Abstract
Huntington's disease (HD) is a neurodegenerative disorder caused by a mutation in the HTT gene (mHTT) encoding the protein huntingtin. An expansion in the gene's CAG repeat length renders a misfolded, dysfunctional protein with an abnormally long glutamine (Q) stretch at the N terminus that often incorporates into inclusion bodies and leads to neurodegeneration in many regions of the brain. HD is characterized by motor and cognitive decline as well as mood disorders, with depression being particularly common. Approximately 40% of the HD population suffers from depressive symptoms. Because these symptoms often manifest a decade or more prior to the knowledge that the person is at risk for the disease, a portion of the early depression in HD appears to be a consequence of the pathology arising from expression of the mutant gene. While the depression in HD patients is often treated with serotonin agonists, there is scant experimental evidence that the depression in HD responds well to these serotonin treatments or in a similar manner to how non-HD depression tends to respond. Additionally, at very early sub-threshold depression levels, abnormal changes in several neuronal populations are already detectable in HD patients, suggesting that a variety of brain structures may be involved. Taken together, the serotonin system is a viable candidate. However, at present there is limited evidence of the precise nuclei or circuits that play a role in HD depression. With this in mind, the current study was designed to control for the widespread brain neuropathology that occurs in HD and in transgenic mouse models of HD and focuses specifically on the influence of the midbrain dorsal raphe nucleus (DRN). The DRN provides the majority of the serotonin to the forebrain and exhibits cell loss in non-HD depression. Therefore, we employed a viral vector delivery system to investigate whether the over-expression of mHTT in the DRN's ventral sub-nuclei alone is sufficient to produce depressive-like behaviors. Wildtype mice were injected with an adeno-associated virus (AAV2/1) encoding HTT containing either a pathogenic (N171-82Q) or control (N171-16Q) CAG repeat length into the ventral DRN and depressive-like behaviors and motor behaviors were assessed for 12 weeks post-surgery. Quantitative PCR and immunohistochemistry (IHC) verified positive transduction in the ventral aspects of the DRN, including the ventral sub-nucleus (DRv) and interfascicular sub-nucleus (DRif). IHC demonstrated microgliosis in and around the injection site and mHTT-positive inclusions in serotonin-producing neurons and a small percentage of astrocytes in animals injected with N171-82Q compared to controls. Moreover, N171-82Q injected mice showed a 75% reduction in cells that stained positive for the serotonin synthesis enzyme, tryptophan hydroxylase-2 (TPH2) compared to controls (p <0.05). Despite mHTT-mediated pathology in the DRv and DRif, no significant changes in depressive-like behavior were detected. Consequently, we conclude that 12 weeks of N171-82Q expression in the ventral sub-nuclei of the DRN of wildtype mice causes characteristic disease-related cellular neuropathology but is not sufficient to elicit depressive-like behaviors. Ongoing studies are investigating whether a larger injection volume that transfects a larger percentage of the DRN and/or a longer time course of mHTT expression might elicit depressive-like behaviors. Moreover, mHTT expression in other regions of the brain, such as the hippocampal dentate gyrus and/or the frontal cortex might be necessary to elicit HD depression. Together, these results may prove helpful in addressing which therapeutic and/or pharmacological strategies might be most efficacious when treating depressive symptomology in patients suffering from HD.
Keywords: Huntington's disease, Depression, Tryptophan hydroxylase-2, TPH2
1. Introduction
Huntington's disease (HD) is a progressive, autosomal dominant, neurodegenerative disorder caused by the inheritance of a CAG repeat expansion mutation in the HTT gene (mHTT) on chromosome 4. mHTT encodes a mutant huntingtin protein (mHTT) that contains an abnormally long glutamine (Q) stretch at the N terminus of the protein. Alleles that contain more than 38 CAG repeats produce a dysfunctional mHTT protein that forms intra-cellular inclusion bodies (Perutz et al., 1994) and causes widespread cellular dysfunction and apoptosis (Adam and Jankovic, 2008). HD is characterized by motor dysfunction, cognitive decline and severe mood disorders (Bates et al., 2002). While the involuntary motor movements, referred to as chorea, are frequently the most outwardly distinguishing symptoms of the disease, psychological manifestations including depression, irritability, and apathy often arise prior to motor changes (Folstein and Folstein 1983; Kingma et al., 2008). Forty percent of HD patients exhibit depressive symptomology at a rate that is approximately twice that of the general population (Paulsen et al., 2005). Suicidal ideation, which is strongly associated with depression, is also prevalent with 10% of HD patients reporting at least one suicide attempt during their disease progression (Paulsen et al. 2005; Wetzel et al., 2011). Historically, it was thought that depressive symptoms in HD resulted from the diagnosis of a debilitating and fatal disease. However, more recent studies demonstrate that these psychiatric symptoms often manifest before the person is cognizant that they are at risk of the disease (Duff et al., 2007; Julien et al., 2007; Paulsen et al., 2008), suggesting that these symptoms result from intrinsic mHTT-related neuropathology.
Progressive neurodegeneration in the HD brain is considerable, particularly in the striatum and cortex. However, mHTT is expressed ubiquitously and inclusions, cellular dysfunction and cell death occur throughout the brain, including structures mediating mood such as the midbrain dorsal raphe nucleus (DRN; Krogias et al., 2011). The DRN is the largest of the eight raphe nuclei, projecting to forebrain structures including the striatum, hippocampus, prefrontal cortex, nucleus accumbens and the amygdala (Michelsen et al., 2007). In the normal population, depression is associated with altered serotonin signaling (Matthews and Harrison, 2012) and a loss of neurons in the DRN (Baumann et al., 2002). The role of serotonin and the DRN in HD is less well understood. Transcranial sonography of the brains of living HD participants indicates that 71% of HD patients suffering from depression exhibit DRN pathology, as evidenced by hyperechogenecity in this brain region. In contrast, no DRN abnormalities are found in those HD patients without depression (Krogias et al., 2011). However, there are widespread changes that occur in the CNS of these patients. Moreover, studies employing diffusion tensor imaging show that there are structural changes in areas of the frontal, anterior cingulate cortex and insular cortices as well as the cerebellum in HD patients with sub-threshold depression (Sprengelmeyer et al., 2014).
Studies that have sought to elucidate serotonin's functional role in HD depression have largely employed mouse models of the disease. Transgenic HD mice, which possess a fragment of human mHTT throughout each cell of the body, exhibit depressive-related behaviors which correlate with decreased levels of 5-HT and its metabolite 5-HIAAaswell asareductionin5-HT turnover in the striatum, hippocampus and prefrontal cortex (Mochel et al., 2011) and a reduction of serotonin transporter (SerT) in the cortex (Pang et al., 2009). Similarly, large transient reductions of serotonin synthesis throughout the entire normal rat brain via systemic administration of para-chlorophenylalanine (PCPA) reduce whole brain of 5-HT levels by inhibiting the rate limiting enzyme tryptophan hydroxylase-2 (TPH2; Koe and Weissman, 1966). PCPA treatments that reduce whole brain 5-HT levels more than 85% can produce depressive-like behavior in rodents as measured by the Forced Swim Task (Page et al., 1999).
Taken together, there are multiple lines of evidence suggesting an association among 5-HT synthesis and depressive-like behaviors in humans without HD and in transgenic HD mouse models that express mHTT throughout the entire brain. However, it is essential to highlight that this evidence supporting an association between serotonin and depressive behaviors was gleaned in subjects that have either widespread neuropathology or whole brain depletions of serotonin. Thus, at present, it remains unclear which specific brain structure(s), circuits or ligands are mediating the depressive-like behaviors observed in HD. The DRN is an attractive candidate because it supplies much of the serotonin to the forebrain and its association with depressive behaviors in non-HD patients. Consequently, here we take advantage of a unique viral vector-mediated delivery system which allows us to restrict mHTT expression within the cells of the DRN alone. Specifically, we used adeno-associated viral vectors (AAV2/1) that express constructs which encode a fragment of the human huntingtin gene, 171 amino acid long N-terminus of the human huntingtin gene. The constructs encoded either 82 CAG repeats (N171-82Q), known to be pathogenic, or the non-pathogenic construct that encodes 16 CAG repeats (N171-16Q). Either N171-82Q, N171-16Q or saline were infused into the ventral DRN of wildtype mice and depressive-like behaviors were assessed over the subsequent 12 weeks. The goal of this study was to determine whether the DRN serotonin system might be a key player in the depression observed in HD. The results from these experiments might provide insight into therapies for the depression that strikes early in persons with HD.
2. Results
2.1. Stereotaxic intra-DRN injections of N171-82Q or N171-16Q constructs encapsulated within AAV2/1
To address whether mHTT expression in the cells of the DRN alone produced HD-related neuropathological changes and depressive-like behaviors, we delivered a single, 0.5 ul injection into the ventral DRN of male and female wildtype mice of either PBS (n=12) or AAV2/1 which expressed one of two versions of the N-terminus fragment of the human mHTT driven a CAG promoter (Fig. 1A). More specifically, the 171 amino acid long constructs expressed either 82 CAG repeats (N171-82Q; n=13) or 16 CAG repeats (N171-16Q; n=13). Vector injected mice received a total of 2.5e8 total viral genomes injected stereotaxically at a 20 degree angle targeting the center of the DRv (Fig. 1B). Post-necropsy, injection sites were verified using either immunohistochemistry (IHC) or quantitative PCR (qPCR). Half the animals were perfused and brain tissue stained using antibodies to identify soluble and aggregated huntingtin fragments (anti-1-82Q and anti-EM48). We then assessed the extent that our constructs were expressed within the borders of the DRN and also the ventral sub-nucleus (DRv) and the interfascicular sub-nucleus (DRif) as defined by the mouse atlas from Franklin and Paxinos (2008) by performing an area fraction fractionator analysis using Microbrightfield Unbiased Stereology Software and assessing cells that stained positive for 1-82Q (identifies both soluble and aggregated huntingtin). The analysis indicated that our injections filled 29.1% of the entire DRN volume, primarily in the ventral aspects of the DRN. Successful injections were identified as positive staining throughout both the DRv and DRif sub-nuclei as identified by Franklin and Paxinos (2008) mouse atlas. In animals allocated for molecular analyses, the entire DRN was immediately microdissected under a dissecting microscope from saline-perfused brain and the expression of the human mHTT transgene was verified by qPCR. Six animals and their data were eliminated from the study due to injections that were located outside of these parameters. Consequently, the data from a total of 32 animals (both male and female) were used in behavioral, histochemical and molecular analyses.
Fig. 1.
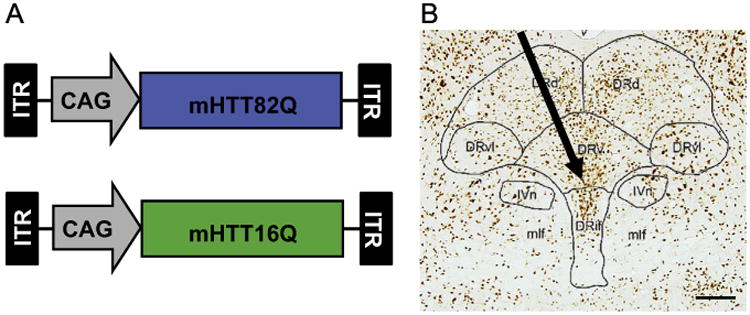
(A) Schematics of the two AAV2/1 vector constructs. Both the mHTT with 82 glutamine repeats (blue) and the mHTT with 16 repeats (green) are driven by a chicken beta-actin promoter containing a CMV enhancer element (CAG). B: A representative coronal section of a mouse midbrain stained for the neuronal nuclear protein NeuN. A schematic overlay (modified from Franklin and Paxinos (2008)) illustrates the subnuclei of the dorsal raphe nucleus. The arrow shows the approximate 20 degree path of the injection needle, targeting the DRv and DRif. The injection angle was used to avoid damaging the superior sagittal sinus. (Abbreviations: DRd: Dorsal subnucleus of the DRN, DRvl: Ventrolateral sub-nucleus, DRv: Ventral sub-nucleus, DRif: Interfascicular sub-nucleus, mfl: Medial longitudinal fasciculus, IVn: Trochlear nucleus; Scale bar=100 μm).
2.2. Over-expression of mHTT in the DRv and DRif causes disease-associated neuropathology
In order to assess cellular pathology in and around the injection site, 20um coronal, midbrain sections were stained for activated microglia. Using antibodies against ionized calcium binding adapter molecule 1 (Iba-1), we employed standard immunohistochemical procedures and identified resting and activated microglia in the brains of mice that were sacrificed 12 weeks post-surgery. PBS (Fig. 2A) and N171-16Q (Fig. 2B) injected mice exhibited only resting microglia. In contrast, the N171-82Q injected mice exhibited enlarged and darkly-stained Iba-1 positive reactive microglia in the region of the injection (Fig. 2C). To determine the possibility that the microglia were in a reactive state due to being transduced by the viral vector and expressing the N171-82Q constructs, we double-labeled tissue sections against Iba-1 and 1-82Q, which detects soluble and accumulated mutant huntingtin. We found no evidence of mutant huntingtin expression in microglia throughout the injected areas (Fig. 5), suggesting that the microglial response near the injection sites was due to the ongoing expression of N171-82Q in nearby neurons or astrocytes rather than a response to the microglia being transduced by AAV2/1 vectors. Interestingly, at 12 weeks post-injection, the bulk of reactive microglia was observed in the DRd, adjacent to regions containing 1-82Q- positive cells in the DRv and DRif. While the reasoning for this is unclear, we hypothesize that there may have been cells in the DRd that were also transduced with vector that had already undergone degeneration at the time of necropsy. A future timecourse study will be necessary in determining the spatio-temporal development of reactive microgliosis that results from the overexpression of mutant HTT in the DR.
Fig. 2.
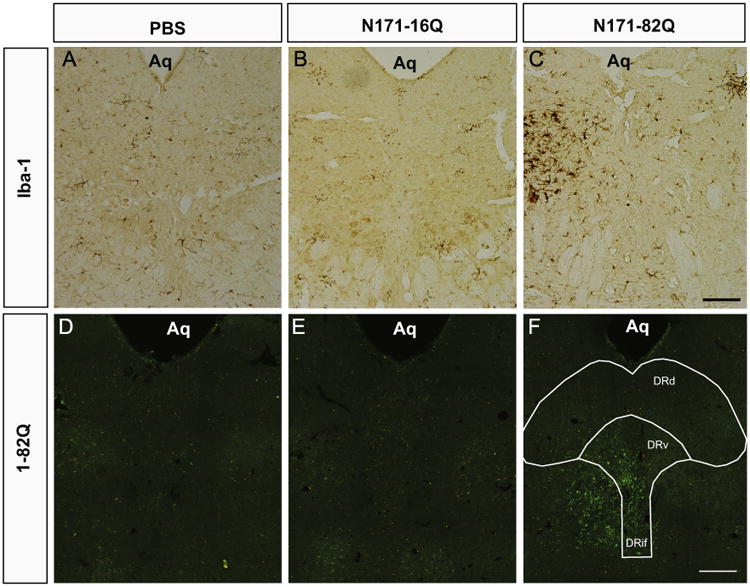
Representative photomicrographs of midbrain coronal sections stained with anti-Iba-1 to identify resting and activated microglia 12 weeks post-surgery (A–C). In the PBS (A) and N171-16Q (B) sections, microglia are lightly stained and display a resting morphology. In contrast, the N171-82Q midbrain section (C) exhibits darkly stained cell bodies, thickened extensions and ameboid shaped cells. The darkest stained microglia are distributed laterally on both sides of the midline injection site. Midbrain sections were also stained for human mutant huntingtin inclusions using antibodies against EM48 (D, E, and F). Punctate green fluorescent staining is absent in animals that received intra-DRN PBS (A) and extremely limited in animals infused with AAV2/1 encapsulating N171-16Q (B). By contrast, EM48-positive inclusions, as indicated by green fluorescence are found in the animals infused with N171-82Q. Positive staining is observed in the ventral DRN, including the DRif and DRV and surrounding the medial longitudinal fasciculus and laterally into the adjacent midbrain (Aq=Aqueduct of Sylvius; Scale bar=100 um).
Fig. 5.
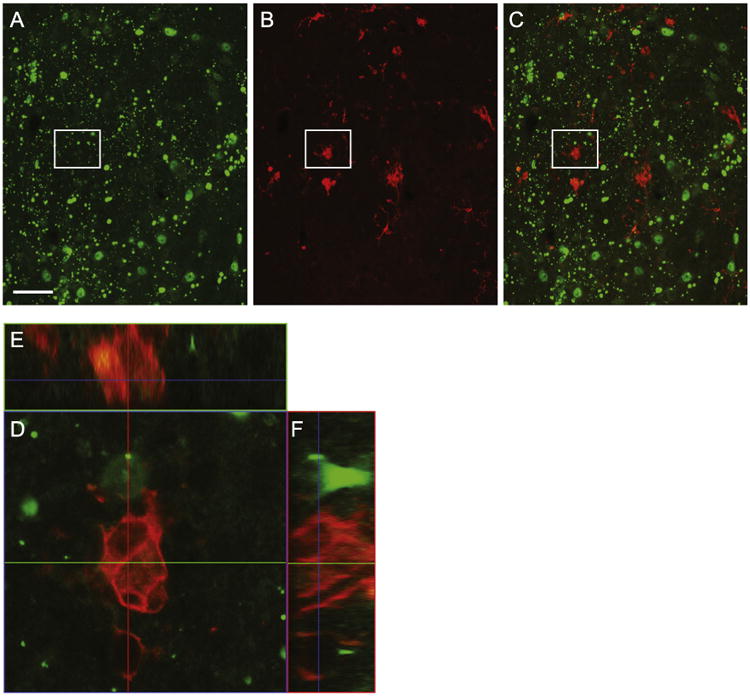
To assess the possibility that microglia were transduced and expressing N171-82Q, 20 μm sections of the DRif were stained for Iba-1 and 1-82Q. Iba-1-positive microglia (red, B) and anti-1-82Q (green, A) soluble and aggregations of mutant huntingtin (Fig. 5C). We evaluated each reactive microglia for 1-82Q-positive staining (an example is Fig. 5D and corresponding orthogonal images E and F). However, no colocalization was detected (Scale bar=50 μm).
A hallmark of human HD brain pathology is the development of mHTT-positive inclusion bodies throughout many regions of the brain. While mHTT-positive inclusion bodies have yet to be described in the DRN of human HD patients, transgenic HD mice expressing the N171-82Q fragment of human mHTT, in addition to their two endogenous mouse HTT alleles, exhibit inclusion bodies in the DRN (unpublished data from the McBride laboratory). In the current study, wildtype mice injected with AAV2/1 expressing N171-82Q constructs exhibit EM48-positive and 1-82Q-positive inclusion bodies in the nuclei of cells in the DRN at 12 weeks post-surgery (Fig. 2F). By contrast, PBS (Fig. 2D) and N171-16Q (Fig. 2E) injected mice, show no or few 1-82Q-positive inclusions, respectively. In N171-82Q injected animals, 1-82Q-positive inclusions were primarily distributed in and around the interfascicular sub-nucleus (DRif), the DRN ventral sub-nucleus (DRv), the DRN lateral (DRl) and along the injection track in the adjacent midbrain areas.
To investigate whether inclusion bodies were located in neurons that stained positive for TPH2, the rate limiting enzyme in serotonin production, relevant sections from N171-82Q injected mice were double-labeled with antibodies against both EM48 (Fig. 3A) and TPH2 (Fig. 3B). Confocal microscopy of cells in the DRif and DRv show that EM48-positive inclusions are located within TPH2-positive neurons, primarily in the nucleus (Fig. 3C). A pair of positively stained cells, outlined in Fig. 3C, are enlarged in Fig. 3D. The enlarged confocal image, in conjunction with the two corresponding orthogonal views positioned above (E) and to the right (F), strongly suggest that the EM48-positive nuclei are located within the TPH2-positive neurons.
Fig. 3.
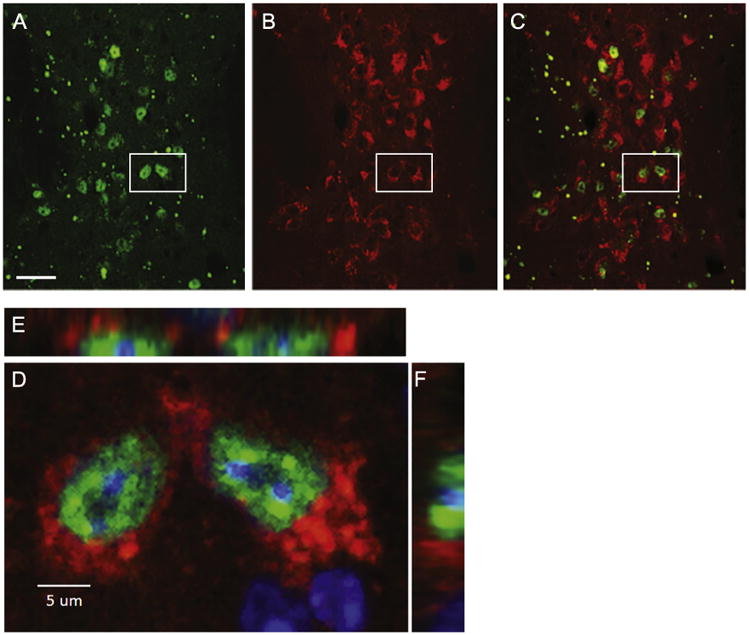
Confocal images of the DRif sub-nuclei of an animal injected with the N171-82Q construct twelve weeks prior to necropsy. Green EM48-positive inclusions of human mutant huntingtin (A) and red TPH2-postive cell bodies with blank nuclei are shown in B. These images, merged in C, exhibit green EM48-positive huntingtin accumulations surrounded by red TPH2-positive cell bodies. To better assess the possibility of colocalization, the pair of cells outlined by white rectangles in A, B and C are enlarged in D. Images E and F are orthogonal views of the cells. The orthogonal views show the EM48-positive inclusions in the nuclei, also fluorescing blue for the nuclear stain Hoechst, surrounded by red TPH2-positive staining. Taken together, the three views suggest that the positively stained huntingtin inclusions are colocalized in TPH2-positive cells. (scale bar in A=30 um)
We also double-labeled tissue sections against glial fibrillary acidic protein (GFAP) and 1-82Q to assess whether astrocytes in the injection area also expressed mutant huntingtin. As compared to neurons, a relatively small percentage of astrocytes also expressed mutant huntintin (Fig. 4C). An enlarged confocal image of a GFAP-positive cell (D) was included to show an example of mutant huntingtin in the nucleus of a GFAP-positive astrocyte. Two corresponding orthogonal images are included to verify co-localization (Figs. 4E and F).
Fig. 4.
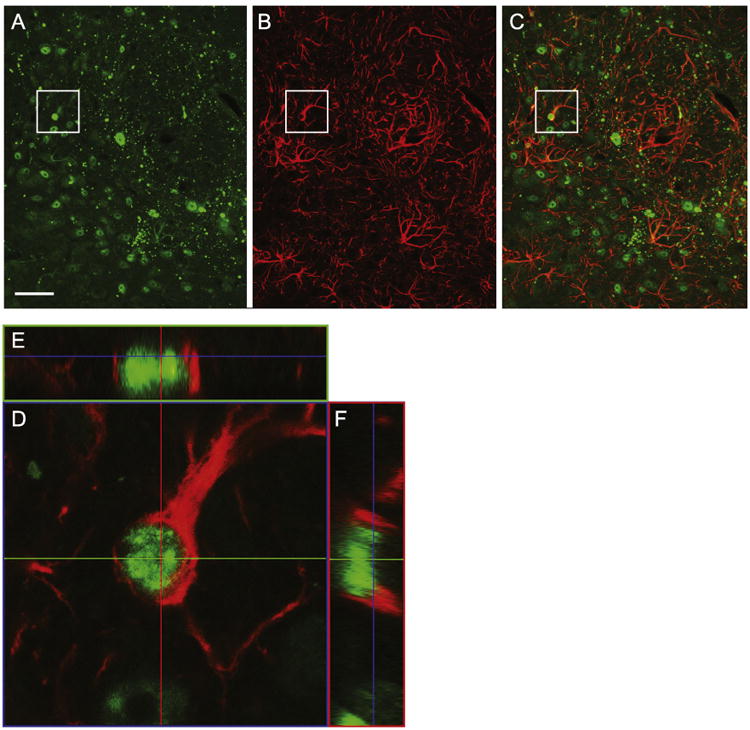
Confocal images of the DRv and DRif of a 20 μm coronal section double labeled against GFAP (red) and 1-82Q (green) to assess the extent that astrocytes were transduced and expressing N171-82Q. As compared to neurons, very few GFAP-positive cells exhibited evidence of N171-82 protein. One cell, identified in the A–C has been enlarged in D to better illustrate that the green mutant huntingtin appears to be positioned within the GFAP-positive astrocyte. Two corresponding orthogonal images (E and F) are included, providing more support that they are colocalized (Scale bar=50 μm).
2.3. Overexpression of N171-82Q in the DRN reduces THP2
Approximately 75% of neurons in the DRN contain serotonin and utilize TPH2 as the rate limiting synthesizing enzyme. R6/1 transgenic HD mice show a significant reduction in DRN TPH2 expression that corresponds with depressive-like behavioral symptoms (Renoir et al., 2012). To address whether expression of N171-82Q in the ventral DRN alone causes a disruption in the serotonin synthesis pathway, we used standard immunohistochemical techniques to visualize TPH2-positive cells. Whereas PBS and N171-16Q injected mice demonstrated robust TPH2-postive staining throughout the DRN, including the DRif and DRv (Fig. 6A and B), N171-82Q injected mice showed a significant reduction in TPH2-positive staining in the DRif and DRv (Fig. 6C). We used ImageJ/Fiji to quantify the number of TPH2-positive neurons in the DRif and DRv and a One–Way ANOVA for Independent Groups indicated a significant difference in the number of TPH2-positive cells (F(2,33)=5.11, p<0.01). Tukeys HSD pairwise comparisons indicated that N171-82Q injected mice displayed a significant reduction in the number of TPH2-positive cells compared to PBS (71% reduction) and N171-16Q (75% reduction) injected controls (Fig. 6G). We were surprised to observe such a dramatic loss of TPH2 expression, despite transducting only approximately 30% of the DRN with vector. It is possible that the AAV2/1 vector transduced an even larger percentage of cells in the DRN than the anti-1-82Q antibody was able to detect using standard ICH techniques.
Fig. 6.
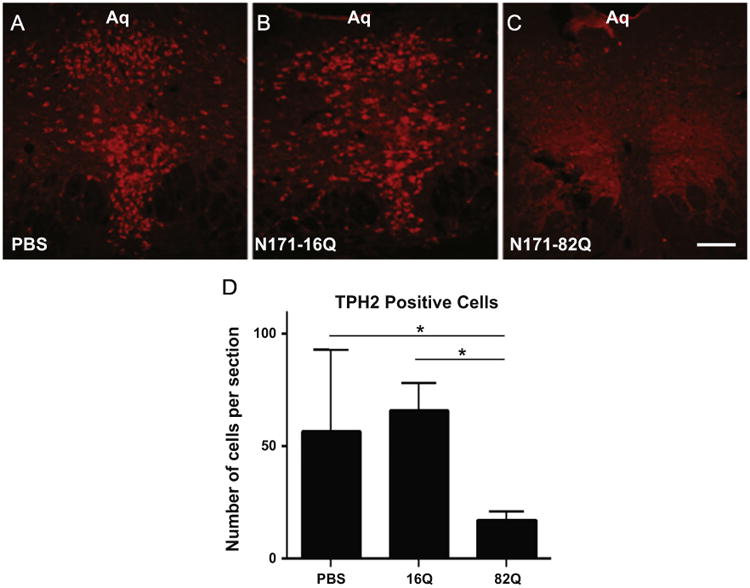
Representative 20 μ thick, coronal, sections through the DRN immunostained to visualize TPH2, the rate limiting enzyme for serotonin in the brain. A dense pattern of red fluorescently stained cells are seen in the DRN of PBS and N171-16Q injected mice (A, B respectively). In contrast, relatively few TPH2 positive cells are observed in the DRN of mice injected with N171-82Q (C). The corresponding graph displays mean positively stained cell counts+SEM from the three groups (D). The data indicate a 75% reduction in TPH2 positive staining in the N171-82Q injected animals compared to the N171-16Q injected animals and a 71% reduction as compared to PBS controls (both p<0.05).
2.4. DRN neuropathology is not associated with changes in behavior or loss of body weight
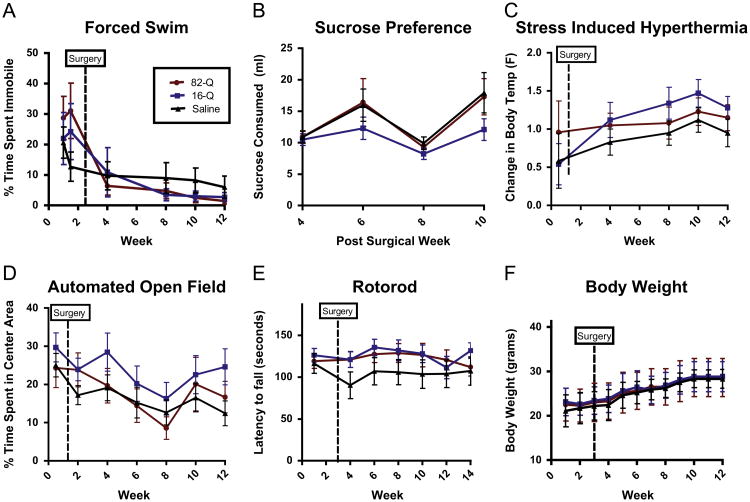
To assess potential depressive-like behaviors elicited by intra-DRN expression of N171-82Q, all the mice were evaluated on the Forced Swim task, the Sucrose Preference task and the Stress Induced Hyperthermia test (Fig. 7A–C). Repeated measures ANOVA's indicated no differences among groups for the Forced Swim task (p>0.05), the Sucrose Preference test (p>0.05) or the Stress-Induced Hyperthermia test (p>0.05). To assess changes in anxiety-like behavior, the time spent in the open area of the automated open field apparatus was measured and no difference was detected between groups (p>0.05; Fig. 7D). In order assess whether general motor dysfunctions were occurring, which might confound the depressive-like and anxiety-like behavioral measures, we also assessed the latency to fall from the accelerated rotarod. No behavioral differences were detected (p>0.05; Fig. 7E). Finally, no differences were detected in body weights between groups (p>0.05; Fig. 7F).
Fig. 7.
Graphs A–C display behavioral measurements on commonly used depressive-like behaviors tests. No significant differences were detected between the animals that received intra-DRv injections of N171-82Q (Red), N171-16Q (Blue) or PBS (Black). Similarly, no significant differences were detected between groups in the open field (D) which assesses anxiety-like behavior or the accelerated rotarod task (E) which assesses general motor and coordination. No significant differences were detected in body weights between groups (All tests p>0.05).
3. Discussion
Depressive symptoms in HD patients frequently manifest prior to both motor disturbances and the knowledge of being a carrier of the mutant gene. While depressive symptomology has been well documented in HD, the neuroanatomical and neurochemical correlates that mediate these early depressive symptoms in HD patients are unknown. The current experiment took advantage of a viral vector-mediated delivery system to express a fragment of the human mHTT (N171-82Q) in the cells of ventral DRN of wildtype mice. We selected the N171 fragment based upon previous studies that have demonstrated that similar fragment lengths lead to pathology in brain tissue 1–2 months post-injection (Franich et al., 2008). Thus, injections of AAV2/1 delivering N171-82Q directly in to the DRN, enabled us to assess whether mHTT in the DRN alone can alter the serotonin synthesis pathway and/or elicit depressive-like behaviors. Here we show that 12 weeks of expression of N171-82Q produced a 75% reduction in the number of TPH2-positive cells detected in the DRN interfasciculus (DRif) and DRN ventral (DRv) sub-nuclei of wildtype mice as compared to animals expressing the control construct bearing 16 glutamine repeats (N171-16Q). Additionally, soluble mutant huntingtin and cellular inclusions were detected in the remaining TPH2-positive cells and in a small percentage of astrocytes, but not within reactive microglia. Despite this significant pathology of the DRif and DRv of N171-82Q treated mice, no alterations in depressive- or anxiety- like behaviors were detected in these animals as compared to controls. Moreover, evaluation of rotarod data showed no development of a motor dysfunction that would have inhibited performance on depressive- and anxiety-like tests. However, assessment of the relevent literature suggests that in order to produce behavioral deficits, a large majority of the cells in the DRN must be disrupted either via lesions or neurotoxins (Lieben et al., 2006; Cervo and Samanin, 1987; Cervo et al., 1991; Lucki, 1998; Soubrié, 1986). Similarly, studies that disrupt TPH2 with PCPA, often find that whole brain 5-HT levels must be reduced by more than 85% to produce depressive-like behavior in rodents as measured by the Forced Swim Task (Page et al., 1999). Consequently, our injection parameters that resulted in expression of N171-82 in only the DRif and DRv are likely to be too limited to alter depressive and anxiety-like behavior.
Previous studies have reported a sexual dimorphism in serotonin dysfunction in R6/1 transgenic HD mice (Pang et al., 2009; Renoir et al., 2011). Renoir observed a global reduction of serotonergic transmission in female R6/1 HD mice only, evidenced by decreased levels of serotonin (5-HT) and its metabolite (5-HIAA) in various brain areas. Additionally, only females displayed deficits in the forced swim task and sucrose preference test. In the current study, we did not observe sex differences in any of the neuropathological or behavioral findings. Consequently, rather than a gender specific effect, we hypothesize that the lack of behavioral change in our animals is likely due to the limited transfection in our mice.
Nevertheless, the current findings are a proof of concept that the expression of mHTT in the DRN can alter TPH2 expression. This is important because TPH2 is found not only in the neurons of the DRN, but also in the remaining raphe nuclei (B1-B8) and a wide array of cells and fibers of the hypothalamus, pituitary, hippocampus, pineal gland, ventral tegmental area and the prefrontal cortex (Carkaci-Salli et al., 2011; Clark et al., 2007; Gutknecht et al., 2009; Sakowski et al., 2006). Thus, our findings suggest the possibility that 5-HT synthesis might be altered not only in the DRN in HD, but throughout the CNS. 5-HT turnover in the striatum, known to be associated with motor and cognitive symptoms of HD, may also play a role in depressive-like behaviors in R6/2 mice (Renoir et al., 2011). Additionally, recent evidence suggests that SSRI's efficacy in treating depressive symptoms may be influenced by the serotonin-mediated potentiation in the hippocampus (Cai et al., 2013).
HD patients with depressive symptoms are frequently prescribed SSRIs that increase 5-HT availability by blocking serotonin transporters and result in changes in serotonin signaling. While SSRI's are widely prescribed, there is currently a dearth of double blind studies assessing the efficacy of antidepressant treatments for depressed HD patients (Holl et al., 2010). It is plausable that if mHTT expression reduces TPH2 efficacy and, as a consequence, reduces 5-HT synthesis, SSRI administration at the normally prescribed doses may prove to be less effective in treating the depressive symptoms in HD patients as compared to the non-HD population. Rodent studies indicate that large decreases in TPH2 activity with PCPA attenuates the depressive-like behavioral benefits of several SSRI's (fluoxetine, citalopram and paroxetine) while leaving general motor behaviors such as locomotion and open field activity unaffected (Guzzetti et al., 2008; Kulikov et al., 2011; O'Leary et al., 2007). Similarly, PCPA-induced inhibition of TPH2 in non-HD depressed patients thwarts the benefits of the tricyclic antidepressant imipramine and the monoamine oxidase inhibitor tranylcypromine in the early stages of treatment (Shopsin et al., 1975, 1976). Thus, it is plausible that non-traditional SSRI doses or non-serotonin treatments such as drugs that alter norepinephrine or dopamine (Holl et al., 2010), or other treatments may prove beneficial.
In addition to 5-HT associated circuits, due to widespread mHTT expression and neuronal pathology throughout the brain, it is likely that there are multiple neuronal circuits that mediate depression in people with HD. A variety of studies suggest an association between depression in people with HD and changes in the frontal cortices, anterior cingulate cortex, insular cortex and the cerebellum (Hobbs et al., 2011; Mayberg et al., 1992; Paulsen et al., 2006; Sprengelmeyer et al., 2014). More recent studies of BACHD transgenic mice that carry the human full length mutant huntingtin suggests that gene expression dysregulation in the hypothalamus may be a primary mediator in the depressive-like symptoms (Hult Lundh et al., 2013). This study also demonstrated that AAV-Cre injections into the hypothalamus of BACHD transgenic mice, wherein the mHTT gene is floxed, significantly lowered mHTT expression and reduced the development of depressive-but not anxiety-like behaviors. Other reports suggest that the documented reduction in hippocampal neurogenesis in HD transgenic mice may be involved in both cognitive and depressive symptoms in HD (Ransome et al., 2012).
Ongoing studies in our laboratory are evaluating whether RNAi mediated reduction of mHTT in the hypothalamus or dentate gyrus of the hippocampus prevent depressive- and anxiety- like behaviors in HD mice. Taken together, using a single treatment that targets any single structure or ligand may prove inadequate. As with treatments for movement dysfunctions and cognitive dysregulation, a more global treatment may be necessary to increase the quality of life of the HD patient.
In conclusion, we found that the accumulation of mHTT in cells of the DRif and DRv disrupts TPH2-positive staining, causes intracellular inclusion formation in neurons and astrocytes and leads to the formation of reactive microglia. Thus, mHTT could play a role in behaviors affected by alterations in serotonin signaling. Taken together, these results suggest that the expression of mutant huntingtin in the brains of healthy mice is capable of disrupting the serotonin synthesis pathway, and therefore, when ubiquitously expressed, as is the case in the HD brain, mutant huntingtin protein accumulation could potentially influence serotonin circuits throughout the CNS and alter serotonin-mediated behaviors in HD patients. The current findings may have implications when seeking better treatment strategies for treating the early depression that frequently strikes people with HD.
4. Experimental procedure
4.1. Animals
The 32 B6C3F2 wildtype mice (22 male, 10 female) used in these experiments were the offspring of B6C3 breeding pairs obtained from Jackson Laboratories (Maine, USA). All were housed in the Small Laboratory Animal Unit (SLAU) at the Oregon National Primate Research Center in Beaverton, OR and maintained on a 12-hour light-dark cycle and given food and water ad libitum. Prior to intra-cranial surgeries, all mice were group-housed, with 2–5 same-sex animals per cage. After the two-week post-surgical period, the mice were separated to perform a sucrose preference test and remained individually housed for the remainder of the study. All experiments were performed with Institutional Animal Care and Use Committee approval from the Oregon Health & Science University/Oregon National Primate Research Center and in accordance with both federal and institutional guidelines.
4.2. Viral vector production
Shuttle plasmids (pAAV-HTT82Q and pAAV-HTT16Q) were created that contain the respective transcriptional units flanked at each end by AAV serotype 2145-bp inverted terminal repeat sequences. Each human transgene contained the first three exons (171 amino acids) of human HTT containing either 82 (pathogenic) or 16 (control) CAG repeats. Respective transgenes were driven from a CAG promoter (chicken beta actin promoter with a cytomegalovirus enhancer element). rAAV2/1-HTT82Q and rAAV2/1HTT-16Q vectors were generated by the viral vector core at the Children's Hospital of Philadelphia. All vectors were injected at a titer of 5e11 vg/ml (diluted prior to infusion with phosphate-buffered saline containing 0.001% v/v Pluronic F-68).
4.3. Intra-cranial stereotaxic surgeries
Intra-cranial stereotaxic surgeries were performed under isoflurane gas vaporized in 100% oxygen. To avoid damaging the superior sagittal sinus, a 33-guage blunt needle connected to a 10 μl Hamilton syringe was lowered stereotaxically at a 20 degree angle into the midbrain's dorsal raphe nucleus. One injection (0.5 μl over 5 min) of either (1) AAV1-HTT82Q, (2) AAV1-HTT16Q or (3) phosphate buffered saline (PBS) was made into the dorsal raphe (Coordinates at the level of the skull: Anterior/Posterior: −4.1mm, Medial/Lateral: +1.4 mm, Dorsal/Lateral: −4.1mm). The needle was left in situ for an additional 5 min to aid diffusion from the needle tip. The needle was then slowly retracted and the wound closed with 5-0 polyvicryl suture. Each animal received carprofen (s.c. 5 mg/kg) for pain management for the subsequent 4 days.
4.4. Behavioral testing
The five behavioral tasks were selected in an attempt to assess (1) depression-related, (2) stress-related, and (3) coordinated locomotor behaviors. The tests included the forced swim test, the sucrose preference test, accelerating rotarod, stress-induced hyperthermia and the tail suspension test. Each test was administered once during every two week period, while body weight was measured every week. All behavioral testing took place during the light cycle (between 8:00 am and 5:00 pm), with the exception of the sucrose preference test, which ran continuously for 48-hour and 96-hour testing periods. Experimenters were blinded to the treatment group during the course of the study.
4.5. Forced swim test
Depressive-related behavior was evaluated using the forced swim test as previously described by Porsolt et al. (1977, 1978). Mice were gently lowered into a 3.7 L glass container (height: 18.5 cm; diameter: 18 cm) filled with 13cm of water (24 ±1 °C). Subsequent to the initial 60 s of the 6 min trial, the experimenter recorded bouts of immobility during every 5 s period. Immobility was identified as the cessation of limb movements, with the exception of minor involuntary movements of the hind limbs or those movements necessary to stay afloat. After the trial, mice were gently dried and returned to their home cage. Immobility was scored as a percentage of the number of instances of immobility ([number of immobility observations/total number of observations [=60]] · 100) (Carroll et al., 2007; Ihne et al., 2012).
4.6. Stress-induced hyperthermia
Reaction to stress was assessed using the stress-induced hyperthermia and the tail suspension tests. Body temperature was taken rectally using a RET-3 thermocouple probe and BAT-12 thermometer (Physitemp, New Jersey). Mice were removed from their home cages and gently restrained by holding the mouse at the base of the tail. Temperature was taken by inserting a lubricated (petroleum jelly) probe 2 cm into the rectum. A constant reading was obtained in 10-15 s. Then, to induce stress, mice were suspended by the tail from a height of 30cm for 6 min. Following the tail suspension, temperature readings were taken at 0 min [T1], 6 min [T2], and 21 min [T3]. Mice were then returned to their home cage. The difference in temperature between T3 and T1 (T3-T1) was used to measure stress-induced hyperthermia (van Der Heyden et al., 1997; Slawinska et al., (2013)).
4.7. Sucrose preference test
The sucrose preference test was used to assess changes in hedonic preference using the two bottle paradigm as previously described by Frenois et al. (2007). Individually housed mice were given access to two bottles which hung from the wire cage tops above each individually housed animal's home cage. Food pellets were placed on the cage floor. During an initial 96 h habituation period, the two bottles were filled with water. Then, to assess any possible preference for drinking from the right or left bottle, two new bottles filled with water were placed on the cage and amount of water ingested was measured after 48 h (no side bias was detected in any of the mice). Finally, to assess preference of the sucrose solution over water, one water bottle (the side was randomly determined) was replaced with a bottle containing 2% sucrose. Ingestion of each fluid was measured after 48 h. Bottle position was switched for each trial. Total sucrose consumption was calculated from the amount of sucrose consumed and expressed as a percentage of the total amount of fluid consumed (amount of sucrose consumed/total fluid consumption · 100) (Frenois et al., 2007; Bechtholt et al., 2008).
4.8. Accelerating rotarod
Motor coordination and balance were assessed using an accelerating rotarod (Ugo Basile, Comerio, Italy). To obtain baseline data, mice underwent three test trials prior to surgery. Subsequent to surgery, mice were tested once every two week period throughout the duration of the study. The rotating rod accelerated from 5 to 40 rpm over a 4 min period. Each mouse received three consecutive trials separated by a 30- min inter-trial interval. The average latency to fall from the rotating rod was calculated from the latencies obtained from three trials. On the rare occasion that a mouse would cling to the rotating rod, three consecutive rotations would immediately end the trial (van Raamsdonk et al., 2005; Cummings et al., 2012).
4.9. Necropsy
Animals were anaesthetized with an overdose of ketamine/xylazine solution. Those mice designated for immunohisto-chemical analysis were perfused trans-cardially with 15 ml of 0.9% sterile sodium chloride saline followed by 15 ml of 4% paraformaldehyde. Brains were then removed and post-fixed in 4% paraformaldehyde for 24 h and then switched to 30% sucrose solution. Brains were subsequently sliced at 20 u thick sections on a sliding microtome. Those animals designated for qPCR analysis were perfused with 10 ml of cold, sterile, 0.9% saline. Brains were rapidly removed and sliced in an ice cold brain matrix. Punches of the DRN were taken and subsequently stored in a -80 °C freezer prior to RNA isolation, reverse transcription and Real-Time PCR analysis.
4.10. Injection placement
Two strategies were used to identify successful injections into the ventral sub-nuclei of the DRN (DRif and DRv). Subsequent to all behavioral testing, approximately half of the animals were perfused, the brains fixed in 4% paraformaldehyde, frozen and sliced on a sliding microtome into 20 μm sections. This tissue was then accessed via light and fluorescent microscopy following basic immunohistochemical staining procedures (see details below) to visualize the soluble huntingtin fragment (anti-1-82 huntingtin) or aggregations of the huntingtin fragment (anti-EM48). Sub-nuclei were identified by first counterstaining with Hoechst or Nissl and then making careful comparisons with the relevant images in Franklin and Paxinos (2008) mouse atlas. A successful injection was defined as when expression was visualized in the interfascicular sub-nuclei (DRif) and the ventral DRN sub-nuclei (DRv). Those animals that did not show expression in both of these sub-nuclei were dropped from all analyses. The remaining brains were assessed using qPCR and, as a consequence, perfused with ice cold saline, the brains rapidly removed and the DRN carefully dissected out and snap frozen on dry ice. At a later time, mutant huntingtin fragment was assessed by qPCR (see protocol below). Those animals that did not show expression were dropped from all analyses.
4.11. Immunohistochemistry
Free floating, coronal, brain sections (20 μ) were processed for light microscopy and for fluorescent detection. DAB based reactions were used to visualize cells containing mutant Htt aggregates (anti-EM48, 1:250, Millipore), neurons (anti-NeuN; 1:1000, Millipore, MAB377) and stable and activated microglia (IBA-1, 1:1000, Wako 019-19741). All sections received endogenous peroxidase inhibition and were thoroughly washed between each step. Sections were blocked for 1 h in 5% donkey serum and incubated in primary antibody for 24 h at room temperature. Sections were then incubated in donkey anti-rabbit or anti-mouse biotinylated IgG secondary antibodies (1:200; Vector Laboratories, Burlingame, CA) for 1 h at room temperature. In all staining procedures, deletion of the primary antibody served as a control. Sections were mounted onto gelatin-coated slides and coverslipped with Cytoseal 60 (Thermo Scientific, Waltham, MA).
Double label fluorescent staining procedures were used to visualize and assess whether neurons that contained the serotonin synthesis enzyme tryptophan hydroxylase 2 (TPH2; Millipore ABN60, 1:200), might also contain accumulations of the mutant human huntingtin protein (EM48; Millipore, Billerica, MA, 1:125) or both soluble and aggregations (anti-1-82Q: 1:400, Millipore, MAB5492). Sections were also stained for stable and activated microglia (IBA-1, 1:1000, Wako 019-19741) and for astrocytes (anti-GFAP; 1:100, Dako, Z0334). Following washes, sections were blocked for 1 h in 5% donkey serum, and primary antibody incubations were carried out for 24 h at room temperature. Sections were incubated in goat anti-rabbit Alexa Fluor 546 (Invitrogen A11036, 1:500) or anti-mouse Alexa-488 (Invitrogen A11029, 1:500) conjugated secondary antibodies (Carlsbad, CA) for 1 h at room temperature. All sections were then mounted onto SuperFrost Plus slides and coverslipped using SlowFade Gold Antifade mounting media. Images were captured with a DP72 digital color camera with Olympus DP controller software via an Olympus BX51 light microscope. Confocal images were captured at X20 using a Leica SP5 confocal microscope. Cell counts of the DR interfasciculus (DRif) and DR ventral (DRv) as depicted in the mouse atlas by Franklin and Paxinos (2008) were made blindly and analyzed with ImageJ/Fiji software.
4.12. Quantitative real-time PCR
Twelve weeks after intra-cranial surgery, a subset of the experimental mice were overdosed with ketamine/xylazine and perfused through the left ventricle with 15 ml of ice-cold 0.9% saline. The unfixed brains were removed from the skull, placed into an ice-cold, steel brain matrix and blocked into 1 or 2-mm-thick coronal slabs. Tissue punches and microdissections were made to isolate the DRN. Tissue samples were immediately frozen in dry ice to preserve DNA, RNA, and protein. RNA was isolated from the DRN tissue using a Qiagen RNeasy kit per the manufacturer's instructions, and reverse transcribed with random primers and Multiscribe reverse transcriptase (Applied Biosystems, Carlsbad, CA). Relative mHTT transgene expression was assessed for each animal by quantitative polymerase chain reaction (QPCR) using SYBR Green detection. Primers were specific for Exon 2 of the truncated human HTT transgene (Exons 1–3) (Forward: 5′–GCCGCTGCACCGACCAAAGAA-3′, Reverse: 5′–AGTTCCATAGCGATGCCCAGAAGTT–3′).Relative gene expression was determined by using the ΔΔCT method, normalizing to 18 S mRNA levels. At the conclusion of the QPCR run, dissociation curve (melting curve) analysis was performed to confirm specific amplification.
4.13. Cell counts
Counts of TPH2-positive DRN cells were made by capturing images of the relevant mounted coronal slices using DP72 digital color camera with Olympus DP Controller software. All analyses were made by researchers blind to experimental groups. The digital images were analyzed using Weka Segmentation in the ImageJ/Fiji image analysis software and with templates adapted from Franklin and Paxinos (2008). Positive-stained cells were only counted if located within the borders of the ventral DR sub-nucleus (DRv) and the interfascicular subnucleus (DRif). We also assessed the volume that the positive-stained cells were found in the entire DRN by performing an area fraction fractionator analysis using Microbrightfield Unbiased Stereology Software. Six coronal sections were analyzed per animal (every sixth 20 μm section).
4.14. Statistical analysis
One way ANOVAs were employed to analyze cell count and qPCR data. Tukey's post-hoc analyses were used to assess differences between specific groups of animals. Repeating measure ANOVAS were used to analyze all behavioral and body weight data.
4.15. Image production
Images were captured with a Leica SP5 confocal microscope or an Olympus BX51 light microscope and an Elyra–Zeiss Super-Resolution Microscope (Elyra). Figures were created using Adobe Photoshop and Illustrator.
Acknowledgments
We thank the MJ Murdock Charitable Trust (Grant # 2022272: HVP) for generously funding this research and Dr. Anda Cornea at the Oregon National Primate Research Center and Dr. Stefanie Kaech at OHSU's Advanced Light Microscopy Core for confocal imaging. NINDS: P30NS061800. We also thank Brett Dufour, Catherine Smith, and Randy Clark for their assistance in open field and rotarod and the SLAU staff for all their assistance and support in the health of the animals. Finally, we thank Megan Biggi for her help with ImageJ/Fiji and Nicole Stucky for her help on the manuscript.
References
- Adam OR, Jankovic J. Symptomatic treatment of Huntington disease. Neurotherapeutics. 2008;5(2):181–197. doi: 10.1016/j.nurt.2008.01.008. http://dx.doi.org/10.1016/j.nurt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G, Harper PS, Jones L, editors. Huntington's Disease. 3rd. Oxford University Press; Oxford; 2002. [Google Scholar]
- Baumann B, Bielau H, Krell D, Agelink MW, Diekmann S, Wurthmann C, Trübner K, Bernstein HG, Danos P, Bogerts B. Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychol Med. 2002;32(1):93–103. doi: 10.1017/s0033291701004822. http://dx.doi.org/10.1017/S0033291701004822. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Smith K, Gaughan S, Lucki I. Sucrose intake and fasting glucose levels in 5-ht1a and 5-ht1b receptor mutant mice. Physiol Behav. 2008;93(4–5):659–665. doi: 10.1016/j.physbeh.2007.11.006. http://dx.doi.org/10.1016/j.physbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Kallarackal AJ, Kvarta MD, Goluskin S, Gaylor K, Bailey AM, Lee HK, Huganir RL, Thompson SM. Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci. 2013;16:464–472. doi: 10.1038/nn.3355. http://dx.doi.org/10.1038/nn.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carkaci-Salli N, Salli U, Kuntz-Melcavage K, Hande Ozgen M, Freeman WI, Vrana K. TPH2 in the ventral tegmental area of the male rat brain. Brain Res Bull. 2011;84(6):376–380. doi: 10.1016/j.brainresbull.2011.01.006. http://dx.doi.org/10.1016/j.brainresbull.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. Effects of mild early life stress on abnormal emotion-related behaviors in 5-htt knockout mice. Behav Genet. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. http://dx.doi.org/10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Potential antidepressant properties of 8-hydroxy-2-(di-n-propylamino)tetralin, a selective serotonin1A receptor agonist. Eur J Pharmacol. 1987;144(2):223–229. doi: 10.1016/0014-2999(87)90523-1. [DOI] [PubMed] [Google Scholar]
- Cervo L, Grignaschi G, Rossi C, Samanin R. Role of central serotonergic neurons in the effect of sertraline in rats in the forced swimming test. Eur J Pharmacol. 1991;196(3):217–222. doi: 10.1016/0014-2999(91)90433-q. [DOI] [PubMed] [Google Scholar]
- Clark JA, Flick RB, Pai LY, Szalayova LY, Key I, Conley S, Deutch RK, Hutson AY, Mezey EPH. Glucocorticoid modulation of tryptophan hydroxylase-2 protein in raphe nuclei and 5-hydroxytryptophan concentrations in frontal cortex of C57/Bl6 mice. Mol Psychiatry. 2007;13(5):498–506. doi: 10.1038/sj.mp.4002041. http://dx.doi.org/10.1038/sj.mp.400204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Alaghband Y, Hickey MA, Joshi PR, Hong S, Zhu C, Ando TK, André VM, Cepeda C, Watson JB, Levine MS. A critical window of cag repeat-length correlates with phenotype severity in the r6/2 mouse model of huntington's disease. J Neurophysiol. 2012;107:677–691. doi: 10.1152/jn.00762.2011. http://dx.doi.org/10.1152/jn.00762.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC. Psychiatric symptoms in huntington's disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62:1341–1346. doi: 10.1016/j.biopsych.2006.11.034. http://dx.doi.org/10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Franich NR, Fitzsimons HL, Fong DM, Klugmann M, During MJ, Young D. AAV vector-mediated RNAi of mutant huntingtin expression is neuroprotective in a novel genetic rat model of Huntington's disease. Mol Ther. 2008;16(5):947–956. doi: 10.1038/mt.2008.50. http://dx.doi.org/10.1038/mt.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Rat Brain in Stereotaxic Coordinates. Vol. 2008. Academic Press; New York: 2008. [Google Scholar]
- Folstein SE, Folstein MF. Psychiatric features of Huntington's disease: recent approaches and findings. Psychiatr Dev. 1983;1(2):193–205. [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lippolysaccharide induces delayed fosb/deltafosb immunostaining within mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. http://dx.doi.org/10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L, Kriegebaum C, Waider J, Schmitt A, Lesch KP. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: Convergent data from Tph2 knockout mice. Eur Neuropsychopharmacol. 2009;19(4):266–282. doi: 10.1016/j.euroneuro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Calcagno E, Canetta A, Sacchetti G, Fracasso C, Caccia S, Cervo L, Invernizzi RW. Strain differences in paroxetine-induced reduction of immobility time in the forced swimming test in mice: role of serotonin. Eur J Pharmacol. 2008;594(1-3):117–124. doi: 10.1016/j.ejphar.2008.07.031. http://dx.doi.org/10.1016/j.ejphar.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Holl AK, Wilkinson L, Painold A, Holl EM, Bonelli RM. Combating depression in Huntington's disease: effective antidepressive treatment with venlafaxine XR. Int Clin Psychopharmacol. 2010;25(1):46–50. doi: 10.1097/YIC.0b013e3283348018. http://dx.doi.org/10.1097/YIC.0b013e3283348018. [DOI] [PubMed] [Google Scholar]
- Hobbs NZ, Pedrick AV, Say MJ, Frost C, Dar Santos R, Coleman A, Sturrock A, Craufurd D, Stout JC, Leavitt BR, Barnes J, Tabrizi SJ, Scahill RI. The structural involvement of the cingulate cortex in premanifest and early huntington's disease. Mov Disord. 2011;26(9):1684–1690. doi: 10.1002/mds.23747. http://dx.doi.org/10.1002/mds.23747. [DOI] [PubMed] [Google Scholar]
- Ihne JL, Fitzgerald PJ, Hefner KR, Holmes A. Pharmacological modulation of stress-induced behavioral changes in the light/dark exploration test in male c57bl/6j mice. Neuropharmacology. 2012;62(1):464–473. doi: 10.1016/j.neuropharm.2011.08.045. http://dx.doi.org/10.1016/j.neuropharm.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, Yardumian P, Snowden JS, Turner G, Craufurd D. Psychiatric disorders in preclinical huntington's disease. J Neurol Neurosci Psychiatry. 2007;78(9):939–943. doi: 10.1136/jnnp.2006.103309. http://dx.doi.org/10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma EM, Van Duijn E, Timman R, Van Der Mast RC, Roos RA. Behavioural problems in Huntington's disease using the problem behaviours assessment. Gen Hosp Psychiatry. 2008;30(2):155–161. doi: 10.1016/j.genhosppsych.2007.11.005. http://dx.doi.org/10.1016/j.genhosppsych.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Koe B, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154(3):499–516. [PubMed] [Google Scholar]
- Krogias C, Strassburger K, Eyding J, Gold R, Norra C, Juckel G, Saft C, Ninphius D. Depression in patients with huntington's disease correlates with alterations of the brain stem raphe depicted by transcranial sonography. J Psychiatry Neurosci. 2011;36(3):187–194. doi: 10.1503/jpn.100067. http://dx.doi.org/10.1503/jpn.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikov AV, Tikhonova MA, Osipova DV, Kulikov VA. Association between tryptophan hydroxylase-2 genotype and the antidepressant effect of citalopram and paroxetine on immobility time in the forced swim test in mice. Pharmacol Biochem Behav. 2011;99(4):683–687. doi: 10.1016/j.pbb.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Lieben CK, Steinbusch HW, Blokland A. 5,7-dht lesion of the dorsal raphe nuclei impairs object recognition but not affective behavior and corticosterone response to stressor in the rat. Behav Brain Res. 2006;168(2):197–207. doi: 10.1016/j.bbr.2005.11.003. http://dx.doi.org/10.1016/j.bbr.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. http://dx.doi.org/10.1016/S0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Hult Lundh S, Nilsson N, Soylu R, Kirik D, Petersén Å. Hypothalamic expression of mutant huntingtin contributes to the development of depressive-like behavior in the BAC transgenic mouse model of Huntington's disease. Hum Mol Genet. 2013;22(17):3485–3497. doi: 10.1093/hmg/ddt203. http://dx.doi.org/10.1093/hmg/ddt203. [DOI] [PubMed] [Google Scholar]
- Matthews PR, Harrison PJ. A morphometric, immunohistochemical, and in situ hybridization study of the dorsal raphe nucleus in major depression, bipolar disorder, schizophrenia, and suicide. J Affect Disord. 2012;137(1–3):125–134. doi: 10.1016/j.jad.2011.10.043. http://dx.doi.org/10.1016/j.jad.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE. Paralimbic frontal lobe hypometabolism in depression associated with huntington's disease. Neurology. 1992;42(9):1791–1797. doi: 10.1212/wnl.42.9.1791. http://dx.doi.org/10.1212/WNL.42.9.1791. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleu-from silver stainings to a role in depression. Brain Res Rev. 2007;55(2):329–342. doi: 10.1016/j.brainresrev.2007.01.002. http://dx.doi.org/10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Mochel F, Durant B, Durr A, Schiffmann R. Altered dopamine and serotonin metabolism in motorically asymptomatic r6/2 mice. PloS ONE. 2011;6(3):1–7. doi: 10.1371/journal.pone.0018336. http://dx.doi.org/10.1371/journal.pone.0018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007;192(3):357–371. doi: 10.1007/s00213-007-0728-9. Epub 2007 Feb 21. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology. 1999;147(2):162–167. doi: 10.1007/s002130051156. http://dx.doi.org/10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Pang TYC, Du X, Zajac MS, Howard ML, Hannan AJ. Altered serotonin receptor expression is associated with depression-related behavior in the R6/1 transgenic mouse model of huntington's disease. Hum Mol Genet. 2009;18(4):753–766. doi: 10.1093/hmg/ddn385. http://dx.doi.org/10.1093/hmg/ddn385. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Magnotta VA, Mikos AE, Paulson HL, Penziner E, Andreasen NC, Nopoulos PC. Brain structure in preclinical Huntington's disease. Biol Psychiatry. 2006;59(1):57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kranz JE, Benjamin M, Conybeare R, McDowell B, Turner B. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005;17(4):496–502. doi: 10.1176/jnp.17.4.496. http://dx.doi.org/10.1176/appi.neuropsych.17.4.496. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Detection of Huntington's disease decades before diagnosis: the predict-hd study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. http://dx.doi.org/10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz MF, Johnson T, Suzuki M, Finch JT. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proceedings of the National Academy of Sciences in the United States of America. 1994;91(12):5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. http://dx.doi.org/10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Ransome MI, Renoir T, Hannan AJ. Hippocampal neurogenesis, cognitive deficits and affective disorder in Huntington's disease. Neural Plast. 2012;874387 doi: 10.1155/2012/874387. http://dx.doi.org/10.1155/2012/874387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir T, Chevarin C, Lanfumey-Mongredien L, Hannan AJ. Effect of enhanced voluntary physical exercise on brain levels of monoamines in Huntington's disease mice. PLOS Currents Huntington's Disease. 2011;1 doi: 10.1371/currents.RRN1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir T, Pang T, Zajac MS, Chan G, Du X, Leang L, Chevarin C, Lanfumey L, Hannan AJ. Treatment of depressive-like behaviour in Huntington's disease mice by chronic sertraline and exercise. Br J Pharmacol. 2012;165(5):1375–1389. doi: 10.1111/j.1476-5381.2011.01567.x. http://dx.doi.org/10.1111/j.1476-5381.2011.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowski SA, Geddes Thomas TJ, Levi DM, Hatfield E, Kuhn DMJS. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006;1085(1):11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Shopsin B, Gershon S, Goldstein M, Friedman E, Wilk S. Use of synthesis inhibitors in defining a role for biogenic amines during imipramine treatment in depressed patients. Psychopharmacol Commun. 1975;1(2):239–249. [PubMed] [Google Scholar]
- Shopsin B, Friedman E, Gershon S. Parachlorophenylalanine reversal of tranylcypromine effects in depressed patients. Arch Gen Psychiatry. 1976;33(7):811–819. doi: 10.1001/archpsyc.1976.01770070041003. [DOI] [PubMed] [Google Scholar]
- Slawinska A, Wieronska JM, Stachowicz K, Palucha-Poniewiera A, Uberti MA, Bacolod MA, Doller D, Pilc A. Anxiolytic – but not antidepressant-like activity of lu af21934, a novel, selective positive allosteric modulator of the mglu4 receptor. Neuropharmacology. 2013;66:225–235. doi: 10.1016/j.neuropharm.2012.05.001. http://dx.doi.org/10.1016/j.neuropharm.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Soubrié P. Reconciling the role of central serotonin neurones in human and animal behaviour. Behav Brain Res. 1986;9:319–364. [Google Scholar]
- Sprengelmeyer R, Orth M, Muller HP, Wolf RC, Gron G, Depping MS, Kassubek J, Justo D, Rees EM, Haider S, Cole JH, Hobbs NZ, Roos RAC, Durr A, Tabrizi SJ, Sussmuth SD, Landwehrmeyer GB. The neuroanatomy of subthreshold depressive symptoms in Huntinton's disease: a combined diffusion tensor imaging (DTI) and voxel-based morphometry (VBM) study. Psychol Med. 2014;44(9):1867–1878. doi: 10.1017/S003329171300247X. http://dx.doi.org/10.1017/S003329171300247X. [DOI] [PubMed] [Google Scholar]
- van Der Heyden JA, Zethof TJ, Olivier B. Stress-induced hyperthermia in singly housed mice. Physiol Behav. 1997;62(3):463–470. doi: 10.1016/s0031-9384(97)00157-1. http://dx.doi.org/10.1016/S0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk JM, Pearson J, Rogers DA, Bissada N, Vogl AW, Hayden MR, Leavitt BR. Loss of wild-type huntingtin influences motor dysfunction and survival in the yac128 mouse model of Huntington's disease. Hum Mol Genet. 2005;14(10):1379–1392. doi: 10.1093/hmg/ddi147. http://dx.doi.org/10.1093/hmg/ddi147. [DOI] [PubMed] [Google Scholar]
- Wetzel HH, Gehl CR, Dellefave-Castillo L, Schiffman JF, Shannon KM, Paulsen JS. Suicidal ideation in Huntington's disease: the role of comorbidity. Psychiatry Res. 2011;188(3):372–376. doi: 10.1016/j.psychres.2011.05.006. http://dx.doi.org/10.1016/j.psychres.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]