Abstract
Herein we disclose a novel method for the facile transfer of primary (–NH2) and secondary amino groups (–NHR) to heteroaryl- as well as arylcuprates at low temperature without the need for precious metal catalysts, ligands, excess reagents, protecting and/or directing groups. This one-pot transformation allows unprecedented functional group tolerance and it is well-suited for the amination of electron-rich, electron-deficient as well as structurally complex (hetero)arylmetals. In some of the cases, only catalytic amounts of a copper(I) salt is required.
Anilines are substructures in a large number of industrially and commercially important organic compounds such as dyes, pesticides, active pharmaceutical ingredients and functional materials.1 Therefore, the development of efficient methods for the synthesis of structurally diverse anilines has been a key research area for the past 20 years.2 Currently, the most general synthesis of anilines is achieved via the palladium-catalyzed cross-coupling of aryl halides with amines (i.e., Buchwald–Hartwig coupling).3 Because of their impressive scope, palladium-catalyzed C–N bond-forming reactions have become an indispensable tool for medicinal as well as synthetic organic chemists. However, high reaction temperatures and time-consuming ligand/catalyst optimizations are often required, sometimes hindering their effective utilization. Recently, a number of direct catalytic C–H amination methods have emerged that usually do not require complex optimization.4 Although these methods represent a welcome new direction that improves overall efficiency and atom-economy, lingering issues with regio- and chemoselectivity must be adequately addressed before this strategy can be routinely and reliably deployed.
It has been our desire to find a complementary and general C–N bond-forming method that can operate at or below room temperature and does not require precious metal catalysis. Such a method obviates the need for ligand screening and thus it would operationally simplify the reaction. A promising approach is electrophilic amination in which an aryl nucleophile is directly reacted with an electrophilic nitrogen source to generate the desired aniline.5 The greatest advantage of this approach is its simplicity, both mechanistically and operationally as it significantly reduces the burden of reaction optimization. However, most existing methods can only install fully substituted or protected amino groups (e.g., –NR2), whereas frequently primary and secondary amines (–NH2, –NHR) are preferred for further functional group elaboration.
Over 1 decade ago, Johnson et al. reported the efficient N,N-dialkyl-amination of organozinc nucleophiles (R2Zn) with Cu(I) catalysis6 (Figure 1A); however, the lack of suitable electrophilic NH2-sources prevented the utilization of this method for the synthesis of primary anilines. The Uchiyama group described the primary amination of arylcuprates, generated from the directed cupration of arenes, although the requirement for neighboring-group participation severely limits the substrate scope (Figure 1B).7
Figure 1.
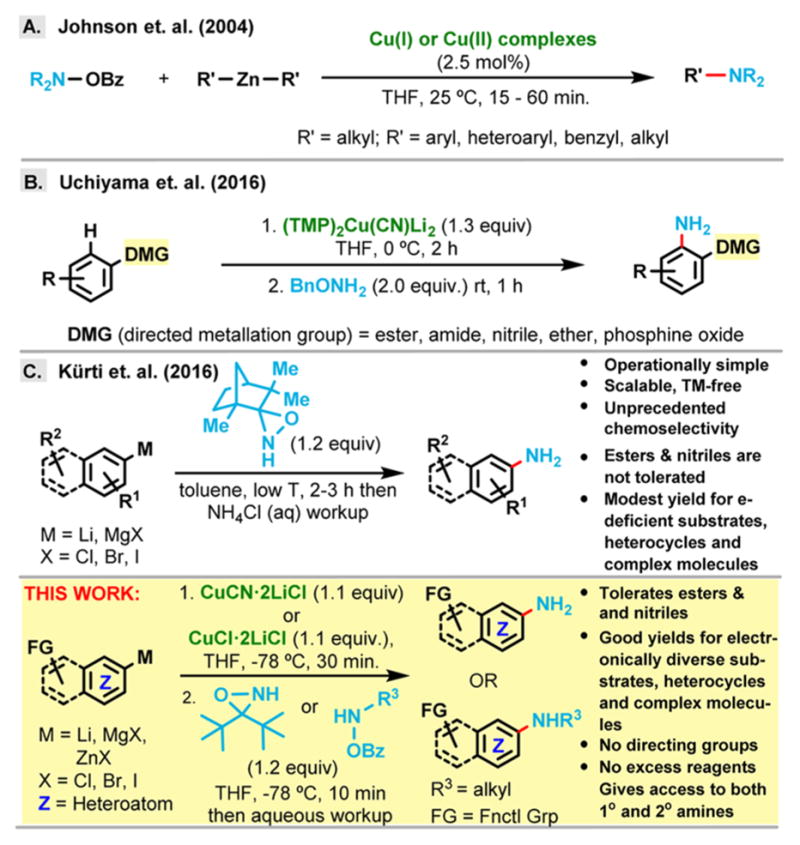
Representative methods for the precious metal-free electrophilic amination of arylmetals.
Recently, we disclosed a novel one-step method (Figure 1C) for the direct primary amination of arylmagnesium halides utilizing a sterically hindered NH-oxaziridine derived from fenchone.8 This efficient method operates at low temperature and does not require metal catalysts, ligands, additives or excess aminating reagents. Although the isolated yields are good to excellent for a large variety of sterically and electronically diverse substrates, it is not without some limitations: (a) electron-deficient substrates generally give lower yields compared to electron-rich substrates; (b) electrophilic functional groups, such as esters and nitriles, cannot be tolerated due to their incompatibility with aryl-magnesium and aryl lithium reagents; (c) many heterocyclic substrates give either small amounts or none of the desired primary amination products due to their unique electronic properties, which results in diminished reactivity; (d) for natural product-like substrates, it is sometimes difficult to transform them efficiently into the corresponding aryl Grignard reagents: this aspect reduces the practicality of this method in the late-stage functionalization of complex molecules.
An in-depth analysis of the reaction mechanism reveals a common cause for these limitations: the success of our recently disclosed method (Figure 1C) depends on a delicate balance between the nucleophilicity and the basicity of the arylmetal reagent. If the arylmetal reagent removes the proton from the NH-oxaziridine (i.e., quenching) instead of directly attacking the nitrogen atom, it will result in unproductive consumption of the arylmetal.
To solve this deprotonation problem and further expand the scope of this method, we explored arylmetals with lower basicity (i.e., containing metals other than Mg and Li) while still possessing sufficient nucleophilicity to react with NH oxaziridines. We initially evaluated monoaryl organozinc reagents (ArZnX). These versatile compounds are less basic than the corresponding aryl-lithium and aryl-magnesium compounds, and are known to tolerate a wider range of functional groups. Recent developments in the direct zincation of arenes by Knochel et al. also provides a practical method for their preparation.9
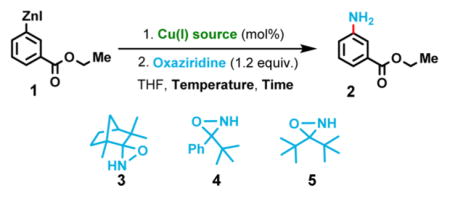
When arylzinc halide 1 was reacted with three different oxaziridines (3–5),8 formation of the desired aniline 2 was not observed (Table 1 entry 1). These results indicated that the balance of arylmetal basicity and nucleophilicity does not fall in the optimal range required for the amination by an NH oxaziridine.10 The aggregation of organozinc molecules in solution likely renders them insufficiently nucleophilic for a successful direct amination, in contrast to the observed behavior of aryl Grignard reagents.8
Table 1.
Optimization of Conditions for the Direct Primary Amination of Arylmetals
| ||||
|---|---|---|---|---|
| Entry | Cu Source | Oxaziridine | Temp, Time | Yield (%) |
| 1 | Not used | 3/4/5 | rt, 16 h | 0% |
| 2 | CuCl (20 mol %) | 3 | rt, 16 h | 11% |
| 3 | CuCl (20 mol %) | 4 | rt, 16 h | 16% |
| 4 | CuCl (100 mol %) | 4 | rt, 16 h | 28% |
| 5 | CuCl (20 mol %) | 4 | 40 °C,16 h | 18% |
| 6 | CuCl (20 mol %) | 5 | rt, 16 h | 34% |
| 7 | CuCN (20 mol %) | 5 | rt, 16 h | 42% |
| 8 | [Cu(OTf)]2 C6H6 (10 mol %) | 5 | rt, 16 h | 13% |
| 9 | Cu(OAc)2 (20 mol %) | 5 | rt, 16 h | 43% |
| 10 | CuCN (110 mol %) | 5 | rt, 16 h | 68% |
| 11 | CuCN-2LiCl (110 mol %) | 5 | rt, 16 h | 66% |
| 12 | CuCN-2LiCI (110 mol %) | 5 | −78 °C, 10 min | 87% |
| 13 | CuCl-2LiCl (110 mol %) | 5 | −78 °C, 10 min | 90% |
Next we examined an organocuprate, which can be easily prepared from the corresponding organolithium, organo-magnesium or organozinc reagents via transmetalation with Cu(I) sources such as CuCN and CuCl. Initially, we looked at using catalytic amounts of Cu(I) salts. To our delight, the anticipated primary amination product (2) was isolated, albeit in low yield (Table 1 entries 2 and 3). Stoichiometric amount of copper afforded much better yield (Table 1, entries 4 vs 3), indicating that the catalytic cycle could not be established for some substrates. This result was not unexpected given the aforementioned issue with deprotonation. Using the less hindered NH-oxaziridine 5 gave better yield than bulkier NH-oxaziridines 3 and 4 under catalytic conditions (entries 6, 7 and 9 vs 3) as well as under stoichiometric conditions (entry 10 vs 4). Many Cu(I) salts efficiently mediate the reaction, although CuCN·2LiCl and CuCl·2LiCl gave the best results and were easy to handle under inert atmosphere using their commercially available THF solutions (Table 1, entries 12 and 13).
Although copper catalyzed/mediated aminations of arylmetals have been reported in the literature,11 the vast majority can only produce tertiary amines due to the lack of suitable electrophilic “NH2” equivalents. As mentioned earlier, the Uchiyama group recently reported a method in which an arylcuprate could be efficiently aminated using O-benzyl-hydroxylamine (BnONH2, Figure 1B).7 Although this innovative approach provides a precious metal-free access to primary anilines with good efficiency and decent scope, it is not without some serious limitations. One of the major issues of this method is the requirement for neighboring group participation, which limits the substrates to those with directing groups (DMG) at the ortho-position of the metal-bearing carbon. This requirement is due to the unique transition state that necessitates coordination to these directing groups to facilitate the formation of the C–N bond.7 Another drawback is the requirement for the use of (TMP)2Cu, which is needed for both the initial deprotonation of the arene and the monodeprotonation of BnONH2. Because direct cupration with (TMP)2Cu also involves some restrictions on the substrates, it is not trivial to expand the scope beyond what has been reported. The aminating reagent BnONH2, of which two equivalents are needed, also requires excessive drying before use because it is very hygroscopic, which further limits the utility of this method especially in large-scale processes.
Using an NH-oxaziridine as the aminating reagent will alleviate many of these limitations. Because the acidity of the N–H bond in oxaziridines (pKa = ~34)12 is much lower than in BnONH2 (pKa = ~20), we anticipated that the unproductive protonation of the cuprate was not going to be a significant problem in our method, therefore neither a base nor excess aminating agent were needed. NH-Oxaziridine 5 also efficiently aminates the arylcuprate in the absence of a directing group, which greatly expands the scope of substrates. Lastly, the cuprates can be readily prepared via transmetalation using a wide range of starting materials such as organolithiums, organozincs or organomagnesiums, which circumvents the limitation that originates from Uchiyama’s directed cupration.
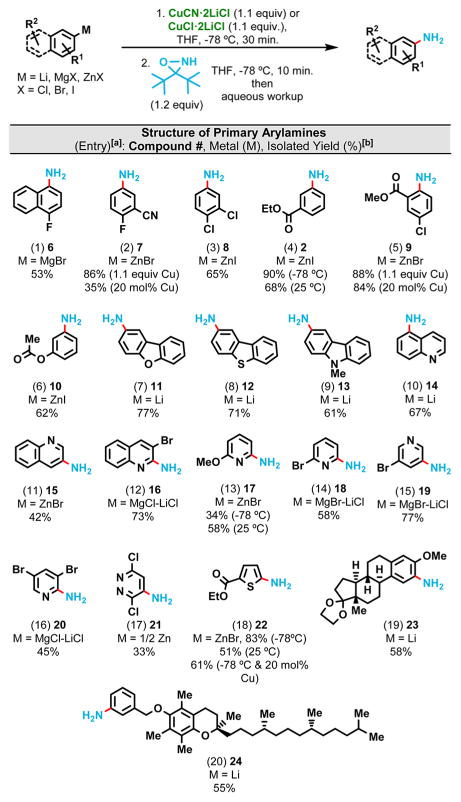
When applied to a wide range of substrates that did not perform well in the direct primary amination of aryl Grignard reagents,8 this novel Cu-mediated method provides not only better yields in all cases but also furnishes many new anilines that were previously inaccessible directly from the corresponding Grignard reagents (Table 2). Electron-deficient arenes (Table 2 entries 1–3) and heterocycles (Table 2 entries 7–20) performed well and afforded isolated yields up to 90%. These conditions are compatible with esters (Table 2 entries 4–6, 18), nitriles (Table 2 entry 2), and give decent yields for complex molecules (Table 2 entries 19 and 20). The reaction is very rapid and in most cases it takes less than 10 min at −78 °C to reach completion. Synthetically useful yields (50–60%) could also be achieved at ambient temperature (Table 2 entries 4, 13 and 18). Both CuCN·2LiCl and CuCl·2LiCl gave similar yields, and control experiments showed that LiCl was not essential for the success of this reaction.
Table 2.
Primary Amination of Structurally Diverse Aryl- and Heteroarylmetals
Reaction conditions: arylmetal (1 mmol) and Cu(l)-reagent (1.1 mmol unless indicated otherwise) were mixed at −78 °C for 30 min in THF followed by the THF solution of the oxaziridine reagent (1.2 mmol) at −78 °C for 10 min unless indicated otherwise.
Isolated yield after column chromatography. Reactions were quenched at the indicated reaction temperature with a ~1:1 mixture of saturated NH4Cl(aq) and saturated Na2S2O3(aq).
When less basic organozincs are used as the arylmetal substrates, this reaction can be rendered catalytic (Table 2, entries 2, 5 and 18). However, the success of the catalytic system largely depends on the electronic properties of the specific organozinc substrate. An ester group at the ortho position greatly improves the yield (Table 2 entry 5): this result is consistent with the findings reported by Uchiyama.
Intrigued by the promising properties of these arylcuprates, we next tried the amination with N-alkyl-O-benzoyl hydroxylamines (BzONHR) expecting to obtain secondary anilines (ArNHR). Johnson et al. reported a few examples where a diarylzinc substrate (R2Zn) could be converted to RNHR’ using BzONHR’ in the presence of catalytic amounts of CuOTf.13 In these cases, one of the R groups in R2Zn presumably first deprotonates the hydroxylamine and facilitates the nitrogen-transfer to the remaining R group, thus allowing catalytic amounts of a Cu(I) salt to drive the amination while sacrificing one of the R groups as a base.
We anticipated that upon complete transmetalation of the arylzinc substrate, the resulting cuprate would undergo exclusive amination instead of protonation, therefore this approach would improve overall atom economy by avoiding the unproductive quenching of the R group. This hypothesis was correct, and arylcuprates (ArCuX) were indeed successfully aminated with good to excellent yields when reacted with 1.1 equiv of BzONHR’ at ambient temperature (Table 3). When the reaction was carried out with TsONHR’, the yield was lower, which is consistent with our hypothesis that the acidity of NH has a major impact on the reaction outcome.
Table 3.
Secondary Amination of Selected Aryl- and Heteroarylmetals
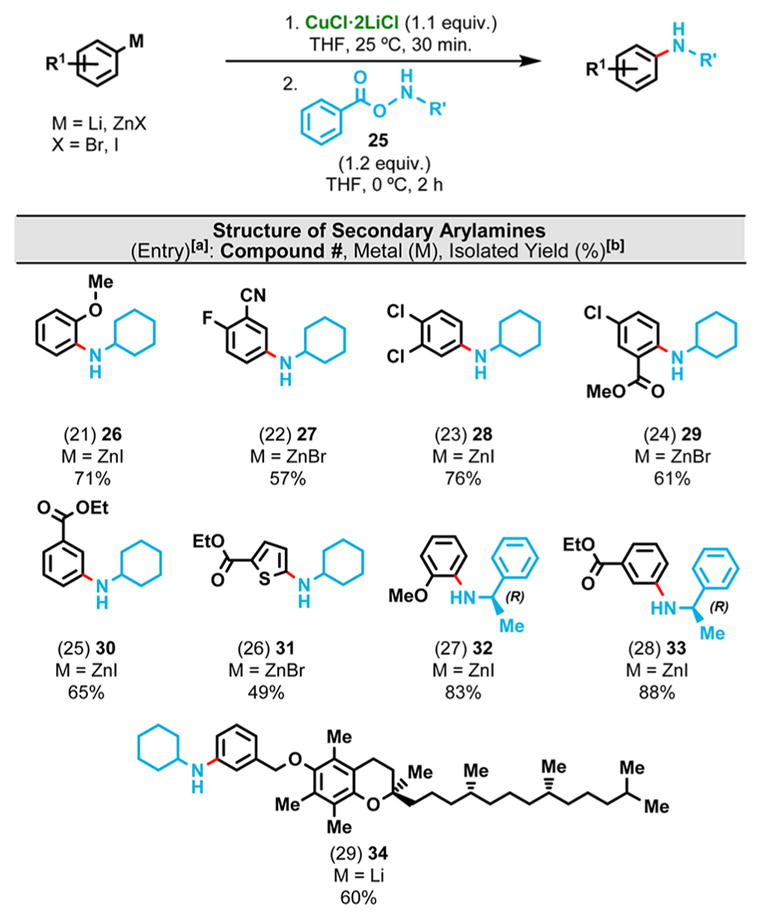
|
Reaction conditions: arylmetal (1 mmol) and Cu(l)-reagent (1.1 mmol) were mixed at 25 °C for 30 min in THF, followed by the THF solution of the hydroxylamine reagent (1.2 mmol) at 0 °C for 2 h.
Isolated yield after column chromatography. Reactions were quenched at the indicated reaction temperature with a ~1:1 mixture of saturated NH4Cl(aq) and saturated Na2S2O3(aq).
Mechanistically, it is possible that the amination of arylcuprates follows the same pathway as the direct amination of Grignard reagents with NH-oxaziridines in which the amination takes place by direct nucleophilic attack on the nitrogen atom (Figure 2A).8 However, if the reaction involved a simple nucleophilic attack, one would also expect the amination to proceed when an organozinc reagent was reacted directly with oxaziridine 5 in the absence of copper complexes. Because the much less nucleophilic reagent (cuprate) yielded the desired aniline, the failed direct amination of organozinc reagents points to a different mechanistic pathway that likely involves the unique ability of copper to undergo oxidation state change [i.e., Cu(I) → Cu(III)]. We propose that the amination starts with the initial oxidative addition of Cu(I) into the N–O bond of oxaziridine 5 to afford intermediate 38, followed by reductive elimination to generate intermediate 35. Finally, facile hydrolysis of 36 gives rise to the product primary arylamine 37. The mechanism that leads to the formation of secondary arylamines involves oxidative addition of the arylcuprate into the N–O bond of aminating agent 25 and the resulting intermediate 39 directly furnishes the secondary arylamine product (40) upon reductive elimination (Figure 2C). These above-mentioned two pathways (Figure 2B,C) are similar to the mechanism proposed by Uchiyama et al.7
Figure 2.
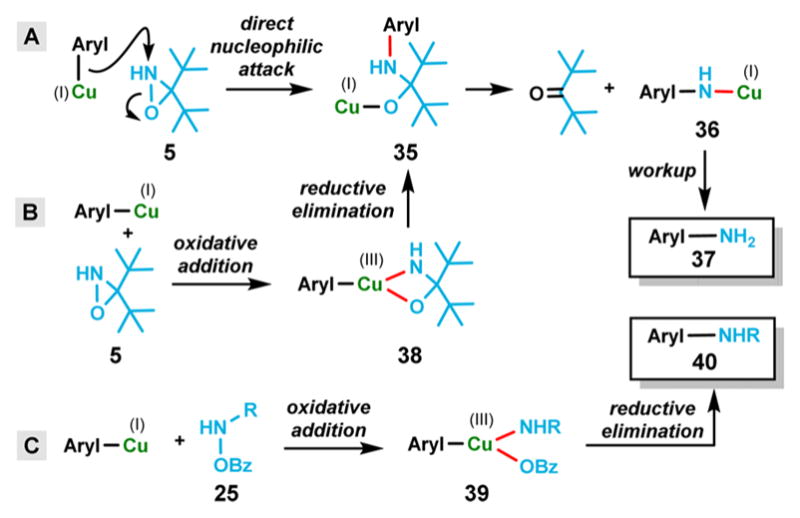
Proposed mechanistic pathways.
We postulate that a successful catalytic cycle demands the coexistence of the initial nontransmetalated arylmetal and the NH-oxaziridine. It is not surprising that only relatively less basic arylmetals (e.g., Table 1, entries 5 and 18) are amenable to catalytic primary amination. Presumably, in all of the other cases, the arylmetals undergo rapid and nonproductive quenching by the NH-oxaziridine reagent before the arylcuprates can form via transmetalation.
In summary, we have developed a novel method for the direct electrophilic primary and secondary amination of arylmetals in the presence of Cu(I) salts. This transformation offers a practical solution for a long-standing synthetic problem of aminating heteroarylmetals directly and efficiently. In addition, the method does not rely on the presence of directing groups and requires only equimolar amounts of the aminating reagent. We anticipate that this general and operationally simple amination approach will find wide utility in the synthesis of structurally complex molecules such as active pharmaceutical ingredients and natural products.
Supplementary Material
Acknowledgments
L.K. gratefully acknowledges the generous financial support of Rice University, National Institutes of Health (R01 GM-114609-01), National Science Foundation (CAREER:SusChEM CHE-1455335), the Robert A. Welch Foundation (Grant C-1764), ACS-PRF (Grant 51707-DNI1), Amgen (2014 Young Investigators’ Award for LK) and Biotage (2015 Young Principal Investigator Award). We thank Professor Daniel H. Ess (BYU) for helpful discussions.
Footnotes
ORCID
Zhe Zhou: 0000-0003-1194-4026
László Kürti: 0000-0002-3412-5894
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b12712.
Complete experimental procedures and characterization data including 1H and 13C NMR spectra (PDF)
References
- 1.Rappoport Z, editor. The Chemistry of Anilines, Parts 1–2. John Wiley & Sons; Chichester: 2007. [Google Scholar]
- 2.Scholz U, Schlummer B. Sci Synth. 2007;31b:1565. [Google Scholar]
- 3.For reviews on the Buchwald-Hartwig amination, see: Hartwig JF. Synlett. 1997;1997:329.Hartwig JF. Acc Chem Res. 1998;31:852.Hartwig JF. Angew Chem, Int Ed. 1998;37:2046. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L.Wolfe JP, Wagaw S, Marcoux JF, Buchwald SL. Acc Chem Res. 1998;31:805.Surry DS, Buchwald SL. Angew Chem, Int Ed. 2008;47:6338. doi: 10.1002/anie.200800497.Surry DS, Buchwald SL. Chem Sci. 2011;2:27. doi: 10.1039/C0SC00331J.
- 4.(a) Romero NA, Margrey KA, Tay NE, Nicewicz DA. Science. 2015;349:1326. doi: 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]; (b) Jiao J, Murakami K, Itami K. ACS Catal. 2016;6:610. [Google Scholar]; (c) Paudyal MP, Adebesin AM, Burt SR, Ess DH, Ma Z, Kürti L, Falck JR. Science. 2016;353:1144. doi: 10.1126/science.aaf8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For reviews on electrophilic amination, see: Daskapan T. ARKIVOC. 2011:230.Jarvo E, Barker T. Synthesis. 2011;2011:3954.Starkov P, Jamison TF, Marek I. Chem - Eur J. 2015;21:5278. doi: 10.1002/chem.201405779.Dong X, Liu Q, Dong Y, Liu H. Chem Eur J. 2016:22. doi: 10.1002/chem.201601607.
- 6.Berman AM, Johnson JS. J Am Chem Soc. 2004;126:5680. doi: 10.1021/ja049474e. [DOI] [PubMed] [Google Scholar]
- 7.Tezuka N, Shimojo K, Hirano K, Komagawa S, Yoshida K, Wang C, Miyamoto K, Saito T, Takita R, Uchiyama M. J Am Chem Soc. 2016;138:9166. doi: 10.1021/jacs.6b03855. [DOI] [PubMed] [Google Scholar]
- 8.Gao H, Zhou Z, Kwon D-H, Coombs J, Jones S, Behnke NE, Ess DH, Kürti L. Nat Chem. 2016 doi: 10.1038/nchem.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Wunderlich SH, Knochel P. Angew Chem, Int Ed. 2007;46:7685. doi: 10.1002/anie.200701984. [DOI] [PubMed] [Google Scholar]; (b) Wunderlich S, Knochel P. Chem Commun. 2008:6387. doi: 10.1039/b815903c. [DOI] [PubMed] [Google Scholar]; (c) Mosrin M, Bresser T, Knochel P. Org Lett. 2009;11:3406. doi: 10.1021/ol901275n. [DOI] [PubMed] [Google Scholar]
- 10.For reviews on the use of oxaziridines as N-transfer reagents, see: Andreae S, Schmitz E. Synthesis. 1991;1991:327.Davis FA, Chen B-C, Zhou P. In: In Compr Heterocycl Chem. Katritzky AR III, editor. 1A. Elsevier; Oxford: 2008. Ch. 559.Williamson KS, Michaelis DJ, Yoon TP. Chem Rev. 2014;114:8016. doi: 10.1021/cr400611n.
- 11.(a) Barton DHR, Ozbalik N, Ramesh M. Tetrahedron Lett. 1988;29:857. [Google Scholar]; (b) Erdik E, Daşkapan T. Synth Commun. 1999;29:3989. [Google Scholar]; (c) del Amo V, Dubbaka SR, Krasovskiy A, Knochel P. Angew Chem, Int Ed. 2006;45:7838. doi: 10.1002/anie.200603089. [DOI] [PubMed] [Google Scholar]; (d) He C, Chen C, Cheng J, Liu C, Liu W, Li Q, Lei A. Angew Chem, Int Ed. 2008;47:6414. doi: 10.1002/anie.200801427. [DOI] [PubMed] [Google Scholar]; (e) Zhang Z, Yu Y, Liebeskind LS. Org Lett. 2008;10:3005. doi: 10.1021/ol8009682. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ciganek E. Org React. 2009;72:1. [Google Scholar]; (g) Zhou S, Yang Z, Chen X, Li Y, Zhang L, Fang H, Wang W, Zhu X, Wang S. J Org Chem. 2015;80:6323. doi: 10.1021/acs.joc.5b00767. [DOI] [PubMed] [Google Scholar]
- 12.Please see the Supporting Information for detailed DFT calculations in Reference 8.
- 13.Berman AM, Johnson JS. J Org Chem. 2006;71:219. doi: 10.1021/jo051999h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.