Abstract
Background
Cobicistat (COBI) is a pharmacoenhancer for antiretroviral therapy.
Objective
The current study was designed to profile the metabolic pathways of COBI and to determine the enzymes that contribute to COBI metabolism.
Method
We screened COBI metabolites in mice and human liver microsomes. We also used cDNA-expressed human cytochromes P450 (CYPs) to explore the role of human enzymes in COBI metabolism.
Results
Twenty new and three known metabolites of COBI were identified in mouse urine and feces. These new metabolic pathways of COBI include glycine conjugation, N-acetyl cysteine conjugation, morpholine ring-opening, and thiazole ring-opening. Twelve of COBI metabolites were further confirmed in mouse and human liver microsomes, including nine new metabolites. Consistent with the previous report, CYP3A4 and CYP2D6 were determined as the major enzymes that contribute to COBI metabolism.
Conclusion
This study provided a full map of COBI metabolism. These results can be used to manage CYP-mediated drug-drug interactions and adverse drug reactions that are associated with COBI-containing regimens in human.
Keywords: Cobicistat, drug metabolism, cytochrome P450, CYP3A4, CYP2D6
Introduction
Cobicistat (COBI) is an emerging pharmacoenhancer that inhibits CYP3A4 (1,2). COBI was approved by the FDA in 2012 as a component of the fixed-dose combination elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate for HIV treatment (3). Elvitegravir, an HIV integrase inhibitor, is a substrate of CYP3A4 (4). By combining elvitegravir with COBI, a low dose of elvitegravir is needed to achieve high levels in the body, thus enhancing the efficacy and minimizing the side effects of elvitegravir (5,6). With the same mechanism, COBI was approved for HIV therapy by the FDA in 2015 for two more combinations with darunavir and atazanavir, respectively.
Although COBI is a CYP3A inhibitor, it is also a substrate of CYP3A4 (7). Three CYP3A4-dependent metabolites have been identified in COBI metabolism (7). The chemical structure and metabolic pathways of COBI are similar to that of ritonavir (RTV), the first generation of pharmacoenhancer (8–10). Recent studies suggest that RTV metabolism and bioactivation are associated with the side effects of RTV (11,12). However, the information of COBI bioactivation remains unclear. The current study was designed to fully profile the metabolic pathways of COBI and to determine the enzymes that contribute to COBI metabolism. We screened COBI metabolism in mice, human liver microsomes and cDNA-expressed human CYPs. Twenty new metabolites of COBI were identified in mice and nine of them were confirmed in human liver microsomes. Most of COBI metabolites are CYP-dependent. The data from this study can be used to predict and prevent CYP-mediated drug-drug interactions and adverse drug reactions that are associated with COBI-containing regimens.
Materials and Methods
Chemicals and Reagents
COBI was purchased from Medchem Express (Monmouth Junction, NJ). Recombinant human CYPs and human liver microsomes (HLM) were purchased from XenoTech (Lenexa, KS). Mouse liver microsomes (MLM) were prepared from the liver of wild-type and Cyp3a-null mice according to the previously established methods (13). Methoxylamine and NADPH were purchased from Sigma-Aldrich (St. Louis, MO). All solvents for metabolite analysis were of the highest grade commercially available.
Animals and Treatments
Rodents are commonly used models for pharmacological studies (14). In the current study, FVB/NJ mice (2–4 months old, male) were maintained under a standard 12 h dark/light cycle with water and chow provided ad libitum. The handling was in accordance with study protocols approved by the Institutional Animal Care and Use Committee. The clinical dose of COBI is 150 mg once daily (3). According to the equivalent surface area dosage conversion factors (14), we set the dose of COBI at 30 mg/kg in mice. The mice were treated orally with either vehicle (corn oil) or COBI. After treatment, the mice were housed individually in a metabolic cage for collection of urine and feces. The metabolites identified in mice were further verified in HLM and recombinant human CYPs. In addition, various organs, including brain, liver, kidney, lung, spleen, heart, and testis, were collected at 1 h after COBI treatment to determine the tissue distribution of COBI and its metabolites.
Sample Preparation
Urinary samples were prepared by mixing 60 µL of urine with 60 µL of methanol. The mixtures were centrifuged at 15,000 g for 10 min. Feces from individual mice were pooled and homogenized in 10 folds of water (1 mg of feces in 10 µL of water). Then, 200 µL of acetonitrile was added to 200 µL of the feces mixture, vortexed and centrifuged at 15,000 g for 10 min. The supernatant was transferred to a new vial and recentrifuged at 15, 000 g for 10 min. Tissue samples were weighted and homogenized in water (100 mg of tissues in 500 µL of water). Two hundred µL of acetonitrile:methanol (1:1, v/v) was added to 100 µL of each homogenate, and followed by vortexing and centrifugation at 15,000 g for 10 min. The supernatant was transferred to a new Eppendorf vial for a second centrifugation (15,000 g for 10 min). Each supernatant was transferred to an autosampler vial and 1 µL was injected to a system combining ultraperformance liquid chromatography (UPLC) and time-of-flight mass spectrometry (TOFMS) (Waters Corporation, Milford, MA) for metabolite analysis.
COBI Metabolism in HLM, MLM and Recombinant Human CYPs
Incubations were conducted in 1 × PBS (pH 7.4), which contained 10 µM COBI and 0.2 mg of liver microsomes (HLM or MLM) or 2 pmol of each cDNA-expressed human CYPs (control, 1A2, 1B1, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, 3A4, and 4F11) in a final volume of 95 µL. After 5 min of preincubation at 37 °C, the reaction was initiated by the addition of NADPH and continued for 45 min with gentle shaking. The same experiment was performed without NADPH as a control. Methoxylamine (2.5 mM) was used to trap aldehydes. Incubations were terminated by adding 100 µL of acetonitrile and vortexing for 30 s and centrifuging at 15,000 g for 10 min. Each supernatant was transferred to an autosampler vial, and 1 µL was injected to the UPLC-TOFMS system for metabolite analysis.
Metabolite Analysis
The same approach has been used in our previous studies on the metabolism of atazanavir and tipranavir (15,16). Briefly, separation of COBI and its metabolites was performed on an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm; Waters Corporation, Milford, MA). The flow rate of the mobile phase was 0.5 ml/min using a gradient ranging from 2% to 98% acetonitrile/water containing 0.1% formic acid in 12 min. The column temperature was maintained at 50 °C. TOFMS was operated in positive mode with electrospray ionization. The source and desolvation temperatures were set at 150 °C and 500 °C, respectively. Nitrogen was applied as cone and desolvation gas. Argon was applied as the collision gas. Capillary and cone voltages were set at 0.8 kV and 40 V. TOFMS was calibrated with sodium formate and monitored by the intermittent injection of lockspray leucine enkephalin. MS data were acquired over a range of 50–1000 Da in centroid format. Tandem mass fragmentation with collision energy ramping from 15 to 45 V was used for structural elucidations of COBI and its metabolites.
Synthesis of M2-1
M2-1 was proposed as a metabolite of COBI. To confirm the structure of M2-1, we synthesized this metabolite. Briefly, thiazole-5-carboxylic acid (1,280 mg), glycine methyl ester (900 mg), dicyclohexylcarbodiimide (DCC) (3,090 mg), 4-Dimethylaminopyridine (DMAP) (1,830 mg) were added to a 50 ml round flask. Then 20 ml dichloromethane (DCM) was added to the reaction system and stirred at room temperature. After 18 h, the solvent in the reaction mixture was removed under vacuum, and the product was purified by flash chromatography on silica gel (petroleum ether/acetone 2:1) to yield (thiazole-5-carboxamido) acetic methyl ester (1000 mg). The obtained methyl ester was dissolved in methanol (30 mL), and then 300 mg of NaOH was added to the reaction mixture with stirring. After 1 h, aqueous HCl (5.0 M) was added dropwise to the reaction mixture till a pH of 4 was obtained, and the mixture was concentrated under vacuum. The product was purified by flash chromatography eluted with (EtOH/CH2Cl2, 50:1) on silica gel to yield M2-1 (600 mg) as a yellow oil. NMR spectra were recorded on Bruker FT 400 MHz. Chemical shifts are reported in ppm and coupling constants (J) are reported in Hz. 1H NMR (DMSO-d6, 400 MHz): δH 9.18 (1H, d, J = 0.4 Hz, CH), 8.51 (1H, s, -NH), 8.47 (1H, s, CH), 3.60 (2H, d, J = 5.2 Hz, CH2).
Synthesis of M9-1
M9-1 was also proposed as a metabolite of COBI. We synthesized M9-1 for structural confirmation. Briefly, 2-isopropylthiazole-4-carboxylic acid (342 mg) was added to 20 mL thionyl chloride, and then the resulting mixture was stirred at reflux for 3 h. After evaporation of thionyl chloride under vacuum, the residue was dissolved in DCM (10 mL) followed by the addition of glycine methyl ester (180 mg) and triethylamine (5 mL). The reaction mixture was stirred at room temperature for 2 h. After solvent removal, the product was purified by flash chromatography on silica gel (petroleum ether/acetone 2:1) to yield 2-(isopropylthiazole-4-carboxamido) acetic methyl ester. The obtained product was dissolved in methanol (10 mL). Afterwards, 100 mg of NaOH was added to the reaction mixture with stirring. After 1 h, the pH of the reaction mixture was adjusted to 4 with aqueous HCl (5.0 M), and the mixture was concentrated under vacuum. The product was purified by flash chromatography with (EtOH/CH2Cl2, 50:1) on silica gel to yield M9-1 (210 mg). 1H NMR (CDCl3, 400 MHz): δH 9.67 (1H, -COOH), 8.15 (1H, s, -NH), 4.29 (2H, d, J = 3.6 Hz, CH2), 8.10 (1H, s, CH), 3.28 (1H, m, CH(CH3)2), 1.39 (6H, d, J = 6.4 Hz, CH(CH3)2); 13C NMR (CDCl3, 100 MHz): δC 178.8 (C), 172.3 (C), 164.4 (C), 147.8 (C), 123.8 (CH), 41.2 (CH2), 33.2 (CH), 23.0 (2 × CH3).
Data Analysis
Centroid and integrated mass chromatographic data were processed by MarkerLynx software (Waters Corporation, Milford, MA) to generate a multivariate data matrix. These data were exported to SIMCA-P+ (Umetrics, Kinnelon, NJ) for multivariate data analysis. Orthogonal projection to latent structures-discriminant analysis (OPLS-DA) was conducted on Pareto-scaled data, which improves group separation and facilitates the identification of drug metabolites (17). Screening and identification of COBI metabolites were performed based on accurate mass measurement (mass errors less than 5 ppm). All quantified data are expressed as means ± S.D. or S.E. Statistical significance was determined by the two-tailed Student’s t test. A P value less than 0.05 is considered as statistically significant.
Results and Discussion
Screening for COBI Metabolites in Mice and HLM
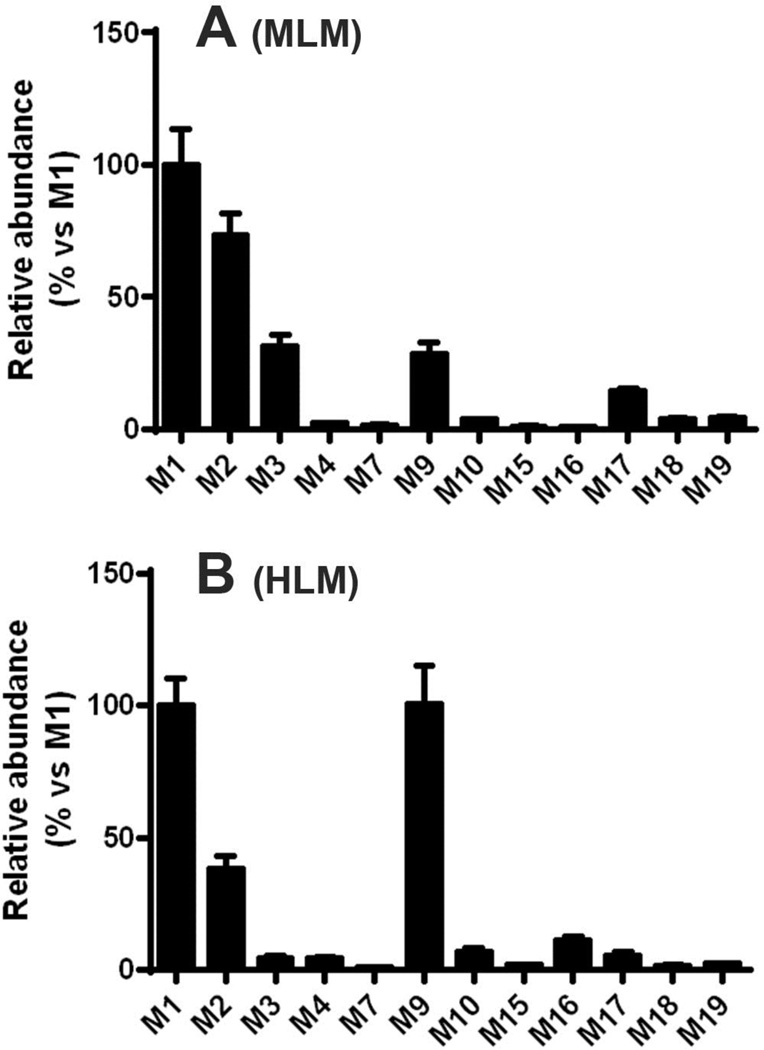
After COBI treatment, COBI and its metabolites were found mainly in mouse feces. The OPLS-DA analysis of mouse feces revealed two clusters corresponding to the control and COBI-treated groups (Figure 1A). The corresponding S-plot indicated the ions contribution to the group separation and the top ranking ions were identified as COBI and its metabolites (Figure 1B). The same methods were used to analyze urine samples (data not shown). The trend-plots showed the relative abundances of the major metabolites of COBI in feces and urine (Figure 1C and 1D). We also explored COBI metabolites in MLM and HLM. We found that the metabolic pathways of COBI in MLM are similar to those in HLM (Figure 2). Overall, twenty-three metabolites of COBI were identified (Table 1), including three previously reported metabolites (M1, M2, and M9) (7) and twenty new metabolites (M2-1, M2-2, M3–M8, M9-1, M10–M20). Most of these metabolic pathways are CYP-dependent. However, we cannot fully exclude the potential contributions of intestinal bacteria to COBI metabolism.
Figure 1. Metabolomic screening for COBI metabolites in mice.
Wild-type mice were treated orally with vehicle or COBI. Feces and urine were collected for metabolites analysis by UPLC-TOFMS. (A) Separation of control and COBI-treated mouse feces in a score plot. (B) Loading S-plot generated by OPLS-DA analysis. The x-axis is a measure of the relative abundance of ions, and the y-axis is a measure of the correlation of each ion to the model. These loading plots represent the relationship between variables (ions) in relation to the first and second components present in the OPLS-DA score plot. Top ranking ions were identified as COBI and its metabolites. (C and D) Trend-plots of major COBI metabolites in feces and urine, respectively. ND, not detected.
Figure 2. Major metabolites of COBI in mouse and human liver microsomes.
Incubations were carried out in 1 × PBS, containing mouse (MLM, A) or human (HLM, B) liver microsomes. The data are expressed as means ± S.D. (n = 3). The abundance of M1 was set as 100%.
Table 1.
COBI metabolites identified in mice and liver microsomes of mice and humans.
| RT (min) |
Observed m/z |
Calculated m/z |
Mass Error (ppm) |
Predicted Molecular Formula |
Identification | Metabolite ID |
Found in mice |
Confirmed in MLM or HLM |
Known or new |
|---|---|---|---|---|---|---|---|---|---|
| 7.74 | 776.3620 | 776.3628 | −1.0 | C40H54N7O5S2 | Cobicistat (COBI) | COBI | Feces | N/A | N/A |
| 6.69 | 792.3567 | 792.3577 | −1.3 | C40H54N7O6S2 | +O | M1 | Feces | HLM, MLM | Known |
| 5.92 | 635.3740 | 635.3743 | −0.5 | C35H51N6O3S | -C5H3NO2S | M2 | Feces, Urine |
HLM, MLM | Known |
| 1.80 | 187.0175 | 187.0177 | −1.1 | C6H7N2O3S | -C34H47N5O2S | M2-1 | Urine | N/A | New |
| 3.13 | 261.0364 | 261.0368 | −1.5 | C9H13N2O3S2 | -C31H41N5O2 | M2-2 | Urine | N/A | New |
| 7.46 | 750.3461 | 750.3471 | −1.3 | C38H52N7O5S2 | -C2H2 | M3 | Feces | HLM, MLM | New |
| 4.95 | 651.3683 | 651.3692 | −1.4 | C35H51N6O4S | -C5H3NOS | M4 | Feces | HLM, MLM | New |
| 7.41 | 778.3421 | 778.3420 | 0.1 | C39H52N7O6S2 | -CH2+O | M5 | Feces | No | New |
| 6.23 | 808.3510 | 808.3526 | −2.0 | C40H54N7O7S2 | +2O | M6 | Feces | No | New |
| 6.46 | 766.3408 | 766.3420 | −1.6 | C38H52N7O6S2 | -C2H2+O | M7 | Feces | HLM, MLM | New |
| 5.82 | 808.3507 | 808.3526 | −2.4 | C40H54N7O7S2 | +2O | M8 | Feces | No | New |
| 6.09 | 637.3165 | 637.3172 | −1.1 | C33H45N6O5S | -C7H9NS | M9 | Feces | HLM, MLM | Known |
| 4.85 | 229.0645 | 229.0647 | −0.9 | C9H13N2O3S | -C31H41N5O2S | M9-1 | Urine | N/A | New |
| 5.65 | 185.0744 | 185.0749 | −2.7 | C8H13N2OS | Trapped by methoxylamine |
M9-0 (trapped) |
N/A | HLM, MLM | New |
| 6.82 | 792.3572 | 792.3577 | −0.6 | C40H54N7O6S2 | +O | M10 | Feces | HLM, MLM | New |
| 6.56 | 808.3528 | 808.3526 | 0.2 | C40H54N7O7S2 | +2O | M11 | Feces | No | New |
| 5.99 | 808.3550 | 808.3526 | 3.0 | C40H54N7O7S2 | +2O | M12 | Feces | No | New |
| 7.07 | 808.3558 | 808.3526 | 4.0 | C40H54N7O7S2 | +2O | M13 | Feces | No | New |
| 7.27 | 808.3511 | 808.3526 | −1.5 | C40H54N7O7S2 | +2O | M14 | Feces | No | New |
| 5.89 | 611.3013 | 611.3016 | −0.5 | C31H43N6O5S | -C9H11NS | M15 | Feces | HLM, MLM | New |
| 4.27 | 496.3277 | 496.3288 | −2.2 | C28H42N5O3 | -C12H12N2O2S2 | M16 | Feces | HLM, MLM | New |
| 7.32 | 736.3834 | 736.3856 | −3.0 | C38H54N7O6S | -C2S+O | M17 | Feces | HLM, MLM | New |
| 6.97 | 764.3812 | 764.3805 | 0.9 | C39H54N7O7S | -CS+2O | M18 | Feces | HLM, MLM | New |
| 7.18 | 764.3799 | 764.3805 | −0.8 | C39H54N7O7S | -CS+2O | M19 | Feces | HLM, MLM | New |
| 5.94 | 808.3528 | 808.3526 | 0.2 | C40H54N7O7S2 | +2O | M20 | Feces | No | New |
RT, retention time; ppm, part per million; MLM, mouse liver microsomes; HLM, human liver microsomes; N/A, not applicable.
Metabolites via Hydroxylation
Two monohydroxylated (M1 and M10) and seven dihydroxylated (M6, M8, M11–M14, and M20) metabolites were detected in the feces of mice treated with COBI (Figure 3A). Most of these hydroxylated metabolites were also identified in the incubation with HLM (Table 1). Among these hydroxylated metabolites, M1 is the most abundant one. MS/MS analysis of M1 indicated that hydroxylation occurred in the isopropylthiazole group (Figure 3B).
Figure 3. Mono- and di-oxidized metabolites of COBI.
(A) The extract ion chromatograms of mono- and di-oxidized metabolites. M1 and M10, monohydroxylated metabolites; M6, M8, M11–M14, M20, dihydroxylated metabolites. (B) MS/MS of M1.
Formation of M2 and M4
M2 and M4 were detected in the feces of mice treated with COBI and in the incubation with HLM (Figure 1C, Table 1, and Figure 4A). M2 was also found to be the most abundant metabolite of COBI in mouse urine (Figure 1D). M2 was eluted at the retention time of 5.92 min, having a protonated molecule [M + H]+ at m/z 635.3740. MS/MS of M2 showed major ions at m/z 465, 448, 361, 252, 214, 197, 127, and 100 (Figure 4B). The protonated molecular ion of M2 was 141 Da lower than that of COBI, suggesting the loss of the thiazol-5-yl-methyl formate moiety in M2. The fragment ions indicate that the remaining parts in M2 are intact, which confirmed the loss of thiazol-5-yl-methyl formate group. M4 had a protonated molecule [M + H]+ at m/z 651.3683, 16 Da higher than that of M2, indicating an additional hydroxylation occurred in M4 (Figure 4C). The major MS/MS fragment ions of M4 at m/z 465, 448, 361, 252, 214, 197, 127, and 100 were the same as those in M2. The ion of m/z 143 suggests the oxidation in M4 occurred in the isopropylthiazole group (Figure 4C).
Figure 4. Chromatograms and MS/MS structural elucidation of M2, M4, M2-1 and M2-2.
(A) The extract ion chromatograms of M2 and M4 in mouse feces. (B) MS/MS of M2. (C) MS/MS of M4. (D) The extract ion chromatograms M2-1 and M2-2 in mouse urine. (E) MS/MS of M2-1. (F) MS/MS of M2-2.
Two small molecules, 2-(thiazole-5-carboxamido) acetic acid (M2-1) and 2-acetamido-3-(thiazol-5-yl-methylthio) propanoic acid (M2-2) (Figure 4D) were identified in the urine of mice treated with COBI. These metabolites were generated from the cleaved thiazol-5-yl-methyl formate moiety in the metabolic pathway of M2. Similar metabolites have been found in metabolism of ritonavir (18), an analog of COBI. Metabolite M2-1 had a protonated molecule [M + H]+ at m/z 187.0175 Da. MS/MS of M2-1 produced major ions at m/z 141, 113, and 86 (Figure 4E). The ion at m/z 141 was produced by the loss of –COOH and the ion at m/z 86 indicated the presence of the thiazole group in M2-1. The structure of M2-1 was further confirmed by NMR. Metabolite M2-2 had a protonated molecule [M + H]+ at m/z 261.0364 Da. The MS/MS of M2-2 showed the major ions at m/z 242, 219, 155, 131, 129, and 98 (Figure 4F).
The detailed mechanism of M2 formation remains unknown. Nevertheless, we propose that M2-1 and M2-2 were generated from 5-(hydroxymethyl)-thiazole rather than thiazole-5-carbaldehyde, since this aldehyde could not be detected in the HLM incubation system containing methoxylamine as a trapping agent. 5-(hydroxymethyl)-thiazole can be further oxidized to form thiazole-5-carboxylic acid and then conjugated with glycine to form M2-1. M2-2 is an N-acetylcysteine conjugated product, generated from a glutathione (GSH)-conjugated metabolite. We proposed an intermediate through sulfation of 5-(hydroxymethyl)-thiazole, in which the sulfate group is easy to leave and forms a reactive cationic intermediate that interacts with GSH (19,20). In addition, our previous study detected both M2-1 and M2-2 in the urine of mice treated with 5-(hydroxymethyl)-thiazole, indicating that 5-(hydroxymethyl)-thiazole is the precursor of M2-1 and M2-2 (18). Uncovering of M2-2 suggests that a reactive intermediate is generated in the metabolic pathway of M2 and it interacts with GSH.
Metabolites via Isopropylthiazole Dealkylation
Two metabolites, M9 and M16 (Figure 5A), were generated through isopropylthiazole moiety cleavage in mice and in HLM (Table 1). The molecular formula of M9 was deduced to be C33H44N6O5S based on its protonated molecule [M + H]+ at m/z 637.3165. MS/MS of M9 produced the major ions at m/z 606, 580, 562, 545, 491, 448, 361, 252, 127, 98, and 84 (Figure 5B). The protonated molecular ion of M9 was 139 Da lower than that of COBI, corresponding to the loss of the 4-methyl-2-isopropylthiazole moiety in M9. The fragment ions at m/z 98 and 84 indicated the presence of the thiazol-5-methyl group in M9, which confirmed the loss of 4-methyl-2-isopropylthiazole moiety. M16 had a protonated molecule [M + H]+ at m/z 496.3277, 280 Da lower than that of COBI, corresponding to the loss of both 4-methyl-2-isopropylthiazole and thiazol-5-yl-methyl formate moieties. The major MS/MS fragment ions of M16 were at m/z 465, 448, 361, 252, 214, 197, and 100 (Figure 5C), which were similar to those in M2 and M9. The protonated molecular ion of M16 was 139 Da lower than that of M2 and 141 Da lower than that of M9, respectively, which further confirmed the loss of the 4-methyl-2-isopropylthiazole and the thiazol-5-yl-methyl formate group in M16.
Figure 5. Chromatograms and MS/MS structural elucidation of the isopropylthiazole dealkylation metabolites of COBI.
Feces and urine samples were analyzed by UPLC-TOFMS. In addition, the incubation of COBI in HLM was conducted to trap 2-isopropylthiazole-4-carbaldehyde, a precursor of M9-1. Methoxylamine was used as a trapping reagent. (A) The extract ion chromatograms of M9 and M16 in mouse feces. (B) MS/MS of M9. (C) MS/MS of M16. (D) The extract ion chromatogram of M9-1 in mouse urine. (E) MS/MS of M9-1. (F) MS/MS of methoxylamine trapped product of 2-isopropylthiazole-4-carbaldehyde.
Together with M9, a glycine conjugated metabolite was identified in the urine of mice treated with COBI and named as M9-1. M9-1 had a retention time of 4.85 min (Figure 5D). The protonated molecule [M + H]+ of M9-1 was at m/z 229.0645 Da. Its MS/MS produced major fragment ions at m/z 183, 154, 126, and 84 (Figure 5E). The ions at m/z 126 and 84 indicated the presence of 4-methyl-2-isopropylthiazole in the molecule. The structure of M9-1 was further confirmed by NMR. M9-1 was proposed as a metabolite of 2-isopropylthiazole-4-carbaldehyde that was generated from the cleavage of the 4-methyl-2-isopropylthiazole moiety in the metabolic pathway of M9. 2-isopropylthiazole-4-carbaldehyde can be oxidized to form an acid that conjugates with glycine to form M9-1. The existence of 2-isopropylthiazole-4-carbaldehyde was confirmed using methoxylamine as a trapping agent in the incubation system with HLM. The trapped product had a mass of [M + H]+ at 185 m/z, and its structure was confirmed by the major MS/MS fragments at m/z 154 (loss of CH3OH) and 127 (loss of C2H4NO) (Figure 5F). Furthermore, M9-1 had been found in the urine of mice treated with 2-isopropylthiazole-4-carbaldehyde (18).
Metabolites via Ring-opening of Morpholine
Three morpholine ring-opening metabolites, M3, M7, and M15, were detected in the feces of mice treated with COBI and in the incubation with HLM (Figure 6 and Table 1). M3 was eluted at 7.46 min (Figure 6A), and had a protonated molecule [M + H]+ at m/z 750.3461 (C38H52N7O5S2). The major MS/MS ions were at m/z 580, 536, 519, 465, 422, 252, 140, 98, and 74 (Figure 6B). The ions at m/z 580, 536, and 519 indicated the loss of C2H2 in the molecule. The key fragment ion at m/z 74 suggested the changes happened in the morpholine ring. M7 had a protonated molecule [M + H]+ at m/z 766.3408 (Figure 6C), 16 Da higher than that of M3. The major MS/MS fragment ions of M7 (at m/z 580, 536, 519, 465, 422, 252, 98, and 74) were similar to those of M3, which indicated that M7 was an oxidized metabolite of M3. M15 had a protonated molecule [M +H]+ at m/z 611.3013, 139 Da lower than that of M3, suggesting the loss of 4-methyl-2-isopropylthiazole moiety (Figure 6D).
Figure 6. Chromatograms and MS/MS structural elucidation of the morpholine ring-opening metabolites of COBI.
(A) The extract ion chromatograms of M3, M7, and M15. (B) MS/MS of M3. (C) MS/MS of M7. (D) MS/MS of M15.
Metabolites via Ring-opening of Thiazole
Three thiazole ring-opening metabolites (M17–M19) were detected in the feces of mice treated with COBI and in the incubation with HLM (Figure 7A and Table 1). M17 had a protonated molecule [M + H]+ at m/z 736.3834, 30 Da lower than that of COBI. The MS/MS of M17 produced the major ions at m/z 649, 606, 567, 545, 491, 448, 361, 278, 214, 197, 171, 140, and 100 (Figure 7B). The fragment ions at m/z 214, 197, 171, 140, and 100 indicated the presence of the 4-methyl-2-propylisothiazole and morpholine ring in the molecule. The ion at m/z 567 indicated the loss of C2HS and addition of one oxygen occurred in the right part of the molecule (Figure 7B). The ions at m/z 491 and 448 suggest the central part of the molecule was intact, thus changes were proposed to occur in the thiazole ring. M18 and M19 had the same protonated molecule [M + H]+ at m/z 764.3812 and 764.3799, corresponding to C39H54N7O7S. They were eluted at 6.97 min and 7.18 min, respectively. M18 and M19 had the same MS/MS fragments at m/z 594, 576, 549, 533, 491, 448, 361, 214, 197, 127, and 100, indicating they had a similar structure (Figure 7C). M17, M18, and M19 might be generated from the oxidation and rearrangement of the thiazole ring. More studies are needed to determine the detailed mechanism of the metabolic pathways M17, M18, and M19.
Figure 7. Chromatograms and MS/MS structural elucidation of thiazole ring-open metabolites of COBI.
(A) The extract ion chromatograms of M17 to M19. (B) MS/MS of M17. (C) MS/MS of M18 and M19.
Tissue Distribution of COBI and its Major Metabolites in Mice
One hour after COBI treatment, the highest concentration of COBI was found in the liver, followed by the kidney, lung, heart, spleen, testis and brain (Figure 8A). A high level of COBI in the liver benefits its efficacy, because CYP3A, the target of COBI (1,2), is highly expressed in the liver. COBI metabolites (M1–M4, and M9) were present predominantly in the liver (Figure 8B–8F), suggesting that COBI metabolism is dependent on the hepatic enzymes. Among these COBI metabolites, a notable level of M2 was found in the kidney (Figure 8C). M2 is also one of the major metabolites found in the urine (Figure 1D).
Figure 8. Tissue distribution of COBI and its major metabolites in mice.
(A) COBI, (B) M1, (C) M2, (D) M3, (E) M4, (F) M9. Mice were treated orally with COBI. Tissues including liver, kidney, lung, spleen, heart, testis, and brain were collected 1 h after treatment. COBI and its metabolites were extracted and analyzed by UPLC-TOFMS. The data are expressed as means ± S.E. (n = 3). ND, not detected.
Role of human CYPs in COBI Metabolism
To determine the role of CYPs in COBI metabolism, cDNA-expressed human CYPs were incubated with COBI. CYP3A4 and 2D6 were found to be the primary enzymes contributing to the major metabolic pathways of COBI (Figure 9A–9E). For the productions of M1–M3, both CYP3A4 and 2D6 are involved, and CYP2D6 shows a higher efficacy than CYP3A4 (Figure 9A–9C). For M9 and M19, their productions are dominantly dependent on CYP3A4 (Figure 9D and 9E). The production of COBI metabolites was also analyzed in the liver microsomes of wild-type (WT) and Cyp3a-null mice. Significant decreases in productions of M2, M3, M9 and M19 were observed in the liver microsomes of Cyp3a-null mice (Figure 9F). However, there was no difference in M1 production between the two genotypes, indicating that CYP2D is important in the M1 pathway. Our data suggest that co-administered drugs that are CYP3A or 2D6 inducers or inhibitors may potentially affect COBI metabolism and lead to drug-drug interactions.
Figure 9. The role of CYPs in COBI metabolism.
The incubation conditions of COBI were detailed in experimental procedures. All samples were analyzed by UPLC-TOFMS. (A–E) The enzymes contributing to the formations of M1 (A), M2 (B), M3 (C), M9 (D) and M19 (E). All data were expressed as means of duplicate incubations. The data from the enzyme with the highest contribution to each metabolic pathway was set as 100%. (F) COBI metabolism in liver microsomes of WT and Cyp3a-null mice. The data are expressed as means ± S.D. (n = 3). The abundances of M1, M2, M3, M9, and M19 were set as 100% in WT. *P < 0.05, **P < 0.01 vs WT.
Conclusions
In summary, COBI metabolism was thoroughly investigated in mice, human liver microsomes and CYPs. We identified twenty new metabolites of COBI and nine of them are confirmed in HLM (Figure 10 and Table 1). We also found that the metabolic pattern of COBI in MLM is similar to that in HLM. Consistent with the previous report (7), CYP3A4 and CYP2D6 were determined as the major enzymes that contribute to COBI metabolism. The results from this study can be used to guide the safe medication of COBI-containing regimens by avoiding potential drug-drug interactions and adverse drug reactions.
Figure 10. A metabolic map of COBI.
Three known metabolites (M1, M2, and M9) and twenty new metabolites (M2-1, M2-2, M3–M8, M9-1, and M10–M20) were identified in mice and human liver microsomes. CYP3A4 and CYP2D6 were determined as the major enzymes that contribute to COBI metabolism.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases [AI095425] and the National Institute of Diabetes and Digestive and Kidney Diseases [DK090305].
List of abbreviations
- COBI
Cobicistat
- CYPs
cytochromes P450
- GSH
glutathione
- HLM
human liver microsomes
- MLM
mouse liver microsomes
- TOFMS
time-of-flight mass spectrometry
- UPLC
ultraperformance liquid chromatography
- OPLS-DA
orthogonal partial least-squares-discriminant analysis.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Xu L, Liu H, Murray BP, Callebaut C, Lee MS, Hong A, Strickley RG, Tsai LK, Stray KM, Wang Y, Rhodes GR, Desai MC. Cobicistat (GS-9350): A Potent and Selective Inhibitor of Human CYP3A as a Novel Pharmacoenhancer. ACS Med. Chem. Lett. 2010;1:209–213. doi: 10.1021/ml1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathias AA, German P, Murray BP, Wei L, Jain A, West S, Warren D, Hui J, Kearney BP. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin. Pharmacol. Ther. 2010;87:322–329. doi: 10.1038/clpt.2009.228. [DOI] [PubMed] [Google Scholar]
- 3.Belavic JM. Drug updates and approvals: 2012 in review. Nurse Pract. 2013;38:24–42. doi: 10.1097/01.NPR.0000425824.44694.e8. quiz 42-23. [DOI] [PubMed] [Google Scholar]
- 4.Ramanathan S, Mathias AA, German P, Kearney BP. Clinical pharmacokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clinical pharmacokinetics. 2011;50:229–244. doi: 10.2165/11584570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Murrell DE, Moorman JP, Harirforoosh S. Stribild: a review of component characteristics and combination drug efficacy. Eur. Rev. Med. Pharmacol. Sci. 2015;19:904–914. [PubMed] [Google Scholar]
- 6.Perry CM. Elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (Stribild(R)): a review of its use in the management of HIV-1 infection in adults. Drugs. 2014;74:75–97. doi: 10.1007/s40265-013-0158-4. [DOI] [PubMed] [Google Scholar]
- 7.Gilead. Elvitegravir/Cobicistat/Emtricitabine/Tenofovir. CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW(S), CENTER FOR DRUG EVALUATION AND RESEARCH, FDA. 2012 [Google Scholar]
- 8.Kuritzkes DR. Drug resistance in HIV-1. Current opinion in virology. 2011;1:582–589. doi: 10.1016/j.coviro.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephson F. Drug–drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. Journal of internal medicine. 2010;268:530–539. doi: 10.1111/j.1365-2796.2010.02301.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsu A, Granneman GR, Cao G, Carothers L, Japour A, El-Shourbagy T, Dennis S, Berg J, Erdman K, Leonard JM. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrobial agents and chemotherapy. 1998;42:2784–2791. doi: 10.1128/aac.42.11.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou S, Chan E, Duan W, Huang M, Chen YZ. Drug bioactivation, covalent binding to target proteins and toxicity relevance. Drug metabolism reviews. 2005;37:41–213. doi: 10.1081/dmr-200028812. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Lu J, Ma X. Metabolomic screening and identification of the bioactivation pathways of ritonavir. Chemical research in toxicology. 2011;24:2109–2114. doi: 10.1021/tx2004147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tien YC, Liu K, Pope C, Wang P, Ma X, Zhong XB. Dose of Phenobarbital and Age of Treatment at Early Life are Two Key Factors for the Persistent Induction of Cytochrome P450 Enzymes in Adult Mouse Liver. Drug metabolism and disposition: the biological fate of chemicals. 2015;43:1938–1945. doi: 10.1124/dmd.115.066316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Wang L, Guo GL, Ma X. Metabolism-mediated drug interactions associated with ritonavir-boosted tipranavir in mice. Drug metabolism and disposition: the biological fate of chemicals. 2010;38:871–878. doi: 10.1124/dmd.109.030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Lu J, Wang L, Ma X. CYP3A-mediated generation of aldehyde and hydrazine in atazanavir metabolism. Drug metabolism and disposition: the biological fate of chemicals. 2011;39:394–401. doi: 10.1124/dmd.110.036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worley B, Powers R. Multivariate Analysis in Metabolomics. Current Metabolomics. 2013;1:92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Lu J, Ma X. Metabolomic screening and identification of the bioactivation pathways of ritonavir. Chemical Res. Toxicol. 2011;24:2109–2114. doi: 10.1021/tx2004147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glatt H. Sulfotransferases in the bioactivation of xenobiotics. Chemico-biological interactions. 2000;129:141–170. doi: 10.1016/s0009-2797(00)00202-7. [DOI] [PubMed] [Google Scholar]
- 20.Jones DP, Moldfus P, Stead AH, Ormstad K, Jornvall H, Orrenius S. Metabolism of glutathione and a glutathione conjugate by isolated kidney cells. J. Biol. Chem. 1979;254:2787–2792. [PubMed] [Google Scholar]