Abstract
Conjugated fatty acid, the general term of positional and geometric isomers of polyunsaturated fatty acids with conjugated double bonds, has attracted considerable attention because of its potentially beneficial biological effects. In the present study, dietary effect of pomegranate seed oil rich in punicic acid (9cis, 11trans, 13cis-conjugated linolenic acid; 9c, 11t, 13c-CLNA) on lipid metabolism was investigated in obese, hyperlipidemic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. After 2 weeks feeding period, OLETF rats revealed obesity and hyperlipidemia compared with their progenitor LETO rats. Feeding of the diet supplemented with 9% safflower oil and 1% pomegranate seed oil (9c, 11t, 13c-CLNA diet) did not affect abdominal white adipose tissue weights and serum lipid levels compared with the diet supplemented with 10% safflower oil (control diet) in OLETF rats. However, the accumulated hepatic triacylglycerol was markedly decreased by 9c, 11t, 13c-CLNA diet in OLETF rats. Activities of hepatic enzymes related to fatty acid synthesis and fatty acid β-oxidation were not altered by 9c, 11t, 13c-CLNA diet. Levels of monounsaturated fatty acid (MUFA), major storage form of fatty acid, in serum triacylglycerol were markedly higher in obese, hyperlipidemic OLETF rats than in lean LETO rats. In addition, 9c, 11t, 13c-CLNA diet significantly decreased MUFA levels in OLETF rats. This is the first study showing that 9c, 11t, 13c-CLNA suppresses delta-9 desaturation in vivo, and we suggest that the alleviation of hepatic triacylglycerol accumulation by 9c, 11t, 13c-CLNA diet was, at least in part, attributable to the suppression of delta-9 desaturation in OLETF rats.
Background
Conjugated fatty acid (CFA) is the general term of positional and geometric isomers of polyunsaturated fatty acids with conjugated double bonds. It has been reported that conjugated linoleic acid (CLA), the CFA form of linoleic acid, has favorable physiological effects, such as anti-atherosclerosis, anti-obesity, anti-tumor, and anti-hypertension [1-9]. There are also other types of CFA in some plant seed oils. Punicic acid (9cis, 11trans, 13cis-conjugated linolenic acid; 9c, 11t, 13c-CLNA) is contained about 72% in pomegranate seed oil [10]. α-Eleostearic acid (9cis, 11trans, 13trans-CLNA) is contained in bitter gourd oil and tung seed oil about 60% and 70%, respectively [10,11]. Catalpa seed oil also contains catalpic acid (9trans, 11trans, 13cis-CLNA) about 31% and pot marigold seed oil contains calendic acid (8trans, 10trans, 12cis-CLNA) about 33% [10]. There are some studies showing that mixtures of CLNA isomers, prepared by alkaline isomerization of α-linolenic acid or plant seed oil, have some physiological functions including body fat reduction and anti-tumor activity [12,13]. In addition, purified α-eleostearic acid (9c, 11t, 13t-CLNA) and α-eleostearic acid rich bitter gourd seed oil also reveal anti-carcinogenesis in vitro and in vivo [10,11,14,15]. However, there are few studies evaluated the physiological function of punicic acid (9c, 11t, 13c-CLNA) [10,16]. Previously, we reported the hypolipidemic effect of purified punicic acid in human liver derived HepG2 cells [17].
In the present study, we investigated the effects of pomegranate seed oil rich in 9c, 11t, 13c-CLNA on lipid metabolism in Otsuka Long-Evans Tokushima fatty (OLETF) rats. OLETF rats develop a syndrome with multiple metabolic and hormonal disorders that shares many features with human obesity [18-21]. OLETF rats have hyperphagia, because they lack receptors for cholecystokinin, and become obese, developing hyperlipidemia, diabetes, and hypertension. To clarify the physiological function of 9c, 11t, 13c-CLNA, we measured hepatic enzyme activities in relation to lipid metabolism and fatty acid composition in plasma of these obese, hyperlipidemic rats.
Results and Discussion
In comparison with their progenitor Long-Evans Tokushima Otsuka (LETO) rats, OLETF rats had increased body weight gain with enhanced food intake during 2 weeks feeding period (Table 1). In OLETF rats, food intake was not different between the groups. There was also no significant difference between groups in the relative liver weights of LETO and OLETF rats. Food efficiency, however, was higher in 9c, 11t, 13c-CLNA group than in other two groups. Chin et al. previously reported that CLA is a growth factor for rats as shown by enhanced weight gain and improved feed efficiency [22]. Thus, we consider that 9c, 11t, 13c-CLNA may have some growth promotional function.
Table 1.
Effect of 9c, 11t, 13c-CLNA on body weight, relative liver weight, food intake, and food efficiency
| LETO | OLETF | ||
| Control | 9c, 11t, 13c-CLNA | ||
| Body weight (g) | |||
| Initial | 223 ± 3a | 266 ± 12b | 265 ± 8b |
| Final | 282 ± 5a | 357 ± 15b | 369 ± 11b |
| Gain | 59.4 ± 2.7a | 91.3 ± 3.6b | 104 ± 6b |
| Relative liver weight (g/100 g BW) | 3.12 ± 0.09 | 3.40 ± 0.11 | 3.35 ± 0.06 |
| Food intake (g) | 17.8 ± 0.4a | 25.8 ± 1.1b | 26.2 ± 1.1b |
| Food efficiency (g BW gain/g intake) | 25.7 ± 1.0a | 27.3 ± 0.6b | 30.4 ± 1.1b |
a,bDifferent superscript letters show significant difference at P < 0.05.
The effect of dietary 9c, 11t, 13c-CLNA on the accumulation of abdominal white adipose tissue (WAT) was investigated (Figure 1). After 2 weeks feeding period, OLETF rats developed marked abdominal obesity. Compared with LETO rats, the control diet increased perirenal, epididymal, and omental WAT weights of OLETF rats to 2.6-, 1.5-, and 2.1-fold, respectively. There was no significant effect of 9c, 11t, 13c-CLNA on the accumulation of abdominal WAT in OLETF rats. However, 2 weeks feeding of the diet supplemented with 5% pomegranate seed oil resulted in a significant reduction of omental WAT weight (by 27%) compared with the feeding of control diet in OLETF rats (unpublished data). These results suggested that 2 weeks feeding of 1% pomegranate seed oil diet might not be enough to reveal anti-obese effect of 9c, 11t, 13c-CLNA.
Figure 1.
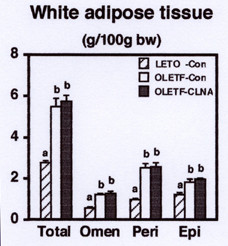
Effect of 9c, 11t, 13c-CLNA on abdominal white adipose tissue weight in LETO and OLETF rats. Rats were fed a control or 9c, 11t, 13c-CLNA diet for 2 weeks. Values are expressed as mean ± SE for 6 rats. a,bDifferent letters show significant differences at P < 0.05. Omen, omental; Peri, perirenal; Epi, epididymal.
After the 2 weeks feeding period, OLETF rats revealed hyperlipidemia. Serum triacylglycerol, phospholipids, and cholesterol levels of OLETF rats fed the control diet were significantly higher than those of LETO rats fed the control diet (Figure 2). However, feeding of 9c, 11t, 13c-CLNA did not affect to serum lipid levels in OLETF rats. Although the present results showing that dietary 1% pomegranate seed oil rich in 9c, 11t, 13c-CLNA could not alleviate hyperlipidemia in OLETF rats, our previous report indicated that purified 9c, 11t, 13c-CLNA suppressed the secretion of apolipoprotein B100 from human liver derived HepG2 cells [17]. Further studies are needed to elucidate the effect of purified 9c, 11t, 13c-CLNA on the pathogenesis of hyperlipidemia in OLETF rats.
Figure 2.
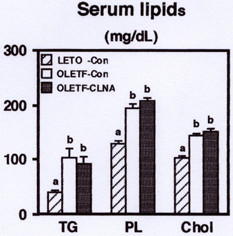
Effect of 9c, 11t, 13c-CLNA on serum lipid levels in LETO and OLETF rats. Rats were fed a control or 9c, 11t, 13c-CLNA diet for 2 weeks. Values are expressed as mean ± SE for 6 rats. a,bDifferent letters show significant differences at P < 0.05. TG, triacylglycerol; PL, phospholipids; Chol, cholesterol.
Next, we investigated the effect of dietary 9c, 11t, 13c-CLNA on the distribution of lipids to the liver. There was no significant difference in relative liver weight between control and 9c, 11t, 13c-CLNA group in OLETF rats. Previous reports indicated that CLA feeding resulted in the development of hepatomegaly and fatty liver in mice [23-25], and a mixture of CLNA also induced hepatic lipid accumulation in rat [13]. In the present study, the triacylglycerol concentration in OLETF rats was significantly higher than that in LETO rats, and the triacylglycerol accumulation in the liver of OLETF rats was markedly alleviated by the 9c, 11t, 13c-CLNA diet (Figure 3). There was no significant difference in liver phospholipids and cholesterol levels among groups in LETO and OLETF rats. These results suggest that 9c, 11t, 13c-CLNA has a preventive effect against the triacylglycerol accumulation in the liver.
Figure 3.
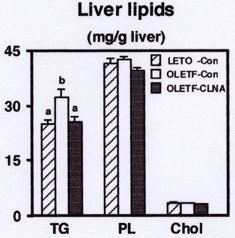
Effect of 9c, 11t, 13c-CLNA on hepatic lipid levels in LETO and OLETF rats. Rats were fed a control or 9c, 11t, 13c-CLNA diet for 2 weeks. Values are expressed as mean ± SE for 6 rats. a,bDifferent letters show significant differences at P < 0.05. TG, triacylglycerol; PL, phospholipids; Chol, cholesterol.
To further investigate the regulation of hepatic lipid metabolism, we analyzed the effect of dietary 9c, 11t, 13c-CLNA on the activities of enzymes related to fatty acid synthesis and fatty acid β-oxidation. As shown in Figure 4A, the activities of glucose-6-phosphate dehydrogenase (G6PDH) and malic enzyme (ME), key enzymes of NADPH production, and fatty acid synthase (FAS), a key enzyme of fatty acid synthesis, were markedly increased in OLETF rats fed the control diet compared with LETO rats. There was no significant effect of dietary 9c, 11t, 13c-CLNA on these enzyme activities in OLETF rats. The activities of carnitine palmitoyltransferase (CPT), a key enzyme of fatty acid β-oxidation, and peroxisomal β-oxidation were not different between OLETF and LETO rats, and 9c, 11t, 13c-CLNA diet did not affect on these activities in OLETF rats (Figure 4B). Koba et al. previously reported that a mixture of CLNA isomers, prepared by alkaline isomerization, enhanced hepatic mitochondrial and peroxisomal β-oxidation compared with linoleic acid, α-linolenic acid, and CLA [13]. Thus, we consider that the effect of 9c, 11t, 13c-CLNA on the fatty acid β-oxidation is weak compared with those of other CLNA isomers. In addition, the alleviation of hepatic triacylglycerol accumulation by 9c, 11t, 13c-CLNA could not be attributed to the regulation of enzyme activities related to the fatty acid synthesis and fatty acid β-oxidation.
Figure 4.
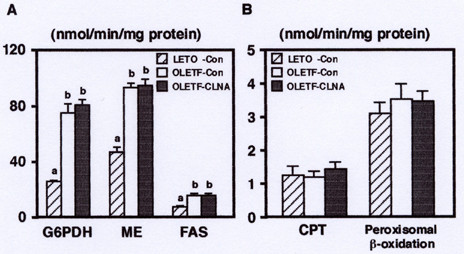
Effect of 9c, 11t, 13c-CLNA on activities of enzymes related to lipid metabolism, (A) G6PDH, ME, FAS (B) CPT, peroxisomal β-oxidation, in the liver of LETO and OLETF rats. Rats were fed a control or 9c, 11t, 13c-CLNA diet for 2 weeks. Values are expressed as mean ± SE for 6 rats. a,bDifferent letters show significant differences at P < 0.05.
To gain insight into the effect of dietary 9c, 11t, 13c-CLNA on lipid metabolism, we analyzed fatty acid composition in serum triacylglycerol. As shown in Table 2, saturated fatty acid (SFA) levels were lower and monounsaturated fatty acid (MUFA) levels were higher in OLETF rats fed the control diet than those in LETO rats. Feeding of 9c, 11t, 13c-CLNA significantly reduced MUFA levels in plasma triacylglycerol of OLETF rats. It has been recognized that MUFAs are the major fatty acid form in fat depots [26]. Alterations in the ratio of SFA to MUFA have been implicated in various disease states including cardiovascular disease, obesity, and diabetes [27-29]. Therefore, the ratio of SFA to MUFA is of physiological importance in normal and disease states. A key enzyme involved in the cellular synthesis of MUFA from SFA is the membrane-bound stearoyl-CoA desaturase (SCD), which inserts a cis-double bond in the delta-9 position of fatty acid substrates. Previous reports indicated that 10t, 12c-CLA, an active isomer of anti-obese effect of CLA, suppresses delta-9 desaturation and SCD activity in vitro and in vivo [30-32]. In the present study, the index of delta-9 desaturation, ratio of oleic acid (18:1) versus stearic acid (18:0), was higher in obese, hyperlipidemic OLETF rats compared with in lean LETO rats, and it was significantly decreased by dietary 9c, 11t, 13c-CLNA in OLETF rats. As far as we know, this is the first study showing that 9c, 11t, 13c-CLNA also suppresses delta-9 desaturation in vivo. We suggest that the alleviation of hepatic triacylglycerol accumulation by dietary 9c, 11t, 13c-CLNA was, at least in part, attributable to the suppression of delta-9 desaturation in OLETF rats.
Table 2.
Effect of 9c, 11t, 13c-CLNA on fatty acid composition in serum triacylglycerol.
| LETO | OLETF | ||
| Control | 9c, 11t, 13c-CLNA | ||
| % | |||
| 14:0 | 2.28 ± 0.23a | 1.05 ± 0.15b | 1.10 ± 0.13b |
| 16:0 | 36.8 ± 0.7a | 28.1 ± 0.7b | 41.2 ± 2.7a |
| 16:1 | 0.492 ± 0.066a | 3.83 ± 0.25b | 2.39 ± 0.20c |
| 18:0 | 5.58 ± 0.63a | 3.29 ± 0.26b | 4.04 ± 0.32b |
| 18:1 | 11.7 ± 0.7a | 24.5 ± 1.4b | 20.4 ± 1.4c |
| 18:2 | 35.5 ± 0.7a | 33.2 ± 0.9a | 27.8 ± 1.4b |
| 20:4 | 7.74 ± 0.76a | 4.80 ± 0.40b | 3.12 ± 0.44c |
| Desaturation index | |||
| Δ9 desaturation | 2.18 ± 0.24a | 7.73 ± 0.83b | 5.34 ± 0.75c |
| Δ6 desaturation | 0.219 ± 0.024a | 0.144 ± 0.009b | 0.110 ± 0.011b |
a,b,cDifferent superscript letters show significant difference at P < 0.05.
Conclusions
Dietary pomegranate seed oil rich in 9c, 11t, 13c-CLNA alleviates hepatic triacylglycerol accumulation in obese, hyperlipidemic OLETF rats. The mechanism of this effect could not be attributed to the regulation of enzyme activity related to fatty acid synthesis and fatty acid β-oxidation. However, suppression of delta-9 desaturation by dietary 9c, 11t, 13c-CLNA may be, at least in part, involved this effect.
Materials and Methods
Animals and diets
All aspects of the experiment were conducted according to the guidelines provided by the ethical committee of experimental animal care at Saga University. Five weeks old male OLETF rats and LETO rats, the progenitor of OLETF rats, were provided by Tokusima Research Institute (Otsuka Pharmaceutical, Tokushima, Japan). Rats were housed individually in metal cages in temperature-controlled room (24°C) under a 12-hour light/dark cycle. After a 1-week adaptation period, OLETF rats were assigned to two groups (six rats each) that were fed with a semisynthetic diet supplemented with 10% safflower oil (the control group) or a semisynthetic diet supplemented with 9% safflower oil and 1% pomegranate seed oil rich in 9cis, 11trans, 13cis-CLNA (the 9c, 11t, 13c-CLNA group). LETO rats were fed the same diet as the OLETF rats in the control group. The pomegranate seed oil rich in 9c, 11t, 13c-CLNA (69.0%) was prepared by Kaneka Co. (Hyogo, Japan). The semisynthetic diet were prepared according to recommendations of the AIN-76 [33] and contained (in weight %): casein, 20; fat, 10; cornstarch, 15; vitamin mixture (AIN-76™), 1; mineral mixture (AIN-76™), 3.5; DL-methionine, 0.3; choline bitartrate, 0.2; cellulose, 5; and sucrose, 45. The rats received different diets for 2 weeks and were killed by aortic exsanguinations under diethyl ether anesthesia. Liver and abdominal (perirenal, epididymal, and omental) WATs were also excised for analysis.
Analysis of lipids
Serum was separated by centrifuging the blood. Triacylglycerol, cholesterol, and phospholipids in serum were measured using enzyme assay kits from Wako Pure Chemicals (Tokyo, Japan). Liver lipids were extracted and purified according to the method of Folch et al [34]. The concentrations of triacylglycerol, cholesterol, and phospholipids were measured according to the methods of Fletcher [35], Sperry and Webb [36], and Bartlett [37]. Measurement of fatty acid composition in plasma was carried out as previously described [38,39].
Preparation of liver subcellular fractions
A piece of liver was homogenized in 6 volumes of a 0.25 M sucrose solution that contained 1 mM EDTA in a 10 mM tris Tris-HCL buffer (pH 7.4). Fractions of mitochondria, microsomes, and cytosol were obtained as previously described[40]. The protein concentration was determined according to the method of Lowry et al [41], with bovine serum albumin used as the standard.
Assays of enzyme activity
The enzyme activities of ME (EC 1.1.1.40) [42], G6PDH (EC1.1.1.49) [43], FAS (EC 2.3.1.85) [44] in the liver cytosol fraction, mitochondrial CPT (EC2.3.1.23) [45] and peroxisomal β-oxidation [46] were determined as described.
Statistical analysis
All values are expressed as means ± SE. Data were analyzed by one-way ANOVA, and all differences were inspected by Duncan's new multiple-range test [47]. Differences were considered to be significant at P<0.05.
List of abbreviations
CFA, conjugated fatty acid; CLA, conjugated linoleic acid; CLNA, conjugated linolenic acid; OLETF rat, Otsuka Long-Evans Tokushima fatty rat; LETO rat, Long-Evans Tokushima Otsuka rat; WAT, white adipose tissue; G6PDH, glucose-6-phosphate dehydrogenase; ME, malic enzyme; FAS, fatty acid synthase; CPT, carnitine palmitoyltransferase; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; SCD, stearoyl-CoA desaturase
Acknowledgments
Acknowledgement
We thank Satoko Ikegami and Masanori Ushijima for technical assistance. This work was supported by a research grant from the Japanese Ministry of Education, Science and Culture.
Contributor Information
Keisuke Arao, Email: k-arao@mm.neweb.ne.jp.
Yu-Ming Wang, Email: a0217@cc.saga-u.ac.jp.
Nao Inoue, Email: 04972004@edu.cc.saga-u.ac.jp.
Junichi Hirata, Email: 03am75@edu.cc.saga-u.ac.jp.
Jae-Young Cha, Email: e996390jp@yahoo.co.jp.
Koji Nagao, Email: knagao@cc.saga-u.ac.jp.
Teruyoshi Yanagita, Email: yanagitt@cc.saga-u.ac.jp.
References
- Lee KN, Kritchevsky D, Pariza MW. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis. 1994;108:19–25. doi: 10.1016/0021-9150(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Nicolosi RJ, Rogers EJ, Kritchevsky D, Scimeca JA, Huth PJ. Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery. 1997;22:266–277. [PubMed] [Google Scholar]
- Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW. Evidence that the trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34:235–241. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- Park Y, Albright KJ, Storkson JM, Liu W, Cook ME, Pariza MW. Changes in body composition in mice during feeding and withdrawal of conjugated linoleic acid. Lipids. 1999;34:243–248. doi: 10.1007/s11745-999-0359-7. [DOI] [PubMed] [Google Scholar]
- Ip C, Singh M, Thompson HJ, Scimeca JA. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–1215. [PubMed] [Google Scholar]
- Ip C, Scimeca JA, Thompson HJ. Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources. Cancer. 1994;74:1050–1054. doi: 10.1002/1097-0142(19940801)74:3+<1050::aid-cncr2820741512>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Nagao K, Inoue N, Wang YM, Hirata J, Shimada Y, Nagao T, Matsui T, Yanagita T. The 10trans, 12cis isomer of conjugated linoleic acid suppresses the development of hypertension in Otsuka Long-Evans Tokushima fatty rats. Biochem Biophys Res Commun. 2003;306:134–138. doi: 10.1016/s0006-291x(03)00929-x. [DOI] [PubMed] [Google Scholar]
- Nagao K, Inoue N, Wang YM, Yanagita T. Conjugated linoleic acid enhances plasma adiponectin level and alleviates hyperinsulinemia and hypertension in Zucker diabetic fatty (fa/fa) rats. Biochem Biophys Res Commun. 2003;310:562–566. doi: 10.1016/j.bbrc.2003.09.044. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Noguchi R, Ota T, Abe M, Miyashita K, Kawada T. Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells. Lipids. 2001;36:477–482. doi: 10.1007/s11745-001-0746-0. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Noguchi R, Hosokawa M, Miyashita K, Tanaka T. Dietary conjugated linolenic acid inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Jpn J Cancer Res. 2002;93:133–142. doi: 10.1111/j.1349-7006.2002.tb01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Miyazawa T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 2000;148:173–179. doi: 10.1016/s0304-3835(99)00332-8. [DOI] [PubMed] [Google Scholar]
- Koba K, Akahoshi A, Yamasaki M, Tanaka K, Yamada K, Iwata T, Kamegai T, Tsutsumi K, Sugano M. Dietary conjugated linolenic acid in relation to CLA differently modifies body fat mass and serum and liver lipid levels in rats. Lipids. 2002;37:343–350. doi: 10.1007/s11745-002-0901-7. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T, Tokuyama Y, Igarashi M, Miyazawa T. Tumor growth suppression by α-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation. Carcinogenesis. 2004;25:1417–1425. doi: 10.1093/carcin/bgh109. [DOI] [PubMed] [Google Scholar]
- Kohno H, Yasui Y, Suzuki R, Hosokawa M, Miyashita K, Tanaka T. Dietary seed oil rich in conjugated linolenic acid from bitter melon inhibits azoxymethane-induced rat colon carcinogenesis through elevation of colonic PPARγ expression and alteration of lipid composition. Int J Cancer. 2004;110:896–901. doi: 10.1002/ijc.20179. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T. Pomegranate seed oil rich in conjugated linoleic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004;95:481–486. doi: 10.1111/j.1349-7006.2004.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arao K, Yotsumoto H, Han SY, Nagao K, Yanagita T. The 9cis, 11trans, 13cis isomer of conjugated linolenic acid reduces apolipoprotein B100 secretion and triacylglycerol synthesis in HepG2 cells. Biosci Biotechnol Biochem. 2004;68:395–397. doi: 10.1271/bbb.68.2643. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994;24:S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin-type A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. [DOI] [PubMed] [Google Scholar]
- Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A (CCKA) receptors. Am J Physiol. 1998;274:R618–R625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- Chin SF, Storkson JM, Albright KJ, Cook ME, Pariza MW. Conjugated linoleic acid is a growth factor for rats as shown by enhanced weight gain and improved feed efficiency. J Nutr. 1994;124:694–701. doi: 10.1093/jn/124.12.344. [DOI] [PubMed] [Google Scholar]
- Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, Kim HJ, Tnage T, Okuyama H, Kasai M, Ikemoto S, Ezaki O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 2000;49:1534–1542. doi: 10.2337/diabetes.49.9.1534. [DOI] [PubMed] [Google Scholar]
- Clement L, Pirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, Staels B, Besnard P. Dietary trans-10, cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res. 2002;43:1400–1409. doi: 10.1194/jlr.m20008-jlr200. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kushiro M, Shinohara K, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid synthesis and oxidation in mice fed conjugated linoleic acid. Biochim Biophys Acta. 2003;1631:265–273. doi: 10.1016/s1388-1981(03)00038-6. [DOI] [PubMed] [Google Scholar]
- Insull W, Jr, Bartsch GE. Fatty acid composition of human adipose tissue related to age, sex and race. Am J Clin Nutr. 1967;20:13–23. doi: 10.1093/ajcn/20.1.13. [DOI] [PubMed] [Google Scholar]
- Jones BH, Maher MA, Banz WJ, Zemel MB, Whelan J, Smith PJ, Moustaid N. Adipose tissue stearoyl-CoA desaturase mRNA is increased by obesity and decreased by polyunsaturated fatty acids. Am J Physiol. 1996;271:E44–E49. doi: 10.1152/ajpendo.1996.271.1.E44. [DOI] [PubMed] [Google Scholar]
- Pan DA, Hulbert AJ, Storlien LH. Dietary fats, membrane phospholipids and obesity. J Nutr. 1994;124:1555–1565. doi: 10.1093/jn/124.9.1555. [DOI] [PubMed] [Google Scholar]
- Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- Park Y, Storkson JM, Ntambi JM, Cook ME, Sih CJ, Pariza MW. Inhibition of hepatic stearoyl-CoA desaturase activity by trans-10, cis-12 conjugated linoleic acid and its derivatives. Biochem Biophys Acta. 2000;1486:285–292. doi: 10.1016/s1388-1981(00)00074-3. [DOI] [PubMed] [Google Scholar]
- Choi Y, Park Y, Pariza MW, Ntambi JM. Regulation of stearoyl-CoA desaturase activity by the trans-10, cis-12 isomer of conjugated linoleic acid in HepG2 cells. Biochem Biophys Res Commun. 2001;284:689–693. doi: 10.1006/bbrc.2001.5036. [DOI] [PubMed] [Google Scholar]
- Lin X, Loor JJ, Herbein JH. Trans 10, cis12–18:2 is a more potent inhibitor of de novo fatty acid synthesis and desaturation than cis9, trans11–18:2 in the mammary gland of lactating mice. J Nutr. 2004;134:1362–1368. doi: 10.1093/jn/134.6.1362. [DOI] [PubMed] [Google Scholar]
- American Institute of Nutrition Report of the American Institute of Nutrition of ad hoc Committee on standards for nutritional studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- Folch J, Lee M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fletcher MJ. A colorimetric method for estimation of serum triglycerides. Clin Chim Acta. 1968;22:393–397. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Sperry WM, Webb M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950;187:97–106. [PubMed] [Google Scholar]
- Bartlett GR. Colorimetric assay methods for free and phosphorylated glyceric acids. J Biol Chem. 1959;234:469–471. [PubMed] [Google Scholar]
- Christie WW. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J Lipid Res. 1982;23:1072–1075. [PubMed] [Google Scholar]
- Park Y, Albright KJ, Storkson JM, Liu W, Cook ME, Pariza MW. Changes in body composition in mice during feeding and withdrawal of conjugated linoleic acid. Lipids. 1999;34:243–248. doi: 10.1007/s11745-999-0359-7. [DOI] [PubMed] [Google Scholar]
- Ikeda I, Cha JY, Yanagita T, Nakatani N, Oogami K, Imaizumi K, Yazawa K. Effects of dietary α-linoleic, eicosapentaenoic and docosahexaenoic acids on hepatic lipogenesis and β-oxidation in rats. Biosci Biotechnol Biochem. 1998;62:675–680. doi: 10.1271/bbb.62.675. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Ochoa S. Malic enzyme: malic enzymes from pigeon and wheat germ. In: Colowick SP, Kaplan NO, editor. In Methods in Enzymology. Vol. 1. New York: Academic Press; 1955. pp. 323–326. [Google Scholar]
- Kelley DS, Kletzien RF. Ethanol modulation of the hormonal and nutritional regulation of glucose 6-phosphate dehydrogenase activity in primary cultures of rat hepatocytes. Biochem J. 1984;217:543–549. doi: 10.1042/bj2170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DS, Nelson GJ, Hunt JE. Effect of prior nutritional status on the activity of lipogenic enzymes in primary monolayer cultures of rat hepatocytes. Biochem J. 1986;235:87–90. doi: 10.1042/bj2350087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell MAK, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. J Biol Chem. 1973;248:3426–3432. [PubMed] [Google Scholar]
- Lazarow PB. Assay of peroxisomal β-oxidation of fatty acids. In: Lowenstein JM, editor. Methods in Enzymology. Vol. 72. New York: Academic Press; 1981. pp. 315–319. [DOI] [PubMed] [Google Scholar]
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]


