Abstract
Children who experience early caregiving neglect are very likely to have problems developing and maintaining relationships and regulating their social behavior. One of the earliest manifestations of this problem is reflected in indiscriminate behavior, a phenomenon where young children do not show normative wariness of strangers or use familiar adults as sources of security. To better understand the developmental mechanisms underlying the emergence of these problems, this study examined whether institutionally reared children, who experienced early social neglect, had difficulty associating motivational significance to visual stimuli. Pairing stimuli with motivational significance is presumably one of the associative learning processes involved in establishing discriminate or selective relationships with others. We found that early experiences of neglectful caregiving were associated with difficulties in acquiring such associations, and that delays in this developmental skill were related to children's social difficulties. These data suggest a way in which early social learning experiences may impact the development of processes underlying emotional development.
Research highlights
Typically developing children successfully utilize implicit cue information to direct their goal‐motivated behavior; however, socially neglected children did not make these associations.
The extent to which children had difficulty learning these reward cues was associated with their indiscriminate behavioral problems.
Motivated reward learning may be causally involved in the relationship between socioemotional neglect and indiscriminate behavior problems.
Such an association sheds new light on biobehavioral systems that might be targets of successful interventions for at‐risk children.
Introduction
To promote the survival of vulnerable infants, primates engage in behaviors that promote caregiver comforting, proximity, and protection, especially when infants are faced with threat. When children are reared in neglectful or abusive conditions the development of these biobehavioral processes is hindered. Disruption of these processes is reflected in children's problems forming and maintaining relationships. These outcomes are not surprising in post‐institutionalized children given that institutional settings usually lack personalized, consistent care from an individual caregiver; therefore, a child's opportunities for establishing a stable relationship with a significant adult is limited. However little is currently understood about the mechanisms through which conditions of deprivation may compromise this social and emotional development (Pollak, 2015). Here, we begin to explore the possibility that social deprivation affects children's motivational learning abilities. Motivation, the incentive to act, is generally believed to be a limbic–striatal–pallidal circuit. This circuitry allows organisms to associate information from the environment in order to integrate reward prediction into behavioral goals (Shohamy, 2011). This experiment tests whether disruption of these processes, through aberrant caregiving, undermines children's abilities to develop interpersonal relationships.
Socioemotional deprivation and socioemotional development
Some previously neglected children establish selective relationships with their caregivers, yet many continue to show socioemotional difficulties throughout their lives. These difficulties remain even years after placement in adequate caregiving environments (Lawler, Hostinar, Mliner & Gunnar, 2014). Indiscriminate social behavior is among the most prominent and lasting of the social abnormalities observed in children not reared in family settings (Gleason, Fox, Drury, Smyke, Nelson et al., 2014; Pears, Bruce, Fisher & Kim, 2010; Smyke, Zeanah, Fox, Nelson & Guthrie, 2010). This lack of social selectivity is manifested as children's close physical engagement, seeking comfort from, or trust in going off with strangers – even when familiar caregivers are proximal. These observations are not new. Among young children placed in residential nurseries in London in the 1940s through the 1960s, 4‐year‐old institutionalized children approached strangers with equal frequency as their familiar caregivers (Tizard & Rees, 1974). These socially indiscriminate behaviors are maintained years after children are adopted into stable families, are predictive of psychological problems, and are highly resistant to treatment (Rutter, Kreppner & Sonuga‐Barke, 2009).
Early social neglect is associated with both indiscriminate behavior and attachment problems (Love, Minnis & O'Connor, 2015), and we do not yet understand whether disinhibited/indiscriminate social behavior reflects a disorder of attachment or a behavioral problem with a distinct etiology (see Zeanah & Gleason, 2015, and Lyons‐Ruth, 2015, for clear articulation of both sides of this issue). For scholars focusing on indiscriminate behavior, the core deficit reflects unmodulated behavior, and does not necessarily reflect an overall pattern of organization encompassed by attachment theory. Support for this view is that indiscriminate behavior and attachment are not directly related among post‐institutionalized children (Dobrova‐Krol, Bakermans‐Kranenburg, IJzendoorn & Juffer, 2010; Zeanah, Smyke, Koga, Carlson & the BEIP Group, 2005). For attachment researchers, implicit in characterizing a child as displaying indiscriminate behavior is identification that the child is not showing selectivity between classes of adults. On this view, absence of stranger wariness and comfort seeking from strangers suggests a problem in the attachment system (Lyons‐Ruth, 2015).
Mechanisms of socioemotional learning
Although many institutions provide rudimentary medical care and nutrition, children often receive care that is highly regimented rather than personal. This inconsistency limits possibilities to establish stable, personal relationships between children and their caregivers (Bakermans‐Kranenburg, Steele, Zeanah, Muhamedrahimov, Vorria et al., 2011). Nonhuman primates raised in analogous situations display social deficits remarkably similar to the indiscriminate patterns of behavior observed in children who have experienced caregiving neglect (Sanchez & Pollak, 2009). These animal studies provide clues that social contingencies in the early rearing environment are a critical factor for socioemotional development. For example, when infant monkeys are reared by dogs who provide responsive care and protection, the dog‐reared monkeys are socially competent and able to use complex social strategies that monkeys reared without responsive care cannot (Capitanio & Mason, 2000). Similarly, human children who receive sensitive and contingent caregiving show more behavioral adaptiveness to changing environmental situations than children raised in neglectful family environments (Van Den Dries, Juffer, van IJzendoorn, Bakermans‐Kranenburg & Alink, 2012).
During their first months of life, human infants engage in social interaction with almost anyone. But by 7–9 months of age, wariness of strangers is normative. From an evolutionary point of view, apprehension about unfamiliar adults is a good strategy to promote survival (Simpson & Belsky, 2008). Yet Provence and Lipton (1962), who were among the first to observe the development of institution‐reared infants, reported that ‘one saw no evidence of increasing personal attachment to a particular person' (p. 78), and that institutionalized infants ‘responded with equal enjoyment to everyone who came around' (p. 80). Further implicating the role of early social experience, the extent to which previously maltreated children experience responsive care from their adoptive parents predicts their positive social development in adolescence (Jaffari‐Bimmel, Juffer, van IJzendoorn, Bakermans‐Kranenburg & Mooijaart, 2006).
Associative learning and contingent caregiving
A key aspect of indiscriminate social behavior is that the child fails to recognize specific familiar individuals as a source of comfort and protection. In other words, children may not recognize familiar adults as being associated with safety. A candidate neural system likely to support the ability to make such a pairing involves the rhinal cortex and closely connected structures (Liu, Murray & Richmond, 2000). Located in the temporal lobe, with intimate connections to both limbic and frontal regions, the rhinal cortex is critically placed to integrate emotional with perceptual processes, as well as store the emotional significance of prior experience. Much is known about the role of this system from nonhuman animal studies that suggest this system is related to the behavioral observations of post‐institutionalized children.
After removal of the rhinal cortex, monkeys cannot learn the association between new cues and their motivational significance – for example that selective cues are predictive of reward (Liu et al., 2000). Moreover, changes in dopamine levels within the rhinal cortex have a similar effect as lesions to the structure. When rhesus monkeys are injected with an antisense DNA construct that decreases D2 dopamine receptor expression in the rhinal cortex, they similarly do not learn the associations between environmental cues and rewards (Liu, Richmond, Murray, Saunders, Steenrod et al., 2004).
There are a number of other indications that this system may be implicated in children's formation of selective relationships with caregivers. For example, the rhinal cortex has strong reciprocal connections with the basolateral amygdala (BLA), a region important for assessing the valence of a stimulus (Stefanacci, Suzuki & Amaral, 1996) as well as the central nucleus (CeN), which helps give emotional context to external events (Paz, Guillaume Pelletier, Bauer & Pare, 2006; Sugase‐Miyamoto & Richmond, 2005). This connection between the BLA and CeN further coordinates neuroendocrine responses tied to social behaviors such as fear and comforting. These are processes observed to functional atypically in post‐institutionalized children (Wismer Fries, Shirtcliff & Pollak, 2008; Wismer Fries, Ziegler, Kurian, Jacoris & Pollak, 2005).
Based upon these extant data, we tested children on a task known to rely upon healthy functioning of rhinal cortex circuitry. We expected typically developing children to show a decrease in their response time to reward as their distance from the reward (signaled by changes in the visual cue) decreases. In contrast, we hypothesized that children who experienced early social deprivation would have difficulty learning the association between visual cues and their rewarding or motivating significance. If this effect is specific to a problem in learning reward cues, the two groups of children should perform similarly when cues are presented randomly, and therefore do not carry information about reward proximity. To examine whether the ability to associate sensory cues with their motivational or rewarding significance is related to socioemotional behavior, we explored whether impaired learning performance was associated with children's indiscriminate behavior problems. We also evaluated general cognitive abilities to ensure that they were not driving any group differences.
Method
Participants
Fifty‐two children participated in this experiment. Children with histories of severe neglect (N = 26; 15 females, 11 males) were placed in institutions at birth for a minimum of 8 months before being adopted (M length of orphanage stay = 16.7 months, SD = 7.9 months, maximum = 35 months). The mean age of the post‐institutionalized group was 6.32 years (SD = 7.66 months). All children came from Eastern European orphanages: 19 from Russia, 6 from a former Russian republic (i.e. Moldova, Kazakhstan), and 1 from Romania. These children were recruited via adoption agencies.
We compared the children who experienced early neglect to a comparison group consisting of children who had always resided with their birth parents (N = 26; 10 females, 16 males; age: M = 6.32 years, SD = 5.35 months).
The two groups of participants did not differ on child age and family socioeconomic status. Children were not recruited for this experiment if they had a developmental disorder, known or suspected fetal alcohol exposure, or fetal alcohol syndrome. A pediatric geneticist who specializes in fetal alcohol syndrome assessed: (1) distance between the endocanthion and exocanthion landmarks, (2) philtrum smoothness, and (3) upper lip thinness. This screening has demonstrated high sensitivity and specificity for prenatal alcohol exposure (Astley, Stachowiak, Clarren & Clausen, 2002). Not reported here are data from eight previously institutionalized children who were not included in this study because the screening suggested pre‐natal alcohol exposure and four children excluded because they did not complete both laboratory visits (one post‐institutionalized and three non‐adopted). No children who completed the task were excluded from analyses.
Associative learning task
Overview
The experimental task measures the child's ability to predict rewards from environmental stimuli and adjust behavior accordingly (Liu et al., 2000). Children were instructed to indicate a change in a stimulus and had to correctly complete a full schedule of trials before receiving a reward. Implicit visual cues indicate how close participants are to reward delivery, and changes in participants' behavior reflect the degree to which they are motivated by these cues.
As a control condition, children completed another, perceptually identical, version of the task. The control task was identical to the experimental task except that the cues were presented in a random fashion. In other words, the implicit reward cues no longer carried any predictive value in terms of the reward. Children performed the experimental and control tasks in random order; order of presentation was not related to task performance.
Task
Details of the task are depicted in Figure 1. To provide a stringent test of learning we used two different versions of the experimental task and two different kinds of cues. Children were randomly assigned to either a color or a shape discrimination task and within each of these tasks children were randomly assigned to either a length or brightness visual cue. The cue was a rectangle located at the top of the computer monitor that became either longer or brighter as the child moved closer to receiving a reward.
Figure 1.
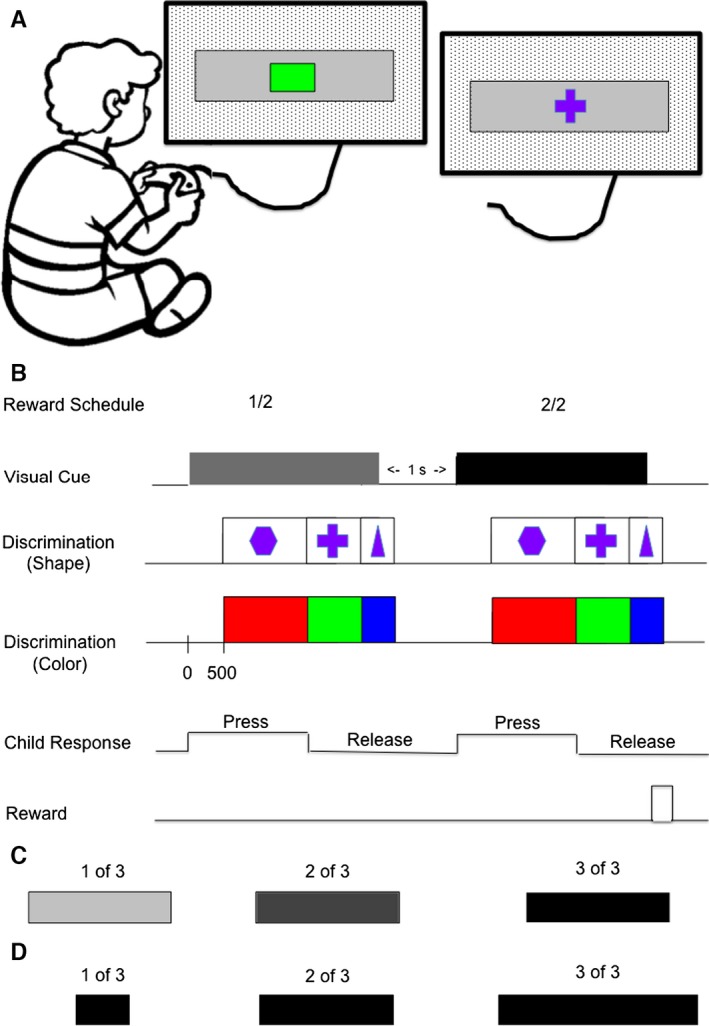
Visually cued reward schedule task. (A) Behavioral testing situation. The child sat facing a rear projection screen (90° °— 90°) located 57 cm away. A touch lever registered responses. A black and white random dot background covered the whole screen. A visual cue (shown here is a gray rectangle, but see C and D, below) and either a colored dot (left example) or a geometric shape (right example) were displayed at the center of the screen. (B) Detailed timing of the sequence of events in a visually cued two‐trial reward schedule. In each trial, the child was required to release a touch lever when the dot on the screen changed color from red to green. The child was required to perform this correctly twice consecutively to obtain a reward. Each trial was assigned a ‘schedule state' (current trial number/schedule length, such as 1 out of 2, or 2 out of 3). The child started each trial by contacting a touch lever. Immediately after the lever was contacted, a visual cue was displayed and remained on without changing throughout the whole trial. The gray rectangle was the cue for schedule state 1/2, whereas the dark rectangle was the cue for 2/2. The cue was displayed for 900–1000 ms before the trial progressed to the color discrimination phase. In the color discrimination phase, a red dot appeared at the center of the screen. After a randomly selected wait time (400, 600, 800, 1000 or 1200 ms), the color of the dot changed from red to green, indicating that the child could release the lever to complete a trial. If the child released the lever within 1000 ms, the dot changed from green to blue, signaling the child that a correct trial had been performed. After the blue dot was displayed for 150 ms, all stimuli disappeared. If the trial was the last trial in a schedule, a coin was delivered to the child's piggy bank. If the child released the lever in the red dot period or in less than 200 ms after the onset of the green dot, or if the child did not release the lever within 1000 ms after the onset of the green dot, all stimuli disappeared, the trial was terminated, and an error was registered. Each trial was separated by a 1000‐ms intertrial interval. The shape condition was identical in every respect with the exception that a stop sign replaced the red dot, a plus sign replaced the green dot, and a triangle replaced the blue dot. Children were randomly assigned to the color or shape discrimination conditions, each randomly paired with either brightness or length cues. (C) Brightness cues. (D) Length cues. Shown in parentheses are the schedule states indicated by each brightness and length cue. From Liu et al. (2000).
The child began each trial by pressing down a button, which caused a stimulus to appear on the screen and then change. The child was required to release the button when she/he detected a change in the stimulus. After 500 ms a small red circle (in the color discrimination version of the task) or small purple octagon (in the shape discrimination version of the task) appeared on the screen. The target stimulus changed after a randomized delay of 400, 600, 800, 1000, or 1200 ms. In the color discrimination task, a circle changed from red to green. In the shape discrimination task an octagon changed to a plus sign. The green circle (or plus sign) remained on the screen until the child released the space bar. Reaction time was recorded as the amount of time the child took to release the space bar following the onset of a change in the stimuli (e.g. the circle turning green or the octagon turning to a plus sign).
Children had to complete a schedule of trials correctly to receive a reward. Each schedule consisted of one, two, or three trials. Each trial in a schedule is referred to by its state within the schedule: 1/3, 2/3, 3/3 for a three‐trial schedule, 1/2, 2/2 for a two‐trial schedule, and 1/1 for a one‐trial schedule. Only the third in a three trial schedule (3/3), the second in a two‐trial schedule (2/2), and the single trial schedules (1/1) were rewarded. Each trial in the two‐ and three‐trial reward schedules was presented 22 times (110 trials) and 1/1 trials were presented 37 times (147 total trials).
Errors and rewards
If an error was made anywhere in the schedule, the child repeated that particular trial until it was successfully completed. Errors reflected the child: (1) releasing the space bar prior to the change from red to green (or octagon to plus sign), (2) releasing the space bar less than 50 ms after the appearance of the green circle (or plus sign), or (3) not responding 2000 ms after the appearance of the green circle (or plus sign).
If a child performed the trial correctly, the circle changed from green to blue (or the plus sign changed to a triangle). The blue circle (or triangle) was displayed for 150 ms and then all stimuli disappeared. When the child performed the last trial in a schedule correctly, the child received an immediate reward. Rewards were delivered via a Davis Scientific Universal Primate Feeder loaded with a variety of coins. The coins dropped into clear plexiglass piggy banks that the children had personalized prior to the start of the experiment.
Assessment of indiscriminate behavior
We used information from the Disturbances of Attachment Interview (Smyke, Dumitrescu & Zeanah, 2002) to assess children's indiscriminate behavior. The first author conducted the interviews, and a panel of three graduate‐level researchers scored the data. Individuals scoring the interviews were unaware of the group status of each child and the hypotheses of this experiment. Items from this semistructured interview are coded 0 = none/little, when there is no evidence of disturbance (e.g. the child clearly differentiates among adults and selectively seeks that person for comfort, support or nurturance). Items are coded 1 = sometimes/somewhat, when there is sometimes evidence of behaviors that may be consistent with disturbance (e.g. the child sometimes or somewhat seeks comfort preferentially from a preferred caregiver). Items are coded 2 = rarely/minimally, when there is evidence of behaviors consistent with indiscriminate behavior (e.g. the child rarely or minimally differentiates among adults as caregiving figures, or the child is willing to go off readily with relative strangers). Ratings are made on the basis of responses to a series of probes that tap the construct of indiscriminate behavior. For example, parents were asked how well the child differentiated among adults and demonstrated a clear preference for a particular caregiver and how much the child sought comfort from a preferred caregiver. Other aspects of the interview captured broader aspects of emotion regulation and attachment security. Details of the scoring criteria and properties of the interview are detailed in Smyke et al. (2002). Of a possible rating of 10, 26 children had scores of less than 2 (Control N = 21, Neglect N = 5, 19 children had scores between 3 and 5 (Control N = 4, Neglect N = 15), and 7 children had scores above 6 (Control N = 1, Neglect N = 6). Neither child sex, IQ, nor length of institutionalization (among the post‐institutionalized group) affected any of the dependent variables (all ps > .1). We selected a sample with longer histories of institutional care, which may account for the lack of effect of length of neglect.
Procedure
Children were tested on two days (7–14 days apart) in their homes. They completed either the experimental or control version of the task at each session. Children were randomly assigned to one of four different versions of the task that varied the implicit cue (a rectangle that either got brighter or longer), and the discrimination to be made (either the color or shape of the changed stimulus). Whatever version of the task children completed on their first session, they were presented with a different version of the task on their second session. For example, if a child was assigned to the length cue‐color discrimination version for their first session, then they completed the brightness cue‐shape discrimination version for their second session. We varied the cues and stimuli for two reasons. First, to minimize carry‐over effects across testing days and, second, to increase the likelihood that any resulting effects reflected learning rather than features of particular stimuli. There was no difference in children's performance across the two cue (length and brightness) and discrimination (color and shape) conditions, γ11 = −1.84, t(60) = −.06, ns.
Participants were instructed only on how to perform the color or shape discrimination tasks; no mention of the reward or the visual cues was made. Children completed 10 practice trials for which no reward was dispensed and no visual cues were presented to familiarize them with the procedures. The experiment did not begin until it was clear to the experimenter that the child understood the discrimination task (this occurred within 3–5 practice trials for all participants). The task was divided into three blocks of approximately 49 trials each, and children took a 5‐minute break between blocks. Children chose a piggy bank that was attached to the pellet dispenser and were told that they could keep the coins they earned during the task.
Data analyses
Hierarchical linear modeling (Snijders & Bosker, 1999) was used to understand the relationships between performance on the associative reward learning task, group status, and indiscriminate behavior problems. We selected this technique because it is well suited to the small and unequal sample sizes and varying conditions in this experiment. The main hypothesis to be tested was that children's performance (based upon reaction time) would improve as they approached receipt of a reward. Therefore, we used a ‘time to reward' model to investigate patterns of change in reaction times relative to the distance from the reward. This type of linear model is based upon extant primate data (Liu et al., 2000, 2004), which indicates a strong linear relationship between time until reward delivery and reaction times. Specifically, the model fit to the data was a two‐level model with trials (i) nested within child (j) of the form:
where TIMEREW ij denotes the time‐to‐reward for child j on trial i, PI j denotes the post‐institutionalized status of child j (0 = not institutionalized, 1 = institutionalized), γ 10 denotes the mean effect of time‐to‐reward for a non‐ institutionalized child, and γ 11 denotes the difference in effect of time‐to‐reward for an institutionalized child.
Negative slopes for time‐to‐reward reflect learning (i.e. as reward grows closer reaction times decrease) and less negative/flat slopes represent difficulties in learning. Our decision to model reaction time in relation to a single time‐to‐reward composite was based on the observation of consistent effects across trials in relation to this factor. As expected based upon extant literature, children responded similarly to the last trials in each reward schedule (i.e. 1/1, 2/2, 3/3): reaction time: F(2, 124) = 1.17, ns; error rate: F(2, 124) = .639, ns.
We also analyzed individual difference predictors of reaction times and error rates at the between‐individual level including group status, cue condition (brightness vs. length), discrimination (color vs. shape), sex, IQ, indiscriminate behavior problems, and length of institutionalization. These models were of the same form as that shown above, but with the relevant studied variable substituted for PI.
Results
Motivated reward learning
We first tested whether the post‐institutionalized children differed from age‐matched peers in terms of their ability to use motivational cues to guide their learning. Consistent with the extant primate literature, typically developing children learned efficiently across trials. These children responded more quickly (reflected in a significantly negative slope) as the reward became closer, γ10 = −56.66, t(61) = −2.90, p = .006. However, the post‐institutionalized children's learning slope was essentially flat and differed from that of typically developing children, γ11 = 63.77, t(61) = 2.10, p = .04 (Figure 2, panel A). These data indicate that post‐institutionalized children had difficulty pairing the significance of the cues with potential reward. The effect size, estimated using Cohen's d as defined by the estimate of γ11 and the residual variance of the slopes (i.e. the estimated variance of U 1j), revealed a large between‐group effect of learning, d = 1.02.
Figure 2.
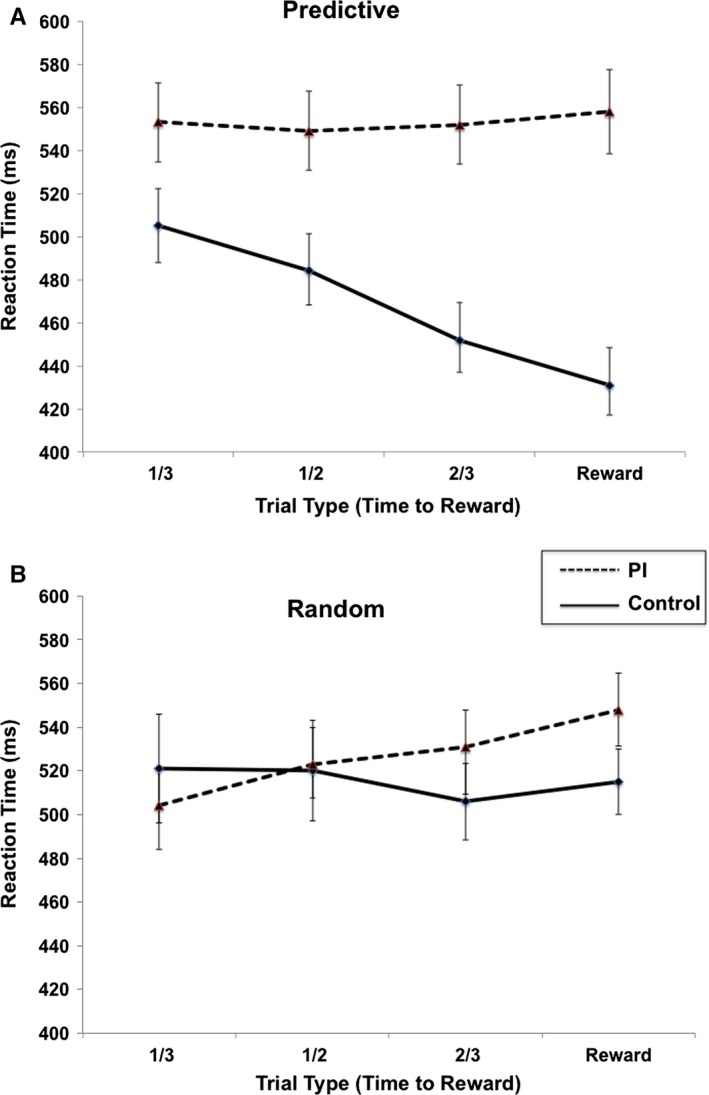
Mean reaction time (standard error) by trial type for post‐institutionalized and comparison children in the Predictive (top panel) and Random (bottom panel) conditions.
Relationship between motivated reward learning and social behavior
Next, we tested a model to determine whether individual differences in children's reward learning were associated with their indiscriminate social behavior. Poor performance on the reward learning task was related to problems with indiscriminate behavior, estimated γ11 = 20.11, t(61) = 3.01, p = .004. As the indiscriminate behavior variable ranges from 0 to 6 (mean = 1.23; SD = 1.67), we examine its effect by considering the slope estimates for several different levels of the variable. This effect can be seen in Figure 3 for three different levels of indiscriminate behavior, illustrating that flatter slopes (reflecting a child's poorer learning performance) are associated with increasing levels of indiscriminate behavior problems.
Figure 3.
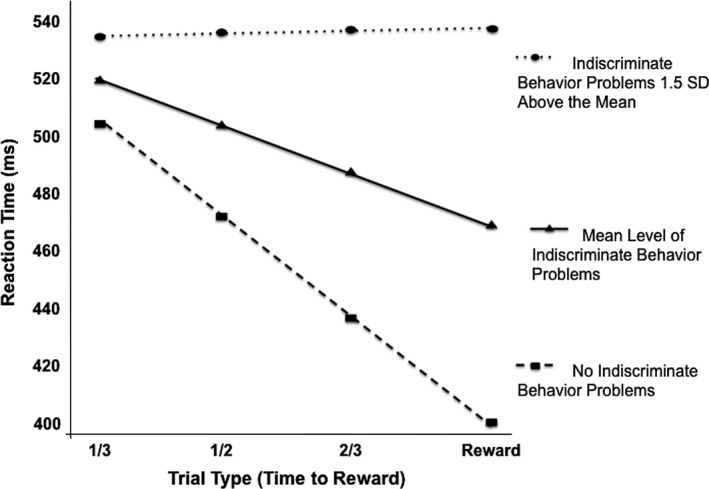
Predicted reaction time (ms) values based on HLM analyses in the predictive condition by indiscriminate behavior problems at, above, and below the sample mean. The mean indiscriminate behavior score for post‐institutionalized children was 1.81 (SD = 1.93; range = 0–6) and for comparison children was 0.90 (SD = 1.20; range = 0–4).
Specificity of this learning effect
There are many cognitive sequelae associated with child neglect; therefore we considered the possibility that we were capturing generalized learning deficits among the post‐institutionalized children. First, there was no evidence that post‐institutionalized children had difficulty understanding or performing the task based on their performance during practice trials, nor were there IQ differences between the two groups. Second, we tested all of the children in a non‐predictive control version of the same task, where reward cues were presented randomly. This allowed us to ensure that there were not performance differences between the post‐institutionalized and comparison children in the absence of associative learning demands. The number of trials and rewards in this condition was identical to the predictive condition. Both groups of children performed similarly, F(1, 60) = .086, ns, further suggesting that the post‐institutionalized children understood and were able to complete the basic task comparably to typically developing children (Figure 2, panel B).
Finally, we examined the errors children made on the task to determine whether post‐institutionalized children's performance was undermined by impulsivity. Because children's response times were measured from the onset of stimuli, two types of errors would reflect impulsivity: responses prior to the appearance of the ‘go' signal and responses less than 50 ms after the appearance of the ‘go' signal. The post‐institutionalized children did not make more of these errors (mean error rate for comparison children = 15%, mean error rate for post‐institutionalized children = 11%), F(1, 60) = 1.85, ns. Neither group of children made many omission errors, defined as failing to respond within 2000 ms (error rates for both groups < 3%). This suggests that the post‐institutionalized children were just as attentive to the task.
Discussion
This experiment sought to better understand how early caregiving neglect is associated with children's difficulties discriminating their caregivers from unfamiliar adults. To do so, we assessed the efficiency of children's motivated reward learning. The experimental task required children to modify their behavior in relation to the learned motivational significance conveyed by implicit sensory cues. Our rationale for targeting this learning process is the idea that children must translate information about the identity of a stimulus (such as a familiar caregiver, a facial expression, a voice) into a signal about its motivational significance (such as a source of safety and security). We found that: (1) typically developing children successfully utilized implicit information to direct their goal‐motivated behavior, whereas post‐institutionalized children did not make these associations and (2) the extent to which children had difficulty learning these cues was associated with their indiscriminate behavioral problems.
The joint significance of these two ‘pathways' is consistent with a mediational explanation (MacKinnon, Lockwood, Hoffman, West & Sheets, 2002); that is, motivated reward learning appears to account, in part, for the relationship between socioemotional neglect and indiscriminate behavior problems. Such behaviors are clearly the end product of multiple interacting cognitive and neural systems; no single measurement or neural structure is likely to account for all of the discrete developmental mechanisms underlying their emergence (Pollak, 2005). Although this experiment is not designed to test causality, the association revealed in these data sheds new light on biobehavioral systems that might be targets of successful interventions for at‐risk children.
It is difficult to excavate neurodevelopmental mechanisms in human populations of children that have had unusual life experiences. Researchers must work with all of the ‘unmeasurable' components of circumstances such as institutional rearing, and many invasive research techniques are not appropriate for use with children. This experiment allowed us to boot‐strap knowledge from nonhuman animal studies. We were able to use an identical paradigm – including stimuli, timing, response mechanisms, and even reward delivery system – across children and rhesus monkeys. The only change in procedures between species was that monkeys were rewarded with food whereas children were rewarded with coins. (This change was made because during pilot testing parents had different views about use of food as a reward or the types of foods they allowed their children to eat.) However, the behavioral data are consistent with the view that the coins did not change task performance. The task was quite repetitive, yet post‐institutionalized children stayed with the task, working as hard and long, and accurately as children in the comparison group. The only difference that emerged was their ability to make use of the implicit cues in the task, which was the point of the experiment. In terms of concurrent validity, the performance of children in this comparison group mirrors the effects observed in typically developing monkeys, and children from our post‐institutionalized group evince performance that is strikingly similar to monkeys with physical and chemical lesions to the rhinal cortex reported by Liu et al. (2000, 2004).
From extant data, we know that the experimental task taps motivational processes and reward expectation tied to the most ventromedial portion of the inferior temporal cortex, namely the rhinal (that is, the perirhinal and entorhinal) cortex. The rhinal cortex is rich in dopaminergic innervations and reaches peak density around the middle of the first year of life in rhesus monkeys, corresponding to 1.5 to 3 years of age in the human child (Erickson, Akil, Levey & Lewis, 1998). This network appears to be an extension of the limbic−striatal−pallidal circuitry involved in translating motivation to action (Baxter, Parker, Lindner, Izquierdo & Murray, 2000). This makes sense given that anatomically, the perirhinal cortex is at the interface of the ventral visual stream (the ‘what' pathway) and the limbic system, implicated in emotion processing (Paz et al., 2006). The basolateral amygdala (BLA), also part of this system, conveys to the ventral striatum associative information about environmental stimuli that predict rewards and constitute goals of behavior; these processes guide adaptive emotional responding to social stimuli (Meunier & Bachevalier, 2002). BLA also projects to the prefrontal cortex, enabling rhinal circuitry to influence the learning of social behavior (Paz, Bauer & Pare, 2008; Pollak, 2013; Wager, Davidson, Hughes, Lindquist & Ochsner, 2008). Convergence across a number of studies links early neglect to BLA circuitry regulating social behavior (Hanson, Nacewicz, Sutterer, Cayo, Schaefer et al., 2015).
We do not suggest that institutionalized settings lesion the rhinal cortex. But the present findings are consistent with reports implicating the importance of the dopamine system in facilitating associative reward learning and incentive motivation. Alterations of the dopamine system and lesions similarly affect circuitry of the rhinal cortex, and dopamine dysregulation has been associated with early child neglect (Naumova, Lee, Koposov, Szyf, Dozier et al., 2012; Wilbarger, Gunnar, Schneider & Pollak, 2010).
Possible alternative explanations
The present study has limitations, but also permits examination of some alternative explanations. It is possible that the post‐institutionalized children had generalized cognitive delays that limited their task performance. For example, the post‐institutionalized children responded more slowly throughout the task than did controls. Yet the post‐institutionalized children were just as accurate on the control condition of the task as the comparison children. In fact, the post‐institutionalized children's discrimination abilities, motivation to succeed at the task, and attentional abilities were equivalent to the comparison children. The critical component of the task is improvement over time, not overall reaction time. Another possibility is that the post‐institutionalized children's indiscriminate behavior simply reflects impulsivity. Yet, analyses of errors did not show any indication that they responded impulsively on this task. In fact, the present data are consistent with a recent report that post‐institutionalized children do not show generalized deficits in executive functioning, but rather have problems in learning (Pollak, Nelson, Schlaak, Roeber, Wewerka et al., 2010). Finally, it is possible children with pre‐existing cognitive problems were surrendered to institutional care and that their behavior is unrelated to post‐natal experience. As is the case with all research on this type, we cannot quantify the prenatal care that the children received. Therefore we included only children that were placed in institutions at or near birth, before many disabilities could be detected by parents. Second, we had a pediatric geneticist screen study participants.
Conclusion
Almost all human infants form some kind of selective relationship with their caregivers, even under abusive conditions. Although institutionalized children do learn to recognize displays of emotion (Mesquita, Belsky, Crego, Fachada, Oliveira et al., 2015), they often have difficulties recognizing specific adults as secure caregivers. Many aspects of primate socioemotional development appear dependent on response‐contingent interactions during infancy (Lamb, 1981). We speculate that the disorganization, regimented care, high child to caregiver ratios, and frequent disruptions in the constancy of caregivers in institutionalized settings all undermine the availability of the contingencies necessary for healthy socioemotional development. For example, children living in Romanian institutions characterized by high child to caregiver ratios (approximately 20 infants to one adult) are not able to identify a preferred caregiver, whereas children from Romanian institutions with lower ratios (four infants to one adult) consistently favor a preferred caregiver (Zeanah et al., 2005). Within institutionalized settings, a friendly approach to any willing adult might enhance opportunities for care. But this does not explain why indiscriminate behavior persists after children have been adopted into family environments. The present data suggest a candidate developmental mechanism underlying these problems. Socioemotional neglect may leave children with impoverished learning opportunities and experiences, making it difficult for them to confront increasingly challenging and complex social interactions.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
This project was supported by grants from the US National Institute of Health to SDP (MH068858 and MH061285). Infrastructure support was provided by the Waisman Center, University of Wisconsin through the National Institute of Child Health and Human Development (HD03352). Anna Bechner, Barb Roeber, and Dana May Albright provided invaluable assistance for this project. We acknowledge Dan Bolt for assistance with data analyses, Chuck Snowdon for providing necessary primate equipment, and Barry Richmond for making software available and providing consultation on translating this task for use with children. We also greatly appreciate the children and their families whose participation made this research possible.
References
- Astley, S.J. , Stachowiak, J. , Clarren, S.K. , & Clausen, C. (2002). Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. Journal of Pediatrics, 141 (5), 712–717. [DOI] [PubMed] [Google Scholar]
- Bakermans‐Kranenburg, M.J. , Steele, H. , Zeanah, C.H. , Muhamedrahimov, R.J. , & Vorria, P. et al (2011). Attachment and emotional development in institutional care: characteristics and catch‐up. Monographs of the Society for Research in Child Development, 76 (4, Serial No. 201), 62–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, M.G. , Parker, A. , Lindner, C.C. , Izquierdo, A.D. , & Murray, E.A. (2000). Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience, 20, 4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio, J.P. , & Mason, W.A. (2000). Cognitive style: problem solving by rhesus macaques (Macaca mulatta) reared with living or inanimate substitute mothers. Journal of Comparative Psychology, 114, 115–125. [DOI] [PubMed] [Google Scholar]
- Dobrova‐Krol, N.A. , Bakermans‐Kranenburg, M.J. , van IJzendoorn, M.H. , & Juffer, F. (2010). The importance of quality of care: effects of perinatal HIV infection and early institutional rearing on preschoolers' attachment and indiscriminate friendliness. Journal of Child Psychology and Psychiatry, 51, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Erickson, S.L. , Akil, M. , Levey, A.I. , & Lewis, D.A. (1998). Postnatal development of Tyrosine Hydroxylase‐ and dopamine transporter‐immunoreactive axons in the money rostral entorhinal cortex. Cerebral Cortex, 8, 415–427. [DOI] [PubMed] [Google Scholar]
- Gleason, M.M. , Fox, N.A. , Drury, S.S. , Smyke, A.T. , Nelson, C.A. et al (2014). Indiscriminate behaviors in previously institutionalized young children. Pediatrics, 133 (3), 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffari‐Bimmel, N. , Juffer, F. , van IJzendoorn, M.H. , Bakermans‐Kranenburg, M.J. , & Mooijaart, A. (2006). Social development from infancy to adolescence: longitudinal and concurrent factors in an adoption sample. Developmental Psychology, 42, 1143–1153. [DOI] [PubMed] [Google Scholar]
- Hanson, J.L. , Nacewicz, B.M. , Sutterer, M.J. , Cayo, A.A. , Schaefer, S.M. et al (2015). Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry, 77 (4), 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, M.E. (1981). The development of social expectations in the first year of life In Lamb M.E. & Sherrod L.R. (Eds.), Infant social cognition: Empirical and theoretical considerations (pp. 155–175). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Lawler, J.M. , Hostinar, C.E. , Mliner, S.B. , & Gunnar, M.R. (2014). Disinhibited social engagement in postinstitutionalized children: differentiating normal from atypical behavior. Development and Psychopathology, 26 (02), 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Murray, E.A. , & Richmond, B.J. (2000). Learning motivational significance of visual cues for reward schedules requires rhinal cortex. Nature Neuroscience, 3, 1307–1315. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Richmond, B.J. , Murray, E.A. , Saunders, R.C. , Steenrod, S. et al (2004). DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward. Proceedings of the National Academy of Sciences, USA, 101, 12336–12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, L. , Minnis, H. , & O'Connor, S. (2015). Factors associated with indiscriminate friendliness in high‐risk children. Infant Mental Health Journal, 36 (4), 427–445. [DOI] [PubMed] [Google Scholar]
- Lyons‐Ruth, K. (2015). Should we move away from an attachment framework for understanding disinhibited social engagement disorder (DSED)? A commentary on Zeanah and Gleason (2015). Journal of Child Psychology and Psychiatry, 56 (3), 223–227. [DOI] [PubMed] [Google Scholar]
- MacKinnon, D.P. , Lockwood, C.M. , Hoffman, J.M. , West, S.G. , & Sheets, V. (2002). A comparison of methods to test mediation and other intervening variable effects. Psychological Methods, 7, 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita, A.R. , Belsky, J. , Crego, A. , Fachada, I. , Oliveira, P. et al (2015). Neural correlates of face familiarity in institutionally reared children with distinctive, atypical social behavior. Child Development, 86, 1262–1271. [DOI] [PubMed] [Google Scholar]
- Meunier, M. , & Bachevalier, J. (2002). Comparison of emotional responses in monkeys with rhinal cortex or amygdala lesions. Emotion, 2, 147–161. [DOI] [PubMed] [Google Scholar]
- Naumova, O.Y. , Lee, M. , Koposov, R. , Szyf, M. , Dozier, M. et al (2012). Differential patterns of whole‐genome DNA methylation in institutionalized children and children raised by their biological parents. Development and Psychopathology, 24 (01), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz, R. , Bauer, E.P. , & Pare, D. (2008). Theta synchronizes the activity of medial prefrontal neurons during learning. Learning & Memory, 15, 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz, R. , Guillaume Pelletier, J. , Bauer, E.P. , & Pare, D. (2006). Emotional enhancement of memory via amygdala‐driven facilitation of rhinal interactions. Nature Neuroscience, 9, 1321–1329. [DOI] [PubMed] [Google Scholar]
- Pears, K.C. , Bruce, J. , Fisher, P.A. , & Kim, H.K. (2010). Indiscriminate friendliness in maltreated foster children. Child Maltreatment, 15, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak, S.D. (2005). Early adversity and mechanisms of plasticity: integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology, 17, 735–752. [DOI] [PubMed] [Google Scholar]
- Pollak, S.D. (2013). Emotion and learning: new approaches to the old nature–nurture debate In Gelman S. & Banaji M. (Eds.), Navigating the social world: What infants, children, and other species can teach us (pp. 54–57). New York: Oxford University Press. [Google Scholar]
- Pollak, S.D. (2015). Developmental psychopathology: recent advances and future challenges. World Psychiatry, 14, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak, S.D. , Nelson, C.A. , Schlaak, M.F. , Roeber, B.J. , Wewerka, S.S. et al (2010). Neurodevelopmental effects of early deprivation in post‐institutionalized children. Child Development, 81 (1), 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provence, S. , & Lipton, R.C. (1962). Infants in institutions. New York: International Universities Press. [Google Scholar]
- Rutter, M. , Kreppner, J. , & Sonuga‐Barke, E. (2009). Emanuel Miller Lecture: Attachment insecurity, disinhibited attachment, and attachment disorders: where do research findings leave the concepts? Journal of Child Psychology and Psychiatry, 50, 529–543. [DOI] [PubMed] [Google Scholar]
- Sanchez, M.M. , & Pollak, S.D. (2009). Socio‐emotional development following early abuse and neglect: challenges and insights from translational research In de Haan M. & Gunnar M.R. (Eds.), Handbook of developmental social neuroscience (pp. 497–520). New York: Guilford Press. [Google Scholar]
- Shohamy, D. (2011). Learning and motivation in the human striatum. Current Opinion in Neurobiology, 21 (3), 408–414. [DOI] [PubMed] [Google Scholar]
- Simpson, J.A. , & Belsky, J. (2008). Attachment theory within a modern evolutionary framework In Cassidy J. & Shaver P.R. (Eds.), Handbook of attachment: Theory, research, and clinical applications (2nd edn.) (pp. 131–157). New York: Guilford Press. [Google Scholar]
- Smyke, A.T. , Dumitrescu, A. , & Zeanah, C.H. (2002). Attachment disturbances in young children: I. The continuum of caretaking casualty. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 972–982. [DOI] [PubMed] [Google Scholar]
- Smyke, A.T. , Zeanah, C.H. , Fox, N.A. , Nelson, C.A. , & Guthrie, D. (2010). Placement in foster care enhances quality of attachment among young institutionalized children. Child Development, 81, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders, T.A.B. , & Bosker, R.J. (1999). Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Stefanacci, L. , Suzuki, W.A. , & Amaral, D.G. (1996). Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. Journal of Comparative Neurology, 375, 552–533. [DOI] [PubMed] [Google Scholar]
- Sugase‐Miyamoto, Y. , & Richmond, B.J. (2005). Neuronal signals in the monkey basolateral amygdala during reward schedules. Journal of Neuroscience, 25, 11071–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard, B. , & Rees, J. (1974). A comparison of the effects of adoption, restoration to the natural mother, and continued institutionalization on the cognitive development of four‐year‐old children. Child Development, 45, 92–99. [PubMed] [Google Scholar]
- Van Den Dries, L. , Juffer, F. , van IJzendoorn, M.H. , Bakermans‐Kranenburg, M.J. , & Alink, L.R.A. (2012). Infants' responsiveness, attachment, and indiscriminate friendliness after international adoption from institutions or foster care in China: application of Emotional Availability Scales to adoptive families. Development and Psychopathology, 24 (1), 49–64. [DOI] [PubMed] [Google Scholar]
- Wager, T.D. , Davidson, M.L. , Hughes, B.L. , Lindquist, M.A. , & Ochsner, K.N. (2008). Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbarger, J. , Gunnar, M. , Schneider, M. , & Pollak, S. (2010). Sensory processing in internationally adopted, post‐institutionalized children. Journal of Child Psychology and Psychiatry, 51 (10), 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer Fries, A.B. , Shirtcliff, E.A. , & Pollak, S.D. (2008). Neuroendocrine dysregulation following early social deprivation in children. Developmental Psychobiology, 50, 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer Fries, A.B. , Ziegler, T.E. , Kurian, J.R. , Jacoris, S. , & Pollak, S.D. (2005). Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences, USA, 102, 17237–17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah, C.H. , & Gleason, M.M. (2015). Annual research review: Attachment disorders in early childhood – clinical presentation, causes, correlates, and treatment. Journal of Child Psychology and Psychiatry, 56, 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah, C.H. , Smyke, A.T. , Koga, S.F. , & Carlson, E.A. , & the BEIP Group (2005). Attachment in institutionalized and community children in Romania. Child Development, 76, 1015–1528. [DOI] [PubMed] [Google Scholar]


