3‐wall vertical bone defects in patients with periodontitis and demonstrated that an FGF‐2 plus hydroxypropyl cellulose (FGF‐2 HPC) formulation showed significant superiority over vehicle alone in terms of the percentage of bone filling in modified Widman periodontal surgery (Kitamura et al. 2008, 2011). Therefore, we considered that application of the FGF‐2 HPC formulation would be efficacious for acquisition of osseointegration even for implants with insufficient primary stability.
The aim of this study was to examine the effect of the FGF‐2 HPC formulation on the osseointegration and stability of implants in a canine low primary stability model by histological analysis and resonance frequency analysis. As a consequence, we revealed that FGF‐2 promoted the acquisition of osseointegration and enhanced the stability of implants with low primary stability.
Material and methods
Preparation of test substances
An FGF‐2 HPC formulation containing 0.3% FGF‐2 was prepared by dissolving freeze‐dried human recombinant FGF‐2 (Kaken Pharmaceutical Co. Ltd., Tokyo, Japan) in 3% HPC solution. The 3% HPC solution alone was used as the vehicle. Either vehicle alone or 0.3% FGF‐2 solution was administered to the control and FGF‐2 groups, respectively.
Animals
Beagle dogs were obtained from Ridglan Farms Inc. (Mt. Horeb, WI) and Kitayama Labes Co. Ltd. (Nagano, Japan). Seven male beagle dogs (age: 37 months; weight: 8.8–13.8 kg) for Study 1 and six female dogs (age: 78–87 months; weight: 11.9–17.0 kg) for Study 2 were used. The dogs were housed individually and allowed to move freely in stainless steel cages under conditions of 18–26°C temperature, 30–70% humidity, and 12‐h lighting (07:00–19:00). All animals were provided with 230 g of solid food (LABO D STOCK; Nihon Nosan Kogyo Co., Yokohama, Japan) per day and filtered tap water ad libitum. This study was approved by the Animal Experiments Ethics Committee of Kaken Pharmaceutical Co. Ltd. and the Ethical Review Board of Dentistry, Osaka University. Kaken Pharmaceutical Co. Ltd. follows in‐house regulations that comply with Japan's Act on Welfare and Management of Animals as well as international and domestic guidelines. In addition, Kaken Pharmaceutical Co. Ltd. has been certified as a qualified institution for Laboratory Animal Care and Use by The Japan Health Sciences Foundation (Tokyo, Japan).
Implantation procedure
The purpose of Study 1 was to examine the effect of FGF‐2 on the bone‐forming ability around the implants at 4, 8, and 12 weeks after installation. To reduce contact with the existing bone and quantify the amount of bone formation, customized titanium implants with measurement regions were prepared (Fig. 1a; diameter: 3.2 mm; length: 6 mm; Kyocera Medical Corporation, Osaka, Japan). After the dogs were anesthetized subcutaneously with xylazine (20 mg/dog; Celactal; Bayer Yakuhin Ltd., Tokyo, Japan), intravenously with pentobarbital (10 mg/kg), and intragingivally with 2% lidocaine with 0.00125% adrenaline (Xylocaine; Dentsply‐Sankin K.K., Tokyo, Japan), the third and fourth premolars on both sides in the mandible were removed. After the wounds had healed, a mucoperiosteal flap was raised, and two bone sockets (diameter: 3.2 mm; depth: 6 mm) on each side were surgically prepared using a twist drill (Implantor Neo BRIGHT; Kyocera Medical Corporation, Osaka, Japan) under anesthesia as described above. To degrade the primary stability of the implants, the diameters of the sockets were spread to 3.7 mm with a depth of 4 mm using a taper drill (Fig. 1b). Four implants per animal were installed after the test solutions (20 μl/site) were administered into the sockets (Fig. 1c). In each dog, the 0.3% FGF‐2 solution was administered into the two sockets on one side and the vehicle solution was administered into the two sockets on the other side. The gingival flaps were sutured, and penicillin (40,000 U/dog) and streptomycin (200 mg/dog) were administered subcutaneously to prevent infection after surgery. The animals were given about 400 g of soft food (Pedigree; Mars Japan Ltd., Tokyo, Japan) per day during the first 7 postoperative days.
Figure 1.
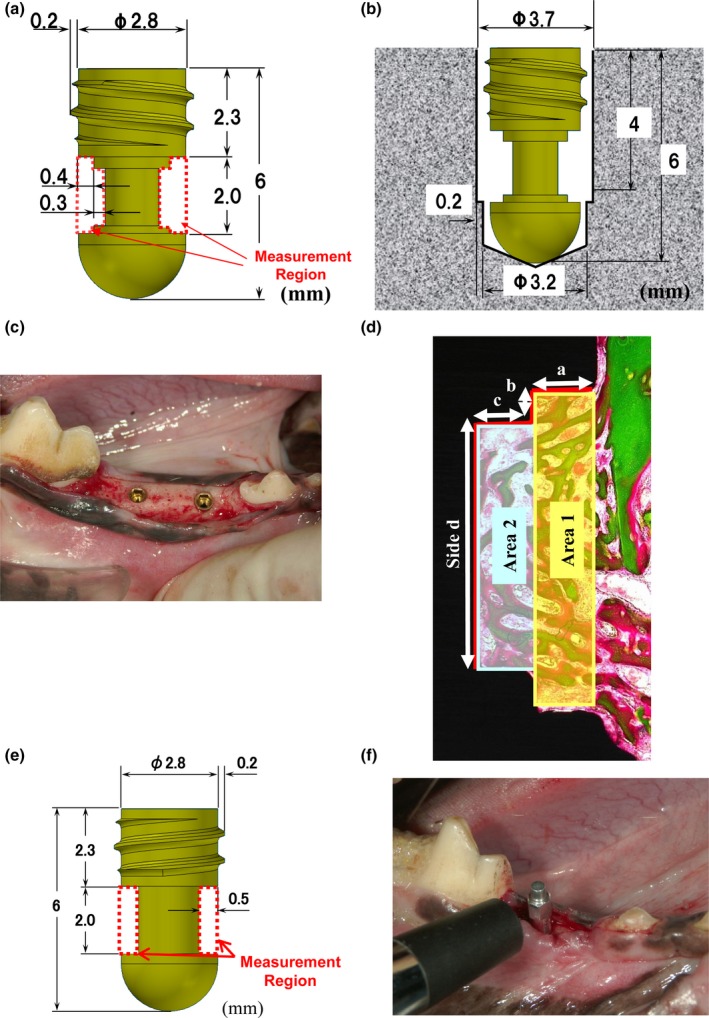
Schematic drawings of the customized implants, postoperative views, and methods for histomorphometric analysis. (a) Design, size, and measurement region of the customized titanium implant in Study 1. (b) Schematic drawing of the installed implant. (c) Postoperative view of the installed titanium implants in Study 1. (d) Schematic drawing of the measurement regions for the histomorphometric analysis in Study 1. (e) Design, size, and measurement region of the customized implant in Study 2. (f) Customized transducer attached to the installed implant for measurement of the ISQ value in Study 2.
Histological analysis
In Study 1, three dogs each were euthanized by exsanguination under general anesthesia at 4 and 8 weeks after installation. Tissues containing the implants (n = 6 per group at each time point) were extracted and fixed with neutral‐buffered 10% formalin. Non‐decalcified histological sections were prepared by grinding after the tissues were embedded with resin, and mesiodistal sections of the specimens were stained with Villanueva Goldner stain. The new bone area and length of new bone‐to‐implant contact (BIC) in the measurement regions (Fig. 1a) were determined with WinRoof image analysis software (ver.5.03; Mitani Co., Tokyo, Japan). To examine the relationship between bone formation and distance from the existing bone, the new bone area was measured in two regions, comprising an area near the existing bone (Area 1) and an area further from the existing bone (Area 2), and the length of BIC was measured on each side (Side a, Side b, Side c, Side d) (Fig. 1d). The individual values of each implant were calculated as the means in two measurement regions because of mesiodistal sections of the specimens (Fig. 1a). The new bone area and length of BIC were shown as percentages calculated as follows: new bone area/area of measurement region × 100 or length of BIC/entire length of each side × 100.
Electron microscopic observations
Under general anesthesia, one dog was euthanized by exsanguination at 12 weeks in Study 1. Mesiodistal sections of the specimens were prepared as described above. The sections were coated with platinum using a plasma multicoater (PMC‐5000; Meiwafosis Co. Ltd., Tokyo, Japan) and observed under a scanning electron microscope (JSM‐6390LV; JEOL Ltd., Tokyo, Japan).
Implant stability measurements
The purpose of Study 2 was to determine whether FGF‐2 enhanced the stability of the implants. The total implant stability was evaluated by the implant stability quotient (ISQ) value derived from the resonance frequency given by an Osstell system (Osstell AB, Gothenburg, Sweden). Six beagle dogs were implanted with customized titanium implants that allowed measurements by the Osstell system (Fig. 1e, f). The implantation was performed as described above with the exception of the bone socket number. In Study 2, only the fourth premolars on both sides were removed and one bone socket on each side was created (Fig. 1b). The ISQ values were recorded at the time of implant placement (0 weeks) and at 4, 6, 8, 12, and 16 weeks after installation by attaching a customized transducer (Kyocera Medical Corporation, Osaka, Japan) to the implant (Fig. 1f). The ISQ values at 0 weeks in both groups were confirmed to be 60 or less, because ISQ values of 60 or less are considered to reflect low stability (Rodrigo et al. 2010). To estimate the relationship between the ISQ value and the histology, the tissues containing the implants (n = 6 per group at each time point) were extracted at 16 weeks and the sections were stained with Villanueva Goldner stain as described above. The final data were shown as the percentages of new bone area and length of BIC in the entire measurement region with WinRoof image analysis software.
Statistical analysis
The mean, standard deviation (SD) and median were calculated for each measurement, and the differences between treatments were analyzed by a Wilcoxon signed‐rank test. The level of significance was set at 5%. All statistical analyses were performed using PASW Statistics 18 (ver. 18.0.0; IBM Japan Services Company Ltd., Tokyo, Japan).
Results
Histomorphometric analysis of new bone formation (Study 1)
Histologic images of the measurement regions at 4 and 8 weeks are shown in Fig. 2a–d. Villanueva Goldner staining distinguishes between uncalcified osteoid (red) and calcified bone (green). In the control group, new bone including osteoid and calcified bone was formed in the measurement region at 4 weeks, and the trabeculae of the new bone were thin and immature (Fig. 2a). From 4 to 8 weeks, the area of new bone expanded, the thickness of the new bone trabeculae increased, and the new bone matured into calcified bone at 8 weeks (Fig. 2c). In the FGF‐2 group, the maturity of the new bone was similar to that in the control group at 4 and 8 weeks (Fig. 2b, d). The amount of new bone in the FGF‐2 group was larger than that in the control group at each time point.
Figure 2.
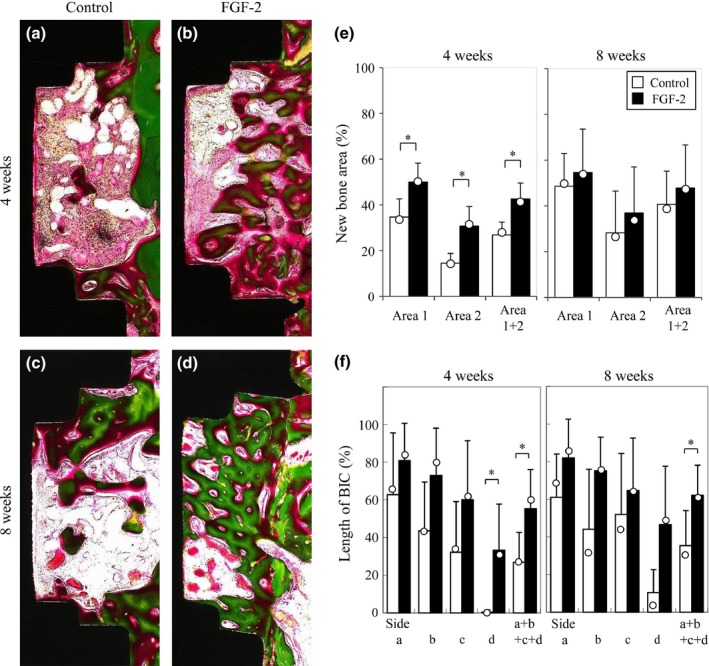
Histomorphometric analysis of new bone formation in Study 1. (a–d) Representative histologic overview of the measurement region of the implants. Specimens were harvested 4 (a, b) and 8 (c, d) weeks after installation in the control (a, c) and FGF‐2 (b, d) groups. Green, calcified bone; red, osteoid (Villanueva Goldner stain). (e) Histomorphometric analysis of the rate of new bone area in the measurement regions. The area of new bone was measured in two areas, comprising an area near the existing bone side (Area 1) and an area further from the existing bone (Area 2). The data are shown as percentages calculated as new bone area/area of measurement region × 100. The results are presented as means (column) ± SD (bar) and median (open circle). (n = 6; *P < 0.05, vs. the control group by a Wilcoxon signed‐rank test). (f) Histomorphometric analysis of the rate of contact between the new bone and the implant in the measurement regions. The length of BIC was measured on each side (Side a, Side b, Side c, and Side d). The data are shown as percentages calculated as length of BIC/entire length of each side × 100. The results are presented as means (column) ± SD (bar) and median (open circle) (n = 6; *P < 0.05, vs. the control group by a Wilcoxon signed‐rank test).
To ascertain the bone‐forming ability of FGF‐2, we first evaluated the area of new bone including osteoid and calcified bone in the measurement regions (Fig. 2e). The new bone area in Area 2 was smaller than that in Area 1 at 4 and 8 weeks in the control group, indicating that bone formation was delayed in the area further from the existing bone. In the FGF‐2 group, the new bone area was significantly larger than that in the control group in both Area 1 and Area 2 at 4 weeks. In particular, in the region further from the existing bone (Area 2), there was a large difference between the FGF‐2 group and the control group. These data indicated that FGF‐2 was effective for bone formation around the implants where bone formation was delayed. From 4 to 8 weeks, the new bone area in the FGF‐2 group did not change dramatically. The new bone area in the FGF‐2 group at 4 weeks was similar to that in the control group at 8 weeks.
To examine the effect of FGF‐2 on osseointegration, we investigated the lengths of BIC in the measurement regions (Fig. 2f). The lengths of BIC were in the order of Side a > Side b > Side c > Side d at 4 weeks in the control group. The length of BIC on Side d was very low even at 8 weeks. These results suggested that the contact appeared to proceed from the side near the existing bone (Side a) to the far side (Side d) and that the far side was slow to acquire contact. In the FGF‐2 group, the total contact length (Side a + Side b + Side c + Side d) was significantly greater than that in the control group at 4 and 8 weeks. In particular, the length on Side d in the FGF‐2 group was higher than that in the control group.
Electron microscopic observation (Study 1)
The contact conditions between the new bone and the implants were observed at 12 weeks after installation (Fig. 3). Osseous matrix adhered to the implant surfaces, and no connective tissue between the bone and the implant surfaces was observed on any side in both groups. Therefore, successful osseointegration was visualized at the ultrastructural level.
Figure 3.
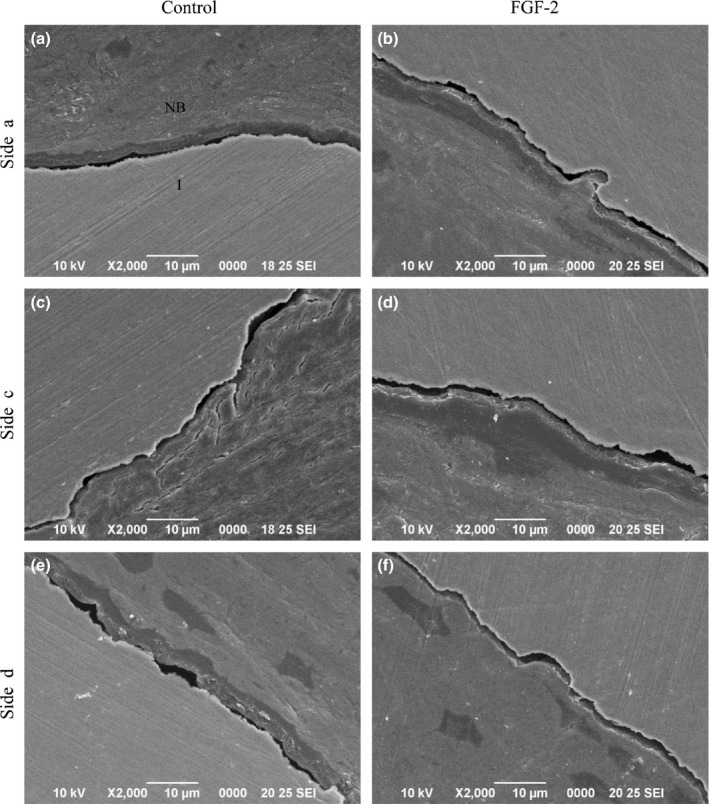
Electron microscopic observation in Study 1. (a–f) Electron microscopic photomicrographs of BIC in the control (a, c, e) and FGF‐2 (b, d, f) groups at 12 weeks. NB, new bone; I, implant. Bars: 10 μm.
Implant stability measurements (Study 2)
To examine the effect of FGF‐2 on implant stability, we evaluated the ISQ values derived from the resonance frequencies (Fig. 4a). The ISQ values of around 50 at 0 weeks (low implant stability) increased to 80 at 4 weeks in both groups. From 4 to 8 weeks, the ISQ values in both groups gradually increased. The ISQ values in the FGF‐2 group were significantly higher than those in the control group at 6 and 8 weeks. The ISQ values in both groups barely changed after 8 weeks, and the difference between the two groups was maintained. At 16 weeks, the new bone area in the entire measurement regions in the FGF‐2 group was higher compared with those in the control group (Fig. 4b). Interestingly, the length of BIC in the entire measurement regions in the FGF‐2 group was significantly higher compared with those in the control group (Fig. 4c). Thus, FGF‐2 was considered to enhance the implant stability.
Figure 4.
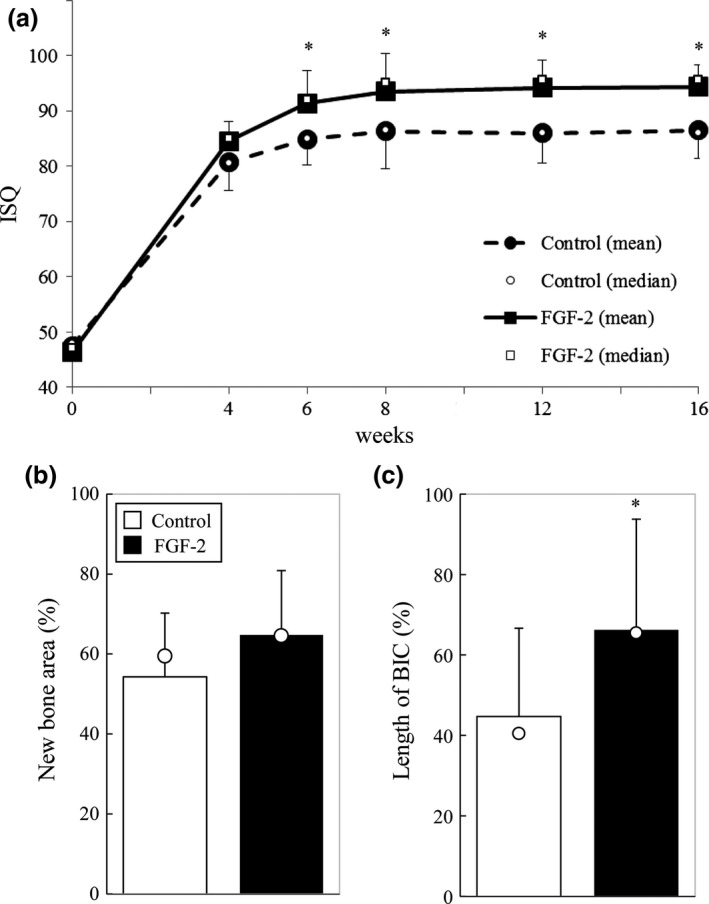
Implant stability measurements and histomorphometric analysis in Study 2. (a) Time‐dependent changes in the ISQ value. The results are presented as means ± SD (n = 6; *P < 0.05, vs. the control group by a Wilcoxon signed‐rank test). (b) Histomorphometric analysis of the rate of new bone area in the entire measurement regions at 16 weeks after installation. The data are shown as percentages calculated as new bone area/area of measurement region × 100. The results are presented as means (column) ± SD (bar) and median (open circle) (n = 6; not significant, vs. the control group by a Wilcoxon signed‐rank test). (c) Histomorphometric analysis of the rate of contact between the new bone and the implant in the entire measurement regions at 16 weeks after installation. The data are shown as percentages calculated as length of BIC/entire length of each side × 100. The results are presented as means (column) ± SD (bar) and median (open circle) (n = 6; *P < 0.05, vs. the control group by a Wilcoxon signed‐rank test).
Discussion
We previously clarified that the FGF‐2 HPC formulation is efficacious for periodontal regeneration accompanied by neogenesis of alveolar bone and cementum (Murakami et al. 1999, 2003; Takayama et al. 2001). In the present study using the same formulation, we demonstrated that FGF‐2 alone promoted the acquisition of osseointegration and enhanced the stability of implants with low primary stability (ISQ values of about 50).
Our histomorphometric analysis demonstrated that FGF‐2 significantly promoted bone formation at 4 weeks. Notably, even in the region far from the existing bone, there was a large difference between the FGF‐2 group and the control group. We also showed that FGF‐2 markedly promoted the acquisition of osseointegration even on the furthest side. These data indicated that FGF‐2 was effective for bone formation and osseointegration around implants with low primary stability and that the promotional effect of FGF‐2 was remarkable in the region where bone formation was delayed by gaps between the implant and the existing bone. Furthermore, the new bone area in the FGF‐2 group at 4 weeks was similar to that in the control group at 8 weeks. This finding suggests that FGF‐2 accelerated bone formation. A covering of the implant surface with bone at the early phase is thought to play an important role in preventing the penetration of soft tissue, because the soft tissues forming at the BIC interface have a negative influence on the fixation (Soballe et al. 1993). The facilitatory effects of FGF‐2 on the new bone formation are considered to be attributable to its strong mitogenic activity toward undifferentiated cells of the osteoblast lineage in bone marrow Martin et al. 1997; Hanada et al. 1997; Dupree et al. 2006) as well as endothelial cells (Gospodarowicz et al. 1987), fibroblasts, and osteoblasts (Rodan et al. 1989). These effects can be exerted even on the titanium surface of implants, because FGF‐2 was reported to effectively enhance the proliferation of mesenchymal stem cells on titanium particles (Jeong et al. 2008).
We showed the promotional effect of FGF‐2 on implant stability by resonance frequency analysis, which was introduced for monitoring of implant stability (Meredith 1998). The ISQ values in the FGF‐2 group from 6 to 16 weeks were significantly higher than those in the control group, indicating that FGF‐2 enhanced the acquisition of stability. However, the ISQ values were similar between the two groups at 4 weeks, although the new bone area in the FGF‐2 group was significantly larger than that in the control group at the same time point. The reason for this might be that the stability was not fully correlated with the amount of new bone formation, because the new bone was immature at 4 weeks. From 4 to 8 weeks, as the new bone matured, the stability of the implants increased in accordance with the amount of new bone. At 16 weeks, the length of BIC in the entire measurement regions in the FGF‐2 group was significantly elevated compared with those in the control group. Furthermore, the length of BIC all around the implants in the FGF‐2 group was significantly higher than those in the control group at 16 weeks (data not shown). These findings suggest that the bone induced by FGF‐2 was maintained all around the implants, thus contributing to the enhancement of implant stability. Therefore, we considered that the enhanced formation of osseointegration and acquisition of high stability of the implants by FGF‐2 were supported by the histomorphometric analysis as well as the resonance frequency analysis.
The acquisition of primary stability depends on the surrounding bone quality and quantity (Oue et al. 2015). As the surrounding bone quality was lowered, the resonance frequency decreased (Huang et al. 2002). Rodrigo et al. (2010) reported that 19% of implants with ISQ<60 failed and that even if the implants did not fail, the unstable implants did not reach the same ISQ values compared with the stable implants at the time of the placement of the prosthetic restoration (2–4 months after the installation). To improve these issues, local administration of FGF‐2 would shorten the treatment period and reduce the failure of implant with low primary stability based on the results in this study that FGF‐2 accelerated bone formation and enhanced the implant stability.
Furthermore, osteoporosis and diabetes mellitus may have insufficient of the primary stability and impairment for bone healing adjacent to dental implants (Nevins et al. 1998; Becker et al. 2000; Oue et al. 2015). ISQ value of the glucocorticoid‐induced osteoporotic animals was lower than normal at the installation in the distal epiphysis of the rabbit femur (Oue et al. 2015). Osseointegration measured by BIC was significantly reduced for diabetic compared to control rats (Nevins et al. 1998). Several previous studies have reported that FGF‐2 enhanced bone formation in animals with impaired ability of bone formation such as streptozotocin‐induced diabetic rats (Kawaguchi et al. 1994) and ovariectomized osteoporotic rabbits and rats (Nakamura et al. 1997; Gao et al. 2009). Based on these reports, even in patients with low bone mass and/or low bone‐forming activity, FGF‐2 is more likely to promote the acquisition of osseointegration.
The present study revealed that FGF‐2 alone increased and accelerated bone formation and promoted the osseointegration of implants with low primary stability, and resulted in the acquisition of high stability. These findings suggest that application of FGF‐2 would increase the success rate in cases with a high probability of implant failure. Interestingly, we previously found that combined usage of FGF‐2 with β‐TCP significantly stimulated periodontal regeneration at 1‐walled periodontal defects in the beagle dog model (Anzai et al. 2010). Thus, it would be possible to achieve osseointegration with a higher probability by topically applying FGF‐2 with various osteoconductive materials.
Acknowledgements
We express our gratitude to J. Anzai, N. Shiraishi, and A. Terashima for technical assistance. Funding: This study was supported by Kaken Pharmaceutical Co., Ltd. and Osaka University Graduate School of Dentistry. This research is partially supported by Grants‐in‐Aid for Scientific Research (No. 24592956). Competing Interests: SM received research grants from Kaken Pharmaceutical Co., Ltd and accepted a position as a medical advisor. TNT is employees and stockholders of Kaken Pharmaceutical Co., Ltd.
Nagayasu‐Tanaka Toshie, Nozaki Takenori, Miki Koji, Sawada Keigo, Kitamura Masahiro, Murakami Shinya. FGF‐2 promotes initial osseointegration and enhances stability of implants with low primary stability. Clin. Oral Impl. Res. 28, 2017, 291–297
References
- Anzai, J. , Kitamura, M. , Nozaki, T. , Nagayasu, T. , Terashima, A. , Asano, T. & Murakami, S. (2010) Effects of concomitant use of fibroblast growth factor (FGF)‐2 with beta‐tricalcium phosphate (β‐TCP) on the beagle dog 1‐wall periodontal defect model. Biochemical and Biophysical Research Communications 403: 345–350. [DOI] [PubMed] [Google Scholar]
- Becker, W. , Hujoel, P.P. , Becker, B.E. & Willingham, H. (2000) Osteoporosis and implant failure: an exploratory case‐control study. Journal of Periodontology 71: 625–631. [DOI] [PubMed] [Google Scholar]
- Branemark P.I., Zarb G.A. & Albrektsson T. (Eds) (1985) Tissue‐Integrated Prostheses‐ Osseointegration in Clinical Dentistry, Vol 11, Chicago: Quintessence. [Google Scholar]
- Brunski, J.B. (1992) Biomechanical factors affecting the bone dental implant interface. Clinical Materials 10: 153–201. [DOI] [PubMed] [Google Scholar]
- Chen, W.J. , Jingushi, S. , Aoyama, I. , Anzai, J. , Hirata, G. , Tamura, M. & Iwamoto, Y. (2004) Effects of FGF‐2 on metaphyseal fracture repair in rabbit tibiae. Journal of Bone and Mineral Metabolism 22: 303–309. [DOI] [PubMed] [Google Scholar]
- Dupree, M.A. , Pollack, S.R. , Levine, E.M. & Laurencin, C.T. (2006) Fibroblast growth factor 2 induced proliferation in osteoblasts and bone marrow stromal cells: a whole cell. Biophysical Journal 91: 3097–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Luo, E. , Hu, J. , Xue, J. , Zhu, S. & Li, J. (2009) Effect of combined local treatment with zoledronic acid and basic fibroblast growth factor on implant fixation in ovariectomized rats. Bone 44: 225–232. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz, D. , Ferrara, N. , Schweigerer, L. & Neufeld, G. (1987) Structural characterization and biological functions of fibroblast growth factor. Endocrine Reviews 8: 95–114. [DOI] [PubMed] [Google Scholar]
- Hanada, K. , Dennis, J.E. & Caplan, A.I. (1997) Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein‐2 on osteogenic differentiation of rat bone marrow‐derived mesenchymal stem cells. Journal of Bone and Mineral Research 12: 1606–1614. [DOI] [PubMed] [Google Scholar]
- Huang, H.M. , Lee, S.Y. , Yeh, C.Y. & Lin, C.T. (2002) Resonance frequency assessment of dental implant stability with various bone qualities: a numerical approach. Clinical Oral Implants Research 13: 65–74. [DOI] [PubMed] [Google Scholar]
- Jeong, W.K. , Park, S.W. & Im, G.I. (2008) Growth factors reduce the suppression of proliferation and osteogenic differentiation by titanium particles on MSCs. Journal of Biomedical Materials Research, Part A 86: 1137–1144. [DOI] [PubMed] [Google Scholar]
- Kato, T. , Kawaguchi, H. , Hanada, K. , Aoyama, I. , Hiyama, Y. , Nakamura, T. , Kuzutani, K. , Tamura, M. , Kurokawa, T. & Nakamura, K. (1998) Single local injection of recombinant fibroblast growth factor‐2 stimulates healing of segmental bone defects in rabbits. Journal of Orthopaedic Research 16: 654–659. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, H. , Kurokawa, T. , Hanada, K. , Hiyama, Y. , Tamura, M. , Ogata, E. & Matsumoto, T. (1994) Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin‐diabetic rats. Endocrinology 135: 774–781. [DOI] [PubMed] [Google Scholar]
- Kitamura, M. , Akamatsu, M. , Machigashira, M. , Hara, Y. , Sakagami, R. , Hirofuji, T. , Hamachi, T. , Maeda, K. , Yokota, M. , Kido, J. , Nagata, T. , Kurihara, H. , Takashiba, S. , Sibutani, T. , Fukuda, M. , Noguchi, T. , Yamazaki, K. , Yoshie, H. , Ioroi, K. , Arai, T. , Nakagawa, T. , Ito, K. , Oda, S. , Izumi, Y. , Ogata, Y. , Yamada, S. , Shimauchi, H. , Kunimatsu, K. , Kawanami, M. , Fujii, T. , Furuichi, Y. , Furuuchi, T. , Sasano, T. , Imai, E. , Omae, M. , Yamada, S. , Watanuki, M. & Murakami, S. (2011) FGF‐2 stimulates periodontal regeneration: results of a multi‐center randomized clinical trial. Journal of Dental Research 90: 35–40. [DOI] [PubMed] [Google Scholar]
- Kitamura, M. , Nakashima, K. , Kowashi, Y. , Fujii, T. , Shimauchi, H. , Sasano, T. , Furuuchi, T. , Fukuda, M. , Noguchi, T. , Shibutani, T. , Iwayama, Y. , Takashiba, S. , Kurihara, H. , Ninomiya, M. , Kido, J. , Nagata, T. , Hamachi, T. , Maeda, K. , Hara, Y. , Izumi, Y. , Hirofuji, T. , Imai, E. , Omae, M. , Watanuki, M. & Murakami, S. (2008) Periodontal tissue regeneration using fibroblast growth factor‐2: randomized controlled phase II clinical trial. PLoS ONE 3: e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall, N. , Dhanasekar, B. & Aparna, I.N. (2011) Validation of implant stability: a measure of implant permanence. Indian Journal Dental Research 22: 462–467. [DOI] [PubMed] [Google Scholar]
- Martin, I. , Muraglia, A. , Campanile, G. , Cancedda, R. & Quarto, R. (1997) Fibroblast growth factor‐2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology 138: 4456–4462. [DOI] [PubMed] [Google Scholar]
- Meredith, N. (1998) Assessment of implant stability as a prognostic determinant. Int J Prosthodont 11: 491–501. [PubMed] [Google Scholar]
- Murakami, S. , Takayama, S. , Ikezawa, K. , Shimabukuro, Y. , Kitamura, M. , Nozaki, T. , Terashima, A. , Asano, T. & Okada, H. (1999) Regeneration of periodontal tissues by basic fibroblast growth factor. Journal of Periodontal Research 34: 425–430. [DOI] [PubMed] [Google Scholar]
- Murakami, S. , Takayama, S. , Kitamura, M. , Shimabukuro, Y. , Yanagi, K. , Ikezawa, K. , Saho, T. , Nozaki, T. & Okada, H. (2003) Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. Journal of Periodontal Research 38: 97–103. [DOI] [PubMed] [Google Scholar]
- Nakamura, T. , Hara, Y. , Tagawa, M. , Tamura, M. , Yuge, T. , Fukuda, H. & Nigi, H. (1998) Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. Journal of Bone and Mineral Research 13: 942–949. [DOI] [PubMed] [Google Scholar]
- Nakamura, K. , Kawaguchi, H. , Aoyama, I. , Hanada, K. , Hiyama, Y. , Awa, T. , Tamura, M. & Kurokawa, T. (1997) Stimulation of bone formation by intraosseous application of recombinant basic fibroblast growth factor in normal and ovariectomized rabbits. Journal of Orthopaedic Research 15: 307–313. [DOI] [PubMed] [Google Scholar]
- Nevins, M.L. , Karimbux, N.Y. , Weber, H.P. , Giannobile, W.V. & Fiorellini, J.P. (1998) Wound healing around endosseous implants in experimental diabetes. The International journal of oral & maxillofacial implants 13: 620–629. [PubMed] [Google Scholar]
- Oue, H. , Doi, K. , Oki, Y. , Makihara, Y. , Kubo, T. , Perrotti, V. , Piattelli, A. , Akagawa, Y. & Tsuga, K. (2015) Influence of implant surface topography on primary stability in a standardized osteoporosis rabbit model study. Journal of Functional Biomaterials 6: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra, S. , Wood, M.C. & Taylor, T.D. (2005) Early wound healing around endosseous implants: a review of the literature. The International journal of oral & maxillofacial implant 20: 425–431. [PubMed] [Google Scholar]
- Rodan, S.B. , Wesolowski, G. , Thomas, K.A. , Yoon, K. & Rodan, G.A. (1989) Effects of acidic and basic fibroblast growth factors on osteoblastic cells. Connective Tissue Research 20: 283–288. [DOI] [PubMed] [Google Scholar]
- Rodrigo, D. , Aracil, L. , Martin, C. & Sanz, M. (2010) Diagnosis of implant stability and its impact on implant survival: a prospective case series study. Clinical Oral Implants Research 21: 255–261. [DOI] [PubMed] [Google Scholar]
- Salgado, A.J. , Coutinho, O.P. & Reis, R.L. (2004) Bone tissue engineering: state of the art and future trends. Macromolecular Bioscience 4: 743–765. [DOI] [PubMed] [Google Scholar]
- Sennerby, L. & Roos, J. (1998) Surgical determinants of clinical success of osseointegrated oral implants: a review of literature. Int J Prosthodont 11: 491–501. [PubMed] [Google Scholar]
- Soballe, K. , Hansen, E.S. , Brockstedt‐Rasmussen, H. & Bünger, C. (1993) Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. The Journal of Bone and Joint Surgery British volume. 75: 270–278. [DOI] [PubMed] [Google Scholar]
- Takayama, S. , Murakami, S. , Shimabukuro, Y. , Kitamura, M. & Okada, H. (2001) Periodontal regeneration by FGF‐2 (bFGF) in primate models. Journal of Dental Research 80: 2075–2079. [DOI] [PubMed] [Google Scholar]


