Supplemental Digital Content is available in the text.
Key Words: chronic pain, low birth weight, follow-up study
Abstract
Objective:
To investigate self-reported pain in young adults with a low birth weight.
Materials and Methods:
This study was a part of a long-term follow-up study of preterm very low birth weight (VLBW; birth weight ≤1500 g), term small for gestational age (SGA; birth weight <10th percentile adjusted for sex and parity), and control young adults born during 1986 to 1988. Of the 300 individuals invited, 216 (62 VLBW, 67 term SGA, and 87 controls) completed a pain questionnaire. Of these, 151 (70%) had answered a pain severity question at 19 years. Chronic pain was defined as pain lasting for >6 months and being moderate, severe, or very severe during the past 4 weeks.
Results:
The prevalence of chronic pain at 26 years was 16% in the VLBW group, 21% in the term SGA group, and 7% in the control group. The VLBW and the term SGA groups had higher odds ratios for chronic pain (crude OR, 2.6; 95% CI, 0.9-7.6 for the VLBW group and crude OR, 3.6; 95% CI, 1.3-9.9 for the term SGA group vs. controls). The main results remained after adjusting for potential confounding factors. Some attenuation was observed when adjusting for anxiety and depressive problems. Moderate to very severe pain increased from 16% to 41% in the term SGA group from 19 to 26 years, whereas less changes were seen in the VLBW and the control groups.
Discussion:
Results of our study imply that pain should be in focus when conducting long-term follow-up programs of individuals with a low birth weight.
Chronic nonspecific pain is a common health problem with substantial consequences for the individual, affecting one’s self-perceived health and social and economic well-being.1,2 The etiology of chronic nonspecific pain is not fully understood, although some occupational and psychosocial risk factors are known, such as poor ergonomy3 and depressive problems.4 Known biological mechanisms include central sensitization and lower pain thresholds.5,6 Speculations have been put forward on whether early life conditions may influence the development of chronic pain.
Children and adolescents born preterm with a very low birth weight (VLBW: birth weight <1500 g) are at risk for neurodevelopmental disability. Some have hypothesized that preterm birth and painful sensory input in the neonatal period lead to changes in the pain-processing system, resulting in long-term changes in pain processing.7 Experimental studies investigating this hypothesis have shown decreased pain thresholds8 and pain tolerance9 in children and adolescents born preterm with neonatal intensive care unit (NICU) experience. Others have reported findings consistent with central sensitization,10,11 a result of enhanced activity in pain pathways leading to pain hypersensitivity.5 These changes in pain processing are regarded as central mechanisms in chronic pain development.6,12 Furthermore, repeated painful procedures in the neonatal period are associated with aberrant brain and cognitive development,13,14 which in turn may have an impact on psychological factors. Anxiety and depressive symptoms are closely associated with chronic pain,15 and a higher prevalence of anxiety and depressive problems has been found among young adults born with VLBW.16–19 This warrants investigation on the occurrence of chronic pain in these individuals. However, few studies have investigated chronic pain in individuals with VLBW. Experimental studies that have also included pain questionnaires have not found increased self-reported pain.8,9 Contradictory to this, some epidemiological studies have indicated a slightly higher prevalence of chronic pain in adults born with a low birth weight.20,21
Individuals born small for gestational age (SGA) may also be at risk for chronic pain. There is evidence that suboptimal conditions in fetal life may alter the susceptibility to adult disease.22 Particularly, a low birth weight and being born SGA has been shown to be associated with an altered hypophysis-pituitary-adrenal (HPA) axis activity.23–25 Others have linked a dysfunctional HPA axis activity to stress-related diseases, including chronic pain.26–28 Moreover, it has been shown that young adults born SGA at term have more mental health problems, including anxiety and depressive problems.16,17,29 This may also have an impact on the susceptibility to chronic pain. However, self-reported chronic pain in adults born SGA at term has not been investigated previously.
The aim of this study was to investigate the occurrence of chronic pain in young adults with a low birth weight. As a cross-sectional self-report including pain duration and pain severity has been shown to be adequate to single out adults with persistent chronic pain,30 we used a pain questionnaire designed for this purpose. We hypothesized that young adults born either preterm with VLBW or term SGA would have a higher prevalence of self-reported chronic pain compared with term-born normal-birth weight controls.
MATERIALS AND METHODS
Study Design
This study is a part of a long-term multidisciplinary follow-up study of preterm VLBW, term SGA, and controls born during 1986 to 1988. The preterm infants with VLBW were admitted to the NICU at the University Hospital in Trondheim, Norway. They were either born at this hospital or transferred from a local hospital after birth. All infants with VLBW, without syndromes or congenital malformations, were considered eligible for the follow-up. Term SGA and control participants were born by mothers living in the Trondheim region in the same time period. Women pregnant with their second or third child carrying singletons were eligible (n=1249). All infants born by eligible mothers at term in the enrollment period with a birth weight <10th percentile adjusted for sex and parity were recruited for follow-up. Exclusion criteria at birth were congenital syndromes or malformations. A 10% random sample of the 1249 eligible women was followed in pregnancy (n=129). From this random sample, all infants born at term with a birth weight ≥10th percentile without congenital syndromes or malformations were recruited as controls. The study groups have been examined previously with multidisciplinary assessments in preschool age, adolescence, and young adulthood.16,31–35 The present study was carried out during 2013 to 2014, when the participants were 25 to 28 (mean age 26) years old.
Study Population
The VLBW Group
In total, 121 children with a birth weight ≤1500 g were admitted to the NICU in the enrollment period. Of these, 2 were excluded due to a congenital syndrome or malformation at birth and 33 died. For the present study, 2 participants were regarded as not testable on the basis of previous examinations. Hence, 84 individuals were eligible and invited to participate. Of these, 62 participated (74%; 32 men, 30 women), and 22 did not respond or declined participation.
The Term SGA Group
Of the 1249 eligible pregnant women, 104 (8%) gave birth to an SGA child at term. One was excluded at baseline due to a congenital malformation, and for the present study, one participant was regarded as not testable on the basis of previous examinations. Of 102 eligible individuals, 2 were not invited due to unknown address, 67 participated (66%; 32 men, 35 women), and 33 did not respond or declined participation.
The Control Group
Of the remaining term-born normal–birth weight infants in the 10% random sample, 120 children were recruited for follow-up. Two were excluded due to a congenital syndrome or malformation. Of the 118 eligible individuals, 2 were not invited due to unknown address, 87 participated (74%; 37 men, 50 women), and 29 did not respond or declined participation.
Measures
Perinatal Measures
Perinatal data, including the birth weight, the gestational age, and 1- and 5-minute Apgar scores, were available in the study database. For the VLBW group, additional NICU and perinatal variables were also available, which included the following; the number of days in the NICU, the number of days on ventilator and days with supplemental O2, and the presence of intraventricular hemorrhage.
Outcome Measures
At the current follow-up at 26 years, participants were asked “How much bodily pain have you had during the past 4 weeks?” with the following response options: “none,” “very mild,” “mild,” “moderate,” “severe,” or “very severe.” This verbal pain rating scale used in the Short-Form 36 health survey (SF-36)36 was used in the Norwegian population-based Nord-Trøndelag Health Study (HUNT),37 and has been recommended as a global measurement of pain severity.38 A cutoff in the middle of this verbal rating intensity scale (none to mild vs. moderate to very severe) has been shown to be adequate to single out a group with more complex pain problems,39 and thus, we used moderate, severe, or very severe pain during the past 4 weeks as the pain severity outcome measure.
The pain duration was assessed by the question “Do you have bodily pain which has lasted for more than 6 months?” with yes and no options. Chronic pain was defined as pain lasting for >6 months and being moderate, severe, or very severe during the past 4 weeks.
In a previous follow-up visit at age 19 years, the same participants completed the SF-36.36 The SF-36 contains 2 items on bodily pain, where the pain severity measure is identical to the pain severity item used at the current follow-up. The answers to the pain severity question at age 19 years were compared with the answers from the current follow-up to measure pain persistency and to study the longitudinal aspect of self-reported pain from late adolescence to young adulthood. Of the 214 participants with answers to the pain severity question at the current follow-up, 151 (71%) had also answered the pain severity question at 19 years.
Covariates
The parental socioeconomic status (SES) was calculated according to Hollingshead’s Two-Factor Index of Social Position,40 based on the education and the occupation of both parents (adapted to today’s categories). The SES score was rated from 1 (lowest) to 5 (highest). Data were obtained through a short interview with the parents at the 14 years’ follow-up, and further supplemented at the age of 19 years in 8 participants who had missing data from age 14 years. Data on the parental SES were unavailable for 11 participants in the VLBW group, 12 in the SGA group, and 14 in the control group at the current follow-up.
At the 14 years’ follow-up, the adolescents and their parents were interviewed about the adolescent’s surgical history after the neonatal period. Surgeries were specified by type, and we added the number of surgeries for each participant into a continuous variable, excluding minor surgeries such as ear tube insertions and skin surgery. Data on surgical history from the 14 years’ follow-up were unavailable for 17 participants in the VLBW group, 19 in the SGA group, and 20 in the control group.
The maternal age at birth was available in the study database for all participants, and was used as a continuous measure. At the 14 years’ follow-up, the mother had been asked if she smoked, and if yes to specify the frequency or the number of cigarettes smoked per day with the following alternatives: “not daily,” “<10 per day,” “10-20 per day,” “>20 per day,” and “unknown/other.” Maternal smoking at 14 years was dichotomized into smoking or no smoking, and data were available for 48 participants in the VLBW group, 53 in the SGA group, and 69 in the control group. Data on maternal smoking at conception were available in the database for the term SGA and the control groups. At enrollment, before week 20 of pregnancy, the woman had been asked if she smoked cigarettes on a daily basis at conception, and if yes to indicate the number of cigarettes smoked per day. Maternal smoking at conception was dichotomized into no smoking and smoking ≥1 cigarette per day, and data were available for 57 participants in the SGA group and 83 in the control group. Data on maternal smoking at conception were not available for the VLBW group as the mothers were not followed in pregnancy.
At 26 years, self-reported mental health was measured using the Achenbach System of Empirically Based Assessment—Adult Self-Report (ASEBA-ASR; age range 18 to 59 years). The ASEBA-ASR assesses behavioral and emotional problems during the past 6 months. After computerization, the ASEBA database encodes items that are consistent with the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for different diagnoses into DSM-oriented subscales.41,42 DSM-oriented subscales reflect anxiety and depressive problems rather than disorders. In this pain study, we chose to use the subscales for anxiety and depressive problems as covariates. The anxiety scale ranges from 0 to 14, and the depressive problems scale ranges from 0 to 28, with higher scores indicating more problems in these areas.42 DSM scales were used as continuous measures. In a somatic health questionnaire completed at the current follow-up, participants were asked whether they smoked currently, with yes and no alternatives. The Wechsler Abbreviated Scale of Intelligence IV administered by trained study personnel under supervision from a neuropsychologist was used to measure the intelligence quotient (IQ). IQ was used as a continuous measure.
Statistical Analysis
Analyses of nonparticipants and differences in group characteristics were conducted with the Wilcoxon rank-sum test. The McNemar’s test for paired nominal data was used to indicate the significance of changes in the prevalence of moderate to very severe pain in the past 4 weeks from 19 to 26 years. For the Wilcoxon rank-sum test and the McNemar’s test, a 2-tailed P-value of <0.05 was considered statistically significant. Binary logistic regression was used to estimate the crude odds ratios (OR) and 95% confidence intervals for the association between a low birth weight and self-reported chronic nonspecific pain. In multivariable analyses, we adjusted for the following potential confounding factors chosen by a priori knowledge43; sex and maternal age; and in subanalyses for those with available data, parental SES and maternal smoking at conception and at 14 years were also included. Separate analyses for men and women and a likelihood-ratio test after estimation was performed to investigate possible effect modification by sex. In separate models, anxiety and depression, IQ, current smoking status, and the number of surgeries among participants were added to investigate possible mediation by these factors. In subanalyses on the VLBW group, logistic regression was used to examine possible associations between perinatal factors and pain reports. Data were analyzed with STATA 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Ethical Considerations
The Regional Committee for Health Research Ethics approved the study (2013/636/REK midt). All participants received written and oral information, and signed a consent form. This consent included linking data from the current study with data from previous follow-up studies.
RESULTS
Analysis of Nonparticipants
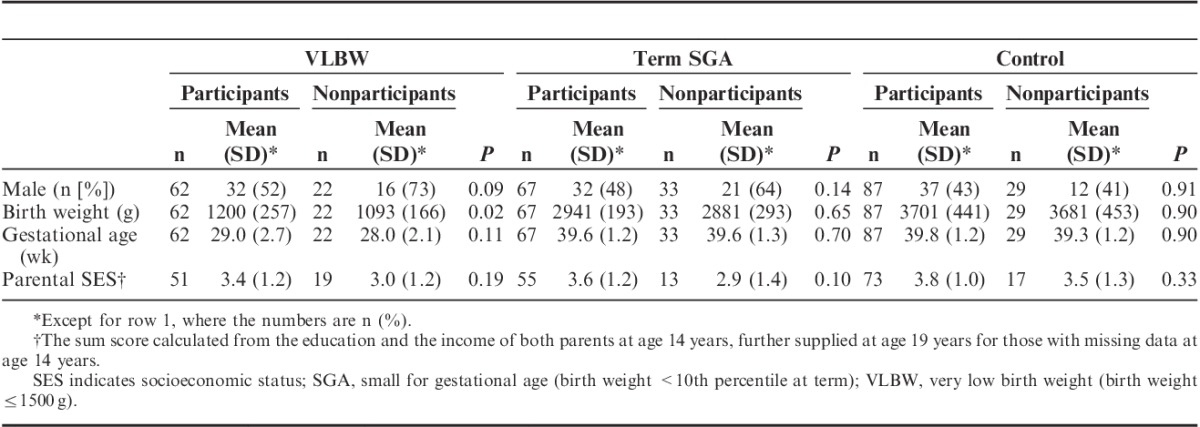
More men than women in the VLBW and term SGA groups did not participate (Table 1). In the VLBW group, nonparticipants had a slightly lower birth weight and on average a one-week shorter gestational age (Table 1). Among participants with available parental SES from the 14 or the 19 years’ follow-up, nonparticipants in the current follow-up tended to have a lower SES score.
TABLE 1.
Description of Participants and Nonparticipants
Characteristics of the Study Participants and Parents
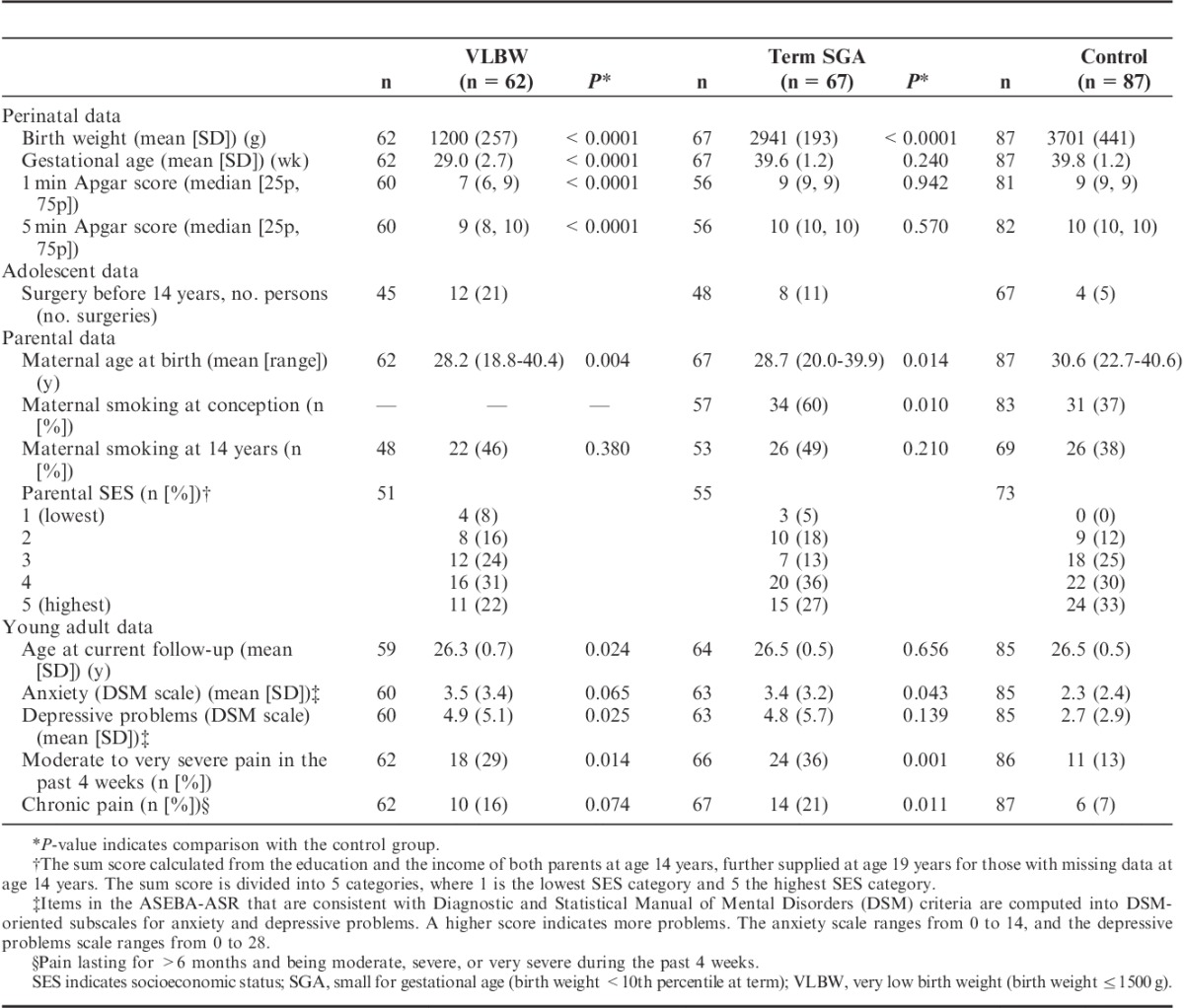
Key perinatal, parental, and demographic data of the 3 groups are summarized in Table 2. The VLBW and the term SGA groups had a lower mean maternal age and more participants in the lower parental SES groups compared with the control group. The vast majority of the term SGA and the control mothers smoking at conception still smoked at 14 years (89%), and only a few (n=5) mothers who did not smoke at conception smoked at 14 years. More mothers of term SGA young adults smoked at conception than mothers of controls (Table 2). The VLBW and the term SGA participants reported more anxiety and depressive problems compared with controls, although only the association between VLBW and depressive problems, and term SGA and anxiety, reached significance. For the VLBW group, the mean NICU stay lasted 75 days (range, 23 to 386 d), and the mean number of days on ventilator was 6 days (range, 0 to 63 d). Eight (13%) of the 60 VLBW participants with available neonatal data had an intraventricular hemorrhage, and 4 of 62 had cerebral palsy. Ten of the VLBW participants were twins.
TABLE 2.
Characteristics of the Study Participants and Parents
Prevalence of Self-reported Pain, Pain Persistence, and the Longitudinal Analysis of Pain Reports
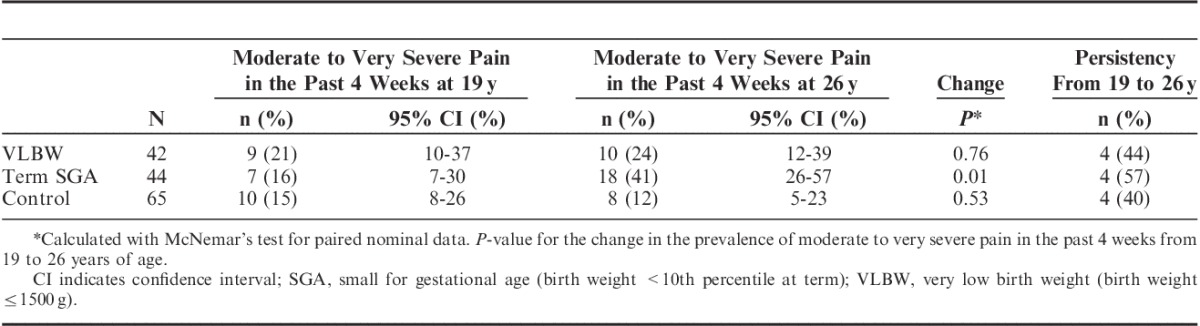
In the control group, 13% of the participants reported moderate to very severe pain during the past 4 weeks, and 7% reported chronic pain at 26 years (Table 2). Compared with controls, the prevalence of moderate to very severe pain in the past 4 weeks was higher in the VLBW and the term SGA groups. Participants in the low–birth weight groups also reported more chronic pain compared with controls. Among participants answering pain questions at both occasions, an overall high percentage of those reporting moderate to very severe pain in the past 4 weeks at 19 years still did so at 26 years. The highest pain persistency was found in the term SGA group (57%; Table 3). The VLBW group had the highest prevalence of moderate to very severe pain at 19 years, and also had a slight increase in the pain prevalence from 19 years to 26 years. The term SGA group had approximately the same pain prevalence as controls at 19 years, but had a more than 2-fold increase in the pain prevalence from 19 to 26 years. In contrast, fewer controls reported pain at 26 years than at 19 years.
TABLE 3.
Longitudinal Data on Self-reported Pain From 19 to 26 Years in the 2 Low–Birth Weight Groups and a Control Group
Association Between a Low Birth Weight and Chronic Pain
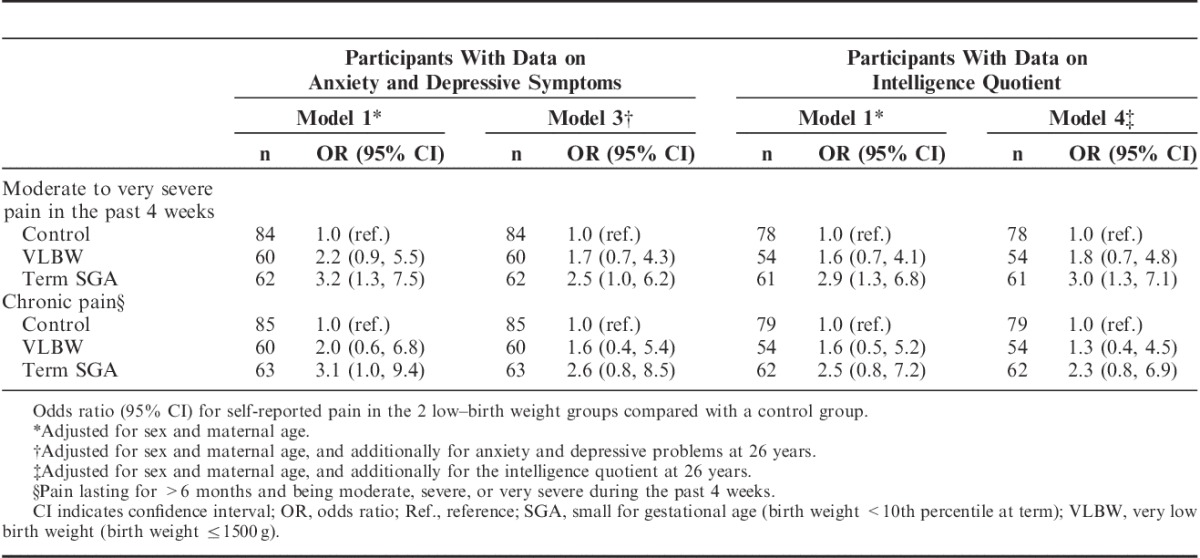
Both the VLBW and the term SGA groups had higher ORs for moderate to very severe pain in the past 4 weeks and chronic pain compared with controls (Table 4). This result was attenuated after adjusting for the potential confounding factors sex and maternal age, although the main results remained. In subanalyses on participants with available data on parental SES and maternal smoking at 14 years, no further attenuation was seen (Table 4). For the term SGA and the control groups, the results were not changed when adjusting for maternal smoking at conception instead of maternal smoking at 14 years of age (data not shown). For both of the low–birth weight groups, no attenuation of the ORs was seen when adjusting for sex only (Supplemental Digital Content, Table 1, Supplemental Digital Content 1, http://links.lww.com/CJP/A366), and testing for interaction did not indicate effect modification by sex (P-values>0.7). When adjusting for possible mediation by anxiety and depressive problems, considerable attenuation of the ORs was seen, but the higher ORs for self-reported pain persisted, especially in the term SGA group (Table 5). When adjusting for the IQ, only minor changes in the ORs were seen (Table 5). In further subanalyses adjusting for current smoking, the associations between a low birth weight and pain remained unchanged (Supplemental Digital Content, Table 2, Supplemental Digital Content 2, http://links.lww.com/CJP/A367). Furthermore, including the number of surgeries before 14 years of age as a covariate in subanalyses on participants with available data did not change the ORs substantially (Supplemental Digital Content, Table 3, Supplemental Digital Content 3, http://links.lww.com/CJP/A368).
TABLE 4.
Odds Ratio (95% CI) for Self-reported Pain in the two Low–Birth Weight Groups Compared With a Control Group
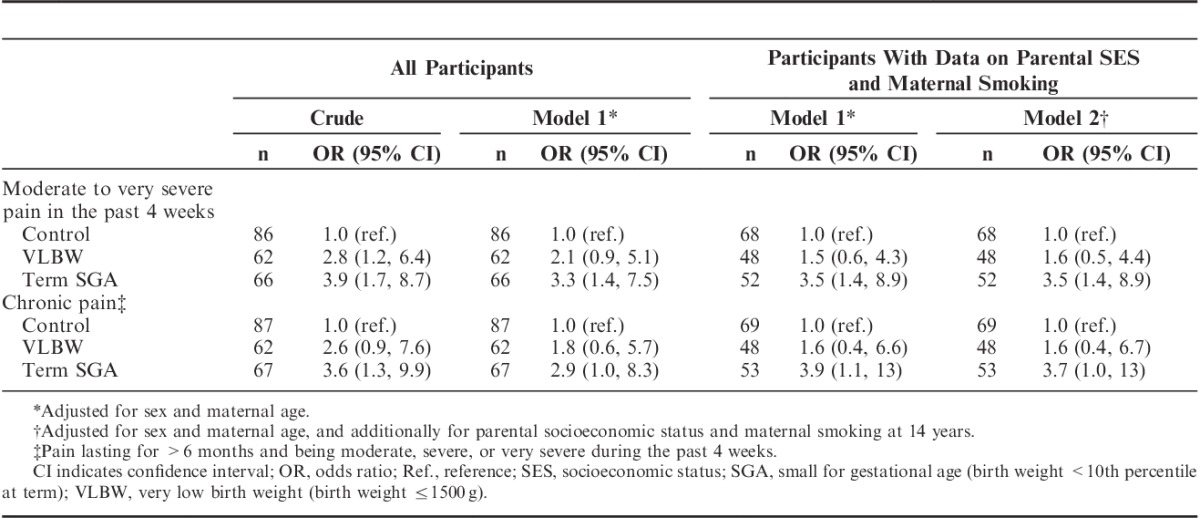
TABLE 5.
Subanalyses on Participants With Available Data on Anxiety and Depressive Problems and Intelligence Quotient
Associations Between Perinatal Factors and Chronic Pain
In the VLBW group with available birth and NICU data, days admitted to the NICU, days on ventilator, days with supplemental O2 treatment, and 1- or 5-minute Apgar scores were not associated with self-reported pain (Supplemental Digital Content, Table 4, Supplemental Digital Content 4, http://links.lww.com/CJP/A369).
DISCUSSION
The main finding in this long-term follow-up study was that young adults born preterm with VLBW or born SGA at term reported moderate to very severe pain and chronic pain more frequently compared with young adults born at term with normal birth weight. The most surprising findings are the substantially higher ORs for self-reported pain in the term SGA group compared with controls, being even higher than in the preterm VLBW group. This is particularly interesting as this group constitutes 10% of the population born at term.
The increased prevalence of chronic pain may contribute to functional limitations and a reduced quality of life in individuals with a low birth weight. Our study indicates that the clearly increased prevalence of self-reported pain in individuals with a low birth weight emerges after the transition to an independent adult life, especially in former SGA individuals. This implies that the negative effects of a low birth weight may increase in adulthood, and is an argument supporting long-term follow-up programs in low–birth weight children. Effective intervention options such as adequate physical activity and stress management programs should be sought to minimize pain and its effects.
The strengths of this study include a high response rate at the current follow-up, the long follow-up time, and the prospective and multidisciplinary design giving well-characterized cohorts. A population-based age-matched and sex-matched control group recruited in the same time period as the low–birth weight groups is a substantial strength. Very few losses to follow-up must be emphasized. Furthermore, available parental data and the possibility to adjust for a range of potentially confounding or mediating factors are advantages of the study. Data on the pain severity from 2 timepoints allows for a longitudinal analysis of self-reported pain.
The higher prevalence of self-reported pain in the VLBW group is in accordance with epidemiological studies examining the relationship between perinatal factors and chronic pain in adults.20,21 The studies indicated a slightly higher prevalence of chronic pain in adults born with a low birth weight, including VLBW.21 The associations were not significant, but these studies were limited by insufficient power with respect to more severe perinatal exposures, and thus further studies on clinical cohorts have been warranted. In contrast to the epidemiological studies on adults, we were not able to find an association between birth weight and chronic pain in a previous population-based study on Norwegian adolescents.44 Very few participants with VLBW might explain the lack of robust associations in that study. Moreover, as many as 44% of the adolescents reported chronic pain,45 and the possible impact of perinatal factors might have been attenuated. In addition to the epidemiological studies, some experimental studies have assessed pain sensitivity in children and adolescents born preterm with neonatal pain exeriences.8–10 The findings include decreased pain thresholds8 and central sensitization,10 indicating an increased susceptibility to chronic pain.5,6 However, experimental studies that also included pain questionnaires have not found indications of increased pain reports,8,9 thus opposing the results of our study. Few participants9 and limited pain questions8 together with a younger age at investigation (adolescence vs. young adulthood) may explain this discrepancy.
In our study, the odds for pain among young adults born SGA at term were clearly higher compared with controls, even after adjustments. To our knowledge, self-reported chronic pain or pain sensitivity has not been investigated previously in young adults born SGA at term. However, it is an established hypothesis that suboptimal conditions in fetal life may permanently modify the susceptibility to disease in adulthood.22 The increased risk for cardiovascular disease is well known, and long-term follow studies have also found enhanced anxiety and depressive symptoms in individuals born SGA.16,17,29 Results of our study indicate that chronic pain may be another condition that adults born SGA may be prone to. However, several confounding factors may explain some or all of the increased prevalence of self-reported pain in the SGA group. A low maternal age, a low SES, and maternal tobacco use in pregnancy affect the birth weight, and may all be risk factors for chronic pain in the offspring. It is known that tobacco exposition is strongly associated with chronic pain,15 and with our perinatal focus, we considered tobacco exposition in utero as a potentially important confounding factor. Still, when adjusting for these potential confounding factors, the main results remained, indicating an effect of a low birth weight on the susceptibility to chronic pain.
In the current study, it is noteworthy that the frequency of reporting moderate to very severe pain in the past 4 weeks differed much more between groups at 26 years than at 19 years. Whereas the prevalence of self-reported pain tended to decrease among controls from 19 to 26 years, the higher prevalence in the VLBW group persisted, and particularly the term SGA group had a clear increase in pain reports. This may indicate that a susceptibility to chronic pain after unfavorable perinatal factors, together with meeting increased demands in adult life, enhances pain reports in the low–birth weight groups. This finding may indicate that individuals born SGA are particularly challenged by the transition from adolescence to an independent adult life.
The etiology of chronic pain in the low–birth weight groups is undoubtedly complex. In the VLBW group, the length of NICU stay and days on ventilator were not associated with chronic pain. The latter is shown to be closely related to the total number of painful procedures during the NICU stay.9 Thus, this finding may suggest that the increased prevalence of self-reported pain is not due to dose-dependent changes in the pain-processing pathways after neonatal pain experience. As the ORs for self-reported pain in the low–birth weight groups were attenuated when adjusting for anxiety and depressive problems, these factors may be mediators in the relationship between a low birth weight and chronic pain. However, it is also possible that a shared sensitivity may underlie both the increased prevalence of anxiety and depressive problems and chronic pain. Thus, adjusting for these mental health factors may induce collider bias,46 and the results must be interpreted with caution. Both a low birth weight and preterm birth are associated with altered HPA activity.23–25,47 Dysfunction in the HPA axis has further been linked to stress-related adult disease, including depression and chronic pain.26–28 Altered function in stress-regulating systems may be a possible mechanism explaining why the young adults with a low birth weight reported more pain in this study. Further speculations on mechanisms underlying our findings require studies designed for this purpose.
Limitations of this study include the relatively small sample size, yielding a low statistical power as indicated by the broad confidence intervals. A general challenge in follow-up studies with many timepoints is that some participants have missing data. We did not have data on all potentially confounding factors in our study database. Particularly, the family history of chronic pain was not available, and thus, we were not able to include this important factor in the analyses. More men than women in the VLBW and the SGA groups did not participate, and the proportion of women was larger in the control group. Despite this, adjusting for sex did not change the associations observed in our study. Several analyses were conducted, and we cannot rule out that some of the observed results in this study were due to chance. Although adjusting for a range of possible confounding factors, other unmeasured confounders may still have affected our results.
Even with a population-based design and a high participation rate, loss to follow-up may have resulted in selection bias. A high and fairly similar participation rate in the three groups and few differences in demographic and perinatal variables among participants and nonparticipants makes significant selection bias less likely. Still, it is noticeable that the prevalence of chronic pain in the control group in the current study is low compared with results from the recent Norwegian population-based HUNT3 study. This study used the same definition of chronic pain, and the prevalence in young adulthood (20 to 34 y) was 15% in women and 11% in men.37 We cannot rule out a selection toward particularly good health with respect to pain reports in the control group in our study with certainty. However, the control group in our study includes only term-born, normal–birth weight individuals, in contrast to the HUNT3 study. Also, a much broader young adult age span in HUNT3 (20 to 34 y in HUNT3 vs. 25 to 28 y in this study) is a plausible explanation for the lower chronic pain prevalence in the control group in our study, as it has been shown that adolescents report more chronic pain than young adults.37,45
In summary, young adults born preterm with VLBW or born SGA at term reported a higher frequency of pain compared with young adults born at term with normal birth weight. Our study provides further evidence of a link between a low birth weight and chronic pain, although larger studies are needed to confirm our results and investigate the degree to which intrauterine factors, immaturity at birth, neonatal pain, or altered function in stress-regulating systems are involved in the mechanisms leading to increased sensitivity to chronic pain in young adulthood. Results of our study warrant increased focus on pain in long-term follow-up programs of individuals with a low birth weight.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.
ACKNOWLEDGMENTS
The authors thank the participants for their use of time and interest in this study. Likewise, the authors thank Research nurse Sigrid Fenne Helgesen, St. Olavs Hospital, Trondheim University Hospital, Trondheim Norway; for her enthusiasm in recruiting the participants. The authors also thank PhD candidate Astrid Merete Winsnes Lærum, MD, Department of Laboratory Medicine, Children's and Women's Health, Norwegian University of Science and Technology, Trondheim, Norway and Department of Pediatrics, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; for inputs on the manuscript.
Footnotes
J.M.I. has made contributions to the design of the study and the analysis and the interpretation of data. She has drafted and revised the article. M.S.I. has contributed to the design of the study and the acquisition and the interpretation of data, has discussed and revised the article critically, and finally given her approval of the version to be published. K.A.I.E. has contributed to the acquisition of data, has discussed and revised the article critically, and given her approval of the version to be published. P.R.R. has made contributions to the analysis and the interpretation of data. He has discussed the results, revised the article, and given his approval of the version to be published. M.R. has contributed to the conception and the design of the study and the interpretation of data. She has discussed and revised the article critically and given her approval of the version to be published.
Supported by the Liaison Committee between Trondheim University Hospital, Trondheim, Norway and the Norwegian University of Science and Technology, Trondheim, Norway. Funding for the PhD candidate was received from the Norwegian University of Science and Technology Trondheim, Norway. The authors declare no conflict of interest.
REFERENCES
- 1.Mantyselka PT, Turunen JH, Ahonen RS, et al. Chronic pain and poor self-rated health. J Am Med Assoc. 2003;290:2435–2442. [DOI] [PubMed] [Google Scholar]
- 2.Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–287. [DOI] [PubMed] [Google Scholar]
- 3.Linton SJ. Risk factors for neck and back pain in a working population in Sweden. Work Stress. 1990;4:41–49. [Google Scholar]
- 4.Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med. 2005;35:1275–1282. [DOI] [PubMed] [Google Scholar]
- 5.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolf CJ, Doubell TP. The pathophysiology of chronic pain—increased sensitivity to low threshold Aβ-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. [DOI] [PubMed] [Google Scholar]
- 7.Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6:971–973. [DOI] [PubMed] [Google Scholar]
- 8.Buskila D, Neumann L, Zmora E, et al. Pain sensitivity in prematurely born adolescents. Arch Pediatr Adolesc Med. 2003;157:1079–1082. [DOI] [PubMed] [Google Scholar]
- 9.Vederhus BJ, Eide GE, Natvig GK, et al. Pain tolerance and pain perception in adolescents born extremely preterm. J Pain. 2012;13:978–987. [DOI] [PubMed] [Google Scholar]
- 10.Hermann C, Hohmeister J, Demirakça S, et al. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–285. [DOI] [PubMed] [Google Scholar]
- 11.Hohmeister J, Kroll A, Wollgarten-Hadamek I, et al. Cerebral processing of pain in school-aged children with neonatal nociceptive input: an exploratory fMRI study. Pain. 2010;150:257–267. [DOI] [PubMed] [Google Scholar]
- 12.Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep. 2002;4:313–321. [DOI] [PubMed] [Google Scholar]
- 13.Vinall J, Miller SP, Bjornson BH, et al. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics. 2014;133:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranger M, Chau CM, Garg A, et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One. 2013;8:e76702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoftun GB, Romundstad PR, Rygg M. Factors associated with adolescent chronic non-specific pain, chronic multisite pain, and chronic pain with high disability: the Young-HUNT Study 2008. J Pain. 2012;13:874–883. [DOI] [PubMed] [Google Scholar]
- 16.Lund LK, Vik T, Lydersen S, et al. Mental health, quality of life and social relations in young adults born with low birth weight. Health Qual Life Outcomes. 2012;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund LK, Vik T, Skranes J, et al. Psychiatric morbidity in two low birth weight groups assessed by diagnostic interview in young adulthood. Acta Paediatr. 2011;100:598–604. [DOI] [PubMed] [Google Scholar]
- 18.Walshe M, Rifkin L, Rooney M, et al. Psychiatric disorder in young adults born very preterm: role of family history. Eur Psychiatry. 2008;23:527–531. [DOI] [PubMed] [Google Scholar]
- 19.Sømhovd MG, Hansen BM, Brok J, et al. Anxiety in adolescents born preterm or with very low birthweight: a meta-analysis of case–control studies. Dev Med Child Neurol. 2012;54:988–994. [DOI] [PubMed] [Google Scholar]
- 20.Mallen CD, Peat G, Thomas E, et al. Is chronic musculoskeletal pain in adulthood related to factors at birth? A population-based case-control study of young adults. Eur J Epidemiol. 2006;21:237–243. [DOI] [PubMed] [Google Scholar]
- 21.Littlejohn C, Pang D, Power C, et al. Is there an association between preterm birth or low birthweight and chronic widespread pain? Results from the 1958 Birth Cohort Study. Eur J Pain. 2012;16:134–139. [DOI] [PubMed] [Google Scholar]
- 22.Barker DJP. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. [DOI] [PubMed] [Google Scholar]
- 23.Schäffer L, Müller-Vizentini D, Burkhardt T, et al. Blunted stress response in small for gestational age neonates. Pediatr Res. 2009;65:231–235. [DOI] [PubMed] [Google Scholar]
- 24.Osterholm EA, Hostinar CE, Gunnar MR. Alterations in stress responses of the hypothalamic-pituitary-adrenal axis in small for gestational age infants. Psychoneuroendocrinology. 2012;37:1719–1725. [DOI] [PubMed] [Google Scholar]
- 25.Phillips DI, Walker BR, Reynolds RM, et al. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301–1306. [DOI] [PubMed] [Google Scholar]
- 26.Kajantie E. Fetal origins of stress-related adult disease. Ann N Y Acad Sci. 2006;1083:11–27. [DOI] [PubMed] [Google Scholar]
- 27.Blackburn-Munro G. Hypothalamo-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr Pain Headache Rep. 2004;8:116–124. [DOI] [PubMed] [Google Scholar]
- 28.McBeth J, Chiu YH, Silman AJ, et al. Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents. Arthritis Res Ther. 2005;7:R992–R1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berle J, Mykletun A, Daltveit A, et al. Outcomes in adulthood for children with foetal growth retardation. A linkage study from the Nord-Trøndelag Health Study (HUNT) and the Medical Birth Registry of Norway. Acta Psychiatr Scand. 2006;113:501–509. [DOI] [PubMed] [Google Scholar]
- 30.Landmark T, Romundstad P, Dale O, et al. Estimating the prevalence of chronic pain: validation of recall against longitudinal reporting (the HUNT pain study). Pain. 2012;153:1368–1373. [DOI] [PubMed] [Google Scholar]
- 31.Skranes J, Evensen KAI, Løhaugen GC, et al. Abnormal cerebral MRI findings and neuroimpairments in very low birth weight (VLBW) adolescents. Eur J Paediatr Neurol. 2008;12:273–283. [DOI] [PubMed] [Google Scholar]
- 32.Rogne T, Engstrøm AA, Jacobsen GW, et al. Fetal growth, cognitive function, and brain volumes in childhood and adolescence. Obstet Gynecol. 2015;125:673–682. [DOI] [PubMed] [Google Scholar]
- 33.Sommerfelt K, Sonnander K, Skranes J, et al. Neuropsychologic and motor function in small-for-gestation preschoolers. Pediatr Neurol. 2002;26:186–191. [DOI] [PubMed] [Google Scholar]
- 34.Evensen KAI, Vik T, Helbostad J, et al. Motor skills in adolescents with low birth weight. Arch Dis Child Fetal Neonatal Ed. 2004;89:F451–F455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Indredavik MS, Vik T, Heyerdahl S, et al. Psychiatric symptoms and disorders in adolescents with low birth weight. Arch Dis Child Fetal Neonatal Ed. 2004;89:F445–F450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992:473–483. [PubMed] [Google Scholar]
- 37.Landmark T, Romundstad PR, Borchgrevink PC, et al. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152:2241–2247. [DOI] [PubMed] [Google Scholar]
- 38.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25:3140–3151. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MK, Sjøgren P, Ekholm O, et al. Identifying a long-term/chronic, non-cancer pain population using a one-dimensional verbal pain rating scale: an epidemiological study. Eur J Pain. 2004;8:145–152. [DOI] [PubMed] [Google Scholar]
- 40.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale University; 1958. [Google Scholar]
- 41.Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont; 2003. [Google Scholar]
- 42.Achenbach TM, Bernstein A, Dumenci L. DSM-oriented scales and statistically based syndromes for ages 18 to 59: linking taxonomic paradigms to facilitate multitaxonomic approaches. J Pers Assess. 2005;84:49–63. [DOI] [PubMed] [Google Scholar]
- 43.Hernán MA, Hernández-Díaz S, Werler MM, et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. [DOI] [PubMed] [Google Scholar]
- 44.Iversen JM, Hoftun GB, Romundstad PR, et al. Adolescent chronic pain and association to perinatal factors: linkage of Birth Registry data with the Young-HUNT Study. Eur J Pain. 2015;19:567–575. [DOI] [PubMed] [Google Scholar]
- 45.Hoftun GB, Romundstad PR, Zwart J-A, et al. Chronic idiopathic pain in adolescence—high prevalence and disability: the Young HUNT Study 2008. Pain. 2011;152:2259–2266. [DOI] [PubMed] [Google Scholar]
- 46.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grunau RE, Haley DW, Whitfield MF, et al. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.


