Abstract
Peak oxygen consumption (VO2peak), as measured by cardiopulmonary exercise testing (CPET), is a powerful independent predictor of cardiovascular disease (CVD) and all-cause mortality in a broad range of populations. We assessed the safety and feasibility of CPET in aging long-term HCT survivors, a population at high risk for premature-onset of CVD. Next we examined how organ-specific impairments (e.g. cardiac, pulmonary, hematologic) impact VO2peak after HCT. Twenty consecutive HCT survivors underwent a comprehensive assessment of cardiopulmonary health that included CPET, echocardiography with strain, pulmonary function testing, six-minute walk test, and timed-up and go. Median age at assessment was 67.4 years (range 42-75 years), and median time from HCT was 9.8 years (range 3-20 years). No adverse events were observed during CPET procedures, and 95% of studies were considered to be at “peak” effort (respiratory exchange ratio ≥1.10). VO2peak was on average 22% less than predicted, and allogeneic HCT survivors had markedly lower VO2peak when compared to autologous HCT survivors (18.2 mL/kg/min vs. 22.2 mL/kg/min; p=0.05). Six (30%) participants had VO2peak ≤16 mL/kg/min, a threshold that is associated with a nine-fold risk of death in patients undergoing HCT. Despite the presence of normal (>50%) resting left ventricular (LV) ejection fraction in all participants, 25% had markedly abnormal LV longitudinal strain, an advanced echocardiographic measure of myocardial dysfunction. These findings highlight the role of stress-based measures and advanced myocardial imaging to characterize CVD risk in HCT survivors, setting the stage for tailored interventions to prevent CVD with its attendant morbidity and mortality.
Keywords: Late Effects, Hematopoietic cell transplantation, Cardiovascular disease, Cardiovascular risk factors, Survivorship, Peak oxygen consumption, Cardiopulmonary exercise testing
Introduction
It is estimated that there are currently >160,000 hematopoietic cell transplantation (HCT) survivors living in the U.S. and that number is expected to exceed 500,000 by the year 2030.1, 2 In this population, cardiovascular diseases (CVDs) such as myocardial infarction, heart failure, and stroke, are leading causes of late-occurring morbidity and mortality.3 HCT survivors have a 4-fold higher risk of developing CVD when compared to the general population;4 median age at first cardiovascular event such as myocardial infarction is 53 years (range 35-66 years),5 which is much lower than would be expected in the general population (67 years).6 The increased risk of CVD, coupled with the recognition that these complications develop earlier than would be expected in the general population, has raised the possibility of an accelerated cardiovascular aging phenotype in HCT survivors.3
The cardiovascular system has an inherent reserve capacity (cardiovascular reserve capacity), which is maintained by cross-organ systems (cardiac, pulmonary, hematologic, musculoskeletal) that adapt to physiologic and/or pathologic perturbations.7, 8 However, this reserve capacity is finite; chronic pathological perturbations to one or more of the organ systems that maintain its integrity lead to cardiovascular aging.7 For HCT survivors, depletion of reserve capacity may be initiated by pre-HCT and HCT-related therapeutic exposures, and worsened by post-HCT complications such as graft vs. host disease (GvHD), comorbidities (e.g., hypertension, diabetes), and lifestyle behaviors (deconditioning).3, 9
Peak oxygen consumption (VO2peak), as measured by cardiopulmonary exercise testing (CPET), is the gold standard measure of aerobic capacity and cardiovascular reserve.10 VO2peak is a powerful independent predictor of CVD and all-cause mortality in a broad range of populations, including breast and lung cancer patients.10-12 Two recent studies 13, 14 in HCT patients reported that low VO2peak prior to HCT was associated with increased risk of non-relapse mortality, independent of other prognostic measures such as age, resting left ventricular ejection fraction (EF), and HCT comorbidity index. There is a paucity of information regarding VO2peak after HCT, and how organ-specific perturbations impact cardiovascular reserve capacity in HCT survivors. Addressing this gap in knowledge is especially important for aging long-term HCT survivors, a population at highest risk for premature onset of CVD.3, 15 Accordingly, we evaluated the safety and feasibility of CPET in survivors who were on average 10 years from HCT. Secondary objectives were to: 1) assess the level of VO2peak impairment, and 2) to examine the impact of organ-specific (e.g. cardiac, pulmonary, hematologic) impairments on VO2peak in these survivors. We hypothesized that CPET would be feasible and safe, and that detailed assessment of specific organ systems would provide useful information regarding the etiology of VO2peak impairment in long-term HCT survivors.
Materials and Methods
Study participants
Patients were identified from an existing electronic database of HCT survivors that included information on age at diagnosis, therapeutic exposures, chronic health conditions, and vital status. Two cohorts were targeted (allogeneic, autologous; 10 participants per cohort). Eligibility criteria included: 1) ≥2y years from HCT, 2) no evidence of active hematologic malignancy, 3) underwent HCT for acute leukemia (lymphoblastic, myeloid) or lymphoma (non-Hodgkin, Hodgkin). Survivors with any of the following conditions were ineligible: acute myocardial Infarction (within 3-5 days of any planned study procedures), unstable angina, uncontrolled arrhythmia causing symptoms or hemodynamic compromise, recurrent syncope, acute myocarditis or pericarditis, symptomatic severe aortic stenosis, uncontrolled heart failure, acute (within 3 months) pulmonary embolus or pulmonary infarction, thrombosis of lower extremities, moderate or severe persistent asthma (as defined by the National Asthma Education and Prevention Program), acute non-cardiopulmonary disorders that may affect exercise performance or be aggravated by exercise (e.g. active systemic infection, renal failure, thyrotoxicosis).
Potentially eligible patients were sequentially recruited by mail or in person using a stratified approach, to ensure a balanced representation across key risk factors: age at recruitment (<65 years, ≥65 years), sex, and type of HCT (allogeneic, autologous). Final eligibility was determined by a cardiologist who performed a detailed history and physical examination and a resting electrocardiogram (ECG). Study participants underwent a comprehensive assessment of cardiopulmonary function that included: 2-dimensional (2D) echocardiography, symptom-limited CPET, and pulmonary function test (PFT). Participants also performed a six-minute walk test (6MWT), timed up and go (TUG), provided information regarding their past medical history and a blood sample to measure hemoglobin (Hgb). The City of Hope institutional review board approved this study and written informed consent was obtained from all participants prior to initiation of study procedures.
CPET
The symptom-limited CPET was conducted on an electronically-braked cycle ergometer (CareFusion Respiratory Technologies; Yorba Linda, CA) with breath-by-breath expired gas analysis (CareFusion V max Encore). All tests were conducted by two certified exercise technicians under the direct supervision of the study pulmonologist (DH); testing was conducted per American Thoracic Society (ATS) guidelines.10, 16 Participants were monitored continuously with 12-lead ECG during exercise and five minutes of recovery. During exercise, oxyhemoglobin saturation was monitored continuously using finger pulse oximetry while blood pressure was measured by an automated sphygmomanometer every two minutes.10 Three minutes of resting metabolic data was collected before participants began cycling at 20 W. Afterwards, workloads were then increased 5–20 W/min until volitional exhaustion or until a symptom limitation was achieved. VO2peak was defined as the highest VO2 value for a given 30 second interval within the last 60 seconds of exercise.17 Age-matched normative VO2peak data for healthy individuals without a history of cancer were calculated from the established sex-specific equations.18, 19
Acceptable test criteria included any of the following: (1) a peak or plateau in oxygen consumption concurrent with increased power output, (2) a respiratory exchange ratio ≥1.1, (3) volitional exhaustion, and (4) a rating of perceived exertion greater than 19.10,13 Severe adverse event (SAE) was defined as the occurrence of a ≥Grade 3 (Common Terminology of Adverse Events [Version 4]) cardiovascular event: (1) sustained ventricular tachycardia, (2) myocardial ischemia, (3) syncope, (4) provision of cardiac life support medications, (5) direct admission to emergency room/equivalent, or (6) death. Criteria for ischemic changes in ECG included 0.1 mV deviation of the ST segment horizontal to or away from the baseline isoelectric line at 0.08 seconds after the J-point in the absence of significant resting ST-T abnormalities, or left bundle branch block.10
Echocardiogram
Resting 2D-Echocardiograms with tissue Doppler strain were performed per the American Society of Echocardiography and the European Association of Cardiovascular Imaging practice guidelines.20 EF was derived from left ventricular volumes in systole (LVESV) and diastole (LVEDV), measured on M-mode recordings obtained from standard left ventricular parasternal long axis view, using the formula: (LVEDV-LVESV/LVEDV) × 100. Left ventricular global longitudinal strain (GLS) was measured manually by tracing the endocardial border in end systole; commercially available analysis software (EchoPAC vBT08; GE Healthcare, USA) was used to automatically trace regions of interest as well as the entire myocardium.
Pulmonary Function Evaluation
PFTs were performed per the ATS recommendations.21-23 Measurements included Spirometry before and after bronchodilators, lung volumes, and diffusion capacity. Total lung capacity (TLC), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), carbon monoxide diffusing capacity of the lungs (DLCO), and DLCO/volume of air (DL/VA). If the DLCO was <80% of predicted, the DLCO corrected for hemoglobin content (DLCOc) was calculated. Percent of predicted normal values were calculated using established reference values.21, 22
Functional Capacity
6MWT was performed in a measured corridor according to ATS guidelines.23 Briefly, study participants were instructed to walk at their fastest pace and to cover the longest possible distance over six minutes under the supervision of trained study staff. Timed Up and Go (TUG) was measured by how many seconds it took an individual to stand up from a standard armchair (approximate seat height: 46 cm), walk a distance of 10 ft, turn, walk back to the chair, and sit down.
Statistical considerations
Descriptive statistics were generated for all outcome measures. VO2peak was analyzed as a continuous and categorical variable (≤16 mL/kg/min, >16 mL/kg/min), based on prognostic significance in patients undergoing HCT.13 Exploratory analyses were performed to describe the association between participant demographics, chronic health conditions, HCT-related exposures and VO2peak. Additionally, the correlation (Spearman) between VO2peak and other prognostic measures (e.g., EF, GLS, 6MWT) was assessed. Data were analyzed by using SPSS Version 18.0 (IBM, Armonk, NY).
Feasibility was defined as: 1) enrollment of ≥50% of eligible patients approached for participation, 2) successful completion of all study measures (history/physical examination, CPET, echocardiography, PFT, 6MWT, TUG) in ≥75% of participants, and 3) capture of all required study CPET measurements in ≥90% of participants.
Results
Twenty three HCT survivors who had undergone HCT between 1995 and 2013 were approached, and 20 (87%) agreed to participate. Due to our a priori recruitment strategy, there was a balanced distribution by sex, type of HCT, and age at assessment (Table 1). Median age at assessment was 67.4 years (range 42-75 years). Median time from HCT was 9.8 years (range 3-20 years), and median Karnofsky Performance Score was 90 (range 70–100). The most common diagnosis was non-Hodgkin lymphoma (55%), followed by acute myeloid leukemia (25%), and acute lymphoblastic leukemia (10%). Fourteen had received myeloablative conditioning, and 6 had received reduced-intensity conditioning (RIC). Among allogeneic HCT survivors, 3 (30%) had active chronic GvHD (2 skin, 1 skin and GI) at the time of assessment. All participants successfully completed study assessments. No adverse events were observed during CPET procedures, and 95% of studies were considered to be at “peak” effort (respiratory exchange ratio [RER] ≥1.10; Table 2).
Table 1. Demographic and Medical Characteristics of Study Participants.
| Characteristics (N=20) | |
|---|---|
| Sex, No. (%) | |
| Female | 10 (50) |
| Type of HCT, No. (%) | |
| Allogeneic | 10 (50) |
| HCT Conditioning, No. (%) | |
| Myeloablative | 14 (70) |
| Underlying Diagnosis, No. (%) | |
| Non-Hodgkin Lymphoma | 11 (55) |
| Acute Myeloid Leukemia | 4 (25) |
| Acute Lymphoblastic Leukemia | 4 (20) |
| Other | 1 (5) |
| Cumulative Anthracycline Dose | |
| Median, mg/m2 (Range) | 200 (0-420) |
| Age at Assessment, No. (%) | |
| ≥65 years old | 10 (50%) |
| Time Since HCT | |
| Median, years (Range) | 9.8 (3-20) |
| Karnofsky Score at Assessment | |
| Median (Range) | 90 (70-100) |
| Cardiovascular Risk Factors at Assessment, No. (%) | |
| Hypertension | 11 (55) |
| Diabetes | 4 (20) |
| Dyslipidemia | 14 (70) |
| Overweight/Obesity (>25 kg/m2) | 7 (35) |
Table 2. Cardiopulmonary function and functional capacity in study participants.
| Variable | Median (Range) |
|---|---|
| Data at rest | |
| Heart rate (beats per minute) | 74 (44-92) |
| Systolic blood pressure (mm Hg) | 131 (119-149) |
| Diastolic blood pressure (mm Hg) | 82 (52-106) |
| LV ejection fraction (%) | 62 (54-68) |
| LV global longitudinal strain (%) | -18.6 (-13.9-[-22.2]) |
| TLC (predicted %) | 100 (67-146) |
| FEV1 (predicted %) | 101 (62-142) |
| FVC (predicted %) | 96 (60-128) |
| DLCO (predicted %) | 85 (50-96) |
| DL/VA (predicted %) | 92 (66-114) |
| Hemoglobin (G/dL) | 13.7 (10.2-15.0) |
| Exercise peak | |
| Heart rate (beats/minute) | 136 (101-179) |
| Predicted %, heart rate (beats/minute) | 88 (71-103) |
| Systolic blood pressure (mm Hg) | 172 (139-204) |
| Diastolic blood pressure (mm Hg) | 93 (63-104) |
| VO2peak (mL/kg/min) | 19.5 (13.1-31.8) |
| VO2peak <16 mL/kg/min, No. (%) | 6 (30) |
| Predicted %, VO2peak (mL/kg/min) | 78 (55-128) |
| Workload (W) | 105 (31-200) |
| Respiratory exchange ratio | 1.2 (1.0-1.4) |
| Functional Capacity | |
| 6MWT Distance (meters) | 474 (244-640) |
| Predicted 6MWT Distance (%) | 72 (38-105) |
Abbreviations: LV, left ventricular; TLC, total lung capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, carbon monoxide diffusing capacity; DL/VA, carbon monoxide diffusing capacity/volume of air; VO2peak, peak oxygen uptake; mL, milliliter; kg, kilogram; 6MWT, six-minute walk test; min, minute; mm, millimeter); Hg, mercury.
Median VO2peak was 19.4 mL/kg/min (range 13.1-26.9 mL/kg/min), corresponding to 78% of predicted (Figure 1). Six (30%) patients had VO2peak ≤16 mL/kg/min. VO2peak was lower in allogeneic patients compared to autologous (18.2 mL/kg/min vs. 22.2 mL/kg/min; p=0.05), and was lowest (16.8 mL/kg/min) in those with active GvHD. Myeloablative conditioning was associated with lower VO2peak compared to RIC (17.2 mL/kg/min vs. 24.4 mL/kg/min; p<0.01). There was no difference in VO2peak by age, sex, time since HCT (≥10 years vs. <10 years), or on the basis of CVD risk factors (data not shown).
Figure 1.
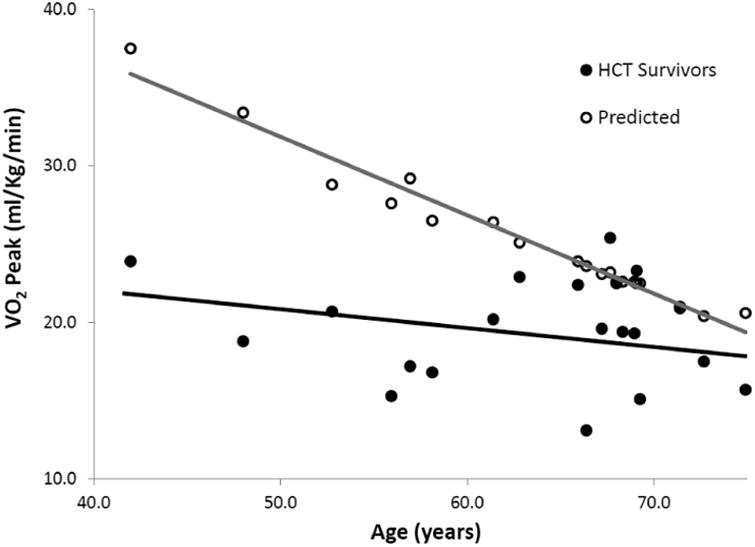
VO2Peak in HCT survivors compared to age- and sex-predicted values
Median distance covered on the 6MWT was 469 meters (range: 243-640 meters), corresponding to 72% of predicted (Table 2); there was a modest correlation between VO2peak and distance covered on the 6MWT (r=0.52, p=0.02). There was no correlation between VO2peak and relevant resting measures of pulmonary (FEV1, TLC, DLCOcorr, DL/VA), hematologic (Hgb), or cardiac (EF) health (Table 3). On the other hand, there was good correlation between VO2peak and echocardiography-derived GLS (r=-0.67, p<0.01). Importantly, five (25%) participants had markedly abnormal (>-16%) GLS despite having a normal (>50%) resting EF.
Table 3.
Spearman correlation between VO2peak (mL/kg/min) and organ-specific and functional measures of health.
| Variable | Correlation coefficient (R) | P-Value |
|---|---|---|
| LV ejection fraction (%) | 0.10 | 0.71 |
| LV global longitudinal strain (%) | -0.67 | <0.01 |
| TLC (predicted %) | 0.33 | 0.16 |
| FEV1 (predicted %) | 0.04 | 0.86 |
| FVC (predicted %) | 0.14 | 0.53 |
| DLCO (predicted %) | 0.32 | 0.17 |
| DL/VA (predicted %) | 0.15 | 0.52 |
| Hemoglobin (G/dL) | 0.28 | 0.23 |
| Predicted 6MWT Distance (%) | 0.52 | 0.02 |
| Timed Up and Go (seconds) | -0.12 | 0.63 |
Abbreviations: VO2peak, peak oxygen uptake; mL, milliliter; kg, kilogram; LV, left ventricular; TLC, total lung capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, carbon monoxide diffusing capacity; DL/VA, carbon monoxide diffusing capacity/volume of air; 6MWT, six-minute walk test; min, minute; mm, millimeter).
Discussion
The principal finding from the current study was that CPET was safe and feasible in an older (median age: 67 years) population of HCT survivors who were on average 10 years from HCT, and had no evidence of clinically evident cardiopulmonary disease. All participants successfully completed the study assessments including CPET, 2D-echocardiogram, PFT, 6MWT, and TUG; 95% of participants had a peak RER ≥1.1 an indication of excellent participant effort during CPET. There were no serious adverse events during CPET, which is important, since CPET required exercise to symptom limitation (volitional exhaustion).
In the current study, exercise capacity (VO2peak) was on average 22% less than predicted for age and sex, and allogeneic HCT survivors had markedly lower VO2peak when compared to autologous HCT survivors. Six (30%) participants had VO2peak ≤16 mL/kg/min, a threshold that is associated with a ninefold risk of death in patients undergoing HCT.13 A recent study24 of 22 patients with multiple myeloma found that median VO2peak was 17.5 mL/kg/min at 17 months post-HCT, corresponding to 38% less than that for age- and sex-predicted normative values. Given these findings, there is a suggestion that a VO2peak may improve over time; in the current study, median VO2peak was 19.5 mL/kg/min (22% predicted) at 10 years post-HCT. On the other hand, we acknowledge that this modest “improvement” may also reflect survival bias, since patients with the greatest VO2peak may not have lived long enough to participate in the current study. Additional studies are needed to describe longitudinal trends in VO2peak over time, to identify subgroups at highest risk of decline as well as those who may have spontaneous improvement in VO2peak, and to describe the prognostic utility VO2peak beyond the immediate post-HCT setting. Also unknown, but highly germane to optimizing outcomes in this group of patients, is whether there could be a subset of patients in whom exercise capacity improves with an exercise training intervention, and whether this translates into a clinically relevant improvement in health related quality of life (HRQOL).
All study participants had a normal (>50%) resting EF, and none had signs or symptoms of cardiopulmonary disease at rest. Importantly, we found no correlation between VO2peak and traditional resting measures of cardiac systolic function (EF) or pulmonary function (e.g. FEV1, DLCOcorr). It is increasingly recognized that a substantial amount of injury needs to occur to the heart and lungs before change in these resting parameters can be detected.3, 7 For example, once a drop in EF (e.g. <50%) is detected in cancer survivors, functional deterioration often proceeds rapidly.25, 26 In non-cancer populations, GLS has emerged as a reproducible measure of early left ventricular dysfunction;27 reduction in GLS is an independent predictor of major cardiac events such as heart failure and cardiac death, independent of EF.27 To date, there has been very little known about the role of echocardiography-derived strain to evaluate left ventricular function in HCT survivors. In the current study, there was a good correlation between GLS and VO2peak, and 25% of participants had markedly abnormal GLS, suggesting that subclinical left ventricular dysfunction may have partly contributed to the decline in VO2peak seen in our study participants. Reduced left ventricular longitudinal strain could contribute to impaired exercise cardiac output, and thus lower VO2peak. Future studies should evaluate the myocardial response to exercise stress in order to elucidate mechanisms contributing to lower VO2peak and establish potential targeted therapies.
Even though functional capacity, as measured by 6MWT, was markedly impaired (72% of predicted) in our study participants, there was only a modest correlation between 6MWT and VO2peak. These findings emphasize the importance of both tests, as the 6MWT and CPET may provide distinct, but complementary information. The 6MWT was designed to assess physical functioning in relatively sedentary and deconditioned populations, while the CPET was designed for more fit populations.10, 14 Considering the heterogeneity in treatment exposures and severity of chronic health conditions in the HCT survivor population, it is important that both measures be integrated into the comprehensive assessment of functional capacity and physiologic reserves in this population.
There are important limitations that need to be considered when interpreting the findings of our study. The small sample size precluded detailed assessment of treatment-related and demographic modifiers of cardiovascular reserve capacity. In addition, the cross-sectional nature of our study limited our ability to describe longitudinal change in VO2peak and its impact on HRQOL over time. Given the long follow-up between HCT and CPET (median time from HCT ∼10 years), it is possible that those with especially low VO2peak after HCT may have died prematurely, and thus unable to participate in the current study. Therefore, any interpretations regarding VO2peak have to be tempered by our limitations of sample size and study design. Future studies will need to describe the natural history and trajectory of change in VO2peak following HCT, setting the stage for interventions to improve VO2peak. To date, there have been no studies to examine whether aerobic training can improve cardiovascular reserve capacity in HCT survivors. This is largely due to the paucity of information on the trajectory of change in VO2peak in these survivors, or subgroups that may be at highest risk for VO2peak impairment. This gap in knowledge is compounded by the recognition that in the general population, effective aerobic training interventions are ones that are able to address specific organ-system impairments, as needed.10, 28 Larger studies are therefore needed to not only characterize the extent of VO2peak impairment in a broader population (malignant, non-malignant diseases) of HCT survivors, but to also understand the organ-specific impairments that may contribute to its decline after HCT. Finally, it is important to note that CPET carries an additional cost as well as competency requirements, and may not be readily available in all clinical settings where HCT survivors are routinely seen. Additional studies are needed to evaluate the prognostic utility and the cost-effectiveness CPET in this population of survivors.
In conclusion, we found that a comprehensive cardiopulmonary evaluation including CPET was safe and feasible in aging long-term HCT survivors. The preliminary findings from this study suggest that a detailed assessment of key organ systems (e.g. cardiac, pulmonary) may provide useful information regarding the etiology of VO2peak impairment in HCT survivors. Information from this and future studies has the potential to facilitate the development of tailored interventions aimed at reversal or arrest of decline in VO2peak,10, 28 and therefore, prevention of overt CVD with its attendant burden of morbidity and mortality.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572 (Rosen). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: NIH/NCI R01 CA196854 (S Armenian), Leukemia and Lymphoma Society Scholar Award in Clinical Research (S Armenian)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altekruse SF, Kosary CL, Krapcho M. SEER cancer statistics review, 1975--2007. http://seer.cancer.gov/csr/1975_2007.2010.
- 2.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armenian SH, Chow EJ. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer. 2014;120:469–479. doi: 10.1002/cncr.28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow EJ, Wong K, Lee SJ, et al. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:794–800. doi: 10.1016/j.bbmt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 7.Koelwyn GJ, Khouri M, Mackey JR, Douglas PS, Jones LW. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012;30:4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brilla CG, Maisch B. Regulation of the structural remodelling of the myocardium: from hypertrophy to heart failure. European heart journal. 1994;15(D):45–52. doi: 10.1093/eurheartj/15.suppl_d.45. [DOI] [PubMed] [Google Scholar]
- 9.Rovo A, Tichelli A. Cardiovascular complications in long-term survivors after allogeneic hematopoietic stem cell transplantation. Seminars in hematology. 2012;49:25–34. doi: 10.1053/j.seminhematol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 11.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood WA, Deal AM, Reeve BB, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone marrow transplantation. 2013;48:1342–1349. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 14.Kelsey CR, Scott JM, Lane A, et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone marrow transplantation. 2014;49:1330–1336. doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol (1985) 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 19.Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. American journal of physiology. 2000;278:H829–834. doi: 10.1152/ajpheart.2000.278.3.H829. [DOI] [PubMed] [Google Scholar]
- 20.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viegi G, Pedreschi M, Pistelli F, et al. Prevalence of airways obstruction in a general population: European Respiratory Society vs American Thoracic Society definition. Chest. 2000;117:339S–345S. doi: 10.1378/chest.117.5_suppl_2.339s. [DOI] [PubMed] [Google Scholar]
- 23.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 24.Tuchman SA, Lane A, Hornsby WE, et al. Quantitative measures of physical functioning after autologous hematopoietic stem cell transplantation in multiple myeloma: a feasibility study. Clinical lymphoma, myeloma & leukemia. 2015;15:103–109. doi: 10.1016/j.clml.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. Journal of the American College of Cardiology. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 26.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–4522. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 27.Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. The international journal of cardiovascular imaging. 2009;25(1):9–22. doi: 10.1007/s10554-008-9414-1. [DOI] [PubMed] [Google Scholar]
- 28.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. The lancet oncology. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]


