Abstract
Background
Coronary artery bypass grafting (CABG) is a common procedure to circumvent the obstruction of coronary arteries when stents are unsuitable. CABG is a very traumatic surgery that requires redirecting blood flow to an external pump. Thus, this procedure has many risks during and after surgery, and minimizing these risks would greatly benefit the patients.
Material/Methods
We selected 126 patients with coronary artery syndrome and who were unsuitable for stent percutaneous coronary intervention. The observation group received minimally invasive direct coronary artery bypass (MIDCAB), while the control group was treated with off-pump CABG.
Results
Blood markers and echocardiography before and after treatment improved equally in both groups. Neither group exhibited obvious adverse reactions, or liver and kidney function damage. However, surgical bleeding and postoperative observation days were significantly reduced in the MIDCAB group. Death and cardiac shock at the end of follow-up were significantly lower in the MIDCAB group.
Conclusions
Overall, the clinical benefits of MIDCAB and OP-CABG were similar, but MIDCAB significantly reduced postoperative hospital stay and intraoperative blood transfusion, and improved clinical prognosis.
MeSH Keywords: Coronary Artery Bypass; Coronary Artery Bypass, Off-Pump; Surgical Procedures, Minimally Invasive
Background
Coronary artery bypass grafting (CABG) is a common procedure to circumvent the obstruction of coronary arteries when stents are unsuitable. CABG is a very traumatic surgery that requires redirecting blood flow to an external pump. Minimally invasive direct coronary artery bypass (MIDCAB) in the left chest is a type of CABG [1]. Bypass from the internal mammary artery (IMA) to the left anterior descending coronary artery (LAD) is an effective technique for the treatment of simple anterior descending artery disease. This surgery is especially recommended for patients with multiple lesions that are not suitable for percutaneous coronary intervention (PCI) or stent stenosis. One of the main advantages of MIDCAB is that there is no need for cardiac arrest and cardiopulmonary bypass (CPB) transfer during surgery [2–4]. MIDCAB patients also benefit from the neurological protection associated with this minimally invasive procedure. According to the methods previously reported in the literature, we analyzed patients with CABG in the Heart Surgery Department in our hospital to further explore the clinical advantages of MIDCAB in the left chest.
Material and Methods
Inclusion and exclusion criteria
Inclusion criteria: 1) All patients enrolled complied with the ACS diagnostic criteria of the American Heart Association (AHA) [5]; 2) All patients enrolled were diagnosed with different degrees of pain or discomfort in the anterior region, and the diagnosis of ACS was confirmed by ECG, myocardial markers, and cardiac troponin; 3) Emergency intravenous thrombolysis in patients undergoing PCI; 4) Patients and their families were willing to accept relevant laboratory tests and signed informed consent [1]; and 5) Patients without other cardiovascular disease. This study was approved by the Ethics Committee of Cangzhou City Central Hospital.
Exclusion criteria were: 1) Application of immunosuppressive agents; 2) Acute and chronic bacterial and/or viral infections; 3) Autoimmune disease; 4) Patients with connective tissue disease; 5) Malignant tumor; 6) Liver or kidney insufficiency; 7) Chronic muscle disease; 8) Allergy to rosuvastatin; 9) Peripheral vascular disease, chronic heart failure, thyroid disease, liver or renal insufficiency, tumor, major trauma in the past 6 months, surgical history; 10) Recent use of adrenal cortex hormone or other immune modulators; and 11) Patients and their families cannot cooperate with doctors and staff, or history of mental illness (6).
Clinical data
We identified 126 cases of acute coronary syndrome as the research objects from March 2014 to January 2015 at the Cangzhou City Central Hospital, including 78 males and 48 females, ages 28–87 years old (Table 1). Patients were randomly divided into the experimental and control groups. The experimental group received MIDCAB while the control group received off-pump coronary artery bypass grafting (OP-CABG). We found no significant differences in age and sex between the 2 groups (Table 1).
Table 1.
Comparison of clinical data between the 2 groups.
| Group | # of cases | Male/female | Age (years) | Course of disease (years) | BMI (kg/m2) |
|---|---|---|---|---|---|
| Observation group | 63 | 34/29 | 61.8±4.6 | 1.7±1.4 | 22.1±0.3 |
| Control group | 63 | 38/25 | 57.8±6.4 | 2.3±1.8 | 21.5±0.7 |
| t/X2 value | – | 0.17 | 0.85 | 0.82 | 0.37 |
| P | – | 0.72 | 0.18 | 0.13 | 0.61 |
Heart rate
We used a diving YX301 finger clip type oximeter to record heart rate at 1, 6, and 12 weeks after PCI. Measurement was performed in a quiet state, each count for 1 minute, measured 3 times, and we calculated the average heart rate.
Imaging diagnosis
Patients in each group were diagnosed by coronary angiography before surgery. Coronary angiography in 1 patient is shown in Figure 1. After surgery, we used a Philips IE33 (probe frequency 2.5 MHZ) color Doppler ultrasound to measure LVEDD, LVESD, LVEDV, LVESV and LVEF at 1, 6, and 12 weeks after the treatment.
Figure 1.
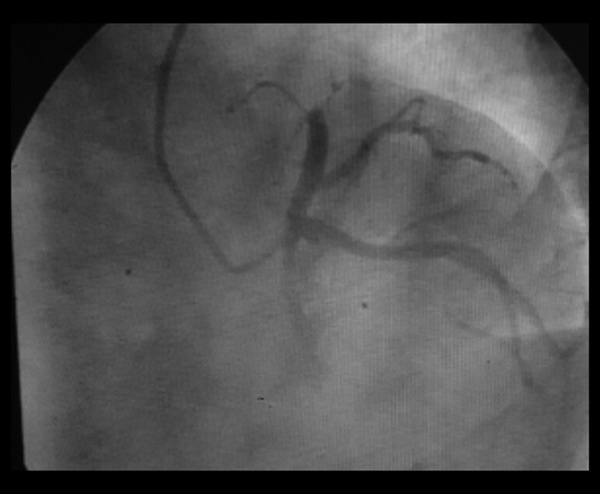
Patient, male, 51 years old, preoperative coronary angiography revealed LAD occlusion of the anterior descending branch.
Serological detection
After fasting for 6–8 h, we extracted 4–6 ml of venous blood from each patient to assess CK-MB, hs-CRP, pro-BNP, matrix metalloproteinase 9 (MMP-9), and other biochemical indicators at 6 and 12 weeks after surgery [3]. At 1 and 12 weeks after treatment, we used 2 ml of blood for detection of pro-BNP levels by rapid fluorescence immunoassay (triage tester); 2–4 ml were centrifuged for 10 min at 1200 rpm, stored at −80°C, and detected hs-CRP, pro-BNP, and MMP-9 levels by enzyme-linked immunosorbent assay (ELISA).
Surgical procedures
We used general anesthesia and double-lumen endotracheal intubation. Patients were in supine position, with left chest pad high at 30°. The front right and left chest wall automatic defibrillation electrode plate was connected with the automatic external defibrillator. We made a 6–8 cm incision in the left chest fourth or fifth intercostal space, with single-lung ventilation in the chest. We suspended the internal mammary artery with a pull-open system (Germany Fehling, State Food and Drug Administration Machinery) with rib retraction, applied moderate stretch, and raised the chest wall, thus providing good visualization. Under direct vision, we moved the left internal mammary artery (LIMA) up to the first rib upper edge, down to the fifth rib. We used titanium clips to cut off the LIMA branch, and washed the LIMA free end with papaverine saline rinse to prevent spasms. After identifying the phrenic nerve, we cut the pericardium, determined the left anterior descending (LAD) artery anastomosis site, estimated that the length of free LAD was sufficient, added heparin (1 mg/kg), and kept intraoperatively activating clotting time ACT over 300 s. We observed the LIMA blood flow in the distal end of the LIMA, placed the rib retractor, and suspended the pericardium. Using a heart stabilizer to fix the LAD after coronary incision, we placed a coronary artery shunt, and used 8-0 PROBLEN line to complete LIMA and LAD anastomosis. We used ultrasonic flowmetry to measure LIMA bridge blood flow after completion of anastomosis. After treatment with protamine-neutralized heparin, we placed a thoracic drainage tube and closed chest. For the prevention and treatment of postoperative incision pain, we use ropivacaine hydrochloride to block the intercostal nerve when closing the chest. For patients with LCX and RCA pathological changes, we started PCI treatment of LCX and (or) RCA at 1 week after surgery.
Statistical methods
We used SPSS19.0 for statistical analysis and processing. Qualitative data were tested by χ2, and for 4-fold table data that did not meet the conditions, we used the Fisher’s exact probability test. Quantitative data were compared and tested by ANOVA. p<0.05 indicated that differences were statistically significant.
Results
Comparison of surgery data
We found no significant differences between the 2 groups in time of admission to the hospital (p>0.05). The surgery time (65.2±12.6 min), the amount of bleeding (56.4±4.7 ml), and the postoperative observation time (6.8±1.3 days) for the MIDCAB group were all significantly lower than for the control group (p<0.05) (Table 2). These results support the benefits of MIDCAB during surgery.
Table 2.
Comparison of operations between the 2 groups.
| Group | Number of cases | Operation time (min) | Bleeding during operation (V/ml) | Postoperative observation (t/d) | Interval of admission to operation (t/min) |
|---|---|---|---|---|---|
| Observation group | 63 | 65.2±12.6 | 56.4±4.7 | 6.8±1.3 | 120.5±63.6 |
| Control group | 63 | 170.3±12.9 | 258.3±13.9 | 12.4±4.2 | 127.4±52.1 |
| T value | – | 30.14 | 11.88 | 20.11 | 0.24 |
| P value | – | 0.008 | 0.005 | 0.002 | >0.05 |
Blood markers before and after surgery
At 6 and 12 weeks after surgery, the serum levels of CK-MB, hs-CRP, pro-BNP, and cTnl were significantly improved by both treatments (Table 3). However, we found no statistically significant differences between the 2 treatments (p>0.05), suggesting that both treatments confer similar clinical benefits.
Table 3.
Comparison of blood markers between the 2 groups before and after surgery.
| Index | Group | # of cases | Before treatment | 6 weeks after treatment | 12 weeks after treatment |
|---|---|---|---|---|---|
| pro-BNP (ng/dl) | Observation group | 63 | 587.8±41.8 | 198.7±23.1 | 157.8±10.2 |
| Control group | 63 | 617.4±54.5 | 198.2±16.5 | 165.4±19.2 | |
| T value | – | 0.76 | 0.62 | 0.81 | |
| P value | 0.527 | 0.33 | 0.18 | ||
| CK-MB (UI/L) | Observation group | 63 | 1.29±0.32 | 0.33±0.11 | 0.24±0.12 |
| Control group | 63 | 1.22±0.47 | 0.38±0.44 | 0.21±0.38 | |
| T value | – | 1.87 | 1.31 | 0.41 | |
| P value | 0.20 | 0.29 | 0.71 | ||
| cTnI (UI/L) | Observation group | 63 | 1.05±0.33 | 0.46±0.11 | 0.97±0.04 |
| Control group | 63 | 1.18±0.12 | 0.87±0.14 | 0.96±0.31 | |
| T value | – | 1.23 | 1.68 | 0.28 | |
| P value | 0.11 | 0.33 | 0.72 | ||
| CRP (μg/L) | Observation group | 63 | 287.4±49.8 | 181.7±11.2 | 108.4±13.6 |
| Control group | 63 | 292.5±24.5 | 194.5±23.2 | 101.4±11.4 | |
| T value | – | 0.42 | 1.27 | 1.81 | |
| P value | 0.87 | 0.14 | 0.12 |
Echocardiography indexes before and after surgery
Comparing echocardiography in the 2 groups before and after treatment, we found significant effects in LVEDD, LVESD, LVEF, and other indicators for both treatments (Table 4). However, we found no statistically significant differences when we compared the 2 groups (p>0.05) (Table 4). This result showed that the clinical benefits of the 2 groups were basically the same.
Table 4.
Comparison of echocardiography indexes between the 2 groups before and after treatment.
| Index | Group | # of cases | Before treatment | 6 weeks after treatment | 12 weeks after treatment |
|---|---|---|---|---|---|
| LVEDD (mm) | Observation group | 63 | 61.4±15.3 | 56.2±12.0 | 51.3±5.1 |
| Control group | 63 | 59.4±12.5 | 59.0±11.8 | 52.2±7.6 | |
| T value | – | 0.38 | 0.52 | 2.48 | |
| P value | 0.37 | 0.51 | 0.71 | ||
| LVESD (mm) | Observation group | 63 | 42.3±7.7 | 38.5±4.3 | 34.2±2.6 |
| Control group | 63 | 40.8±3.2 | 41.6±3.8 | 39.5±5.8 | |
| T value | – | 0.22 | 0.36 | 1.82 | |
| P value | 0.39 | 0.42 | 0.28 | ||
| LVEF (%) | Observation group | 63 | 44.9±7.2 | 48.2±5.5 | 59.2±5.8 |
| Control group | 63 | 43.4±5.6 | 50.3±4.8 | 57.5±2.6 | |
| T value | – | 1.23 | 2.47 | 1.73 | |
| P value | 0.45 | 0.25 | 0.49 |
Prognosis
Neither treatment group showed obvious adverse reactions or liver and kidney function damage. In the MIDCAB group, death at the end of follow-up (1 year after treatment) was significantly lower than in the control group (χ2=24.79, p=0.003) (Table 5). Also, there were significantly fewer patients with cardiac shock in the MIDCAB group than in the control group (χ2=32.45, p=0.002). However, there was no significant difference in congestive heart failure at the end of follow-up between the 2 groups (χ2=1.39, p=0.357) (Table 5).
Table 5.
Comparison of follow-up data of patients of the 2 groups.
| Item | # of follow-up | Control group (n=63) | Observation group (n=63) | χ2 | P |
|---|---|---|---|---|---|
| Death at the end of follow-up | 126 | 9 (0.12) | 2 (0.03) | 24.79 | 0.003 |
| Cardiac shock | 126 | 12 (0.16) | 4 (0.05) | 32.45 | 0.002 |
| Congestive heart failure | 126 | 1 (0.02) | 0 (0.00) | 1.39 | 0.357 |
Discussion
CABG consists of using the patient’s own vein or artery to connect the distal end of the affected coronary artery, bypassing the narrow coronary artery, and restoring the blood supply of the ischemic myocardium [6]. CABG is one of the main methods used to treat coronary heart disease and myocardial infarction [7]. The traditional CABG requires cardiopulmonary bypass, with produces major trauma, requires a long recovery time, and has a high incidence of death and complications, including arrhythmia and bleeding [8,9]. MIDCAB grafting is done through median sternotomy or intercostal incision, without cardiopulmonary bypass or stopping the beating of the heart, and finishes with coronary artery anastomosis. This procedure increases patient safety, and greatly reduces the operative mortality and postoperative complications [10,11].
MIDCAB and TECAB (totally endoscopic coronary artery bypass) technologies can significantly improve clinical outcomes, especially for patients not suitable for coronary stents, and can significantly reduce the surgery rate of CABG. MIDCAB surgery has been gradually accepted by most cardiac surgeons throughout the world [12–15]. MIDCAB treatment of LAD lesions with LIMA has a good long-term patency compared with bare stents and vein grafts [16], does not increase the mortality rate or the incidence of myocardial infarction, and the revascularization rate of the MID group was lower than in the PCI group [17].
In our study, the 2 groups had a similar time of admission to the hospital, but MIDCAB improved operation time, bleeding, and postoperative observation time compared to the control group. These results are similar to a previous report showing that MIDCAB surgery had good outcomes in elderly patients with coronary artery stenosis or occlusion who did not qualify for stent interventional therapy, with the largest benefits coming from avoiding cardiac arrest and cardiopulmonary bypass [18]. MIDCAB reduces surgery time and bleeding, is well tolerated by elderly patients, and demonstrates better postoperative recovery [18].
The blood markers and echocardiography were similarly improved before and after treatment in both groups. Also, the 2 groups showed no obvious adverse reactions or liver and kidney function damage. Heart function and multiple organ function were not severely damaged after the operation. These results indicate that MIDCAB and OP-CABG surgery had basically the same benefits, but 1 year after treatment, number of deaths at the end of follow-up and incidence of cardiac shock were significantly lower in the MIDCAB group. We found no significant differences in congestive heart failure at the end of follow-up between the 2 groups. We think that this result was due to the reduced trauma in minimally invasive surgery. Due to the poor tolerance for surgery in elderly patients, minimally invasive surgery effectively avoids inflammation reaction following the surgical trauma of OP-CABG surgery, especially the effect of inflammatory cytokines IL-6 and 10, TNF-α, and autoimmune reactions on postoperative recovery. Especially, patients with coronary heart disease often have abnormalities of blood lipid and glucose metabolism, and the effect of major trauma after median sternotomy on the immune neural system and the endocrine system further aggravates the existing basic diseases [19–21].
Conclusions
In summary, our data support that although the effect of MIDCAB and OP-CABG surgeries is basically similar, MIDCAB significantly reduces postoperative in-hospital stay and intraoperative blood transfusion volume, and effectively improve the clinical prognosis.
Footnotes
Source of support: Departmental sources
References
- 1.Soylu E, Harling L, Ashrafian H, et al. A systematic review of the safety and efficacy of distal coronary artery anastomotic devices in MIDCAB and TECAB surgery. Perfusion. 2015;31:537–43. doi: 10.1177/0267659115618004. [DOI] [PubMed] [Google Scholar]
- 2.Raja SG, Benedetto U, Alkizwini E, et al. Harefield Cardiac Outcomes Research Group. Propensity Score Adjusted Comparison of MIDCAB Versus Full Sternotomy Left Anterior Descending Artery Revascularization. Innovations (Phila) 2015;10:174–78. doi: 10.1097/IMI.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 3.Zamani P, Schwartz GG, Olsson AG, et al. Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the MIRACL study. J Am Heart Assoc. 2013;2:e3103. doi: 10.1161/JAHA.112.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazek S, Rossbach C, Borger MA, et al. Comparison of sirolimus-eluting stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 7-year follow-up of a randomized trial. JACC Cardiovasc Interv. 2015;8:30–38. doi: 10.1016/j.jcin.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina) J Am Coll Cardiol. 2000;36:970–1062. doi: 10.1016/s0735-1097(00)00889-5. [DOI] [PubMed] [Google Scholar]
- 6.Birla R, Patel P, Aresu G, Asimakopoulos G. Minimally invasive direct coronary artery bypass versus off-pump coronary surgery through sternotomy. Ann R Coll Surg Engl. 2013;95:481–85. doi: 10.1308/003588413X13629960047119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jussli-Melchers J, Haneya A, Hoffmann G, Cremer J. Minimally invasive direct coronary artery bypass in a child with an occlusion of left main coronary artery after arterial switch operation. Interact Cardiovasc Thorac Surg. 2013;17:1040–41. doi: 10.1093/icvts/ivt333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siebert J, Lewicki Ł, Młodnicki M, et al. Atrial fibrillation after conventional and off-pump coronary artery bypass grafting: Two opposite trends in timing of atrial fibrillation occurrence? Med Sci Monit. 2003;9(3):CR137–41. [PubMed] [Google Scholar]
- 9.Raffa GM, Malvindi PG, Ornaghi D, et al. Minimally invasive direct coronary artery bypass in the era of percutaneous coronary intervention. J Cardiovasc Med (Hagerstown) 2015;16(2):118–24. doi: 10.2459/JCM.0b013e3283630c60. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa H, Nabuchi A, Terada H, et al. Minimally invasive direct coronary artery bypass surgery with right gastroepiploic artery for redo patients. Ann Thorac Cardiovasc Surg. 2015;21:378–81. doi: 10.5761/atcs.oa.14-00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng N, Gao CQ, Yang M, Wu Y. Clinical factors influencing surgical approach selection of robotic-enhanced minimally invasive coronary artery bypass grafting. Zhonghua Wai Ke Za Zhi. 2013;51:1016–20. [PubMed] [Google Scholar]
- 12.De Maria R, Repossini A, Dabdoob W, et al. Myocardial perfusion imaging evidence of functionally complete revascularization by minimally invasive direct coronary artery bypass in 2-vessel coronary artery disease. J Nucl Cardiol. 2007;14:860–68. doi: 10.1016/j.nuclcard.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Dehne MG, Meckum J, Dumitriu M, Matheis G. Rupture of the internal thoracic artery 6 weeks after MIDCAB operation. Anaesthesist. 2008;57:1084–86. doi: 10.1007/s00101-008-1431-x. [DOI] [PubMed] [Google Scholar]
- 14.Kettering K. Minimally invasive direct coronary artery bypass grafting: A meta-analysis. J Cardiovasc Surg (Torino) 2008;49:793–800. [PubMed] [Google Scholar]
- 15.Karpuzoglu OE, Ozay B, Sener T, et al. Comparison of minimally invasive direct coronary artery bypass and off-pump coronary artery bypass in single-vessel disease. Heart Surg Forum. 2009;12:E39–43. doi: 10.1532/HSF98.20081068. [DOI] [PubMed] [Google Scholar]
- 16.Samak M, Fatullayev J, Sabashnikov A, et al. Total arterial revascularization: bypassing antiquated notions to better alternatives for coronary artery disease. Med Sci Monit Basic Res. 2016;22:107–14. doi: 10.12659/MSMBR.901508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourad F, Duncan AJ. Tissue stabilizer reverse mounting in minimally invasive direct coronary artery bypass, a simple tool in difficult times. Innovations (Phila) 2009;4:117. doi: 10.1097/IMI.0b013e3181a34740. [DOI] [PubMed] [Google Scholar]
- 18.Sorm Z, Harrer J, Voborník M, et al. Early and long-term results of minimally invasive coronary artery bypass grafting in elderly patients. Kardiol Pol. 2011;69:213–18. [PubMed] [Google Scholar]
- 19.Gorki H, Hoenicka M, Rupp P, et al. Similarity of coagulation and inflammation despite different surgical revascularization strategies – a prospective randomized trial. Perfusion. 2016 doi: 10.1177/0267659116649426. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Parolari A, Poggio P, Myasoedova V, et al. Biomarkers in coronary artery bypass surgery: Ready for prime time and outcome prediction. Front Cardiovasc Med. 2016;2:39. doi: 10.3389/fcvm.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen BA, Fiorentino F, Reeves BC, et al. Mini bypass and proinflammatory leukocyte activation: A randomized controlled trial. Ann Thorac Surg. 2016;101:1454–63. doi: 10.1016/j.athoracsur.2015.09.029. [DOI] [PubMed] [Google Scholar]


