Abstract
Background
Irisin is a novel myokine, secreted from skeletal muscle after exercise. Irisin mediates exercise-related energy expenditure by turning white adipose tissue (WAT) into brown adipose tissue (BAT). Thus, irisin is considered as a potential biomarker for obesity and metabolic syndrome. Infants born small for gestational age (SGA) have increased risk for metabolic syndrome. However, the physiologic role of irisin in neonates remains to be studied.
Objective
To evaluate the association of umbilical cord blood irisin levels with gestational age and birth weight categories in neonates.
Methods
A cross-sectional study of 341 newborns, from 26 to 41 weeks' gestation. We collected umbilical cord blood and analyzed plasma for irisin by ELISA.
Results
Plasma irisin levels were positively correlated with gestational age (r=0.21, p<0.001), and birth weight Z-score (r=0.18, p<0.001). SGA infants had significantly lower irisin (median [interquartile range] 55.38 [46.56 - 65.72] ng/mL) compared to appropriate for gestational age infants (64.41 [53.87 - 76.76] ng/mL) and large for gestational age infants (68.70 [54.78 - 79.09] ng/mL, p<0.01). The association between SGA and lower irisin remained significant in multivariate analysis independent of gestational age, maternal age, maternal BMI, and gestational diabetes (p=0.03). In singleton infants, irisin was also significantly negatively associated with maternal preeclampsia (p=0.01).
Conclusions
Our results support the notion that irisin may have a physiologic role in neonates. We speculate that decreased levels of irisin in SGA infants may contribute to the development of catch up growth and metabolic syndrome later in life.
Keywords: cord blood, irisin, birth weight, newborn
Introduction
Irisin, a novel regulator of metabolic function, is known for its role in exercise-induced energy expenditure and ‘browning’ of white adipose tissue [1]. Irisin is produced by cleavage of its precursor, fibronectin type III domain containing 5 (FNDC 5), in skeletal muscle after exercise-induced stimulation by peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1-alpha (PGC-1α) [1, 2]. Circulating irisin acts on white adipose tissue, an energy-saving organ, and results in its ‘conversion’ to brown adipose tissue (BAT), which is an energy-producing organ. This is mediated by thermogenesis via the uncoupling protein 1 (UCP1) pathway. Animal studies showed that irisin improved glucose tolerance in obese, pre-diabetic mice [1]. In clinical settings, patients with type 2 diabetes mellitus have lower levels of irisin compared to healthy controls [3, 4], and irisin’s precursor, FNDC5, is decreased in patients with obesity [5]. However, circulating irisin is reported to be paradoxically higher in adults with metabolic syndrome [6], which suggests that states of irisin resistance or tolerance may exist [7, 8].
Newborn infants have predominantly high content of brown adipose tissue (BAT) for thermogenesis, and the content of brown fat decreases significantly after infancy [9]. Small for gestational age (SGA) infants have lower percentage of total body fat (% body fat) compared to appropriate for gestational age (AGA) infants [10-12], which may result in lower irisin levels. SGA and intrauterine growth restriction (IUGR) are known risk factors for metabolic syndrome in adult life [13]. However, the mechanism underlying the association between SGA and metabolic syndrome later in life is not fully understood. Thus, we examined the levels of irisin in the umbilical cord blood between different birth weight categories, and examined the association between cord blood irisin levels and birth weight Z-score and gestational age across a range of gestational ages from 26 to 41 weeks.
METHODS
Study population
This is a cross-sectional study of 341 neonates. Neonates included in the study were born at Brigham and Women’s Hospital (BWH) from April 2010 to November 2013. Collection of umbilical cord blood was performed from all vaginal and Cesarian deliveries when the research nurse or an investigator were available, and when the cord blood was not needed for another purpose such as cord blood banking. The study was reviewed and approved by the Institutional Review Board (IRB) of Brigham and Women’s Hospital (BWH). Analysis of discarded material (cord blood) and review of medical record do not require a written consent from the parents. The study adheres to the declaration of Helsinki.
We excluded newborns with major congenital anomalies such as congenital heart and renal anomalies or maternal chronic inflammatory disease, such as Crohn’s disease, ulcerative colitis, systemic lupus erythematosus (SLE) or cancer.
Among the 341 infants included in analysis, 217 were full term (≥37 weeks), and 124 were preterm (35-36 weeks: 51, 32-34 weeks: 29, 29-31 weeks: 26, 26-28 weeks: 18). Determination of gestational age was based on best obstetrical estimate from the medical record. Newborns were categorized as small for gestational age (SGA: Birth weight <10th percentile), appropriate for gestational age (AGA: 10th percentile ≤ Birth weight ≤ 90th percentile), and large for gestational age (LGA: Birth weight>90th percentile) groups based on the intrauterine growth curves [14].
Cord blood collection and measurement of plasma irisin
Umbilical cord blood was collected from the umbilical vein attached to the placenta at the time of delivery. They were centrifuged and the plasma was divided into 0.5 milliliter aliquots, which were stored in Eppendorf tubes at −80°C until analysis. Levels of irisin were measured by ELISA by commercial kits (EK 067-52, Phoenix Pharmaceutical, Burlingame, CA, USA) with 1:2 dilution of plasma sample (70 μL). Intra- and inter-assay variances were <4-6% and <8-10%, and the range of detectable concentration was 0.066-1024 ng/mL.
Clinical data of newborn infants and mothers
We collected clinical information from the electronic medical records. Maternal data included age, race/ethnicity, marital status, parity, multiple gestation versus singleton, insurance, smoking status, pre-pregnancy body mass index (BMI), clinical chorioamnionitis, preeclampsia and gestational diabetes. Infant data included sex, gestational age, mode of delivery, birth weight, 1 and 5 minute Apgar scores. Birth weight Z scores were obtained using a reference by Fenton et al [14]. Diagnosis of maternal preeclampsia was made by systolic blood pressure≥140mmHg and/or diastolic 90mmHg after 20 weeks of gestation, and proteinuria≥0.3g protein in 24 hour urine or spot urine protein/creatinine ratio ≥ 0.3 mg/mg [15]. Diagnosis of gestational diabetes was made by glucose loading test with 50g glucose, and confirmed by subsequent diagnostic oral glucose challenge test (OGTT) with 100g glucose. Gestational diabetes was diagnosed when two or more of the following criteria were met: the glucose level at fasting was >95 mg/dL, 1 hour glucose>180 mg/dL, 2 hour >155 mg/dL, 3 hour >140mg/dL [16].
Clinical chorioamnionitis was diagnosed with maternal fever>100.4°F, and two out of four additional findings (uterine tenderness, maternal tachycardia, fetal tachycardia, foul/purulent amniotic fluid) [17].
Statistical Analysis
Kruskal-Wallis test was used for continuous variables for comparison between the three different birth weight categories (SGA, AGA, LGA). Post-hoc pairwise analyses between two groups were performed by Wilcoxon rank sum test with Bonferroni correction (p<0.017 was considered significant in post-hoc analyses). Chi square test and Fisher’s exact tests were used for categorical variables. Normality of irisin was tested with Shapiro-Wilk test, and the data was transformed to logarithmic scale to obtain normality. Spearman correlation analyses were performed between continuous variables including irisin, gestational age, birth weight Z-score, maternal age and BMI. We performed multiple regression analysis with irisin as an outcome variable including those variables with p<0.1 in univariate analyses and maternal BMI, infant sex. We used SPSS 19 (Chicago, IL, USA) for statistical analysis.
RESULTS
Patient characteristics (Table 1)
Table 1.
Patients Characteristics
| All (n=341) |
SGA (n=60) |
AGA (n=257) |
LGA (n=24) |
P | |
|---|---|---|---|---|---|
| Newborn Characteristics | |||||
| Irisin (ng/mL) a | 63.3 (52.0, 74.6) | 55.4 (46.6, 65.7) | 64.4 (53.9, 76.7) | 68.7 (54.8, 79.1) | <0.001 |
| Gestational Age (weeks) b | 38.1 (35.4, 39.1) | 36.9 (35.1, 38.4) | 38.0 (35.4, 39.1) | 39.4 (39.0, 39.9) | <0.001 |
| Birth Weight (Kg) b | 2.9 (2.2, 3.5) | 2.3 (1.9, 2.6) | 3.1 (2.5, 3.5) | 4.3 (4.2, 4.5) | <0.001 |
| Birth Weight Z-score b | −0.3 (−1.0, 0.3) | −1.6 (−1.8, −1.5) | −0.2 (−0.6, 0.3) | 1.6 (1.4, 1.8) | <0.001 |
| C-section, n (%) | 258 (75.7) | 50 (83.3) | 190 (73.9) | 18 (75.0) | 0.31 |
| Multiple Gestation, n (%) | 105 (30.8) | 35 (58.3) | 69 (26.9) | 1 (4.2) | <0.001 |
| Female Sex, n (%) | 173 (50.7) | 36 (60.0) | 125 (48.6) | 12 (50.0) | 0.28 |
| 1 min Apgar | 8 (8-9) | 8 (7-8) | 8 (8-9) | 8 (8-9) | 0.21 |
| 5 min Apgar | 9 (9-9) | 9 (8-9) | 9 (9-9) | 9 (9-9) | 0.08 |
| Maternal Characteristics | |||||
| Maternal Age (years) | 33 (30, 37) | 34.0 (31.0, 38.0) | 32.0 (30.0, 36.0) | 34.5 (32.0, 37.0) | 0.02 |
| Maternal Race c | (n=337) | (n=59) | (n=254) | (n=24) | |
| White, n (%) | 206 (60.4) | 36 (60.0) | 154 (59.9) | 16 (66.7) | 0.84 |
| Non-White, n (%) | 131 (38.4) | 23 (38.3) | 100 (38.9) | 8 (33.3) | |
| Smoking (yes), n (%) c | (n=329) 7 (2.1) |
(n=56) 1 (1.7) |
(n=256) 6 (2.3) |
(n=23) 0 (0) |
1.00 |
| Primigravida, n (%) | 123 (36.1) | 26 (43.3) | 94 (36.6) | 3 (12.5) | 0.03 |
| Maternal BMI (Kg/m2) c | (n=324) 24.0 (21.6, 28.3) |
(n=59) 24.0 (21.4, 27.7) |
(n=243) 24.0 (21.4, 28.3) |
(n=22) 27.0 (22.7, 33.4) |
0.05 |
| Preeclampsia, n (%) | 31 (9.1) | 8 (13.3) | 23 (9.0) | 0 (0) | 0.15 |
| Chorioamnionitis, n (%) | 10 (2.9) | 0 (0) | 10 (3.9) | 0 (0) | 0.33 |
| Gestational Diabetes, n (%) c | (n=299) 27 (9.0) |
(n=56) 3 (5.4) |
(n=222) 21 (9.5) |
(n=21) 3 (14.3) |
0.42 |
| PROM>18 hrs, n (%) | 14 (6.2) | 3 (5.0) | 17 (6.6) | 1 (4.2) | 0.92 |
Abbreviations: SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; BMI, body mass index; PROM, premature rupture of membrane
Note: Variables are presented with median (interquartile range), or n (%), p<0.05 in bold, Kruskal-Wallis test (continuous variables), Chi-square or Fisher’s exact test (categorical variables)
Post-hoc pairwise analysis between two groups by Wilcoxon rank-sum test with Bonferroni correction ( P<0.017 was considered significant)
Significant difference between SGA group and AGA/LGA groups
Significant difference between all three groups (SGA vs AGA, AGA vs LGA, and SGA vs LGA)
Data with missing values, n is presented
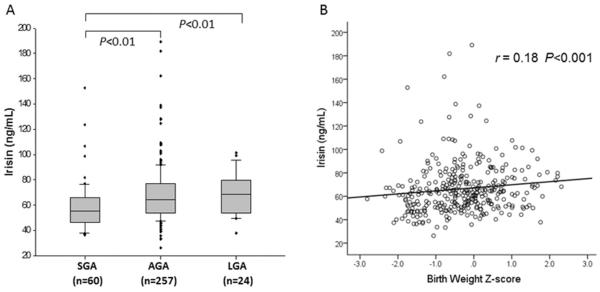
Among 341 newborn infants, 60 (17.6%) were SGA, 257 (75.4%) were AGA, and 24 (7.0%) were LGA infants. The median gestational age and birth weight were lower in the SGA group compared to AGA and LGA groups. Median irisin level was significantly lower in SGA infants (median [interquartile range] 55.4 [46.6-65.7] ng/mL) compared to AGA (64.4 [53.9-76.7] ng/mL) and LGA (68.7 [54.8-79.1] ng/mL) groups (p<0.001, Table 1 and Figure 1A). Gestational age and birth weight were significantly lower in SGA compared with AGA and LGA groups (p<0.001), and there were more infants from multiple gestation pregnancies in the SGA group compared to AGA and LGA groups (p<0.001). Maternal age was lower in AGA group compared to SGA and LGA groups (p=0.02), and there were more primigravid mothers in SGA group (p=0.03). There was no difference in the maternal complications related to pregnancy such as gestational diabetes, preeclampsia, and chorioamnionitis between groups.
Fig. 1.
A. Cord blood irisin levels in relation to birth weight categories. Kruskal-Wallis test reveals a significant difference between the levels of irisin in SGA compared to AGA and LGA infants. Bars represent median, boxes represent 25th and 75th percentile (interquartile range), and whiskers represent 95% confidence interval. Circles represent extreme values. B. Correlation analysis (Spearman correlation coefficient) between plasma irisin and birth weight Z-score.
Correlation between gestational age, birth weight, and circulating irisin levels
Full term infants had higher levels of irisin (67.16 [56.51-83.92] ng/mL) compared to preterm infants (56.9 [48.93-68.32] ng/mL, p=0.001) and there was a significant positive correlation between plasma irisin levels and gestational age (r= 0.21, p<0.001, Table 2).
Table 2.
Correlation analysis of irisin, gestational age, birth weight Z-score, and maternal BMI
| Irisin (ng/mL) |
Gestational age (weeks) |
Birth weight Z-score |
Maternal Age (years) |
Maternal BMI (Kg/m2) |
|
|---|---|---|---|---|---|
| Gestational Age (weeks) |
r = 0.21
p < 0.001 |
1 | |||
| Birth weight Z-score |
r = 0.18
p = 0.001 |
r = 0.25
p < 0.001 |
1 | ||
| Maternal Age (year) |
r = −0.12
p = 0.02 |
r = −0.13
p = 0.01 |
r = 0.06 p = 0.31 |
1 | |
| Maternal BMI (Kg/m2) |
r = 0.02 p = 0.79 |
r = 0.06 p = 0.25 |
r = 0.14
p = 0.01 |
r = 0.02 p = 0.72 |
1 |
Abbreviations: BMI, body mass index
Spearman correlation coefficient
In correlation analysis, we found a significant positive linear correlation between cord blood irisin levels and birth weight Z-score (r= 0.18, p <0.001, Table 2 and Fig. 1B). Maternal age was negatively correlated with irisin level (r=−0.12, p=0.02, Table 2). Maternal BMI was not associated with cord blood irisin (r=0.02, p=0.79, Table 2).
Multivariate regression analysis for factors associated with cord blood irisin levels (Table 3)
Table 3.
A. Multivariate Linear Regression Analysis (Outcome: log(Irisin))
| Variables | Model 1a | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β (95% C.I.) | P | β (95% C.I.) | P | β (95% C.I.) | P | β (95% C.I.) | P | |
| Birth Weight Z-score | 0.05 (0.02 – 0.08) | 0.003 | 0.04 (0.10 – 0.07) | 0.01 | 0.05 (0.01 – 0.08) | 0.007 | 0.05 (0.01 – 0.09) | 0.008 |
| Gestational Age (weeks) | 0.01 (0.001 – 0.02) | 0.03 | 0.005 (−0.005 – 0.01) | 0.31 | 0.005 (−0.01 – 0.02) | 0.43 | ||
| Multiple Gestation | −0.04 (−0.11 – 0.04) | 0.35 | −0.01 (−0.10 – 0.08) | 0.79 | ||||
| Maternal Age (yr) | −0.01 (−0.01 – 0.001) | 0.08 | −0.01 (−0.01 – 0.001) | 0.09 | ||||
| Maternal Race (non-White) | 0.04 (−0.03 – 0.10) | 0.26 | 0.04 (−0.04 – 0.11) | 0.30 | ||||
| Maternal BMI (Kg/m2) | −0.001 (−0.01 – 0.01) | 0.59 | −0.0003 (−0.01 – 0.01) | 0.93 | ||||
| Gestational Diabetes | 0.01 (−0.11– 0.13) | 0.85 | ||||||
| Maternal Preeclampsia | −0.07 (−0.19 – 0.05) | 0.25 | ||||||
| Mode of Delivery (C-section) | −0.05 (−0.14 – 0.04) | 0.26 | ||||||
| Primigravida | −0.04 (−0.11 – 0.03) | 0.22 | ||||||
| Infant Sex (Female) | 0.01 (−0.06 – 0.08) | 0.72 | ||||||
|
| ||||||||
| B. Multivariate Linear Regression Analysis (Outcome: log (Irisin)) | ||||||||
|
| ||||||||
| Variables | Model 1a | Model 2 | Model 3 | Model 4 | ||||
|
| ||||||||
| β (95% C.I.) | P | β (95% C.I.) | P | β (95% C.I.) | P | β (95% C.I.) | P | |
|
| ||||||||
| SGA (Ref: AGA) | −0.15 (−0.23 – −0.07) | <0.001 | −0.15 (−0.23 – −0.07) | <0.001 | −0.12 (−0.20 – −0.04) | 0.005 | −0.10 (−0.19 – −0.01) | 0.03 |
| LGA (Ref: AGA) | 0.04 (−0.09 – 0.17) | 0.52 | 0.02 (−0.12 – 0.15) | 0.82 | 0.06 (−0.08 – 0.19) | 0.40 | 0.09 (−0.06 – 0.24) | 0.25 |
| Gestational Age (weeks) | 0.01 (0.003 – 0.02) | 0.01 | 0.006 (−0.003 – 0.02) | 0.21 | 0.006 (−0.01 – 0.02) | 0.39 | ||
| Multiple Gestation | −0.05 (−0.12 – 0.30) | 0.24 | −0.03 (−0.11 – 0.06) | 0.56 | ||||
| Maternal Age (yr) | −0.004 (−0.10 – 0.002) | 0.17 | −0.005 (−0.01 – 0.002) | 0.16 | ||||
| Maternal Race (non-White) | −0.03 (−0.03 – 0.10) | 0.34 | 0.03 (−0.04 – 0.10) | 0.41 | ||||
| Maternal BMI (Kg/m2) | −0.001 (−0.01 – 0.004) | 0.60 | −0.0004 (−0.01 – 0.01) | 0.90 | ||||
| Gestational Diabetes | 0.02 (−0.10 – 0.14) | 0.74 | ||||||
| Maternal Preeclampsia | −0.08 (−0.20 – 0.04) | 0.18 | ||||||
| Mode of Delivery (C-section) | −0.04 (−0.13 – 0.05) | 0.35 | ||||||
| Primigravida | −0.05 (−0.12 – 0.02) | 0.20 | ||||||
| Infant Sex (Female) | 0.01 (−0.05 – 0.08) | 0.70 | ||||||
Abbreviations: BMI, body mass index; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age
Model 1: unadjusted univariate regression
In univariate linear regression analysis, cord blood levels of irisin were associated with gestational age, birth weight Z-score, maternal age and race/ethnicity, gravidity, delivery by C-section, multiple gestation, and maternal preeclampsia with p<0.1. Multivariate regression analysis was performed in stepwise models as shown in Table 3. Variables listed above and clinically important demographic variables such as maternal BMI and infant sex were included in the final model. Birth weight Z-score was independently associated with cord blood levels of irisin (β=0.05, p=0.008, Table 3A). Gestational age was not independently associated with irisin in multivariate regression analysis (β=0.005, p=0.43). In a model which included SGA, and LGA, with AGA as the reference group instead of using birth weight Z-score to assess the association of SGA and LGA statuses and irisin, the result showed that SGA was an independent factor associated with lower irisin (β= −0.10, p=0.03, Table 3B). LGA status was not associated with cord blood irisin levels (β=0.09, p=0.25, Table 3B).
Sub-group analysis of singleton infants (n=236, Table 4)
Table 4.
Multiple Regression Analysis for a subgroup of singleton Infants(n=236)
| Variables | β (95% C.I.) | SE | P |
|---|---|---|---|
| SGA (Ref: AGA) | −0.14 (−0.26 – −0.03) | 0.06 | 0.02 |
| LGA (Ref: AGA) | −0.04 (−0.11 – 0.19) | 0.08 | 0.58 |
| Gestational Age (weeks) | 0.006 (−0.01 – 0.02) | 0.008 | 0.44 |
| Maternal Age (year) | −0.008 (−0.02 – −0.001) | 0.004 | 0.04 |
| Maternal Race (non-White) | 0.07 (−0.01 – 0.15) | 0.04 | 0.11 |
| Maternal BMI (Kg/m2) | 0.005 (−0.002 – 0.01) | 0.003 | 0.17 |
| Gestational Diabetes | 0.02 (−0.13 – 0.17) | 0.08 | 0.82 |
| Maternal Preeclampsia | −0.23 (−0.40 – −0.06) | 0.09 | 0.01 |
| Mode of Delivery (C-section) | −0.03 (−0.12 – 0.06) | 0.05 | 0.48 |
| Primigravida | −0.06 (−0.15 – 0.02) | 0.04 | 0.14 |
| Infant Sex (Female) | −0.02 (−0.09 – 0.05) | 0.04 | 0.70 |
Abbreviations: SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; LGA, large for gestational age; BMI, body mass index
When we excluded all infants who were products of multiple gestations, SGA remained a significant independent predictor of lower cord blood irisin levels (β= −0.14, p=0.02). Maternal preeclampsia (β =−0.23 p=0.01) and age (β=−0.008, p=0.04) were negatively associated with circulating irisin levels in the cord blood (Table 4).
Discussion
Our cross-sectional study shows that cord blood irisin levels are positively correlated with birth weight Z-scores and gestational age, and the levels are decreased in SGA compared to AGA and LGA infants. To our knowledge, this is the first report of irisin cord blood levels in relation to the birth weight Z-scores and gestational age. The positive correlation between birth weight Z-score and irisin remained significant after controlling for potential confounding variables such as gestational age, maternal age, maternal BMI, and infant sex. Our results also reveal that singleton infants of mothers with preeclampsia had lower cord blood irisin levels compared to infants of mothers without preeclampsia.
A recent small study of irisin levels in umbilical cord arterial blood revealed positive correlation between irisin and fetal weight, and a statistically significant lower levels of irisin in growth restricted infants [18]. The authors did not find a difference between cord blood venous levels and maternal blood levels of irisin in the same study population. Our findings are in agreement with this prior study although our study samples were a mixture of arterial and venous blood. No LGA infants were included in the prior study. In contrast, our study included LGA infants as well as SGA infants for comparison. While it is plausible that the differences in skeletal muscle content between SGA, AGA and LGA infants is the main underlying reason for the observed differences, our results revealed that there is no significant difference in cord blood irisin levels between LGA and AGA/ SGA population. We speculate that cord blood irisin levels are more relevant to the fetal growth restricted state, and may not play an important role in overgrowth, although this may be due to the small number of LGA infants in our sample (n=24).
Another study reported that irisin is detectable in cord blood and correlates with maternal circulating levels [19]. Although we did not examine maternal blood irisin levels, we included important maternal factors such as gestational diabetes, preeclampsia, BMI, and age as potential confounders. Compared to a prior study of circulating levels of irisin in healthy adults (Park et al, n=107) [6], the levels we detected in cord blood in neonates are significantly lower (162.2 [133.5–206.9] ng/mL vs 63.25 [53.99 - 74.60] ng/mL). This needs to be confirmed and its etiology should be explored further, but it may be due to lower body fat mass and/or muscle mass in newborns compared to adults or different regulation of irisin during fetal life.
Garces et al recently reported that irisin’s precursor, FNDC 5, is detectable by immunohistochemistry in placental tissues from normal pregnancies including the decidua, cytotrophoblast, and syncytiotrophoblast cells [20]. They also reported that circulating irisin levels in pregnant women with preeclampsia were lower compared to women with healthy pregnancies in the third trimester. In this previous study, umbilical cord blood irisin levels were not measured. In our current study, we found that circulating cord blood irisin levels were negatively associated with preeclampsia among singleton neonates. The current knowledge of the pathophysiology of preeclampsia includes inadequate trophoblast invasion and abnormal placental development, which lead to hypoperfusion and ischemia to the placenta [21]. Thus, our results suggest that low irisin levels may be related to abnormal placentation and/or placental insufficiency, in the setting of preeclampsia. However, our study does not prove a causal relationship between irisin and preelampsia, and the underlying molecular mechanisms need to be investigated in future studies.
Circulating irisin levels in pediatric populations have not been widely studied. A recently published study in children reported that plasma levels of irisin are higher in females compared to males, are positively correlated with BMI, and are negatively correlated with insulin resistance as assessed by the homeostasis model assessment-estimated insulin resistance (HOMA-IR) [22]. We did not find an association between infant sex and irisin levels in our newborn population. Differences in fat content and distribution of adipose tissue between females and males in older children may account for the discrepancy in sexual dimorphism in irisin levels between these two age groups. In adults, there is a controversial relationship between irisin and BMI. A positive correlation was found among healthy subjects in three studies, but a negative correlation was found in patients with metabolic diseases in two studies [7]. Since BMI does not have important clinical implications in the newborn period, we used birth weight Z-score in our study, and found that irisin is positively correlated with birth weight Z-score independent of gestational age.
Previous studies in adults showed significantly lower circulating irisin levels in patients with type 2 DM compared to healthy controls [3, 4, 7]. Interventional studies have documented an increase in irisin after acute exercise, which may prove to be a therapeutic target in glucose homeostasis [8].Recent reports of irisin in relation to gestational diabetes mellitus (GDM) have shown controversial results between maternal circulating irisin levels and GDM [19, 23, 24]. In our study, offspring of mothers with GDM did not have significantly different circulating irisin levels compared to offspring of mothers without GDM (data not shown). Our findings are in agreement with the reports by Yuksel et al and Piya et al, who also found no difference in circulating irisin levels in cord blood of infants of mothers with GDM and mothers without GDM [19, 23]. In the study of Yusksel et al, in contrast to cord blood, maternal serum irisin was lower in the GDM group compared to the control group. One study measuring CSF irisin levels of mothers at the time of delivery revealed that irisin is significantly elevated in CSF in women with GDM compared to non-GDM [19].
Irisin is also found in human breast milk, but its role in growth and weight gain of neonates and infants is unknown [25]. Animal studies in sheep revealed that browning of white adipose tissue was stimulated by irisin via activation of the Mitogen Activated Protein Kinases (MAP kinase) p38 and Erk signaling and that this pathway was disrupted by maternal nutritional restriction in late pregnancy [26]. In a study of nutritionally deprived newborn sheep, there was decreased expression of brown adipose tissue related genes such as uncoupling protein (UCP)-1 and beta 3 adrenergic receptor (β3ADR), whereas the expression of white adipose tissue related genes were not affected [9]. This suggests that brown adipose tissue may be more significantly affected compared to white adipose tissue in fetal growth restriction.
Differences in white and brown adipose tissue mass between SGA and AGA newborn human infants have not been studied. Although direct measurement of brown fat by 18-FDG PET is not feasible in the newborn population, further studies measuring markers of brown fat, such as uncoupling protein (UCP)-1, could reveal an association between circulating irisin levels and the amount of brown fat in newborns of different birth weight categories more accurately. SGA infants are also known to have lower skeletal muscle mass compared to AGA infants [10] and this may directly affect the levels of irisin through decreased production. Future studies including direct measurement of lean body mass or muscle enzymes such as creatine kinase, aldolase and lactate dehydrogenase will be needed to evaluate this association. With current study design, we cannot conclude whether the difference in circulating irisin levels in SGA and LGA groups is related more to differences in fat content or skeletal muscle content. Low irisin levels in SGA infants may also be related to lower energy expenditure, which could potentially lead to fast weight gain and catch-up growth later in life. Since rapid catch-up growth is a known risk factor for obesity and metabolic syndrome [27], this association may reveal a potentially new pathophysiologic explanation regarding the development of obesity and metabolic syndrome in this vulnerable population. Further prospective studies could reveal a potential association more precisely.
The limitations of our study include a relatively high-risk study population, with a high number of preterm and SGA infants in addition to a high proportion of multiple pregnancies, based in a tertiary medical center, which may limit the generalizability of our findings. Our study population had high proportion of babies born via C-section reflecting high-risk deliveries. However, additional subgroup analysis of babies born via spontaneous vaginal delivery (n=83), also showed the same significant difference between SGA, AGA, and LGA infants (p=0.049) as is shown in the analysis of total population, and multiple linear regression analysis also showed statistically significant association between SGA status and lower level of cord blood irisin (β=-0.30, p=0.02). Although, there have been debates in the literature regarding the optimal methodology for measuring circulating irisin levels, we used the validated ELISA kit and method described in our previous studies as mentioned above [2, 6].
Finally, we did not collect maternal blood for this study, thus, the effect of maternal plasma level on cord blood irisin cannot be evaluated. As previously mentioned, our cord samples are mixed, therefore it is not possible to determine the origin of irisin in the samples. Our study raises a possibility that irisin may mediate the relationship between SGA status at birth and later metabolic syndrome however a direct causal relationship cannot be confirmed by our study. This needs to be addressed in a longitudinal study of irisin levels and postnatal weight gain as well as future onset obesity and metabolic syndrome.
In summary, we found that cord blood irisin levels are positively correlated with birth weight Z-score and are significantly lower in SGA infants compared to AGA, and LGA infants. Cord blood irisin levels are negatively associated with maternal preeclampsia among singleton infants. Decreased irisin levels in SGA infants may be related to low content of brown adipose tissue and irisin may link the association between fetal growth restriction and the higher incidence of obesity and metabolic syndrome in adult life.
Acknowledgement
We acknowledge Marcia Filip, Yvonne Sheldon, Elena Arons, and Deirdre Greene for their assistance with cord blood collection and processing, and Vanessa Gaines and Zoe Michael for data entry. We also thank Emily Oken MD and Gordon Williams MD for critical discussion. This study was funded by Biomedical Research Institute at Brigham and Women’s Hospital, Gerber Foundation, Stork Fund Award and the William F. Milton Fund (to Helen Christou, M.D.), Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources.
Abbreviations
- PPARγ
peroxisome proliferator-activated receptor gamma
- PGC-1α
PPARγ-coactivator 1 alpha
- FNCD5
fibronectin type III domain containing 5
- UCP1
uncoupling protein 1
- BMI
body mass index
- BAT
brown adipose tissue
- GDM
gestational diabetes mellitus
- HOMA-IR
homestasis model assessment-estimated insulin resistance
Footnotes
Disclosure:
The authors do not have any conflicts of interests
Author contribution:
KJ designed study, performed experiments and analysis and drafted the manuscript. HC oversaw umbilical cord blood sample collection and processing and clinical data entry, contributed to study design and edited the manuscript. KH contributed in statistical analysis of data. FD and AF performed laboratory measurements. CM conceptualized the study and supervised all steps including laboratory measurement, analysis of data, and reviewed the final manuscript.
Reference
- [1].Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–38. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100:96–101. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- [4].Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:365–9. doi: 10.1016/j.jdiacomp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- [5].Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–78. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- [6].Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bostrom PA, Fernandez-Real JM, Mantzoros C. Irisin in humans: recent advances and questions for future research. Metabolism. 2014;63:178–80. doi: 10.1016/j.metabol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- [8].Polyzos SA, Kountouras J, Shields K, Mantzoros CS. Irisin: a renaissance in metabolism? Metabolism. 2013;62:1037–44. doi: 10.1016/j.metabol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- [9].Ojha S, Robinson L, Yazdani M, Symonds ME, Budge H. Brown adipose tissue genes in pericardial adipose tissue of newborn sheep are downregulated by maternal nutrient restriction in late gestation. Pediatr Res. 2013;74:246–51. doi: 10.1038/pr.2013.107. [DOI] [PubMed] [Google Scholar]
- [10].Van de Lagemaat M, Rotteveel J, Lafeber HN, van Weissenbruch MM. Lean mass and fat mass accretion between term age and 6 months post-term in growth-restricted preterm infants. Eur J Clin Nutr. 2014;68:1261–3. doi: 10.1038/ejcn.2014.182. [DOI] [PubMed] [Google Scholar]
- [11].Verkauskiene R, Beltrand J, Claris O, Chevenne D, Deghmoun S, Dorgeret S, et al. Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur J Endocrinol. 2007;157:605–12. doi: 10.1530/EJE-07-0286. [DOI] [PubMed] [Google Scholar]
- [12].Harrington TA, Thomas EL, Frost G, Modi N, Bell JD. Distribution of adipose tissue in the newborn. Pediatr Res. 2004;55:437–41. doi: 10.1203/01.PDR.0000111202.29433.2D. [DOI] [PubMed] [Google Scholar]
- [13].Simmons RA. Developmental origins of adult disease. Pediatr Clin North Am. 2009;56:449–66. doi: 10.1016/j.pcl.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Publications Committee SfM-FM. Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011;205:191–8. doi: 10.1016/j.ajog.2011.07.017. [DOI] [PubMed] [Google Scholar]
- [16].Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- [17].Gibbs RS, Duff P. Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol. 1991;164:1317–26. doi: 10.1016/0002-9378(91)90707-x. [DOI] [PubMed] [Google Scholar]
- [18].Caglar M, Goksu M, Isenlik BS, Yavuzcan A, Yilmaz M, Ustun Y, et al. Irisin in idiopathic foetal growth restriction. J Endocrinol Invest. 2014;37:619–24. doi: 10.1007/s40618-014-0078-5. [DOI] [PubMed] [Google Scholar]
- [19].Piya MK, Harte AL, Sivakumar K, Tripathi G, Voyias PD, James S, et al. The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am J Physiol Endocrinol Metab. 2014;306:E512–8. doi: 10.1152/ajpendo.00308.2013. [DOI] [PubMed] [Google Scholar]
- [20].Garces MF, Peralta JJ, Ruiz-Linares CE, Lozano AR, Poveda NE, Torres-Sierra AL, et al. Irisin levels during pregnancy and changes associated with the development of preeclampsia. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2013-4127. jc20134127. [DOI] [PubMed] [Google Scholar]
- [21].Pauli JM, Repke JT. Preeclampsia: Short-term and Long-term Implications. Obstetr Gynecol Clin North Am. 2015;42:299–313. doi: 10.1016/j.ogc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- [22].Al-Daghri NM, Alkharfy KM, Rahman S, Amer OE, Vinodson B, Sabico S, et al. Irisin as a Predictor of Glucose Metabolism in Children: Sexually Dimorphic Effects. Eur J Clin Invest. 2013 doi: 10.1111/eci.12196. [DOI] [PubMed] [Google Scholar]
- [23].Yuksel MA, Oncul M, Tuten A, Imamoglu M, Acikgoz AS, Kucur M, et al. Maternal serum and fetal cord blood irisin levels in gestational diabetes mellitus. Diabetes Res Clin Pract. 2014 doi: 10.1016/j.diabres.2013.12.025. [DOI] [PubMed] [Google Scholar]
- [24].Ebert T, Stepan H, Schrey S, Kralisch S, Hindricks J, Hopf L, et al. Serum levels of irisin in gestational diabetes mellitus during pregnancy and after delivery. Cytokine. 2014;65:153–8. doi: 10.1016/j.cyto.2013.11.009. [DOI] [PubMed] [Google Scholar]
- [25].Aydin S, Kuloglu T. Copeptin, adropin and irisin concentrations in breast milk and plasma of healthy women and those with gestational diabetes mellitus. Peptides. 2013;47:66–70. doi: 10.1016/j.peptides.2013.07.001. [DOI] [PubMed] [Google Scholar]
- [26].Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, et al. Irisin Stimulates Browning of White Adipocytes through Mitogen-Activated Protein Kinase p38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes. 2013 doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- [27].De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology. 2007;148:1350–8. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]