Abstract
To carry epigenetic information, the chromatin structure must be accurately rebuilt after its deconstruction during genomic replication. Using an elegant novel approach, Vasseur et al., [1] reveal that transcription plays a key role in sculpting the chromatin after DNA replication.
The accurate replication of chromosomes is essential for life. This involves not only the replication of the genetic material, but also the faithful replication of the associated proteins that make up the chromatin structure. Nucleosomes are the repeating unit of chromatin, comprising 146bp of relatively inaccessible DNA wrapped around an octamer of histone proteins. Nucleosome are separated a short segment of accessible DNA, called the linker DNA. As such, the exact DNA sequences occupied by the histone octamers influences all our genomic processes. Electron micrographs [2] revealing a ~250bp stretch of nucleosome-free DNA behind the replication fork, indicated that histones are transiently removed during DNA during replication. These chromatin disassembly and reassembly processes are mediated by histone chaperones and ATP-dependent nucleosome remodeling machines [3]. However, many questions remain: How do histone octamers find the same DNA positions on the daughter chromosome that they occupied on the parental chromosome? How quickly does this nucleosome positioning occur after DNA replication? Is nucleosome positioning achieved in the same manner on the lagging and leading strands? What are the proteins and processes that drive nucleosome positioning after DNA replication? A recent study from the Radman-Livaja lab [1] answers these questions using a clever new technology, termed Nascent Chromatin Avidin Pull-down (NChAP).
NChAP enables the genome-wide mapping of nucleosome positions on newly-replicated DNA. Newly-synthesized DNA is labeled with the nucleotide analogue EdU, followed by cutting of the chromatin within the linker regions. The EdU-containing nucleosomal DNA fragments are captured using a Click reaction to add biotin onto EdU, and their sequencing reveals the nucleosome positions genome-wide on newly-replicated DNA [1]. NChAP shows that most yeast nucleosomes are poorly positioned (i.e. occupy variable DNA locations within the population of cells) immediately after DNA replication. An exception is the nucleosome immediately downstream of the transcription start site (TSS), termed the +1 nucleosome, which occupies its mature position at the first measurable time point after DNA replication. The positioning of the +1 nucleosome is established by the nucleosome depleted region (NDR) upstream of the TSS [4]. In agreement, NDR regions are depleted of nucleosomes immediately after DNA replication [1].
Over time, the initially poorly-positioned nucleosomes on the remainder of the newly-synthesized genome adopt the nucleosome positioning pattern of mature chromatin, termed chromatin maturation. However, the rate of chromatin maturation varies over the genome, occurring most rapidly within highly transcribed genes [1] (Fig. 1). An active role for transcriptional elongation was shown using transcription inhibitors. To identify the factors involved in chromatin maturation, Vasseur et al., focused on proteins known to be involved in nucleosome organization over coding sequences. They found that the HIR histone chaperone that mediates histone exchange [5] was required for the shortening of the linker DNA from 20bp in newly-replicated DNA to 13bp in mature chromatin [1]. Meanwhile, inactivation of the ATP-dependent nucleosome remodelers CHD1 and ISW1 blocked chromatin maturation after DNA replication [1]. This role for CHD1 and ISW1 was also shown in another elegant study that examined nucleosome positioning by analysis of Okazaki fragments [4], whose length mirrors the periodicity of the nucleosome repeat [6].
Figure 1. Chromatin maturation is driven by transcription.
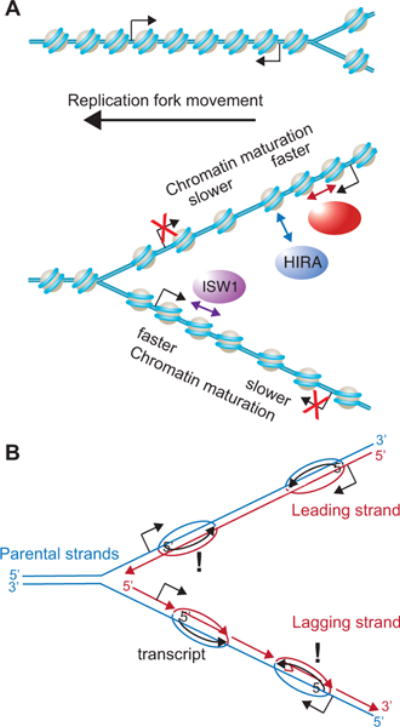
A. The schematic shows the movement of the replication fork on the left side of a replication bubble. The work of Vasseur et al., reveal that nucleosome positioning pattern of mature chromatin is recovered more rapidly within transcribed regions after DNA replication. This is seen in particular when the transcription template strand is on the replication template strand, rather than the newly-synthesized DNA strand, leading to differences in rates of chromatin maturation between the leading and lagging strands for many genes. ISWI and CHD1 promote nucleosome positioning, presumably by helping the histone octamers move along the DNA. HIRA enables nucleosomes to tighten their spacing, presumably via its histone removal and histone assembly activities. B. The schematic represents the same replication fork shown at the bottom of A, but with the chromatin removed, and with the leading and lagging strands indicated. The growing transcripts complementary to the transcription template strand are also shown. When the newly-synthesized DNA strand serves as the transcription template, the transcription machinery would move in the opposite direction to the replication machinery, causing a potential clash, which would be unfavorable to the cell. As a consequence, Vasseur et al., propose that transcription may be suppressed after DNA replication when the newly-synthesized DNA strands serve as transcription template strands.
An ingenious aspect of NChAP is that it differentiates between lagging and leading strands [1]. This is achieved by separating the complimentary DNA strands from the immobilized EdU-containing strands by primer extension in the presence of dUTP, followed by the digestion of dUTP-containing DNA strands. This ensures that only the newly-replicated strand of the DNA duplex gets sequenced. Leading strands map to the Watson DNA strand when downstream of a known origin of replication, while lagging strands map to the Crick DNA strand (Fig. 1). By examining nucleosome positioning around efficient origins of replication, Vasseur et al., found that rates of chromatin maturation on the leading vs. lagging strands differed significantly for about 20% of yeast genes [1]. In these cases, it was not always the leading strand that had faster chromatin maturation, or vice versa. Instead, the difference in the rates of chromatin maturation was a consequence of transcription, because addition of a transcription inhibitor negated these differences. Specifically, the rate of chromatin maturation was slower on which ever of the newly-synthesized strands (lagging or leading) is the template strand for transcription (Fig. 1).
It has recently been shown that transcript levels do not double after DNA replication, despite doubling of the number of gene copies [7, see also Ziva et al., this issue of Trends in Genetics]. This buffering of transcription through replication could be due to either a two-fold decrease in transcription from both gene copies after DNA replication or transcription of only a single gene copy. Given that Vasseur et al., found that transcription promotes chromatin maturation, yet the rate of chromatin maturation was slower when the newly-synthesized strand was also a transcription template strand, they proposed that transcription is suppressed on the gene copy where the newly-synthesized strand is also the transcription template strand (Fig. 1B) [1]. The potential mechanism for this could be related to the fact that the replication machinery and transcription machinery would travel in opposite directions along the same DNA strand (irrespective of lagging or leading) when the newly-synthesized strand is also the transcription template strand (Fig. 1B). Usually the transcription machinery would win out in such a collision, leading to genomic instability [8]. To avoid this unfavorable situation, Vasseur et al., propose that the cell has a mechanism to suppress transcription from the newly-replicated DNA strand when it is also the transcription template strand [1]. This mechanism likely involves acetylation of histone H3 on lysine 56 (H3 K56Ac), given that loss of H3 K56Ac inhibits transcriptional buffering during DNA replication [7] and increases genomic instability [9]. Future analyses will determine whether transcription is blocked from whichever gene copy has the transcription template strand on the newly-synthesized DNA strand, and will reveal the details of such an exciting novel process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vasseur P, Tonazzini S, Ziane R, Camasses A, Rando OJ, Radman-Livaja M. Dynamics of nucleosome positioning maturation following genomic replication. Cell Reports. 2016 doi: 10.1016/j.celrep.2016.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sogo JM, et al. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- 3.Ransom M, Dennehey BK, Tyler JK. Chaperoning histones during DNA replication and repair. Cell. 2010;140(2):183–95. doi: 10.1016/j.cell.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fennessy RT, Owen-Hughes T. Establishment of a promoter-based chromatin architecture on recently replicated DNA can accommodate variable inter-nucleosome spacing. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green EM, et al. Replication-independent histone deposition by the HIR complex and Asf1. Curr Biol. 2005;15(22):2044–9. doi: 10.1016/j.cub.2005.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DJ, Whitehouse I. Intrinsic coupling of lagging-strand synthesis to chromatin assembly. Nature. 2012;483(7390):434–8. doi: 10.1038/nature10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voichek Y, Bar-Ziv R, Barkai N. Expression homeostasis during DNA replication. Science. 2016;351(6277):1087–90. doi: 10.1126/science.aad1162. [DOI] [PubMed] [Google Scholar]
- 8.Brambati A, et al. Replication and transcription on a collision course: eukaryotic regulation mechanisms and implications for DNA stability. Front Genet. 2015;6:166. doi: 10.3389/fgene.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science. 2007;315(5812):649–52. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]


