Abstract
Malignant gliomas are devastating tumors, frequently killing those diagnosed in little over a year. The profuse infiltration of glioma cells into healthy tissue surrounding the main tumor mass is one of the major obstacles limiting the improvement of patient survival. Migration along the abluminal side of blood vessels is one of the salient features of glioma cell invasion. Invading glioma cells are attracted to the vascular network, in part by the neuro-peptide bradykinin, where glioma cells actively modify the gliovascular interface and undergo volumetric alterations to navigate the confined space. Critical to these volume modifications is a proposed hydrodynamic model that involves the flux of ions in and out of the cell, followed by osmotically obligated water. Ion and water channels expressed by the glioma cell are essential in this model of invasion and make opportune therapeutic targets. Lastly, there is growing evidence that vascular-associated glioma cells are able to control the vascular tone, presumably to free up space for invasion and growth. The unique mechanisms that enable perivascular glioma invasion may offer critical targets for therapeutic intervention in this devastating disease. Indeed, a chloride channel-blocking peptide has already been successfully tested in human clinical trials.
Keywords: Glioma, Invasion, Bradykinin, Hydrodynamic glioma invasion, Ion channels
Introduction
Every year, nearly 25,000 Americans are newly diagnosed with a malignant glioma (Ostrom et al. 2015). The World Health Organization (WHO) classifies gliomas on a numeric scale with grade III–IV tumors categorized as malignant gliomas, which include anaplastic astrocytomas and glioblastomas. Although rare, only occurring in 4–8 per 100,000 people, malignant gliomas are devastating to those that are affected (Ostrom et al. 2015). By 5 years post-diagnosis, 67 % of patients succumb to their disease (Ostrom et al. 2015). In patients diagnosed with a glioblastoma, the prognosis is most dire with a median survival time of 14–16 months. This poor outcome is in spite of rigorous therapeutic attempts that include gross resection of the tumor mass, if possible, followed by radiation and chemotherapy treatment. As it stands today, these therapeutic regimens add mere months to patient survival; thus, there is a great need for more effective treatments.
Our cellular, molecular, and genetic understanding of glioma biology has improved considerably in recent years. Notably, a consortium of scientists that make up ‘The Cancer Genome Atlas (TCGA) Research Network’ was able to classify gliomas based on genetic changes that initiate glioma-genesis. Principal among these changes are aberrations in three core signaling pathways: the receptor tyrosine kinase-Ras-phosphoinositide 3-kinase (RTK-RAS-PI3K) signaling pathway and the pathways for cell cycle regulators retinoblastoma protein (Rb) and p53 (Cancer Genome Atlas Research Network 2008). Perhaps the most striking outcome of the TCGA effort has been to showcase the remarkable heterogeneity of these tumors. This heterogeneity—compounded with the presence of a highly intervention-resistant cancer stem cell population and the therapeutic challenges due to the blood-brain barrier (BBB)—understandably makes malignant gliomas very difficult to manage clinically. While all of these aspects of malignant glioma biology limit our ability to treat patients, this review will chiefly detail another salient feature of malignant gliomas, their aggressive invasiveness, and the role that ion channels may play in facilitating this process.
Malignant gliomas are highly invasive
Early attempts to cure patients of solid tumors relied almost exclusively on surgical removal. However, aggressively resecting the main tumor mass and taking wide margins in the surrounding healthy tissue proved to be curative only in cases of locally contained tumors. Thus, early radical surgical attempts that removed an entire brain hemisphere of an individual suffering from a malignant glioma merely resulted in recurrence in the remaining hemisphere (Dandy 1928). Even today, after substantial improvements in surgical techniques have been made, recurrence in patients is almost always inevitable. In most cases recurrence is driven by the diffuse migration of glioma cells away from the main tumor mass, allowing these cells to be insulated from disease management measures such as surgery and radiation.
Gliomas invade and expand into healthy tissue in a manner that is unlike other malignant cancers—rarely using the bloodstream to do so (Beauchesne 2011). Instead, gliomas navigate through extracellular routes and are extremely adept at utilizing existing structures to guide invasion into the surrounding brain tissue. Neuropathologist Hans-Joachim Scherer was the first to describe these routes of invasion, now frequently termed ‘Scherer’s structures’ (Scherer 1938). These patterns of glioma cell invasion roughly compartmentalize the brain into the parenchyma, the vascular network, white matter tracts, and the subarachnoid space. The ability of glioma cells to migrate through these compartments varies drastically. For example, invasion through the brain parenchyma is largely restricted because of the density of extracellular matrix (ECM) proteins, along with the cell bodies and processes of neuronal and glial cells. Although the ECM that constitutes the brain is largely devoid of rigid structural proteins, such as collagen and laminin that are found in other tissues throughout the body, the parenchyma is still saturated with an organized network of ECM proteins (Bellail et al. 2004). The most highly expressed ECM protein in the parenchyma is glycosaminoglycan hyaluronan. Hyaluronan has a high concentration of exposed negative charges that attract oppositely charged ions and cause an influx of water molecules, providing the brain an overall consistency that is elastic and highly hydrated (Bellail et al. 2004). Consequently, this hydration causes the extracellular space to expand to a volume that is approximately ten times greater than the hyaluronan matrix alone (Bellail et al. 2004). As a whole, the brain parenchyma is extremely tortuous, lacking the space—and possibly the ECM proteins—that are most conducive to glioma cell migration.
Ultimately, glioma cells are far more adept at using rigid secondary structures, such as white matter tracts and the vascular network, to invade far into the brain tissue. It is unclear whether this preference is due to parenchymal spatial constraints, the release of chemo-attractive molecules from vascular cells, or potentially the components of the niche environment around these structures. It is apparent, however, that invading glioma cells overwhelmingly associate with blood vessels and white matter tracts, as seen in histological sections and animal models that recapitulate the hallmarks of human gliomas (Giese and Westphal 1996; Watkins and Sontheimer 2011; Hardee and Zagzag 2012; Watkins et al. 2014). While invasion does occur via white matter tracts, the focus of this review will be on the invasion of glioma cells along existing cerebral blood vessels, modifications of the cells’ own shape and volume, and changes imposed on the vasculature by invading cells.
The glioma-vascular relationship and the role of bradykinin
As the most metabolically active organ in the body, the brain is highly vascularized; blood vessels can be found reaching within ~50 μm of every neuron present in the brain. The carotid and vertebral arteries are the major sources of blood supply for the brain. At the base of the brain these arteries merge to form the Circle of Willis, an interconnecting network of pial arteries that travel along the surface of the brain. As the pial arteries dive into the brain parenchyma, they are surrounded by a relatively large space, the Virchow-Robin space, which is continuous with the subarachnoid space of the meninges (Iadecola and Nedergaard 2007). The Virchow-Robin space, also called the perivascular space, is a fluid-filled compartment containing cerebrospinal fluid that comprises the glymphatic system (Jessen et al. 2015). The perivascular space disappears as these arteries travel deeper into the parenchyma and become almost entirely encased by astrocytic protrusions, called endfeet, that are coupled to the vascular basement membrane. The basement membrane that encompasses blood vessels is highly specialized, containing many ECM proteins that cannot be found anywhere else in the brain parenchyma. Instead, the vascular basement membrane contains ECM proteins that are structurally rigid—namely, laminin, collagen IV, heparan sulfate proteoglycans agrin and perlecan, and nidogen (Yousif et al. 2013). These ECM proteins provide structural and functional integrity to the blood vessel, but may also increase glioma cell motility (Ohnishi et al. 1997; Kalluri 2003; Fukushima et al. 2007).
One can therefore argue that glioma cell migration along blood vessels offers two distinct advantages: (1) the spacious, fluid-filled perivascular space presents little physical resistance and (2) the basement membrane has unique ECM proteins that are amenable to migrating glioma cells. Two recent studies sought to quantify the extent to which glioma cells use blood vessels during invasion and the underlying mechanism of this association, using a clinically relevant animal model (Montana and Sontheimer 2011; Watkins et al. 2014). Watkins et al. found that between 65 and 95 % of all glioma cells xenografted into the brains of scid mice eventually associate with blood vessels, but do not have a preference between arterial and venous vessels (Watkins et al. 2014). This study also highlighted that glioma cells may have a preference for vessels that are less than 7 μm in diameter (capillaries), as more than 50 % of glioma cells were found to populate these vessels. However, capillaries accounted for close to 50 % of all cerebral vessels analyzed; thus, this preference could be a result of the high density of capillaries compared to other vessel types. Regardless, this study and those of others (Holash et al. 1999; Zagzag et al. 2000; Winkler et al. 2009; Baker et al. 2014) demonstrate the high occurrence of glioma cells that are associated with the vascular network.
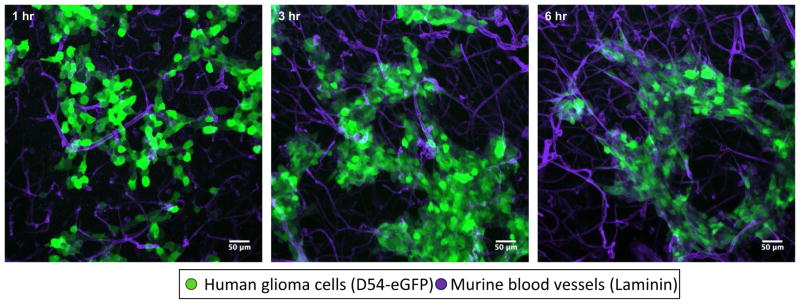
Montana et al., using a brain slice invasion assay, surprisingly found that within four hours, close to 90 % of glioma cells populate vessels, even in the absence of blood flow (Montana and Sontheimer 2011). Figure 1 also supports these findings, displaying the rapid association of glioma cells with laminin-labeled blood vessels within hours. Furthermore, Montana et al. showed that glioma cells are attracted to blood vessels by following a chemotactic gradient of bradykinin, a peptide released in part by vascular endothelial cells. Bradykinin is produced by enzymatic cleavage of high-molecular-weight kininogen by kallikrein and binds G protein-coupled receptors B1R and B2R. While B2R is constitutively expressed on a number of cell types in the brain—including endothelial cells, neurons, and astrocytes—B1R expression is more limited and stimulated during pathological conditions such as inflammation, hypoxia, and tissue damage (Golias et al. 2007). Under physiological conditions, bradykinin is a mediator of the inflammatory response and can activate endothelial cells via B2R, leading to vasodilation of the associated vessel (Dray and Perkins 1993; Hornig et al. 1997). Glioma cells appear to express B2R exclusively, with expression positively correlating with increasing tumor grade (Zhao et al. 2005). Bradykinin signaling mediated through B2R has been shown to be essential in the chemotactic association of glioma cells with the vasculature, as inhibition of B2R or its genetic elimination leads to the failure of glioma cells to associate with blood vessels (Montana and Sontheimer 2011; Cuddapah et al. 2013; Seifert and Sontheimer 2014).
Fig. 1.
Rapid association of glioma cells with blood vessels in situ. Glioma cells were implanted onto murine brain slices and allowed to invade into the tissue. After the set timepoint the slices were fixed, stained for vascular basement membrane component laminin (Sigma- Aldrich L9393), and imaged using confocal microscopy. This series of images shows the association of glioma cells to the vasculature network within a few hours
Another recent publication examined the cellular effects of bradykinin on glioma cells, finding that it mediates an increase in intracellular calcium ( [Ca2+]i ), leading to activation of calcium-sensitive ion channels and increased motility (Cuddapah et al. 2013). Building upon these findings, another study (Seifert and Sontheimer 2014) found that bradykinin-induced [Ca2+]i increases caused contraction of the actomyosin cortex, which led to membrane deformation or ‘blebbing.’ Subsequent activation of calcium-activated chloride and potassium channels was shown to be critical in membrane bleb retraction. Thus, it appears that bradykinin not only acts as a chemo-attractant, attracting glioma cells to blood vessels, but also arbitrates ion channel activity, which is critical for glioma cell volume regulation. This will be discussed in detail later.
These studies clearly point to the significant impact that inhibition of bradykinin signaling potentially has on one of the most prominent features of malignant gliomas—their invasiveness. A number of B2R antagonists have been developed, including the second-generation peptide antagonist HOE-140 (Icatibant, Firazyr) and third-generation antagonist MEN16132 (Fasitibant). However, only HOE-140 has been approved for clinical use in hereditary angioedema (Baş et al. 2015). No clinical trials for these therapies have been initiated for the treatment of malignant gliomas, but with the safety of HOE-140 in humans already being explored, the path to clinical utility may be accelerated if shown to be efficacious. It is also worth noting that a bradykinin analog, RMP-7, was found to induce permeability in tumor-derived blood vessels (Inamura and Black 1994; Nomura et al. 1994). This finding spurred several efforts to use RMP-7 in conjunction with chemotherapeutic drugs, in the hope that better drug delivery could improve patient survival. While none of these attempts have come to fruition in the context of glioma treatment, bradykinin analogs like RMP-7 could improve therapeutic drug delivery in other neurological disorders.
The perivascular space and the gliovascular interface
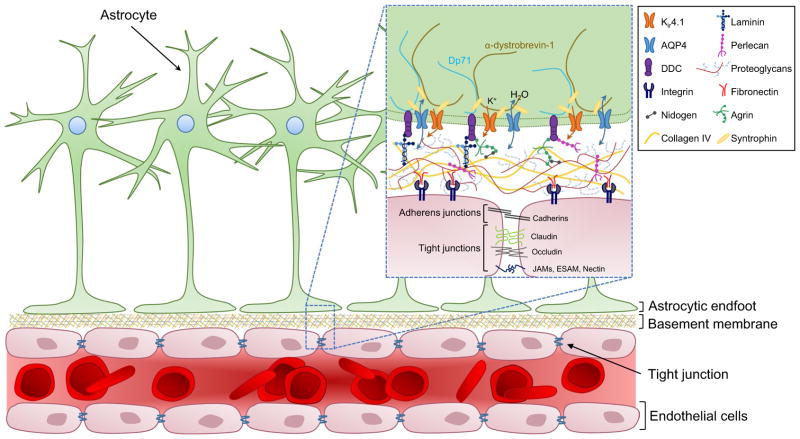
The above section discussed the spatial advantage offered by the perivascular space, but neglects the fact that blood vessels are innervated by astrocytic endfeet that terminate on the vessel surface. As a result, the vascular surface looks much like an obstacle course for an invading glioma cell (Fig. 2). In a healthy adult brain, astrocytic endfeet cover ~99 % of the vascular surface (Iadecola and Nedergaard 2007), where they serve many purposes essential to brain function. These include maintenance of the BBB, ion and volume regulation, and the regulation of blood flow to meet neuronal demands (Abbott et al. 2006; Sofroniew and Vinters 2010)—all of which require the astrocytic endfoot to exchange molecules with either the vessel itself or the perivascular space surrounding it. At this gliovascular interface, the water channel aquaporin 4 (AQP4) is highly expressed and contained at high density in orthogonal arrays of particles (OAPs), along with the inwardly rectifying potassium channel Kir4.1 (Nagelhus et al. 1999; Wolburg et al. 2011). Both channels play important roles in ion and volume regulation and are tethered to the astrocytic endfoot by a plethora of interacting proteins (refer to Fig. 2). The most critical of these interactions appears to be the connection between the heparin sulfate proteoglycan agrin (a component of the vascular basement membrane) and α-dystroglycan (part of the dystrophindystroglycan complex (DDC) within the endfoot) (Noell et al. 2007; Camassa et al. 2015). Internally, AQP4 and Kir4.1 are sequestered to the astrocytic endfoot by interactions with α-syntrophin, as determined by studies in an α-syntrophin-null mouse model (Neely et al. 2001; Bragg et al. 2006). α-Syntrophin also interacts with one of the dystrophin family proteins, Dp71, and α-dystrobrevin-1, which collectively link AQP4, Kir4.1, and the DDC to the ECM and cytoskeleton of the astrocytic endfoot (refer to Fig. 2) (Waite et al. 2009).
Fig. 2.
The gliovascular unit. Astrocytic endfeet are tightly anchored to the vascular surface by interactions between the dystrophrin-dystroglycan complex (DDC) and proteins that make up the basement membrane (refer to insert). Tight and adherens junctions link endothelial cells together to form the blood-brain barrier (BBB), while integrins connect the endothelial cells to the basement membrane
Because of the importance of astrocytic endfeet in vasoregulation and ion homeostasis, any insult that can provoke a change in the polarization or anchoring of the endfoot could have profound pathological consequences. Therefore, it is not surprising that several neurological disorders including temporal lobe epilepsy, stroke, and Alzheimer’s disease have been reported to be associated with disturbances of the gliovascular unit (Seifert et al. 2006; Kimbrough et al. 2015). Malignant gliomas also exhibit focal breaches in the BBB and dysfunctions in gliovascular coupling (Wolburg et al. 2003, 2012; Watkins et al. 2014). Watkins et al. demonstrated that as glioma cells migrate along the abluminal side of a blood vessel, they dislodge astrocytic endfeet from the vascular surface (Watkins et al. 2014). This also disrupts the ability of astrocytes to control blood vessel tone and causes a focal breach in the BBB, presumably by a downregulation of the tight junction proteins zonula occludens-1 and claudin-5. The mechanism(s) by which glioma cells dislodge the astrocytic endfeet has yet to be determined; however, the matrix metalloproteinase (MMP) family of ECM degrading enzymes has long been implicated in ECM remodeling events that occur during tumor expansion (Rao et al. 1996; Sawaya et al. 1996; Nakada et al. 2003). Furthermore, bradykinin signaling is known to promote the release of MMPs (Hsieh et al. 2008). It is therefore reasonable to hypothesize that invading glioma cells release MMPs at the vascular surface that subsequently remodel the gliovascular interface.
Glioma cell volume changes and perivascular invasion
Even with the removal of astrocytic endfeet from the vessel surface, space surrounding the blood vessel is still limited, requiring volume modifications within the glioma cell to navigate through this confined area. Ion channels have been suggested to serve an important role in the regulation of glioma cell volume. Specifically, channels mediate the flux of ions across the cell membrane. This ionic flux is accompanied by a simultaneous movement of water, either into or out of the cell, to maintain osmotic equilibrium. Ultimately, the osmotic content of the cytoplasm is subject to dynamic changes in response to ionic fluxes. The concerted movement of ions and water in glioma cells during migration has been termed the hydrodynamic model of glioma cell invasion (Watkins and Sontheimer 2011). It assumes that glioma cells migrate in an amoebae-like fashion, where the cytosol is contained in a flexible membrane that has the ability to freely flow into narrow spaces. Additionally, in this model, the cytoskeleton is believed to play a limited role, allowing for rapid volume changes without complex cytoskeletal rearrangements. The idea that gliomas invade using hydrodynamic forces is supported by a number of studies (Watkins and Sontheimer 2011, 2012; Cuddapah et al. 2014; Seifert and Sontheimer 2014) that will be expanded upon hereafter.
Hydrodynamic glioma invasion
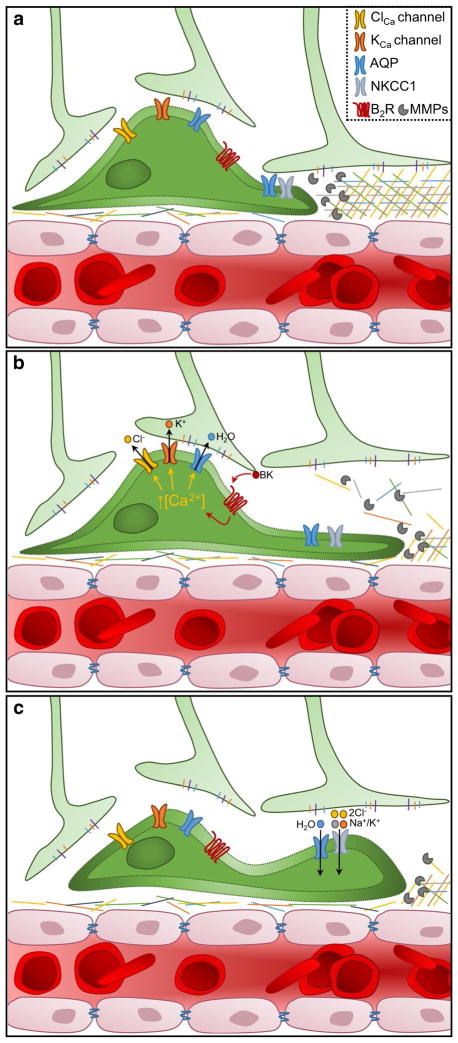
Under normal physiological conditions cells contain high potassium (K+) and low sodium (Na+) ion concentrations, intracellularly. In contrast, the extracellular space is rich in Na+ ions but has a limited concentration of K+ ions. Cations are almost exclusively responsible for setting the resting membrane potential (RMP) within all cells and are the fluxing ions that underlie cellular excitability. The single biologically relevant anion, chloride (Cl−), is typically at equilibrium with the membrane potential; hence, in most cells, it is maintained at low levels inside and high levels outside the cell. From a simplistic perspective, the cell contains a cytosol that is primarily composed of salt (K+Cl−) and water. When a volumetric reduction is required, the cell secretes salt and osmotically obligated water will follow, resulting in a decrease in cytosolic content or cell shrinkage. Any accumulation of salt within the cell achieves the opposite, and the cell will increase in volume. The hydrodynamic model posits that invading glioma cells utilize the secretion and reuptake of salt to dynamically adjust their volume; refer to Fig. 3. Invading glioma cells effectively flow into the available space, occupying it completely.
Fig. 3.
The hydrodynamic model of glioma cell invasion. a Glioma cells encounter spatial barriers during perivascular invasion. Pro-teolytic enzymes, such as matrix metalloproteinases (MMPs), are released from invading glioma cells and degrade components of the basement membrane. In addition, glioma cells express ion channels which allow volumetric changes, including: Ca2+-activated Cl− and K+ channels (ClCa and KCa channels), aquaporins (AQP), G protein-coupled receptors such as bradykinin receptor B2 (B2R), and Na+/K+/Cl− cotransporter 1 (NKCC1). b Bradykinin (BK) binds to B2R, leading to an increase in [Ca2+]i. In response, the ClCa and KCa channels open and release Cl− and K+. Obligate water flows out of the cell via AQPs, causing a volume decrease that enables the glioma cell to squeeze into the small space. c Upon passing a spatial barrier, Cl− and K+ ions are pumped back into the cell by NKCC1 and water flows back into the cell
For this model to be functional, cells must establish and maintain gradients to move ions across the membrane, possess channels to quickly release ions, and have water permeability. Moreover, for cell invasion to be directed, channel activity must be coordinated. The remainder of this review will discuss transporters and channels that support a hydrodynamic model of cell invasion. It is worth noting that this proposed model is consistent with one of the early observations by Scherer, who noted that glioma cells assume the shape of the space they occupy rather than displaying a unique cell-specific shape (Scherer 1938).
Transporters that establish and maintain ionic gradients
The potassium gradient: the Na+/K+-ATPase and Kir4.1
The Na+/K+-ATPase is the fundamental transporter that establishes the RMP in mammalian cells. This transporter requires ATP to pump Na+ against its concentration gradient out of the cell in exchange for the import of K+. The activity of this transporter is largely responsible for the accumulation of K+ intracellularly, where [K+]i is 100–150 mM compared to [K+]o ~ 3 mM. This accumulation of K+ sets the RMP between −60 and −90 mV in almost all resident brain cells. The Na+/K+-ATPase is essential for cell survival and has been found to be involved in a number of other critical cellular mechanisms, such as cell volume regulation and signal transduction through the MAPK pathway (Xie and Askari 2002; Blanco 2005). There have been a number of studies to suggest that the Na+/K+-ATPase is upregulated in malignant cancers, including gliomas (Sakai et al. 2004; Mijatovic et al. 2006; Klatte et al. 2008; Lefranc et al. 2008; Mathieu et al. 2009). In gliomas, the Na+/K+-ATPase α1 subunit, which is responsible for the exchange of Na+ for K+, has been found to be upregulated and localized to the plasma membrane (Lefranc et al. 2008; Chen et al. 2014). It is unclear, however, what effect this upregulation is playing in glioma biology. One interesting hypothesis would be that the excessive expression of Na+/K+-ATPase leads to an accumulation of K+ ions far above what is normal for non-excitatory cells. Although, the RMP of malignant gliomas is around −40 mV, significantly positive of the potassium equilibrium potential (EK+), the high concentration of Cl− present in glioma cells could account for this shift. However, additional evidence is needed to support this hypothesis.
A relatively depolarized RMP appears to be a common feature of cancer cells, which tend to rest somewhere around −40 mV (Cone 1971). In gliomas, this is likely the case for two reasons: limited membrane expression of Kir4.1 (Olsen and Sontheimer 2004) and the unusually high expression of a Ca2+/calmodulin-dependent protein kinase II (CaMKII)-regulated Cl− channel, ClC-3 (discussed in detail later) (Cuddapah and Sontheimer 2010).
Kir4.1 has its highest open probability near the K+ equilibrium potential and is therefore ideally suited to set the RMP near the EK+. This is undeniably the case in establishing the RMP of non-malignant glial cells (Butt and Kalsi 2006; Olsen and Sontheimer 2008). However, in gliomas Kir4.1 is mislocalized to the nucleus, most likely due to a trafficking defect (Olsen and Sontheimer 2004). Whether this is by accident or for a purpose is unclear, but this does appear to provide a survival advantage to glioma cells since Kir4.1 plays a role in suppressing cell division (Higashimori and Sontheimer 2007). Thus, without functional Kir4.1, glioma cells are able to progress through unlimited cycles of cell division. The absence of Kir4.1 and the high expression of the Na+/K+-ATPase together establish a situation where K+ can be accumulated intracellularly. Although the glioma cell’s RMP remains moderately positive of the K+ equilibrium potential, a significant driving force for K+ ions to leave the cell upon the opening of K+ channels is still present.
The chloride gradient: Na+/K+/Cl− cotransporter 1
Unlike most cells in the brain, glioma cells accumulate a high intracellular concentration of Cl− ions. The [Cl−]i in glioma cells has been measured to be as high as 100 mM (Habela et al. 2008). This is in stark contrast to most of the resident cells that make up the adult brain, where in mature neurons and oligodendrocytes there is no appreciable Cl− gradient—here, [Cl−]i lies between 6 and 10 mM. The intracellular Cl− concentration displayed in glioma cells does, however, resemble the [Cl−]i of immature neurons and neural stem cells. In immature neurons, Cl− accumulates intracellularly by the activity of the Na+/K+/2Cl− cotransporter isoform 1 (NKCC1; SLC12A2). NKCC1 employs the considerable Na+ gradient, provided by the Na+/K+-ATPase, to move Cl− ions against their chemical gradient into the cell. The activity of NKCC1 in immature neurons leads to a [Cl−]i, which ranges from 20 to 140 mM and thus explains the excitatory response of immature neurons to the inhibitory neurotransmitter GABA (Owens et al. 1996). As development progresses, the sizable Cl− gradient is diminished as the expression of NKCC1 decreases and K+/Cl− transporter member 5 (known commonly as KCC2; SLC12A5) expression increases—effectively causing Cl− to be at electrochemical equilibrium in mature neurons (Ben-Ari 2002).
NKCC1 is a member of the Na+/K+/Cl− cotransporter family, which comprises one other isoform in addition to NKCC1, NKCC2 (SLC12A1). NKCC2 expression is localized to renal epithelial cells, where it is essential for regulation of extracellular fluid volume and osmolarity (Russell 2000). In contrast, NKCC1 can be found in nearly all cell types, but with the highest expression in exocrine glands (Haas and Forbush 2000). As mentioned above, NKCC1 has limited expression in the brain during adulthood.
NKCC1 has been found to be the primary mechanism for Cl− accumulation in glioma cells (Haas and Sontheimer 2010). Furthermore, NKCC1 was found to be localized to the leading edge of migratory glioma cells, and its pharmacological inhibition led to reduced glioma cell invasion (Haas and Sontheimer 2010). Analysis of human glioma samples have revealed that NKCC1 expression is constitutive and positively correlates with increasing tumor grade (Garzon-Muvdi et al. 2012). Activation of NKCC1 is regulated by its phosphorylation status established by protein kinase With-No-Lysine Kinase 3 (WNK3) (Haas et al. 2011). WNK3 activity lies downstream of epidermal growth factor receptor (EGFR) signaling, which is aberrant in close to 50 % of gliomas, setting up a feed-forward loop of prolonged NKCC1 activation. In addition to accumulating Cl−, which mediates volume changes, NKCC1 can also facilitate changes in migration speed via interactions with the actin cytoskeleton (Garzon-Muvdi et al. 2012). These findings suggest that NKCC1 would be an excellent target to inhibit glioma invasion. In fact, the NKCC1 inhibitor bumetanide (trade name Bumex), already FDA-approved as a diuretic, has shown promising results in preclinical studies (Haas and Sontheimer 2010; Garzon-Muvdi et al. 2012).
Ion channels that mediate volume changes
Utilizing the potassium gradient: Calcium-activated potassium channels
With the Na+/K+-ATPase amassing a high concentration of K+ ions inside the cell, the glioma cell is primed to use this gradient for volumetric changes. First discovered in 1958 by Gyorgi Gardos in human erythrocytes, calcium-activated potassium (KCa) channels are a heterogeneous family of potassium channels that are unified by their activation in response to increases in [Ca2+]i (Gardos 1958). The KCa channel family can be roughly divided into three subtypes based on conductance of potassium: ~250pS (KCa1.1, also known as BK), 20–80pS (KCa3.1 or IK), and 18pS channels (KCa2.1–2.3 or SK1–3) (Latorre et al. 1989). These channels have areas of localized expression throughout the body and play roles in a variety of important physiological functions, a few of which include: regulation of vascular tone, gland secretion, firing of action potentials in neurons, and immune cell migration (Schilling et al. 2004; Melvin et al. 2005; Ledoux et al. 2006; Cruse et al. 2006; Bean 2007). Early studies suggested that KCa channel expression in the brain was restricted and localized to neurons (KCa2.1–2.3) and endothelial cells (KCa1.1); however, more recent studies have detailed the expression of all KCa channel subtypes on a wider subset of cells (Schlichter et al. 2010; Seidel et al. 2011; Turner and Sontheimer 2014). One recent discovery of particular interest is that migrating neuronal precursor cells (NPCs) in the rostral migratory stream express and utilize KCa3.1 during migration (Turner and Sontheimer 2014). The selective inhibition of KCa3.1 with 1-[(2-chlorophenyl)diphenylmethyl)]-1H-pyrazole (TRAM-34) significantly reduced the number of NPCs that successfully reached the olfactory bulb from the stem cell niche in the subventricular zone (SVZ). The similarities between the NPCs in the SVZ and glioma cells has fueled the hypothesis that these cells initiate tumorigenesis. While there is still much debate about the cell type that catalyzes glioma genesis, each of the KCa channel subtypes has been shown to be expressed in glioma cells.
There has been extensive characterization of KCa1.1 in gliomas, where a novel KCa1.1 channel isoform was discovered. This isoform, termed the glioma BK channel or gBK, is the result of alternative splicing of the pore-forming α-subunit gene Slo (Liu et al. 2002). The gBK channel has enhanced sensitivity to Ca2+ at physiologically relevant [Ca2+]i, conferring increased activity in response to relatively small changes in intracellular Ca2+ (Liu et al. 2002; Ransom et al. 2002). This sensitivity provides survival advantages to the glioma cell, such as the abilities to migrate and suppress apoptosis under suboptimal conditions (Weaver et al. 2004, 2006).
Although the gBK channel has been well characterized, there are some discrepancies as to the abundance at which this channel is expressed in vivo in human gliomas relative to the levels seen in in vitro glioma models. A recent study using data from the REpository of Molecular BRAin Neoplasia DaTa (REMBRANDT) database looked at the expression profiles of KCa channels in biopsied human glioma tissue (Turner et al. 2014). This study found that roughly 30 % of glioma patients exhibit overexpression of KCa3.1. Those patients with highly expressed KCa3.1 had a significantly shortened survival compared to patients with- out overexpression. On the other hand, KCa1.1 was found to be expressed in far fewer patients (~10 %), which was not correlated with the survival profile of the patient. Lastly, KCa2.1–2.3 were found to have conflicting correlation to overall patient survival, with the expression of different SK subtypes correlating with both increased or decreased patient survival. It is apparent that the SK channels need to be studied further to clearly define the role they may play in glioma cells. Of all the KCa channels, KCa3.1 has emerged as the predominate potassium channel that modulates migration in glioma cells (Cuddapah et al. 2013; Turner et al. 2014). In these studies, KCa3.1 was found to be present at the leading edge of migrating gliomas cells; specific inhibition of the channel with TRAM-34 significantly impeded migration.
The relatively high expression of KCa3.1 channels in human gliomas (greater than 30 %) and their role in cell invasion make them an attractive target in anti-glioma therapy. Furthermore, there is recent evidence to suggest that radiation causes an elevation in [Ca2+]i that leads to activation of KCa channels, potentially enhancing the migration that shelters glioma cells from treatment measures (Steinle et al. 2011; Stegen et al. 2015). Inhibition of KCa3.1 has previously been proposed for a number of disorders, particularly sickle cell anemia (Ataga et al. 2008). Although the safety of the KCa3.1 inhibitor Senicapoc (ICA-17043) was demonstrated in a phase II clinical trial (NCT00040677), the drug failed in efficacy during phase III (NCT00294541). It would be interesting to determine whether this drug or TRAM-34 would have benefits in glioma treatment, with the added advantage that TRAM-34 is able to cross the BBB (D’Alessandro et al. 2013).
Utilizing the Cl− gradient: Calcium-activated chloride channels
Identifying the Cl− channels that are responsible for the efflux of Cl− necessary to catalyze the movement of cytosolic water has been difficult because of the limited availability of specific inhibitors. Early studies were able to demonstrate the expression of chloride channels ClC-2, ClC-3, and ClC-5 in human glioma tissue biopsies (Olsen et al. 2003), while in normal brain tissue only ClC-2 is abundantly expressed and functional (Günther et al. 1998; Stobrawa et al. 2001). A closer look at the chloride channels that are operational in gliomas using genetic knockdown approaches found that selective loss of inwardly rectifying Cl− currents occurred with knockdown of ClC-2 and ClC-3 (Olsen et al. 2003). No change in the Cl− current occurred upon knockdown of ClC-5. McFerrin and Sontheimer further pursued the identification of the observed Cl− currents in glioma cells, finding ClC-3 expression in lipid-raft domains on invading glioma cell processes (McFerrin and Sontheimer 2006). Upon suppression of ClC-3 expression with antisense oligonucleotides, the Cl− current was significantly diminished, and the small residual Cl− current was no longer sensitive to multi-Cl− channel (including CFTR, ClC-2, and ClC-3) inhibitor 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB). This suggests that ClC-3 is most likely the primary mediator of the inwardly rectifying Cl− currents in glioma cells.
Extensive characterization of the role of ClC-3 in glioma invasion has been pursued since these initial findings. Ca2+/calmodulin-dependent protein kinase II (CaMKII) was found to activate Cl− conductance through ClC-3 in human glioma cells (Cuddapah and Sontheimer 2010; Cuddapah et al. 2012). This finding links an increase in intra-cellular calcium to modulation of ion channel activity. As bradykinin is known to evoke increases in [Ca2+]i, a recent study investigated whether ClC-3 is required for brady-kinin-induced chemotaxis (Cuddapah et al. 2013). It was found that both KCa3.1 and ClC-3 colocalized to the invading processes of glioma cells, and upon inhibition of either channel, bradykinin-induced chemotaxis was abolished. Besides the role that ClC-3 plays in the hydrodynamic model of invasion, this channel is also a critical regulator of cell volume changes associated with the cell cycle (Habela et al. 2008).
Although the availability of selective inhibitors has hindered the molecular characterization of Cl− channels, inhibition of ClC-3 in malignant gliomas has been a surprising success story. Chlorotoxin (Cltx, also known as TM-601), a 36-amino-acid peptide isolated from the venom of the Israeli desert scorpion (Leiurus quinquestriatus), causes an almost complete inhibition of glioma Cl− currents (Ullrich et al. 1998; McFerrin and Sontheimer 2006). Moreover, Cltx inhibits glioma cell migration in several in situ invasion models (Soroceanu et al. 1999; Lui et al. 2010). Interestingly, Cltx appears not only to bind to ClC-3, but also to a complex of MMP-2 and membrane type 1-MMP (MT1-MMP) on the surface of glioma cells (Deshane et al. 2003; McFerrin and Sontheimer 2006). It is still unclear whether this MMP complex is independent or associated with ClC-3. Regardless, Cltx was found to cause internalization of ClC-3 into caveolar rafts after 15 min of application, thus explaining the decrease of glioma cell migration with Cltx application (McFerrin and Sontheimer 2006).
Arguably of more importance is the finding that Cltx binds specifically to human glioma cells and not the surrounding healthy brain tissue (Soroceanu et al. 1998, 1999; Lyons et al. 2002). This selectivity has led to the development of Cltx-derived compounds that have the potential to delineate between normal and cancerous tissue during the surgical removal of a malignant glioma. Three such compounds have been designed with Cltx coupled to: (1) fluorescent indicator Cy5.5 (Veiseh et al. 2007), (2) magnetic resonance imaging (MRI) contrast agent gadolinium (Huang et al. 2011), and (3) nanoparticles (Veiseh et al. 2009). However, all of these applications still remain in the pre-clinical phase. Intracavitary administration of radioactive TM-601 (131I-TM601) in high-grade glioma patients entered clinical trial (NCT00040573) in 2002 (Mamelak et al. 2006). Overall, 131I-TM601 was well tolerated and, as expected, accumulated in the tumor cavity margin. Late in 2008, a phase III trial was approved for 131I-TM601. Results from this trial have yet to be released. Altogether, the targeting of Cl− channels for glioma treatment still has a distance to go before benefits will reach the patient, but this area of exploration still appears to hold much promise.
The movement of water: aquaporins
In the brain, water homeostasis is essential to maintain intracranial pressure (ICP). Any disturbance to the brain, be it from trauma, seizure, ischemia, or stroke, has the potential to affect ICP. Brain tumors are no exception, with almost all malignant gliomas causing tumor-associated edema (Stummer 2007). The aquaporin (AQP) family is solely responsible for water homeostasis in the brain via expression of AQP channels in fluid interfaces including: the subarachnoid space, brain parenchyma, intraventricular compartment, and the perivascular space (Badaut et al. 2002). At these interfaces, and in general, AQPs increase water permeability 5–50 fold (Verkman and Mitra 2000). The AQP family includes 13 known members in mammals. The ‘classic’ aquaporins, those that are believed to act solely as water transporters, include AQP 0, 1, 2, 4, and 5 (Rojek et al. 2008). AQP 3, 7, 9, and 10 are often collectively termed the aquaglyceroporins for their ability to transport water and other small uncharged molecules, such as glycerol. Thorough characterization of AQP 6, 8, 11, and 12 still largely needs to be completed (Rojek et al. 2008).
AQP1 and AQP4 are the main AQPs that facilitate water transport in the brain. In fact, AQP4 is the most highly expressed AQP in the brain, where it can be found in ependymal cells that surround the intraventricular space and in the glial limitans at the perivascular space (refer to Fig. 2) (Jung et al. 1994; Badaut et al. 2002). Because of the high concentration of AQP4 expression in astrocytic endfeet, AQP4 is often used as a marker for an intact glio-vascular interface. In the study by Watkins et al., AQP4 expression was no longer found to be confined to the astrocytic endfoot, suggesting that the gliovascular interface was disturbed (Watkins et al. 2014). While there appears to be a loss of AQP4 expression in the astrocytic endfoot during blood vessel co-option, invading glioma cells have been shown to display an upregulation of AQPs, including AQP1, 4, and 9 (Saadoun et al. 2002a, b, Warth et al. 2007; McCoy and Sontheimer 2007). Furthermore, several studies have found that when AQP expression is knocked down via siRNA, glioma cell migration is slowed (McCoy and Sontheimer 2007; Ding et al. 2011). This solidifies the role that AQPs play in the hydrodynamic model of glioma cell migration. As detailed above, this model of glioma cell migration is critically dependent on the expression and function of water channels to distribute cytoplasmic water in response to the movement of charged ions across the cell membrane.
Therapeutic attempts involving glioma AQPs have largely been unsuccessful (Verkman et al. 2014). The limited reliability of water permeation assays, combined with the small pore diameter of the channel, has made the attempt to find effective inhibitors an up-hill battle. Success may be found in large-scale functional screens, however, and the benefits of finding selective inhibitors for AQPs would not be limited to gliomas alone, as other neurological disease mechanisms, such as stroke, also involve dysfunction of AQPs (Zador et al. 2009).
Glioma vascular regulation: making it all work together
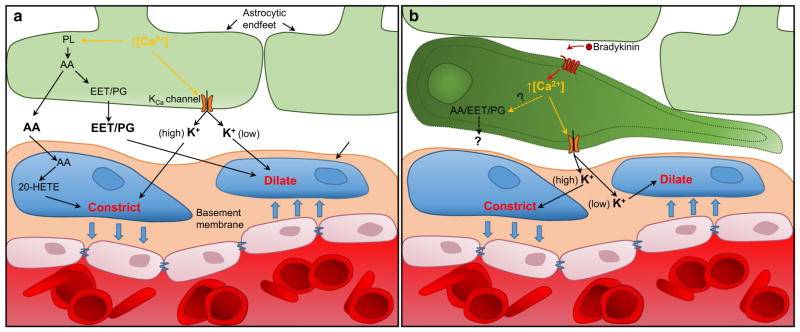
Considerable controversy has surrounded the topic of whether astrocytes and the associated Ca2+ signaling cascade are capable of regulating blood vessel tone (Bazargani and Attwell 2016). However, it now appears that most of the inconsistencies between research groups can be equated to limitations in the IP3R2 receptor knockout mouse model and the resulting persistence of [Ca2+]i increases in the fine processes of astrocytes (Srinivasan et al. 2015; Bazargani and Attwell 2016). Controversies aside, astrocytes are capable of releasing vasoactive molecules—such as K+, prostaglandins (PG), epoxyeicosatrienoic acids (EETs), and arachidonic acid (AA)—which act on vascular smooth muscle cells (VSMCs) present on arterioles (Attwell et al. 2010). While the origins of transient [Ca2+]i changes in astrocytes are still under debate, increases in intracellular Ca2+ cause the activation of phospholipase A2, which converts membrane phospholipids into AA. AA can be released directly or further metabolized within the astrocytes into EETs or PG, which upon release dilate nearby arterioles (refer to Fig. 4). Within VSMCs, AA is converted into 20-hydrox-yeicosatetraenoic acid (20-HETE), which causes constriction of the associated arteriole. Increases in [Ca2+]i can also lead to activation of KCa channels, as detailed extensively above. Astrocytes express and localize KCa1.1 in the end-foot domain, where these channels appear to be functional and mediate dilation of blood vessels (Paulson and Newman 1987; Price et al. 2002; Filosa et al. 2006). Furthermore, there appears to be a switch between dilation and constriction depending on the extracellular K+ concentration following astrocyte activation (refer to Fig. 4) (Girouard et al. 2010).
Fig. 4.
Astrocytes and glioma cells regulate vascular tone a Astro-cytes regulate vascular tone by releasing vasoactive molecules that act on vascular smooth muscle cells (VSCM). The generation of these substrates is dependent on astrocyte activation via increased [Ca2+]i. Raised [Ca2+]i activates phospholipase A2, which converts membrane phospholipids (PL) into arachidonic acid (AA). AA is either released directly or metabolized further into prostaglandins (PG) or epox-yeicosatrienoic acids (EETs). In addition, increased [Ca2+]i causes activation of KCa channels expressed on the astrocyte, leading to the release of potassium (K+). b In the invading glioma cell, K+ release through KCa channels elicits changes in vascular tone. Glioma cells could also be releasing other vasoactive molecules, such as PGs or EETs, that act on VSMCs
Following the observation that glioma cells migrating along blood vessels dislodge astrocytic endfeet from the vascular surface, Watkins et al. questioned whether astro-cytes could still regulate blood vessel tone (Watkins et al. 2014). Norepinephrine (NE) and trans-ACPD (t-ACPD) were used to selectively increase [Ca2+]i in astrocytes with and without perivascular glioma cells present. Vessel responses were significantly decreased when glioma cells were associated with the vasculature compared to areas with intact gliovascular units. Conversely, VSMC function was not found to be compromised during perivascular glioma cell invasion.
As glioma cells express relatively high levels of KCa channels, it is possible that they could assume control of the vasculature in place of the astrocyte. To address this possibility, glioma cells were selectively activated with an agonist for protease-activated receptor 1 (PAR1), which is highly expressed and causes increases in [Ca2+]i in glioma cells. Additionally, selective photo-uncaging of Ca2+ in glioma cells was used to investigate vessel responses. With these experimental paradigms, vessel-associated glioma cells were found to exert control over the vasculature upon stimulation, most likely from the release of K+ from KCa1.1 and KCa3.1 channels expressed on the glioma cell. Interestingly, the glioma cells caused constriction rather than dilation in the associated vessel. Further studies are needed to determine whether KCa channels are the only mediators of the observed vessel response, as glioma cells are also known to express enzymes that are part of the AA pathway as well (De Armas et al. 2010; Omahen 2011).
The idea that glioma cells are able to modulate vascular tone is somewhat surprising, but given the evidence above, it could be a way for glioma cells to modify the vascular niche for further invasion and/or tumor expansion. Furthermore, glioma-vascular regulation could be the culmination of active efforts on the part of the glioma cell to free up needed space for continued invasion. The process first initiated by the chemotactic recruitment of glioma cells to blood vessels, followed by displace of obstacles such as astrocytic endfeet, and lastly the necessary volumetric changes required to facilitate navigation in the limited space.
Conclusion
Two decades of biophysical studies on malignant gliomas have connected old findings with new mechanisms. More specifically, the pathways of glioma invasion first described by Scherer and his observation that glioma cells assume the shape of the space they occupy are now being explained by our understanding of the mechanisms that govern glioma cell migration. A substantial compilation of evidence has proven that glioma cells must repurpose ion channels and transporters for the shape adjustments neccesary to fit the narrow spaces available. This research has identified numerous potential therapeutic targets through which glioma invasion could be reduced. Whether these findings and their application will benefit glioma patients directly has yet to be seen, but several initial pilot studies show great promise.
Acknowledgments
This work was supported by the US National Institutes of Health (NIH) research grants R01-NS036692, R01-NS031234, R01-NS082851, and R01-NS052634 to H.S.. E.G.T was supported by The Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant T32-NS48039.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no conflicts of interest.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Smith WR, De Castro LM, et al. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111:3991–3997. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Baker GJ, Yadav VN, Motsch S, et al. Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia. 2014;16:543–561. doi: 10.1016/j.neo.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372:418–425. doi: 10.1056/NEJMoa1312524. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Beauchesne P. Extra-neural metastases of malignant gliomas: myth or reality? Cancers (Basel) 2011;3:461–477. doi: 10.3390/cancers3010461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellail AC, Hunter SB, Brat DJ, et al. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Blanco G. Na, K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Bragg AD, Amiry-Moghaddam M, Ottersen OP, et al. Assembly of a perivascular astrocyte protein scaffold at the mammalian blood-brain barrier is dependent on alpha-syntrophin. Glia. 2006;53:879–890. doi: 10.1002/glia.20347. [DOI] [PubMed] [Google Scholar]
- Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med. 2006;10:33–44. doi: 10.1111/j.1582-4934.2006.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camassa LM, Lunde LK, Hoddevik EH, et al. Mechanisms underlying AQP4 accumulation in astrocyte endfeet. Glia. 2015 doi: 10.1002/glia.22878. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Song M, Mohamad O, Yu SP. Inhibition of Na+/K+-ATPase induces hybrid cell death and enhanced sensitivity to chemotherapy in human glioblastoma cells. BMC Cancer. 2014;14:716. doi: 10.1186/1471-2407-14-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone CD. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30:151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- Cruse G, Duffy SM, Brightling CE, Bradding P. Functional KCa3.1 K+channels are required for human lung mast cell migration. Thorax. 2006;61:880–885. doi: 10.1136/thx.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah VA, Sontheimer H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J Biol Chem. 2010;285:11188–11196. doi: 10.1074/jbc.M109.097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah VA, Habela CW, Watkins S, et al. Kinase activation of ClC-3 accelerates cytoplasmic condensation during mitotic cell rounding. Am J Physiol Cell Physiol. 2012;302:C527–C538. doi: 10.1152/ajpcell.00248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah VA, Turner KL, Seifert S, Sontheimer H. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J Neurosci. 2013;33:1427–1440. doi: 10.1523/JNEUROSCI.3980-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15:455–465. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro G, Catalano M, Sciaccaluga M, et al. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 2013;4:e773. doi: 10.1038/cddis.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA. 1928;90:823. doi: 10.1001/jama.1928.02690380007003. [DOI] [Google Scholar]
- De Armas R, Durand K, Guillaudeau A, et al. mRNA levels of enzymes and receptors implicated in arachidonic acid metabolism in gliomas. Clin Biochem. 2010;43:827–835. doi: 10.1016/j.clinbiochem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- Ding T, Ma Y, Li W, et al. Role of aquaporin-4 in the regulation of migration and invasion of human glioma cells. Int J Oncol. 2011;38:1521–1531. doi: 10.3892/ijo.2011.983. [DOI] [PubMed] [Google Scholar]
- Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Tamura M, Nakagawa H, Itoh K. Induction of glioma cell migration by vitronectin in human serum and cerebrospinal fluid. J Neurosurg. 2007;107:578–585. doi: 10.3171/JNS-07/09/0578. [DOI] [PubMed] [Google Scholar]
- Gárdos G. The function of calcium in the potassium permeability of human erythrocytes. Biochimica et biophysica acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Garzon-Muvdi T, Schiapparelli P, ap Rhys C, et al. Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biol. 2012;10:e1001320. doi: 10.1371/journal.pbio.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, et al. Astrocytic end-foot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golias CH, Charalabopoulos A, Stagikas D, et al. The kinin system–bradykinin: biological effects and clinical implications. Multiple role of the kinin system–bradykinin. Hippokratia. 2007;11:124–128. [PMC free article] [PubMed] [Google Scholar]
- Günther W, Lüchow A, Cluzeaud F, et al. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci USA. 1998;95:8075–8080. doi: 10.1073/pnas.95.14.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Forbush B. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- Haas BR, Sontheimer H. Inhibition of the Sodium-Potassium-Chloride Cotransporter Isoform-1 reduces glioma invasion. Cancer Res. 2010;70:5597–5606. doi: 10.1158/0008-5472.CAN-09-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BR, Cuddapah VA, Watkins S, et al. With-No-Lysine Kinase 3 (WNK3) stimulates glioma invasion by regulating cell volume. Am J Physiol Cell Physiol. 2011;301:C1150–C1160. doi: 10.1152/ajpcell.00203.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habela CW, Olsen ML, Sontheimer H. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J Neu-rosci. 2008;28:9205–9217. doi: 10.1523/JNEUROSCI.1897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee ME, Zagzag D. Mechanisms of glioma-associated neo-vascularization. Am J Pathol. 2012;181:1126–1141. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimori H, Sontheimer H. Role of Kir4.1 channels in growth control of glia. Glia. 2007;55:1668–1679. doi: 10.1002/glia.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Hornig B, Kohler C, Drexler H. Role of bradykinin in mediating vascular effects of angiotensin-converting enzyme inhibitors in humans. Circulation. 1997;95:1115–1118. doi: 10.1161/01.cir.95.5.1115. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Wu CY, Yang CM. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes. Glia. 2008;56:619–632. doi: 10.1002/glia.20637. [DOI] [PubMed] [Google Scholar]
- Huang R, Han L, Li J, et al. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials. 2011;32:5177–5186. doi: 10.1016/j.biomaterials.2011.03.075. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Inamura T, Black KL. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab. 1994;14:862–870. doi: 10.1038/jcbfm.1994.108. [DOI] [PubMed] [Google Scholar]
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a Beginner’s guide. Neurochem Res. 2015 doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JS, Bhat RV, Preston GM, et al. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Kimbrough IF, Robel S, Roberson ED, Sontheimer H. Vascular amyloidosis impairs the gliovascular unit in a mouse model of Alzheimer’s disease. Brain. 2015;138:3716–3733. doi: 10.1093/brain/awv327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte T, Seligson DB, Leppert JT, et al. The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J Urol. 2008;179:61–66. doi: 10.1016/j.juro.2007.08.148. [DOI] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Lefranc F, Mijatovic T, Kondo Y, et al. Targeting the alpha 1 subunit of the sodium pump to combat glioblastoma cells. Neurosurgery. 2008;62:211–221. doi: 10.1227/01.NEU.0000311080.43024.0E. (discussion 221) [DOI] [PubMed] [Google Scholar]
- Liu X, Chang Y, Reinhart PH, et al. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci. 2002;22:1840–1849. doi: 10.1523/JNEUROSCI.22-05-01840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui VC, Lung SS, Pu JK, et al. Invasion of human glioma cells is regulated by multiple chloride channels including ClC-3. Anticancer Res. 2010;30:4515–4524. [PubMed] [Google Scholar]
- Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39:162–173. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- Mamelak AN, Rosenfeld S, Bucholz R, et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol. 2006;24:3644–3650. doi: 10.1200/JCO.2005.05.4569. [DOI] [PubMed] [Google Scholar]
- Mathieu V, Pirker C, Martin de Lassalle E, et al. The sodium pump alpha1 sub-unit: a disease progression-related target for metastatic melanoma treatment. J Cell Mol Med. 2009;13:3960–3972. doi: 10.1111/j.1582-4934.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E, Sontheimer H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia. 2007;55:1034–1043. doi: 10.1002/glia.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Mijatovic T, Op De Beeck A, Van Quaquebeke E, et al. The cardenolide UNBS1450 is able to deactivate nuclear factor kappaB-mediated cytoprotective effects in human non-small cell lung cancer cells. Mol Cancer Ther. 2006;5:391–399. doi: 10.1158/1535-7163.MCT-05-0367. [DOI] [PubMed] [Google Scholar]
- Montana V, Sontheimer H. Bradykinin promotes the chemotac-tic invasion of primary brain tumors. J Neurosci. 2011;31:4858–4867. doi: 10.1523/JNEUROSCI.3825-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus EA, Horio Y, Inanobe A, et al. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999;26:47–54. doi: 10.1002/(sici)1098-1136(199903)26:1<47::aid-glia5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nakada M, Okada Y, Yamashita J. The role of matrix metallo-proteinases in glioma invasion. Front Biosci. 2003;8:e261–e269. doi: 10.2741/1016. [DOI] [PubMed] [Google Scholar]
- Neely JD, Amiry-Moghaddam M, Ottersen OP, et al. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell S, Fallier-Becker P, Beyer C, et al. Effects of agrin on the expression and distribution of the water channel protein aqua-porin-4 and volume regulation in cultured astrocytes. Eur J Neurosci. 2007;26:2109–2118. doi: 10.1111/j.1460-9568.2007.05850.x. [DOI] [PubMed] [Google Scholar]
- Nomura T, Inamura T, Black KL. Intracarotid infusion of bradykinin selectively increases blood-tumor permeability in 9L and C6 brain tumors. Brain Res. 1994;659:62–66. doi: 10.1016/0006-8993(94)90863-x. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Arita N, Hiraga S, et al. Fibronectin-mediated cell migration promotes glioma cell invasion through chemokinetic activity. Clin Exp Metastasis. 1997;15:538–546. doi: 10.1023/a:1018422926361. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia. 2004;46:63–73. doi: 10.1002/glia.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Schade S, Lyons SA, et al. Expression of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–5582. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omahen DA. Augmentation of chemotherapy-triggered glioma cell apoptosis by blockade of arachidonic acid metabolism–the potential role of ceramide accumulation. Med Hypotheses. 2011;77:726–733. doi: 10.1016/j.mehy.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Ludwig JW, Mi H, et al. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956:183–193. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Liu X, Sontheimer H. BK channels in human glioma cells have enhanced calcium sensitivity. Glia. 2002;38:281–291. doi: 10.1002/glia.10064. [DOI] [PubMed] [Google Scholar]
- Rao JS, Yamamoto M, Mohaman S, et al. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996;14:12–18. doi: 10.1007/BF00157681. [DOI] [PubMed] [Google Scholar]
- Rojek A, Praetorius J, Frøkiaer J, et al. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70:301–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, et al. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatr. 2002a;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, et al. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002b;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Suzuki T, Maeda M, et al. Up-regulation of Na(+), K(+)-ATPase alpha 3-isoform and down-regulation of the alpha1-isoform in human colorectal cancer. FEBS Lett. 2004;563:151–154. doi: 10.1016/S0014-5793(04)00292-3. [DOI] [PubMed] [Google Scholar]
- Sawaya RE, Yamamoto M, Gokaslan ZL, et al. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- Scherer HJ. Structural development in gliomas. The American Journal of Cancer. 1938;34:333–351. [Google Scholar]
- Schilling T, Stock C, Schwab A, Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci. 2004;19:1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- Schlichter LC, Kaushal V, Moxon-Emre I, et al. The Ca2+ activated SK3 channel is expressed in microglia in the rat striatum and contributes to microglia-mediated neurotoxicity in vitro. J Neuroinflammation. 2010;7:4. doi: 10.1186/1742-2094-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel KN, Derst C, Salzmann M, et al. Expression of the voltage- and Ca2+-dependent BK potassium channel subunits BKβ1 and BKβ4 in rodent astrocytes. Glia. 2011;59:893–902. doi: 10.1002/glia.21160. [DOI] [PubMed] [Google Scholar]
- Seifert S, Sontheimer H. Bradykinin enhances invasion of malignant glioma into the brain parenchyma by inducing cells to undergo amoeboid migration. J Physiol (Lond) 2014;592:5109–5127. doi: 10.1113/jphysiol.2014.274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58:4871–4879. [PubMed] [Google Scholar]
- Soroceanu L, Manning TJ, Sontheimer H. Modulation of glioma cell migration and invasion using Cl(−) and K(+) ion channel blockers. J Neurosci. 1999;19:5942–5954. doi: 10.1523/JNEUROSCI.19-14-05942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, et al. Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18:708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen B, Butz L, Klumpp L, et al. Ca2+-activated IK K+ channel blockade radiosensitizes glioblastoma cells. Mol Cancer Res. 2015;13:1283–1295. doi: 10.1158/1541-7786.MCR-15-0075. [DOI] [PubMed] [Google Scholar]
- Steinle M, Palme D, Misovic M, et al. Ionizing radiation induces migration of glioblastoma cells by activating BK K(+) channels. Radiother Oncol. 2011;101:122–126. doi: 10.1016/j.radonc.2011.05.069. [DOI] [PubMed] [Google Scholar]
- Stobrawa SM, Breiderhoff T, Takamori S, et al. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Stummer W. Mechanisms of tumor-related brain edema. Neurosurg Focus. 2007;22:E8. doi: 10.3171/foc.2007.22.5.9. [DOI] [PubMed] [Google Scholar]
- Turner KL, Sontheimer H. KCa3.1 modulates neuroblast migration along the rostral migratory stream (RMS) in vivo. Cereb Cortex. 2014;24:2388–2400. doi: 10.1093/cercor/bht090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KL, Honasoge A, Robert SM, et al. A proinvasive role for the Ca(2+) -activated K(+) channel KCa3.1 in malignant glioma. Glia. 2014;62:971–981. doi: 10.1002/glia.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich N, Bordey A, Gillespie GY, Sontheimer H. Expression of voltage-activated chloride currents in acute slices of human gliomas. Neuroscience. 1998;83:1161–1173. doi: 10.1016/S0306-4522(97)00456-9. [DOI] [PubMed] [Google Scholar]
- Veiseh M, Gabikian P, Bahrami SB, et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- Veiseh O, Sun C, Fang C, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278:F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13:259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite A, Tinsley CL, Locke M, Blake DJ. The neurobiology of the dystrophin-associated glycoprotein complex. Ann Med. 2009;41:344–359. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- Warth A, Simon P, Capper D, et al. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res. 2007;85:1336–1346. doi: 10.1002/jnr.21224. [DOI] [PubMed] [Google Scholar]
- Watkins S, Sontheimer H. Hydrodynamic cellular volume changes enable glioma cell invasion. J Neurosci. 2011;31:17250–17259. doi: 10.1523/JNEUROSCI.3938-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Sontheimer H. Unique biology of gliomas: challenges and opportunities. Trends Neurosci. 2012;35:546–556. doi: 10.1016/j.tins.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Robel S, Kimbrough IF, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AK, Liu X, Sontheimer H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res. 2004;78:224–234. doi: 10.1002/jnr.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AK, Bomben VC, Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia. 2006;54:223–233. doi: 10.1002/glia.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler F, Kienast Y, Fuhrmann M, et al. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia. 2009;57:1306–1315. doi: 10.1002/glia.20850. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Kraus J, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Fallier-Becker P, et al. Structure and functions of aquaporin-4-based orthogonal arrays of particles. Int Rev Cell Mol Biol. 2011;287:1–41. doi: 10.1016/B978-0-12-386043-9.00001-3. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Fallier-Becker P, et al. The disturbed blood-brain barrier in human glioblastoma. Mol Aspects Med. 2012;33:579–589. doi: 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Xie Z, Askari A. Na/K-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- Yousif LF, Di Russo J, Sorokin L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh Migr. 2013;7:101–110. doi: 10.4161/cam.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol. 2009;190:159–170. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Amirnovin R, Greco MA, et al. Vascular apoptosis and involution in gliomas precede neovascularization: a novel concept for glioma growth and angiogenesis. Lab Invest. 2000;80:837–849. doi: 10.1038/labinvest.3780088. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xue Y, Liu Y, et al. Study of correlation between expression of bradykinin B2 receptor and pathological grade in human gliomas. Br J Neurosurg. 2005;19:322–326. doi: 10.1080/02688690500305555. [DOI] [PubMed] [Google Scholar]